The Effect of Increasing the Body’s Core Temperature and Improving Blood Flow by Using Far-Infrared Rays Emitted from Functional Loess Bio-Balls
Abstract
:1. Introduction
2. Materials and Methods
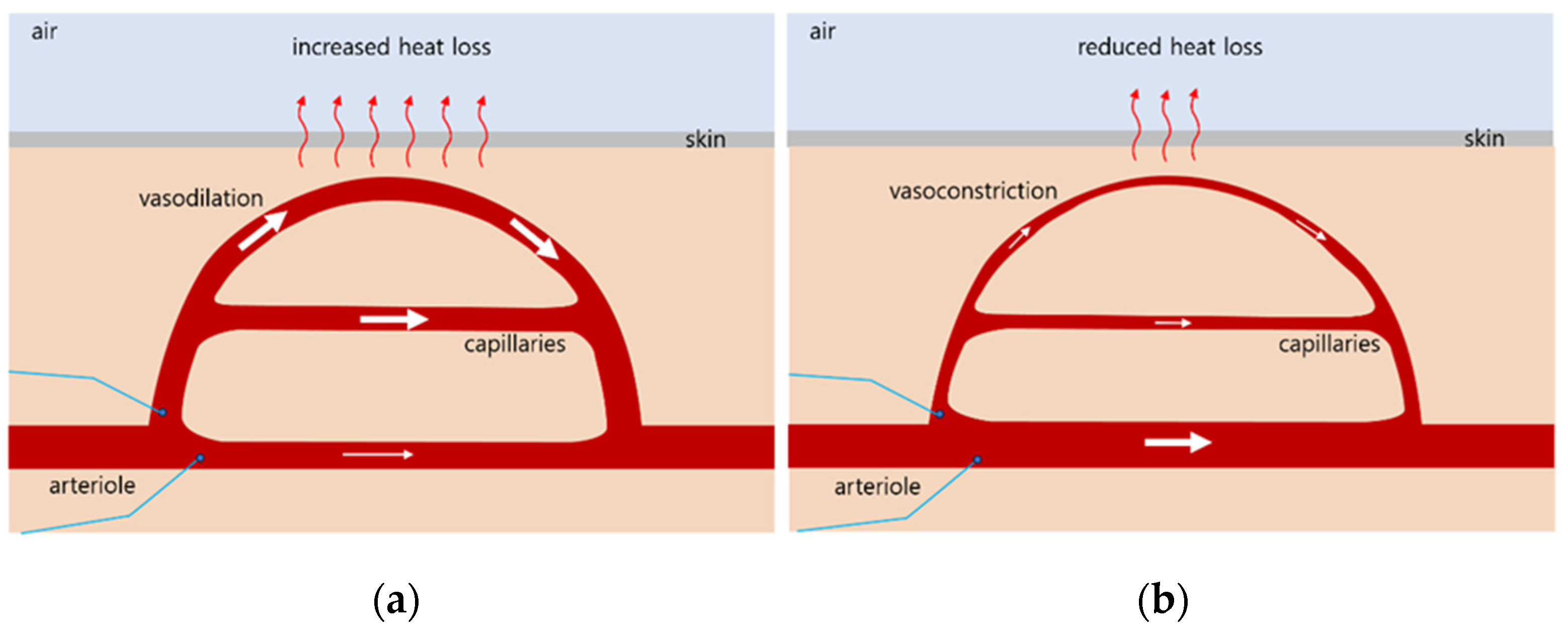
2.1. Mechanisms of Thermoregulation in the Body
2.2. Study Participants
2.3. Trial Design and Setting
2.4. Interventions
2.5. Experimental Measurements
2.5.1. Temperature Changes in the Cushion Placed on the Electric Mat and Loess Bio-Ball Mat
2.5.2. Blood Flow
2.5.3. Epidermal Temperature
2.6. Statistical Analyses
3. Results
3.1. Temperature Change on the Cushion When Heat Was Applied to the Electric Mat and Loess Bio-Ball Mat
3.2. Changes in Blood Flow and Epidermal Temperature Measured in the LMF and RMF When Using the Electric Mat and the Loess Bio-Ball Mat
3.3. Blood Flow and Epidermal Temperature According to the Set Temperature in the LMF When Using the Electric Mat and the Loess Bio-Ball Mat
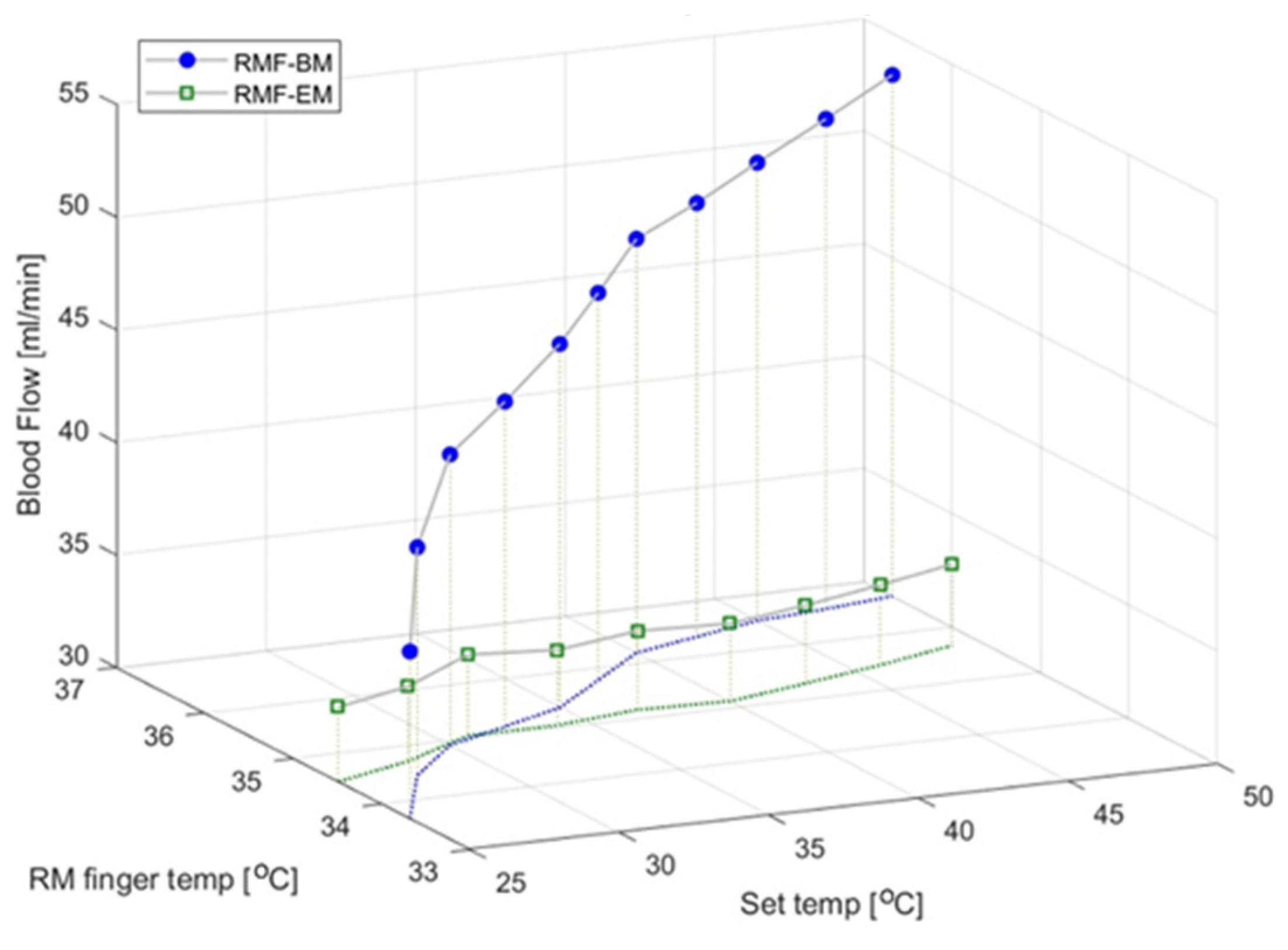
3.4. Blood Flow and Epidermal Temperature According to the Set Temperature in the RMF When Using the Electric Mat and the Loess Bio-Ball Mat
3.5. Scatter Plot Showing the Slope and Offset for Blood Flow in the LMF and RMF for 30 Control and 30 Experimental Subjects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, K.; Zhang, Z.; Liu, N.F.; Feng, S.Q.; Zhang, J.F. Efficacy and safety of far infrared radiation in lymphedema treatment: Clinical evaluation and laboratory analysis. Lasers Med. Sci. 2017, 32, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Vatansever, F.; Hamblin, M.R. Far infrared radiation (FIR): Its biological effects and medical applications. Photonic Lasers Med. 2012, 4, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Leung, T.K.; Lin, S.L.; Yang, T.S.; Lin, Y.S. The influence of ceramic far-infrared ray (cFIR) irradiation on water hydrogen bonding and its related chemo-physical properties. Hydrol. Curr. Res. 2014, 5, 1000174. [Google Scholar] [CrossRef]
- Tsai, S.R.; Hamblin, M.R. Biological effects and medical applications of infrared radiation. J. Photochem. Photobiol. B 2017, 170, 197–207. [Google Scholar] [CrossRef]
- Yen, C.C.; Hsu, P.C.; Lin, C.C.; Chen, S.C.; Hsiao, C.Y.; Hwang, S.J. Effect of far-infrared radiation therapy on von Willebrand factor in patients with chronic kidney disease. Front. Med. 2023, 10, 1268212. [Google Scholar] [CrossRef]
- Carrick, F.R.; Valerio, L.S.A.; Gonzalez-Vega, M.N.; Engel, D.; Sugaya, K. Accelerated wound healing using a novel far-infrared ceramic blanket. Life 2021, 11, 878. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Lung, C.W.; Kuo, F.C. Evaluating the far-infrared radiation bioeffects on micro vascular dysfunction, nervous system, and plantar pressure in diabetes mellitus. Int. J. Low. Extrem. Wounds 2019, 19, 153473461988074. [Google Scholar] [CrossRef]
- Lin, L.C.; Lin, Y.Y. The Biological effectiveness and medical significance of far infrared radiation (FIR). Malays. J. Med. Biol. Res. 2021, 8, 41–46. [Google Scholar] [CrossRef]
- Hsieh, C.C.; Nosaka, K.; Chou, T.Y.; Hsu, S.T.; Chen, T.C. Effects of far-infrared radiation-lamp therapy on recovery from simulated soccer match running activities in elite soccer players. Int. J. Sports Physiol. Perform. 2022, 17, 1432–1438. [Google Scholar] [CrossRef]
- Yu, S.Y.; Chiu, J.H.; Yang, S.D.; Hsu, Y.C.; Lui, W.Y.; Wu, C.W. Biological effect of far-infrared therapy on increasing skin microcirculation in rats. Photodermatol. Photoimmunol. Photomed. 2006, 22, 78–86. [Google Scholar] [CrossRef]
- Athonvarangkul, D.; Wang, K.; Deng, Y.; Inzucchi, S.E.; Mayerson, A. Improved extremity tissue oxygenation with short-term exposure to textiles embedded with far infrared light emitting thermoactive particles in patients with diabetes mellitus. Diab Vasc. Dis. Res. 2023, 20, 14791641231170282. [Google Scholar] [CrossRef] [PubMed]
- Madden, C.J.; Morrison, S.F. Central nervous system circuits that control body temperature. Neurosci. Lett. 2019, 696, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Kuht, J.; Farmery, A.D. Body temperature and its regulation. Anaesth. Intensive Care Med. 2021, 22, 657–662. [Google Scholar] [CrossRef]
- Cramer, M.N.; Gagnon, D.; Laitano, O.; Crandall, C.G. Human temperature regulation under heat stress in health, disease, and injury. Physiol. Rev. 2022, 102, 1907–1989. [Google Scholar] [CrossRef]
- Chen, W. Thermometry and interpretation of body temperature. Biomed. Eng. Lett. 2019, 9, 3–17. [Google Scholar] [CrossRef]
- Lim, C.L. Fundamental Concepts of Human Thermoregulation and Adaptation to Heat: A Review in the Context of Global Warming. Int. J. Environ. Res. Public. Health 2020, 17, 7795. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomized trials. Ann. Intern. Med. 2010, 152, 726–732. [Google Scholar] [CrossRef]
- Choi, W.S.; Toyama, S.; Choi, Y.J.; Woo, B.S. Preclinical efficacy examination on healing practices and experiences of users for pillows and mattresses of loess ball bio-products. Procedia Eng. 2015, 102, 399–409. [Google Scholar] [CrossRef]
- Choi, W.S.; Toyama, S.; Choi, Y.J.; Woo, B.S. Preclinical efficacy examination on healing practices and experiences of users for S4 bio-balls bed of loess bio-balls products. Acad. J. Sci. Res. 2020, 8, 104–113. [Google Scholar]
- Choi, Y.J.; Choi, W.C.; Jeon, G.R.; Kim, J.H.; Kim, M.S.; Kim, J.H. Characteristics of far-infrared ray emitted from functional loess bio-balls and its effect on improving blood flow. Bioengineering 2024, 11, 380. [Google Scholar] [CrossRef]
- Campbell, I. Body temperature and its regulation. Anaesth. Intensive Care Med. 2008, 9, 259–263. [Google Scholar] [CrossRef]
- Hsu, Y.H.; Chen, Y.C.; Chen, T.H.; Sue, Y.M.; Cheng, T.H.; Chen, J.R.; Chen, C.H. Far-infrared therapy induces the nuclear translocation of PLZF which inhibits VEGF-induced proliferation in human umbilical vein endothelial cells. PLoS ONE 2012, 7, e30674. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Xia, L.; Liu, N.F.; Nicoli, F.; Constantinides, J.; D’Ambrosia, C.; Lazzeri, D.; Tremp, M.; Zhang, J.F.; Zhang, Y.X. Far infrared ray (FIR) therapy: An effective and oncological safe treatment modality for breast cancer related lymphedema. J. Photochem. Photobiol. B 2017, 172, 95–101. [Google Scholar] [CrossRef]
- Wang, Y.H.; Cheng, F.Y.; Chao, Y.F.; Liu, C.Y.; Chang, Y. Effects of far-infrared therapy on foot circulation among hemodialysis patients with diabetes mellitus. Biol. Res. Nurs. 2020, 22, 403–411. [Google Scholar] [CrossRef]
- Cristiano, L. Use of infrared-based devices in aesthetic medicine and for beauty and wellness treatments. Infrared Phys. Technol. 2019, 102, 10299. [Google Scholar] [CrossRef]
- Xia, L.; Cui, C.; Nicoli, F. Far infrared radiation therapy for gynecological cancer-related lymphedema is an effective and oncologically safe treatment: A randomized-controlled trial. Lymphat. Res. Biol. 2022, 20, 164–174. [Google Scholar] [CrossRef]
- Jeong, I.S.; Lee, E.J.; Kim, J.H.; Kim, G.H.; Hwang, Y.J. Detection of intravenous infiltration using impedance parameters in patients in a long-term care hospital. PLoS ONE 2019, 14, e0213585. [Google Scholar] [CrossRef]
- Guillermo, M.; Eitner, F.; Floege, J.; Leonhardt, S. 2010 A novel bioimpedance technique to monitor fluid volume state during hemodialysis treatment. ASAIO J. 2010, 56, 215–220. [Google Scholar] [CrossRef]
- Anand, G.; Yu, Y.; Lowe, A.; Kalra, A. Bioimpedance analysis as a tool for hemodynamic monitoring: Overview, methods and challenges. Physiol. Meas. 2021, 42, 03TR01. [Google Scholar] [CrossRef]
- Brix, B.; Apich, G.; Roessler, A.; Ure, C.; Schmid-Zalaudek, K.; Hinghofer-Szalkay, H.; Goswami, N. Fluid shifts induced by physical therapy in lower limb lymphedema patients. J. Clin. Med. 2020, 9, 3678. [Google Scholar] [CrossRef]
- Shin, Y.I.; Kim, M.S.; Yang, Y.A.; Jeon, G.R.; Kim, J.H.; Choi, Y.J.; Choi, W.C.; Kim, J.H. Effects of far-infrared rays emitted from loess bio-balls on lymphatic circulation and reduction of inflammatory fluids. Biomedicines 2024, 12, 2392. [Google Scholar] [CrossRef]
- Lin, Y.W.; Tsai, C.S.; Huang, C.Y.; Tsai, Y.T.; Shih, C.M.; Lin, S.J.; Li, C.Y.; Lin, C.Y.; Sung, Y.S.; Lin, F.Y. Far-infrared therapy decreases orthotopic allograft transplantation vasculopathy. Biomedicines 2022, 10, 1089. [Google Scholar] [CrossRef]





| Variables | Experimental Group (n = 30) | Control Group (n = 30) | |
|---|---|---|---|
| Gender | Female | 14 (46.67%) | 16 (53.33%) |
| Male | 16 (53.33%) | 14 (46.67%) | |
| Age | 60.17 (±10.35) | 57.44 (±8.53) | |
| BMI [kg/m2] | 21.40 (±3.01) | 24.77 (±3.26) | |
| Blood flow | Discomfort or disorder | Discomfort or disorder | |
| Temperature [°C] | 25 | 28 | 31 | 34 | 37 | 40 | 43 | 46 | 49 | |
|---|---|---|---|---|---|---|---|---|---|---|
| LMF | BF [mL/min] | 38.27 ± 4.58 | 38.34 ± 4.46 | 38.53 ± 4.48 | 38.57 ± 4.85 | 38.57 ± 4.65 | 38.62 ± 4.97 | 38.62 ± 4.87 | 38.59 ± 4.87 | 38.62 ± 4.90 |
| Temperature. [℃] | 34.07 ± 1.00 | 34.12 ± 1.02 | 34.28 ± 1.02 | 34.37 ± 1.02 | 34.47 ± 1.07 | 34.54 ± 1.12 | 34.54 ± 1.10 | 34.53 ± 1.16 | 34.53 ± 1.07 | |
| RMF | BF [mL/min] | 38.25 ± 4.56 | 38.22 ± 4.60 | 38.57 ± 4.28 | 38.59 ± 4.47 | 38.62 ± 4.66 | 38.67 ± 4.49 | 38.63 ± 4.42 | 38.70 ± 4.29 | 38.64 ± 4.40 |
| Temperature. [℃] | 34.17 ± 0.92 | 34.39 ± 0.91 | 34.47 ± 0.92 | 34.47 ± 0.93 | 34.49 ± 0.92 | 34.48 ± 0.91 | 34.52 ± 0.94 | 34.58 ± 0.94 | 34.63 ± 0.96 | |
| Temperature [°C] | 25 | 27.5 | 30 | 32.5 | 35 | 37.5 | 40 | 42.5 | 45 | 47.5 | 50 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LMF | BF [mL/min] | 38.14 ±7.30 | 41.08 ±6.77 | 43.11 ±6.64 | 45.03 ±6.79 | 46.49 ±7.01 | 48.07 ±7.27 | 48.96 ±7.22 | 50.01 ±7.33 | 50.78 ±7.38 | 51.82 ±7.43 | 53.32 ±7.51 |
| Temperature. [℃] | 33.72 ±1.02 | 34.10 ±0.97 | 34.43 ±0.89 | 34.69 ±0.86 | 35.00 ±0.76 | 35.34 ±0.67 | 35.73 ±0.51 | 36.00 ±0.43 | 36.27 ±0.34 | 36.50 ±0.25 | 36.68 ±0.25 | |
| RMF | BF [mL/min] | 38.30 ±5.45 | 42.12 ±6.09 | 44.68 ±7.00 | 46.51 ±7.31 | 48.26 ±7.87 | 49.72 ±7.65 | 50.70 ±7.54 | 51.48 ±7.54 | 52.40 ±7.15 | 53.52 ±7.25 | 54.32 ±7.27 |
| Temperature. [℃] | 33.87 ±1.40 | 34.61 ±1.24 | 35.01 ±1.13 | 35.33 ±1.02 | 35.63 ±0.93 | 35.92 ±0.59 | 36.20 ±0.43 | 36.37 ±0.31 | 36.53 ±0.25 | 36.63 ±0.24 | 36.73 ±0.21 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, Y.-I.; Kim, M.-S.; Yang, Y.-A.; Jeon, G.-R.; Kim, J.-H.; Choi, Y.-J.; Choi, W.-C.; Kim, J.-H. The Effect of Increasing the Body’s Core Temperature and Improving Blood Flow by Using Far-Infrared Rays Emitted from Functional Loess Bio-Balls. Biomedicines 2024, 12, 2922. https://doi.org/10.3390/biomedicines12122922
Shin Y-I, Kim M-S, Yang Y-A, Jeon G-R, Kim J-H, Choi Y-J, Choi W-C, Kim J-H. The Effect of Increasing the Body’s Core Temperature and Improving Blood Flow by Using Far-Infrared Rays Emitted from Functional Loess Bio-Balls. Biomedicines. 2024; 12(12):2922. https://doi.org/10.3390/biomedicines12122922
Chicago/Turabian StyleShin, Yong-Il, Min-Seok Kim, Yeong-Ae Yang, Gye-Rok Jeon, Jae-Ho Kim, Yeon-Jin Choi, Woo-Cheol Choi, and Jae-Hyung Kim. 2024. "The Effect of Increasing the Body’s Core Temperature and Improving Blood Flow by Using Far-Infrared Rays Emitted from Functional Loess Bio-Balls" Biomedicines 12, no. 12: 2922. https://doi.org/10.3390/biomedicines12122922
APA StyleShin, Y.-I., Kim, M.-S., Yang, Y.-A., Jeon, G.-R., Kim, J.-H., Choi, Y.-J., Choi, W.-C., & Kim, J.-H. (2024). The Effect of Increasing the Body’s Core Temperature and Improving Blood Flow by Using Far-Infrared Rays Emitted from Functional Loess Bio-Balls. Biomedicines, 12(12), 2922. https://doi.org/10.3390/biomedicines12122922






