Bile Acids in Inflammatory Bowel Disease: From Pathophysiology to Treatment
Abstract
1. Introduction
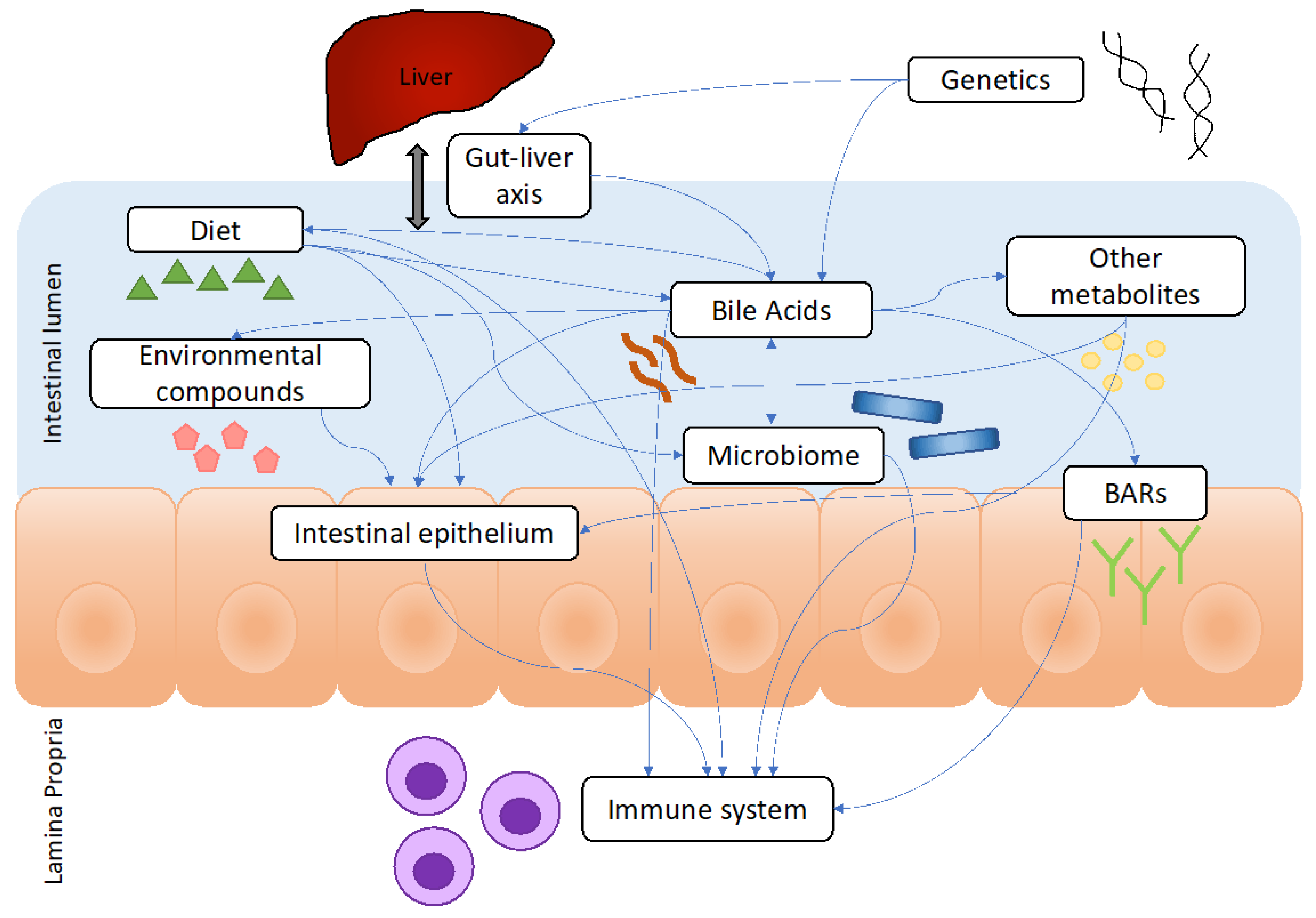
2. Overview of BAs and Their Emerging Roles in IBD
2.1. Synthesis of BAs
2.2. BA Alterations in Patients with IBD
2.3. Roles of BAs in IBD Pathogenesis
2.4. BAs as Biomarkers of IBD
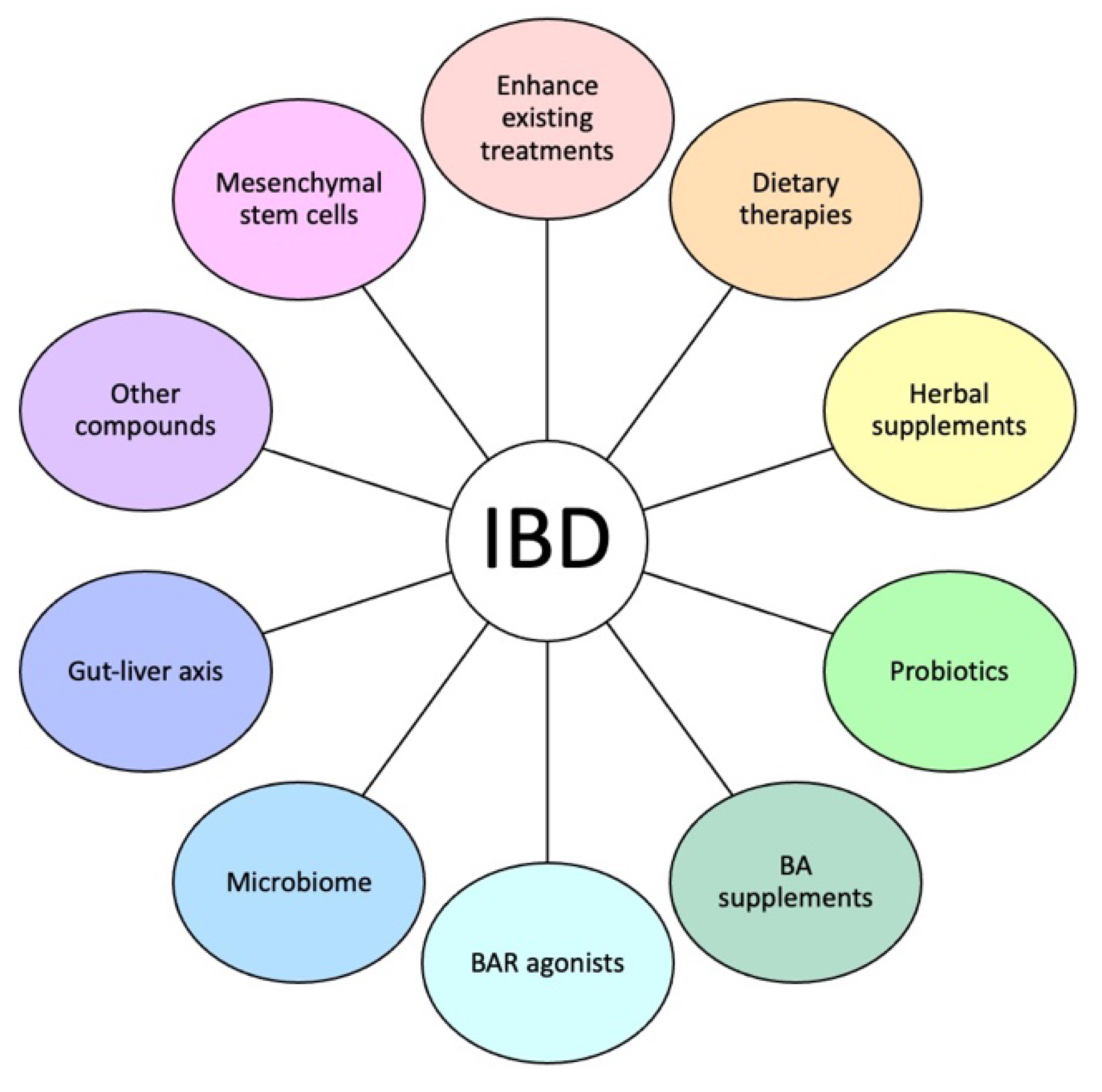
2.5. BA-Based IBD Therapies
2.5.1. Enhancement of Existing IBD Treatments
2.5.2. Diets That Modify BAs
2.5.3. Herbal/Natural Supplements
2.5.4. Probiotics
2.5.5. BA Supplements
2.5.6. BAR Agonists
2.5.7. Microbiome and the Gut–Liver Axis
2.5.8. Other BA-Related Therapeutic Targets
2.5.9. Mesenchymal Stem Cells
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thomas, J.P.; Modos, D.; Rushbrook, S.M.; Powell, N.; Korcsmaros, T. The Emerging Role of Bile Acids in the Pathogenesis of Inflammatory Bowel Disease. Front. Immunol. 2022, 13, 829525. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Kriaa, A.; Mariaule, V.; Jablaoui, A.; Rhimi, S.; Mkaouar, H.; Hernandez, J.; Korkmaz, B.; Lesner, A.; Maguin, E.; Aghdassi, A.; et al. Bile Acids: Key Players in Inflammatory Bowel Diseases? Cells 2022, 11, 901. [Google Scholar] [CrossRef] [PubMed]
- Syed, S.; Boland, B.S.; Bourke, L.T.; Chen, L.A.; Churchill, L.; Dobes, A.; Greene, A.; Heller, C.; Jayson, C.; Kostiuk, B.; et al. Challenges in IBD Research 2024: Precision Medicine. Inflamm. Bowel Dis. 2024, 30 (Suppl. 2), S39–S54. [Google Scholar] [CrossRef]
- Long, X.Q.; Liu, M.Z.; Liu, Z.H.; Xia, L.Z.; Lu, S.P.; Xu, X.P.; Wu, M.H. Bile acids and their receptors: Potential therapeutic targets in inflammatory bowel disease. World J. Gastroenterol. 2023, 29, 4252–4270. [Google Scholar] [CrossRef]
- Di Ciaula, A.; Bonfrate, L.; Khalil, M.; Portincasa, P. The interaction of bile acids and gut inflammation influences the pathogenesis of inflammatory bowel disease. Intern. Emerg. Med. 2023, 18, 2181–2197. [Google Scholar] [CrossRef]
- Pratt, M.; Forbes, J.D.; Knox, N.C.; Bernstein, C.N.; Van Domselaar, G. Microbiome-Mediated Immune Signaling in Inflammatory Bowel Disease and Colorectal Cancer: Support from Meta-omics Data. Front. Cell Dev. Biol. 2021, 9, 716604. [Google Scholar] [CrossRef]
- Camilleri, M. Bile acid detergency: Permeability, inflammation, and effects of sulfation. Am. J. Physiol. Gastrointest. Liver Physiol. 2022, 322, G480–G488. [Google Scholar] [CrossRef]
- Xiang, D.; Yang, J.; Liu, L.; Yu, H.; Gong, X.; Liu, D. The regulation of tissue-specific farnesoid X receptor on genes and diseases involved in bile acid homeostasis. Biomed. Pharmacother. 2023, 168, 115606. [Google Scholar] [CrossRef]
- Kwon, S.J.; Khan, M.S.; Kim, S.G. Intestinal Inflammation and Regeneration-Interdigitating Processes Controlled by Dietary Lipids in Inflammatory Bowel Disease. Int. J. Mol. Sci. 2024, 25, 1311. [Google Scholar] [CrossRef]
- Thibaut, M.M.; Bindels, L.B. Crosstalk between bile acid-activated receptors and microbiome in entero-hepatic inflammation. Trends Mol. Med. 2022, 28, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhang, H.; Li, M.; He, T.; Guo, S.; Zhu, L.; Tan, J.; Wang, B. Novel approaches in IBD therapy: Targeting the gut microbiota-bile acid axis. Gut Microbes 2024, 16, 2356284. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, T.; Gu, Y.; Wang, X.; Xie, R.; Sun, Y.; Wang, B.; Cao, H. Regulation of gut microbiota-bile acids axis by probiotics in inflammatory bowel disease. Front. Immunol. 2022, 13, 974305. [Google Scholar] [CrossRef] [PubMed]
- Fiorucci, S.; Carino, A.; Baldoni, M.; Santucci, L.; Costanzi, E.; Graziosi, L.; Distrutti, E.; Biagioli, M. Bile Acid Signaling in Inflammatory Bowel Diseases. Dig. Dis. Sci. 2021, 66, 674–693. [Google Scholar] [CrossRef]
- Poland, J.C.; Flynn, C.R. Bile Acids, Their Receptors, and the Gut Microbiota. Physiology 2021, 36, 235–245. [Google Scholar] [CrossRef]
- Zhang, J.; Lyu, A.; Wang, C. The molecular insights of bile acid homeostasis in host diseases. Life Sci. 2023, 330, 121919. [Google Scholar] [CrossRef]
- Cai, J.; Sun, L.; Gonzalez, F.J. Gut microbiota-derived bile acids in intestinal immunity, inflammation, and tumorigenesis. Cell Host Microbe 2022, 30, 289–300. [Google Scholar] [CrossRef]
- Gadaleta, R.M.; Cariello, M.; Crudele, L.; Moschetta, A. Bile Salt Hydrolase-Competent Probiotics in the Management of IBD: Unlocking the “Bile Acid Code”. Nutrients 2022, 14, 3212. [Google Scholar] [CrossRef]
- Guo, X.; Okpara, E.S.; Hu, W.; Yan, C.; Wang, Y.; Liang, Q.; Chiang, J.Y.L.; Han, S. Interactive Relationships between Intestinal Flora and Bile Acids. Int. J. Mol. Sci. 2022, 23, 8343. [Google Scholar] [CrossRef]
- Bromke, M.A.; Krzystek-Korpacka, M. Bile Acid Signaling in Inflammatory Bowel Disease. Int. J. Mol. Sci. 2021, 22, 9096. [Google Scholar] [CrossRef]
- Sun, R.; Xu, C.; Feng, B.; Gao, X.; Liu, Z. Critical roles of bile acids in regulating intestinal mucosal immune responses. Ther. Adv. Gastroenterol. 2021, 14, 17562848211018098. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Al-Hassi, H.O.; Steed, H.; Phipps, O.; Brookes, M.J. Bile Acids and the Microbiome: Making Sense of This Dynamic Relationship in Their Role and Management in Crohn’s Disease. Can. J. Gastroenterol. Hepatol. 2022, 2022, 8416578. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, C.D.; Trauner, M. Role of bile acids and their receptors in gastrointestinal and hepatic pathophysiology. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 432–450. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, L.R.; Jenabzadeh, P. IBD and Bile Acid Absorption: Focus on Pre-clinical and Clinical Observations. Front. Physiol. 2020, 11, 564. [Google Scholar] [CrossRef]
- Li, M.; Yang, L.; Mu, C.; Sun, Y.; Gu, Y.; Chen, D.; Liu, T.; Cao, H. Gut microbial metabolome in inflammatory bowel disease: From association to therapeutic perspectives. Comput. Struct. Biotechnol. J. 2022, 20, 2402–2414. [Google Scholar] [CrossRef]
- Guzior, D.V.; Quinn, R.A. Review: Microbial transformations of human bile acids. Microbiome 2021, 9, 140. [Google Scholar] [CrossRef]
- Fu, Y.; Lyu, J.; Wang, S. The role of intestinal microbes on intestinal barrier function and host immunity from a metabolite perspective. Front. Immunol. 2023, 14, 1277102. [Google Scholar] [CrossRef]
- Di Vincenzo, F.; Puca, P.; Lopetuso, L.R.; Petito, V.; Masi, L.; Bartocci, B.; Murgiano, M.; De Felice, M.; Petronio, L.; Gasbarrini, A.; et al. Bile Acid-Related Regulation of Mucosal Inflammation and Intestinal Motility: From Pathogenesis to Therapeutic Application in IBD and Microscopic Colitis. Nutrients 2022, 14, 2664. [Google Scholar] [CrossRef]
- Su, X.; Gao, Y.; Yang, R. Gut microbiota derived bile acid metabolites maintain the homeostasis of gut and systemic immunity. Front. Immunol. 2023, 14, 1127743. [Google Scholar] [CrossRef]
- Gasaly, N.; de Vos, P.; Hermoso, M.A. Impact of Bacterial Metabolites on Gut Barrier Function and Host Immunity: A Focus on Bacterial Metabolism and Its Relevance for Intestinal Inflammation. Front. Immunol. 2021, 12, 658354. [Google Scholar] [CrossRef]
- Funabashi, M.; Grove, T.L.; Wang, M.; Varma, Y.; McFadden, M.E.; Brown, L.C.; Guo, C.; Higginbottom, S.; Almo, S.C.; Fischbach, M.A. A metabolic pathway for bile acid dehydroxylation by the gut microbiome. Nature 2020, 582, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Ning, S.; Zhang, Z.; Zhou, C.; Wang, B.; Liu, Z.; Feng, B. Cross-talk between macrophages and gut microbiota in inflammatory bowel disease: A dynamic interplay influencing pathogenesis and therapy. Front. Med. 2024, 11, 1457218. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Wang, S.; Wang, P.; Tang, C.; Wang, Z.; Chen, L.; Luo, G.; Chen, H.; Liu, Y.; Feng, B.; et al. Bile acids and their receptors in regulation of gut health and diseases. Prog. Lipid Res. 2023, 89, 101210. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Wang, D.; Li, X.; Cao, Y.; Yi, C.; Wiredu Ocansey, D.K.; Zhou, Y.; Mao, F. Farnesoid-X receptor as a therapeutic target for inflammatory bowel disease and colorectal cancer. Front. Pharmacol. 2022, 13, 1016836. [Google Scholar] [CrossRef] [PubMed]
- Calzadilla, N.; Comiskey, S.M.; Dudeja, P.K.; Saksena, S.; Gill, R.K.; Alrefai, W.A. Bile acids as inflammatory mediators and modulators of intestinal permeability. Front. Immunol. 2022, 13, 1021924. [Google Scholar] [CrossRef]
- Lavelle, A.; Sokol, H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 223–237. [Google Scholar] [CrossRef]
- Quinn, R.A.; Melnik, A.V.; Vrbanac, A.; Fu, T.; Patras, K.A.; Christy, M.P.; Bodai, Z.; Belda-Ferre, P.; Tripathi, A.; Chung, L.K.; et al. Global chemical effects of the microbiome include new bile-acid conjugations. Nature 2020, 579, 123–129. [Google Scholar] [CrossRef]
- Xu, R.H.; Shen, J.N.; Lu, J.B.; Liu, Y.J.; Song, Y.; Cao, Y.; Wang, Z.H.; Zhang, J. Bile acid profiles and classification model accuracy for inflammatory bowel disease diagnosis. Medicine 2024, 103, e38457. [Google Scholar] [CrossRef]
- Paik, D.; Yao, L.; Zhang, Y.; Bae, S.; D’Agostino, G.D.; Zhang, M.; Kim, E.; Franzosa, E.A.; Avila-Pacheco, J.; Bisanz, J.E.; et al. Human gut bacteria produce Tau(Eta)17-modulating bile acid metabolites. Nature 2022, 603, 907–912. [Google Scholar] [CrossRef]
- Jangi, S.; Zhao, N.; Hsia, K.; Park, Y.S.; Michaud, D.S.; Yoon, H. Specific bacterial co-abundance groups are associated with inflammatory status in patients with ulcerative colitis. J. Crohns Colitis 2024, jjae125. [Google Scholar] [CrossRef]
- Yang, M.; Gu, Y.; Li, L.; Liu, T.; Song, X.; Sun, Y.; Cao, X.; Wang, B.; Jiang, K.; Cao, H. Bile Acid-Gut Microbiota Axis in Inflammatory Bowel Disease: From Bench to Bedside. Nutrients 2021, 13, 3143. [Google Scholar] [CrossRef] [PubMed]
- Vijayvargiya, P.; Gonzalez Izundegui, D.; Calderon, G.; Tawfic, S.; Batbold, S.; Saifuddin, H.; Duggan, P.; Melo, V.; Thomas, T.; Heeney, M.; et al. Increased Fecal Bile Acid Excretion in a Significant Subset of Patients with Other Inflammatory Diarrheal Diseases. Dig. Dis. Sci. 2022, 67, 2413–2419. [Google Scholar] [CrossRef] [PubMed]
- Effinger, A.; McAllister, M.; Tomaszewska, I.; O’Driscoll, C.M.; Taylor, M.; Gomersall, S.; Heaton, J.; Smith, K.L.; Sarcevica, I.; Young, S.L.; et al. Investigating the Impact of Crohn’s Disease on the Bioaccessibility of a Lipid-Based Formulation with an In Vitro Dynamic Gastrointestinal Model. Mol. Pharm. 2021, 18, 1530–1543. [Google Scholar] [CrossRef] [PubMed]
- Gentry, E.C.; Collins, S.L.; Panitchpakdi, M.; Belda-Ferre, P.; Stewart, A.K.; Carrillo Terrazas, M.; Lu, H.H.; Zuffa, S.; Yan, T.; Avila-Pacheco, J.; et al. Reverse metabolomics for the discovery of chemical structures from humans. Nature 2024, 626, 419–426. [Google Scholar] [CrossRef]
- Reiter, S.; Dunkel, A.; Metwaly, A.; Panes, J.; Salas, A.; Haller, D.; Hofmann, T. Development of a Highly Sensitive Ultra-High-Performance Liquid Chromatography Coupled to Electrospray Ionization Tandem Mass Spectrometry Quantitation Method for Fecal Bile Acids and Application on Crohn’s Disease Studies. J. Agric. Food Chem. 2021, 69, 5238–5251. [Google Scholar] [CrossRef]
- Wilson, A.; Almousa, A.; Teft, W.A.; Kim, R.B. Attenuation of bile acid-mediated FXR and PXR activation in patients with Crohn’s disease. Sci. Rep. 2020, 10, 1866. [Google Scholar] [CrossRef]
- Liu, C.; Zhan, S.; Li, N.; Tu, T.; Lin, J.; Li, M.; Chen, M.; Zeng, Z.; Zhuang, X. Bile acid alterations associated with indolent course of inflammatory bowel disease. Scand. J. Gastroenterol. 2023, 58, 988–997. [Google Scholar] [CrossRef]
- Kiasat, A.; Prast-Nielsen, S.; Rautiainen, S.; Engstrand, L.; Andersson, F.; Lindberg, J.; Schuppe-Koistinen, I.; Lof Granstrom, A.; Gustafsson, U.O. Plasma bile acids in association with Crohn’s disease. Scand. J. Gastroenterol. 2024, 59, 674–682. [Google Scholar] [CrossRef]
- Feng, L.; Zhou, N.; Li, Z.; Fu, D.; Guo, Y.; Gao, X.; Liu, X. Co-occurrence of gut microbiota dysbiosis and bile acid metabolism alteration is associated with psychological disorders in Crohn’s disease. FASEB J. 2022, 36, e22100. [Google Scholar] [CrossRef]
- Sommersberger, S.; Gunawan, S.; Elger, T.; Fererberger, T.; Loibl, J.; Huss, M.; Kandulski, A.; Krautbauer, S.; Muller, M.; Liebisch, G.; et al. Altered fecal bile acid composition in active ulcerative colitis. Lipids Health Dis. 2023, 22, 199. [Google Scholar] [CrossRef]
- Liu, H.; Xu, M.; He, Q.; Wei, P.; Ke, M.; Liu, S. Untargeted serum metabolomics reveals specific metabolite abnormalities in patients with Crohn’s disease. Front. Med. 2022, 9, 814839. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, C.G.; Mills, R.H.; Zhu, Q.; Sauceda, C.; Knight, R.; Dulai, P.S.; Gonzalez, D.J. Location-specific signatures of Crohn’s disease at a multi-omics scale. Microbiome 2022, 10, 133. [Google Scholar] [CrossRef] [PubMed]
- Battat, R.; Scherl, E.J.; Lukin, D.; Charilaou, P.; Mahtani, P.; Gerber, J.; Gandara, J.A.; Consortium, J.I.L.C.B.; Dundar, F.; Zumbo, P.; et al. Increased Primary Bile Acids with Ileocolonic Resection Impact Ileal Inflammation and Gut Microbiota in Inflammatory Bowel Disease. J. Crohns Colitis 2023, 17, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.D.; Daniel, S.G.; Li, H.; Hao, F.; Patterson, A.D.; Hecht, A.L.; Brensinger, C.M.; Wu, G.D.; Bittinger, K.; Dine, C.D.; et al. Surgery for Crohn’s Disease Is Associated with a Dysbiotic Microbiome and Metabolome: Results from Two Prospective Cohorts. Cell Mol. Gastroenterol. Hepatol. 2024, 18, 101357. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.H.; Liu, F.; Zhu, X.R.; Suo, F.Y.; Jia, Z.J.; Yao, S.K. Altered profiles of fecal bile acids correlate with gut microbiota and inflammatory responses in patients with ulcerative colitis. World J. Gastroenterol. 2021, 27, 3609–3629. [Google Scholar] [CrossRef]
- Li, N.; Ma, P.; Li, Y.; Shang, X.; Nan, X.; Shi, L.; Han, X.; Liu, J.; Hong, Y.; Li, Q.; et al. Gut microbiota-derived 12-ketolithocholic acid suppresses the IL-17A secretion from colonic group 3 innate lymphoid cells to prevent the acute exacerbation of ulcerative colitis. Gut Microbes 2023, 15, 2290315. [Google Scholar] [CrossRef]
- Nystrom, N.; Prast-Nielsen, S.; Correia, M.; Globisch, D.; Engstrand, L.; Schuppe-Koistinen, I.; Halfvarson, J. Mucosal and Plasma Metabolomes in New-onset Paediatric Inflammatory Bowel Disease: Correlations with Disease Characteristics and Plasma Inflammation Protein Markers. J. Crohns Colitis 2023, 17, 418–432. [Google Scholar] [CrossRef]
- Schirmer, M.; Strazar, M.; Avila-Pacheco, J.; Rojas-Tapias, D.F.; Brown, E.M.; Temple, E.; Deik, A.; Bullock, K.; Jeanfavre, S.; Pierce, K.; et al. Linking microbial genes to plasma and stool metabolites uncovers host-microbial interactions underlying ulcerative colitis disease course. Cell Host Microbe 2024, 32, 209–226.e7. [Google Scholar] [CrossRef]
- Misra, R.; Sarafian, M.; Pechlivanis, A.; Ding, N.; Miguens-Blanco, J.; McDonald, J.; Holmes, E.; Marchesi, J.; Arebi, N. Ethnicity Associated Microbial and Metabonomic Profiling in Newly Diagnosed Ulcerative Colitis. Clin. Exp. Gastroenterol. 2022, 15, 199–212. [Google Scholar] [CrossRef]
- Aboud Syriani, L.; Parsana, R.; Durazo-Arvizu, R.A.; Michail, S. Differences in gut microbiota and fecal bile acids between Caucasian and Hispanic children and young adults with ulcerative colitis. Physiol. Rep. 2023, 11, e15752. [Google Scholar] [CrossRef]
- Dubinsky, V.; Reshef, L.; Rabinowitz, K.; Yadgar, K.; Godny, L.; Zonensain, K.; Wasserberg, N.; Dotan, I.; Gophna, U. Dysbiosis in Metabolic Genes of the Gut Microbiomes of Patients with an Ileo-anal Pouch Resembles That Observed in Crohn’s Disease. mSystems 2021, 6, e00984-20. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.R.; Haileselassie, Y.; Nguyen, L.P.; Tropini, C.; Wang, M.; Becker, L.S.; Sim, D.; Jarr, K.; Spear, E.T.; Singh, G.; et al. Dysbiosis-Induced Secondary Bile Acid Deficiency Promotes Intestinal Inflammation. Cell Host Microbe 2020, 27, 659–670.e5. [Google Scholar] [CrossRef] [PubMed]
- Yan Ang, Q.; Plichta, D.; Kim, S.; Hyun, A.K.I.; Gregory, S.; Xia, Y.; Lau, H.; Xavier, R.; Ananthakrishnan, A.N. Differential Impact of Smoking on Methylome and Transcriptome in Crohn’s Disease and Ulcerative Colitis. Inflamm. Bowel Dis. 2024, 30, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.P.; Sauk, J.S.; Ahdoot, A.I.; Liang, F.; Katzka, W.; Ryu, H.J.; Khandadash, A.; Lagishetty, V.; Labus, J.S.; Naliboff, B.D.; et al. Microbial and Metabolite Signatures of Stress Reactivity in Ulcerative Colitis Patients in Clinical Remission Predict Clinical Flare Risk. Inflamm. Bowel Dis. 2024, 30, 336–346. [Google Scholar] [CrossRef]
- Leibovitzh, H.; Nayeri, S.; Borowski, K.; Hernandez-Rocha, C.; Lee, S.H.; Turpin, W.; Stempak, J.M.; Sandhu, I.; Milgrom, R.; Smith, M.I.; et al. Inflammatory bowel disease associated with primary sclerosing cholangitis is associated with an altered gut microbiome and bile acid profile. J. Crohns Colitis 2024, 18, 1957–1966. [Google Scholar] [CrossRef]
- Santiago, P.; Quinn, K.P.; Chen, J.; Friton, J.J.; Rypstra, C.R.; Kashyap, P.C.; Raffals, L.E. Altered Bile Acid and Pouch Microbiota Composition in Patients with Chronic Pouchitis. Inflamm. Bowel Dis. 2024, 30, 1062–1070. [Google Scholar] [CrossRef]
- Liu, L.; Yang, M.; Dong, W.; Liu, T.; Song, X.; Gu, Y.; Wang, S.; Liu, Y.; Abla, Z.; Qiao, X.; et al. Gut Dysbiosis and Abnormal Bile Acid Metabolism in Colitis-Associated Cancer. Gastroenterol. Res. Pract. 2021, 2021, 6645970. [Google Scholar] [CrossRef]
- Collins, S.L.; Stine, J.G.; Bisanz, J.E.; Okafor, C.D.; Patterson, A.D. Bile acids and the gut microbiota: Metabolic interactions and impacts on disease. Nat. Rev. Microbiol. 2023, 21, 236–247. [Google Scholar] [CrossRef]
- Jia, B.; Park, D.; Hahn, Y.; Jeon, C.O. Metagenomic analysis of the human microbiome reveals the association between the abundance of gut bile salt hydrolases and host health. Gut Microbes 2020, 11, 1300–1313. [Google Scholar] [CrossRef]
- Deng, Z.L.; Pieper, D.H.; Stallmach, A.; Steube, A.; Vital, M.; Reck, M.; Wagner-Dobler, I. Engraftment of essential functions through multiple fecal microbiota transplants in chronic antibiotic-resistant pouchitis-a case study using metatranscriptomics. Microbiome 2023, 11, 269. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, X.; Zhu, X.; Jiao, J.; Wu, Y.; Li, Y.; Zhao, L. Fusobacterium nucleatum aggravates ulcerative colitis through promoting gut microbiota dysbiosis and dysmetabolism. J. Periodontol. 2023, 94, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Jiang, L.; Fang, X.; Guo, Z.; Wang, X.; Shi, B.; Meng, Q. Host-microbiota interaction-mediated resistance to inflammatory bowel disease in pigs. Microbiome 2022, 10, 115. [Google Scholar] [CrossRef] [PubMed]
- Galler, A.I.; Suchodolski, J.S.; Steiner, J.M.; Sung, C.H.; Hittmair, K.M.; Richter, B.; Burgener, I.A. Microbial dysbiosis and fecal metabolomic perturbations in Yorkshire Terriers with chronic enteropathy. Sci. Rep. 2022, 12, 12977. [Google Scholar] [CrossRef] [PubMed]
- Briggs, K.; Tomar, V.; Ollberding, N.; Haberman, Y.; Bourgonje, A.R.; Hu, S.; Chaaban, L.; Sunuwar, L.; Weersma, R.K.; Denson, L.A.; et al. Crohn’s Disease-Associated Pathogenic Mutation in the Manganese Transporter ZIP8 Shifts the Ileal and Rectal Mucosal Microbiota Implicating Aberrant Bile Acid Metabolism. Inflamm. Bowel Dis. 2024, 30, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, M.; Lang, M.; Holley, H.; Crepaz, D.; Hausmann, B.; Pjevac, P.; Moser, D.; Haller, F.; Hof, F.; Beer, A.; et al. Mucosal Biofilms Are an Endoscopic Feature of Irritable Bowel Syndrome and Ulcerative Colitis. Gastroenterology 2021, 161, 1245–1256.e20. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Z.; Yue, W.; Zhu, L.; Zhong, H.; Yang, C.; He, T.; Wan, P.; Geng, J. Mucosa-Colonizing Microbiota Correlate with Host Autophagy Signaling in Patients with Inflammatory Bowel Disease. Front. Microbiol. 2022, 13, 875238. [Google Scholar] [CrossRef]
- Mohanty, I.; Allaband, C.; Mannochio-Russo, H.; El Abiead, Y.; Hagey, L.R.; Knight, R.; Dorrestein, P.C. The changing metabolic landscape of bile acids—Keys to metabolism and immune regulation. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 493–516. [Google Scholar] [CrossRef]
- Zhang, S.; Zhong, R.; Tang, S.; Chen, L.; Zhang, H. Metabolic regulation of the Th17/Treg balance in inflammatory bowel disease. Pharmacol. Res. 2024, 203, 107184. [Google Scholar] [CrossRef]
- Hang, S.; Paik, D.; Yao, L.; Kim, E.; Trinath, J.; Lu, J.; Ha, S.; Nelson, B.N.; Kelly, S.P.; Wu, L.; et al. Bile acid metabolites control T(H)17 and T(reg) cell differentiation. Nature 2019, 576, 143–148. [Google Scholar] [CrossRef]
- Arifuzzaman, M.; Won, T.H.; Yano, H.; Uddin, J.; Emanuel, E.R.; Hu, E.; Zhang, W.; Li, T.T.; Jin, W.B.; Grier, A.; et al. Dietary fiber is a critical determinant of pathologic ILC2 responses and intestinal inflammation. J. Exp. Med. 2024, 221, e20232148. [Google Scholar] [CrossRef]
- Song, X.; Sun, X.; Oh, S.F.; Wu, M.; Zhang, Y.; Zheng, W.; Geva-Zatorsky, N.; Jupp, R.; Mathis, D.; Benoist, C.; et al. Microbial bile acid metabolites modulate gut RORgamma(+) regulatory T cell homeostasis. Nature 2020, 577, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.; Zhang, X.; Zhang, Q.; Xia, J.; Zhang, Y.; Ma, C.; Liu, K.; Li, H.; Hong, Y.; Xie, Z. Gallic acid attenuates murine ulcerative colitis by promoting group 3 innate lymphocytes, affecting gut microbiota, and bile acid metabolism. J. Nutr. Biochem. 2024, 131, 109677. [Google Scholar] [CrossRef] [PubMed]
- Couto, M.; Andrade, N.; Magro, F.; Martel, F. Taurocholate uptake by Caco-2 cells is inhibited by pro-inflammatory cytokines and butyrate. Cytokine 2023, 169, 156307. [Google Scholar] [CrossRef] [PubMed]
- Biagioli, M.; Marchiano, S.; Carino, A.; Di Giorgio, C.; Santucci, L.; Distrutti, E.; Fiorucci, S. Bile Acids Activated Receptors in Inflammatory Bowel Disease. Cells 2021, 10, 1281. [Google Scholar] [CrossRef] [PubMed]
- Negroni, A.; Fiaschini, N.; Palone, F.; Vitali, R.; Colantoni, E.; Laudadio, I.; Oliva, S.; Aloi, M.; Cucchiara, S.; Stronati, L. Intestinal Inflammation Alters the Expression of Hepatic Bile Acid Receptors Causing Liver Impairment. J. Pediatr. Gastroenterol. Nutr. 2020, 71, 189–196. [Google Scholar] [CrossRef]
- Sorrentino, G.; Perino, A.; Yildiz, E.; El Alam, G.; Bou Sleiman, M.; Gioiello, A.; Pellicciari, R.; Schoonjans, K. Bile Acids Signal via TGR5 to Activate Intestinal Stem Cells and Epithelial Regeneration. Gastroenterology 2020, 159, 956–968.e8. [Google Scholar] [CrossRef]
- Wilson, A.; Wang, Q.; Almousa, A.A.; Jansen, L.E.; Choi, Y.H.; Schwarz, U.I.; Kim, R.B. Genetic variation in the farnesoid X-receptor predicts Crohn’s disease severity in female patients. Sci. Rep. 2020, 10, 11725. [Google Scholar] [CrossRef]
- Cheung, K.C.P.; Ma, J.; Loiola, R.A.; Chen, X.; Jia, W. Bile acid-activated receptors in innate and adaptive immunity: Targeted drugs and biological agents. Eur. J. Immunol. 2023, 53, e2250299. [Google Scholar] [CrossRef]
- Ma, C.; Han, M.; Heinrich, B.; Fu, Q.; Zhang, Q.; Sandhu, M.; Agdashian, D.; Terabe, M.; Berzofsky, J.A.; Fako, V.; et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science 2018, 360, eaan5931. [Google Scholar] [CrossRef]
- Guo, C.; Xie, S.; Chi, Z.; Zhang, J.; Liu, Y.; Zhang, L.; Zheng, M.; Zhang, X.; Xia, D.; Ke, Y.; et al. Bile Acids Control Inflammation and Metabolic Disorder through Inhibition of NLRP3 Inflammasome. Immunity 2016, 45, 802–816, Erratum in Immunity 2016, 45, 944. [Google Scholar] [CrossRef]
- Hao, H.; Cao, L.; Jiang, C.; Che, Y.; Zhang, S.; Takahashi, S.; Wang, G.; Gonzalez, F.J. Farnesoid X Receptor Regulation of the NLRP3 Inflammasome Underlies Cholestasis-Associated Sepsis. Cell Metab. 2017, 25, 856–867.e5. [Google Scholar] [CrossRef] [PubMed]
- Mendler, A.; Pierzchalski, A.; Bauer, M.; Roder, S.; Sattler, A.; Standl, M.; Borte, M.; von Bergen, M.; Rolle-Kampczyk, U.; Herberth, G. MAIT cell activation in adolescents is impacted by bile acid concentrations and body weight. Clin. Exp. Immunol. 2020, 200, 199–213. [Google Scholar] [CrossRef]
- Fu, T.; Li, Y.; Oh, T.G.; Cayabyab, F.; He, N.; Tang, Q.; Coulter, S.; Truitt, M.; Medina, P.; He, M.; et al. FXR mediates ILC-intrinsic responses to intestinal inflammation. Proc. Natl. Acad. Sci. USA 2022, 119, e2213041119. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yang, H.; Wang, X.; Huang, Y.; Li, Y.; Pan, G. Bile acids as signaling molecules in inflammatory bowel disease: Implications for treatment strategies. J. Ethnopharmacol. 2024, 337 Pt 3, 118968. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Chen, X.; Ferrari, M.; Walvoort, M.T.C.; de Vos, P. Gut Epithelial Barrier Function is Impacted by Hyperglycemia and Secondary Bile Acids In Vitro: Possible Rescuing Effects of Specific Pectins. Mol. Nutr. Food Res. 2024, 68, e2300910. [Google Scholar] [CrossRef]
- O’Guinn, M.L.; Handler, D.A.; Hsieh, J.J.; Mallicote, M.U.; Feliciano, K.; Gayer, C.P. FXR deletion attenuates intestinal barrier dysfunction in murine acute intestinal inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2024, 327, G175–G187. [Google Scholar] [CrossRef]
- Lukawska, A.; Mulak, A. Serum fibroblast growth factor 19 level correlates inversely with clinical and endoscopic activity of inflammatory bowel disease. Adv. Clin. Exp. Med. 2024. [Google Scholar] [CrossRef]
- Jyotsna; Sarkar, B.; Yadav, M.; Deka, A.; Markandey, M.; Sanyal, P.; Nagarajan, P.; Gaikward, N.; Ahuja, V.; Mohanty, D.; et al. A hepatocyte-specific transcriptional program driven by Rela and Stat3 exacerbates experimental colitis in mice by modulating bile synthesis. Elife 2024, 12, RP93273. [Google Scholar] [CrossRef]
- Kwon, S.J.; Kim, Y.S.; Tak, J.; Lee, S.G.; Lee, E.B.; Kim, S.G. Hepatic Galpha13 ablation shifts region-specific colonic inflammatory status by modulating the bile acid synthetic pathway in mice. Sci. Rep. 2024, 14, 19580. [Google Scholar] [CrossRef]
- Quraishi, M.N.; Acharjee, A.; Beggs, A.D.; Horniblow, R.; Tselepis, C.; Gkoutos, G.; Ghosh, S.; Rossiter, A.E.; Loman, N.; van Schaik, W.; et al. A Pilot Integrative Analysis of Colonic Gene Expression, Gut Microbiota, and Immune Infiltration in Primary Sclerosing Cholangitis-Inflammatory Bowel Disease: Association of Disease with Bile Acid Pathways. J. Crohns Colitis 2020, 14, 935–947. [Google Scholar] [CrossRef]
- Kwon, J.; Lee, C.; Heo, S.; Kim, B.; Hyun, C.K. DSS-induced colitis is associated with adipose tissue dysfunction and disrupted hepatic lipid metabolism leading to hepatosteatosis and dyslipidemia in mice. Sci. Rep. 2021, 11, 5283. [Google Scholar] [CrossRef]
- Gui, W.; Hole, M.J.; Molinaro, A.; Edlund, K.; Jorgensen, K.K.; Su, H.; Begher-Tibbe, B.; Gassler, N.; Schneider, C.V.; Muthukumarasamy, U.; et al. Colitis ameliorates cholestatic liver disease via suppression of bile acid synthesis. Nat. Commun. 2023, 14, 3304. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Wang, L.; Zou, T.; Lian, S.; Luo, J.; Lu, Y.; Hao, H.; Xu, Y.; Xiang, Y.; Zhang, X.; et al. Ileitis promotes MASLD progression via bile acid modulation and enhanced TGR5 signaling in ileal CD8(+) T cells. J. Hepatol. 2024, 80, 764–777. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chu, Q.; Dong, W.; Wang, X.; Zhao, W.; Dai, X.; Liu, W.; Wang, B.; Liu, T.; Zhong, W.; et al. Microbial metabolite deoxycholic acid-mediated ferroptosis exacerbates high-fat diet-induced colonic inflammation. Mol. Metab. 2024, 84, 101944. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Tan, H.; Song, M.; Liu, K.; Liu, H.; Wang, J.; Shi, Y.; Hou, F.; Zhou, Q.; Huang, R.; et al. Maternal Western diet mediates susceptibility of offspring to Crohn’s-like colitis by deoxycholate generation. Microbiome 2023, 11, 96. [Google Scholar] [CrossRef]
- Zheng, M.; Zhai, Y.; Yu, Y.; Shen, J.; Chu, S.; Focaccia, E.; Tian, W.; Wang, S.; Liu, X.; Yuan, X.; et al. TNF compromises intestinal bile-acid tolerance dictating colitis progression and limited infliximab response. Cell Metab. 2024, 36, 2086–2103.e9. [Google Scholar] [CrossRef]
- Wang, L.; Gong, Z.; Zhang, X.; Zhu, F.; Liu, Y.; Jin, C.; Du, X.; Xu, C.; Chen, Y.; Cai, W.; et al. Gut microbial bile acid metabolite skews macrophage polarization and contributes to high-fat diet-induced colonic inflammation. Gut Microbes 2020, 12, 1–20. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, X.; He, J.; Xu, X.; Ju, C.; Luo, S.; Lu, X.; Du, P.; Chen, Y. High-fructose corn syrup aggravates colitis via microbiota dysbiosis-mediated Th17/Treg imbalance. Clin. Sci. 2023, 137, 1619–1635. [Google Scholar] [CrossRef]
- Li, S.; Zhuge, A.; Wang, K.; Lv, L.; Bian, X.; Yang, L.; Xia, J.; Jiang, X.; Wu, W.; Wang, S.; et al. Ketogenic diet aggravates colitis, impairs intestinal barrier and alters gut microbiota and metabolism in DSS-induced mice. Food Funct. 2021, 12, 10210–10225. [Google Scholar] [CrossRef]
- Liu, T.C.; Kern, J.T.; Jain, U.; Sonnek, N.M.; Xiong, S.; Simpson, K.F.; VanDussen, K.L.; Winkler, E.S.; Haritunians, T.; Malique, A.; et al. Western diet induces Paneth cell defects through microbiome alterations and farnesoid X receptor and type I interferon activation. Cell Host Microbe 2021, 29, 988–1001.e6. [Google Scholar] [CrossRef]
- O’Mahony, C.; Clooney, A.; Clarke, S.F.; Aguilera, M.; Gavin, A.; Simnica, D.; Ahern, M.; Fanning, A.; Stanley, M.; Rubio, R.C.; et al. Dietary-Induced Bacterial Metabolites Reduce Inflammation and Inflammation-Associated Cancer via Vitamin D Pathway. Int. J. Mol. Sci. 2023, 24, 1864. [Google Scholar] [CrossRef] [PubMed]
- Fart, F.; Salihovic, S.; McGlinchey, A.; Gareau, M.G.; Oresic, M.; Halfvarson, J.; Hyotylainen, T.; Schoultz, I. Perfluoroalkyl substances are increased in patients with late-onset ulcerative colitis and induce intestinal barrier defects ex vivo in murine intestinal tissue. Scand. J. Gastroenterol. 2021, 56, 1286–1295. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Ren, T.; Yu, G.; Meng, X.; Feng, L.; Li, F.; Zhang, J.; Wang, C. Unraveling the long-term gastrointestinal impact of perinatal perfluorobutane sulfonate exposure on rat offspring: Intestinal barrier dysfunction and Th17/Treg imbalance. Sci. Total Environ. 2024, 955, 176858. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Tang, L.; Wang, J.S. Aflatoxin B1 Induces Gut-Inflammation-Associated Fecal Lipidome Changes in F344 Rats. Toxicol. Sci. 2021, 183, 363–377. [Google Scholar] [CrossRef]
- Couto, M.R.; Andrade, N.; Magro, F.; Martel, F. Bile salts and proinflammatory cytokines inhibit MCT1-mediated cellular uptake of butyrate and interfere with its antiproliferative properties. Exp. Cell Res. 2023, 429, 113670. [Google Scholar] [CrossRef]
- Chen, W.; Wang, D.; Deng, X.; Zhang, H.; Dong, D.; Su, T.; Lu, Q.; Jiang, C.; Ni, Q.; Cui, Y.; et al. Bile acid profiling as an effective biomarker for staging in pediatric inflammatory bowel disease. Gut Microbes 2024, 16, 2323231. [Google Scholar] [CrossRef]
- Connors, J.; Dunn, K.A.; Allott, J.; Bandsma, R.; Rashid, M.; Otley, A.R.; Bielawski, J.P.; Van Limbergen, J. The relationship between fecal bile acids and microbiome community structure in pediatric Crohn’s disease. ISME J. 2020, 14, 702–713. [Google Scholar] [CrossRef]
- Sun, Q.; Tang, Y.; Dai, L.; Tang, Z.; Zhou, W.; Wu, T.; Ji, G. Serum Bile Acid Metabolites Predict the Therapeutic Effect of Mesalazine in Patients with Ulcerative Colitis. J. Proteome Res. 2023, 22, 1287–1297. [Google Scholar] [CrossRef]
- Lee, J.W.J.; Plichta, D.; Hogstrom, L.; Borren, N.Z.; Lau, H.; Gregory, S.M.; Tan, W.; Khalili, H.; Clish, C.; Vlamakis, H.; et al. Multi-omics reveal microbial determinants impacting responses to biologic therapies in inflammatory bowel disease. Cell Host Microbe 2021, 29, 1294–1304.e4. [Google Scholar] [CrossRef]
- Ding, N.S.; McDonald, J.A.K.; Perdones-Montero, A.; Rees, D.N.; Adegbola, S.O.; Misra, R.; Hendy, P.; Penez, L.; Marchesi, J.R.; Holmes, E.; et al. Metabonomics and the Gut Microbiome Associated with Primary Response to Anti-TNF Therapy in Crohn’s Disease. J. Crohns Colitis 2020, 14, 1090–1102. [Google Scholar] [CrossRef]
- Han, B.; Tang, D.; Lv, X.; Fan, J.; Li, S.; Zhu, H.; Zhang, J.; Xu, S.; Xu, X.; Huang, Z.; et al. Integrated multi-omics reveal gut microbiota-mediated bile acid metabolism alteration regulating immunotherapy responses to anti-alpha4beta7-integrin in Crohn’s disease. Gut Microbes 2024, 16, 2310894. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Lv, X.; Liu, G.; Li, S.; Fan, J.; Chen, L.; Huang, Z.; Lin, G.; Xu, X.; Huang, Z.; et al. Gut microbiota-related bile acid metabolism-FXR/TGR5 axis impacts the response to anti-alpha4beta7-integrin therapy in humanized mice with colitis. Gut Microbes 2023, 15, 2232143. [Google Scholar] [CrossRef] [PubMed]
- Lyutakov, I.; Lozanov, V.; Sugareva, P.; Valkov, H.; Penchev, P. Serum 7-alfa-hydroxy-4-cholesten-3-one and fibroblast growth factor-19 as biomarkers diagnosing bile acid malabsorption in microscopic colitis and inflammatory bowel disease. Eur. J. Gastroenterol. Hepatol. 2021, 33, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.L.; Mullish, B.H.; Danckert, N.P.; Liu, Z.; Olbei, M.L.; Saifuddin, A.; Torkizadeh, M.; Ibraheim, H.; Blanco, J.M.; Roberts, L.A.; et al. The gut microbiota and metabolome are associated with diminished COVID-19 vaccine-induced antibody responses in immunosuppressed inflammatory bowel disease patients. eBioMedicine 2023, 88, 104430. [Google Scholar] [CrossRef]
- Wu, X.; Li, P.; Wang, W.; Xu, J.; Ai, R.; Wen, Q.; Cui, B.; Zhang, F. The Underlying Changes in Serum Metabolic Profiles and Efficacy Prediction in Patients with Extensive Ulcerative Colitis Undergoing Fecal Microbiota Transplantation. Nutrients 2023, 15, 3340. [Google Scholar] [CrossRef]
- Ramos, R.J.; Zhu, C.; Joseph, D.F.; Thaker, S.; Lacomb, J.F.; Markarian, K.; Lee, H.J.; Petrov, J.C.; Monzur, F.; Buscaglia, J.M.; et al. Metagenomic and bile acid metabolomic analysis of fecal microbiota transplantation for recurrent Clostridiodes difficile and/or inflammatory bowel diseases. Med. Res. Arch. 2022, 10. [Google Scholar] [CrossRef]
- Chen, L.A.; Oliva-Hemker, M.; Radin, A.; Weidner, M.; O’Laughlin, B.D.; Sears, C.L.; Javitt, N.B.; Hourigan, S.K. Longitudinal Bile Acid Composition Changes Following Faecal Microbiota Transplantation for Clostridioides difficile Infection in Children with and Without Underlying Inflammatory Bowel Disease. J. Crohns Colitis 2023, 17, 1364–1368. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, X.; Zhang, X.; Xiao, F.; Hu, H.; Li, X.; Dong, F.; Sun, M.; Xiao, Y.; Ge, T.; et al. Microbial and metabolic features associated with outcome of infliximab therapy in pediatric Crohn’s disease. Gut Microbes 2021, 13, 1–18. [Google Scholar] [CrossRef]
- Manka, P.; Sydor, S.; Wase, N.; Best, J.; Brandenburg, M.; Hellbeck, A.; Schanzer, J.; Vilchez-Vargas, R.; Link, A.; Figge, A.; et al. Anti-TNFalpha treatment in Crohn’s disease: Impact on hepatic steatosis, gut-derived hormones and metabolic status. Liver Int. 2021, 41, 2646–2658. [Google Scholar] [CrossRef]
- Diederen, K.; Li, J.V.; Donachie, G.E.; de Meij, T.G.; de Waart, D.R.; Hakvoort, T.B.M.; Kindermann, A.; Wagner, J.; Auyeung, V.; Te Velde, A.A.; et al. Exclusive enteral nutrition mediates gut microbial and metabolic changes that are associated with remission in children with Crohn’s disease. Sci. Rep. 2020, 10, 18879. [Google Scholar] [CrossRef]
- Xiao, F.; Gao, X.; Hu, H.; Le, J.; Chen, Y.; Shu, X.; Liang, Z.; Xu, Y.; Wang, Y.; Zhang, T. Exclusive Enteral Nutrition Exerts Anti-Inflammatory Effects through Modulating Microbiota, Bile Acid Metabolism, and Immune Activities. Nutrients 2022, 14, 4463. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Lou, Y.; Liu, A.; Cheng, Q.; Yang, G.; Xu, C.; Luo, Y.; Lou, J.; Yu, J.; Fang, Y.; et al. The impact of exclusive enteral nutrition on the gut microbiome and bile acid metabolism in pediatric Crohn’s disease. Clin. Nutr. 2023, 42, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Man, D.; Shi, D.; Wu, W.; Wang, S.; Wang, K.; Li, Y.; Yang, L.; Bian, X.; Wang, Q.; et al. Intermittent Fasting Alleviates Risk Markers in a Murine Model of Ulcerative Colitis by Modulating the Gut Microbiome and Metabolome. Nutrients 2022, 14, 5311. [Google Scholar] [CrossRef] [PubMed]
- Ke, J.; Li, Y.; Han, C.; He, R.; Lin, R.; Qian, W.; Hou, X. Fucose Ameliorate Intestinal Inflammation Through Modulating the Crosstalk Between Bile Acids and Gut Microbiota in a Chronic Colitis Murine Model. Inflamm. Bowel Dis. 2020, 26, 863–873. [Google Scholar] [CrossRef]
- Bretin, A.; Zou, J.; San Yeoh, B.; Ngo, V.L.; Winer, S.; Winer, D.A.; Reddivari, L.; Pellizzon, M.; Walters, W.A.; Patterson, A.D.; et al. Psyllium Fiber Protects Against Colitis Via Activation of Bile Acid Sensor Farnesoid X Receptor. Cell Mol. Gastroenterol. Hepatol. 2023, 15, 1421–1442. [Google Scholar] [CrossRef]
- Yang, L.; Lin, Q.; Han, L.; Wang, Z.; Luo, M.; Kang, W.; Liu, J.; Wang, J.; Ma, T.; Liu, H. Soy hull dietary fiber alleviates inflammation in BALB/C mice by modulating the gut microbiota and suppressing the TLR-4/NF-kappaB signaling pathway. Food Funct. 2020, 11, 5965–5975. [Google Scholar] [CrossRef]
- Tian, M.; Li, D.; Ma, C.; Feng, Y.; Hu, X.; Chen, F. Barley Leaf Insoluble Dietary Fiber Alleviated Dextran Sulfate Sodium-Induced Mice Colitis by Modulating Gut Microbiota. Nutrients 2021, 13, 846. [Google Scholar] [CrossRef]
- Zhang, J.; Zhong, Y.; Wang, D.; Zhu, J.; Deng, Y.; Li, Y.; Liu, C.; Wang, J.L.; Zhang, M. Wallace melon juice fermented with Lactobacillus alleviates dextran sulfate sodium-induced ulcerative colitis in mice through modulating gut microbiota and the metabolism. J. Food Sci. 2024, 89, 2450–2464. [Google Scholar] [CrossRef]
- Zhang, L.; Miao, C.; Wang, Z.; Guan, X.; Ma, Y.; Song, J.; Shen, S.; Song, H.; Li, M.; Liu, C. Preparation and characterisation of baicalin magnesium and its protective effect in ulcerative colitis via gut microbiota-bile acid axis modulation. Phytomedicine 2024, 126, 155416. [Google Scholar] [CrossRef]
- Wang, L.; Tao, J.H.; Chen, Y.F.; Shen, Y.M.; Jiang, S. Lizhong Decoction Ameliorates Ulcerative Colitis in Mice via Regulation of Plasma and Urine Metabolic Profiling. Chin. J. Integr. Med. 2022, 28, 1015–1022. [Google Scholar] [CrossRef]
- Han, Z.; Wang, H.; Guo, D.; Zhang, J. Integrative transcriptomic and metabonomic profiling analyses reveal the molecular mechanism of Chinese traditional medicine huankuile suspension on TNBS-induced ulcerative colitis. Aging 2021, 13, 5087–5103. [Google Scholar] [CrossRef] [PubMed]
- Nong, F.; Luo, S.; Liang, Y.; Zhao, Z.; Xing, S.; Wen, B.; Zhou, L. Evaluation of the effect of Dahuang-Mudan decoction on TNBS-induced colitis using UPLC-QTOF/MS-based metabolomic analysis. Biomed. Chromatogr. 2021, 35, e5003. [Google Scholar] [CrossRef] [PubMed]
- Nguepi Tsopmejio, I.S.; Yuan, J.; Diao, Z.; Fan, W.; Wei, J.; Zhao, C.; Li, Y.; Song, H. Auricularia polytricha and Flammulina velutipes reduce liver injury in DSS-induced Inflammatory Bowel Disease by improving inflammation, oxidative stress, and apoptosis through the regulation of TLR4/NF-kappaB signaling pathways. J. Nutr. Biochem. 2023, 111, 109190. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Tao, Q.; Li, X.; Han, Y.; Du, H.; Hu, Q.; Xiao, H. Metabolomics study of dietary Pleurotus eryngii beta-type glycosidic polysaccharide on colitis induced by dextran sodium sulfate in mice—Exploration for the potential metabolic indicators in urine and serum. Food Chem. 2024, 458, 140195. [Google Scholar] [CrossRef]
- Fan, A.; Hou, B.L.; Tang, Z.; Wang, T.; Zhang, D.; Liang, Y.; Wang, Z. Liquid Chromatography-Tandem Mass Spectrometry-Based Metabolomics Analysis of Indigo Naturalis Treatment of Ulcerative Colitis in Mice. J. Med. Food 2023, 26, 877–889. [Google Scholar] [CrossRef]
- Cheng, H.; Zhang, D.; Wu, J.; Liu, J.; Tan, Y.; Feng, W.; Peng, C. Atractylodes macrocephala Koidz. volatile oil relieves acute ulcerative colitis via regulating gut microbiota and gut microbiota metabolism. Front. Immunol. 2023, 14, 1127785. [Google Scholar] [CrossRef]
- Wu, Y.; Zheng, Y.; Wang, X.; Tang, P.; Guo, W.; Ma, H.; Zhang, A.; Li, D.; Xie, Y.; Wang, C.Z.; et al. Ginseng-Containing Sijunzi Decoction Ameliorates Ulcerative Colitis by Orchestrating Gut Homeostasis in Microbial Modulation and Intestinal Barrier Integrity. Am. J. Chin. Med. 2023, 51, 677–699. [Google Scholar] [CrossRef]
- Li, S.; Tan, H.Y.; Wang, N.; Hong, M.; Li, L.; Cheung, F.; Feng, Y. Substitutes for Bear Bile for the Treatment of Liver Diseases: Research Progress and Future Perspective. Evid. Based Complement. Altern. Med. 2016, 2016, 4305074. [Google Scholar] [CrossRef]
- Wang, A.; Yang, X.; Lin, J.; Wang, Y.; Yang, J.; Zhang, Y.; Tian, Y.; Dong, H.; Zhang, Z.; Song, R. Si-Ni-San alleviates intestinal and liver damage in ulcerative colitis mice by regulating cholesterol metabolism. J. Ethnopharmacol. 2025, 336, 118715. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, J.; Wang, F.; Tang, X. Modified Gegen Qinlian Decoction modulated the gut microbiome and bile acid metabolism and restored the function of goblet cells in a mouse model of ulcerative colitis. Front. Immunol. 2024, 15, 1445838. [Google Scholar] [CrossRef]
- Li, Q.; Cui, Y.; Xu, B.; Wang, Y.; Lv, F.; Li, Z.; Li, H.; Chen, X.; Peng, X.; Chen, Y.; et al. Main active components of Jiawei Gegen Qinlian decoction protects against ulcerative colitis under different dietary environments in a gut microbiota-dependent manner. Pharmacol. Res. 2021, 170, 105694. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Mai, T.; Wang, Z.; Zeng, Z.; Shi, J.; Zhang, F.; Kong, N.; Jiang, H.; Guo, L.; Xu, M.; et al. The improvement of intestinal dysbiosis and hepatic metabolic dysfunction in dextran sulfate sodium-induced colitis mice: Effects of curcumin. J. Gastroenterol. Hepatol. 2023, 38, 1333–1345. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xu, K.; Cai, X.; Wang, C.; Cao, Y.; Xiao, J. Rosmarinic Acid Restores Colonic Mucus Secretion in Colitis Mice by Regulating Gut Microbiota-Derived Metabolites and the Activation of Inflammasomes. J. Agric. Food Chem. 2023, 71, 4571–4585. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Tang, S.; Dong, X.; Dong, M.; Shao, R.; Liu, R.; Li, T.; Zhang, X.; Wong, Y.H.; Xie, Q. Analysis of metagenome and metabolome disclosed the mechanisms of Dendrobium officinale polysaccharide on DSS-induced ulcerative colitis-affected mice. Int. J. Biol. Macromol. 2024, 277 Pt 2, 134229. [Google Scholar] [CrossRef]
- Feng, P.; Li, Q.; Liu, L.; Wang, S.; Wu, Z.; Tao, Y.; Huang, P.; Wang, P. Crocetin Prolongs Recovery Period of DSS-Induced Colitis via Altering Intestinal Microbiome and Increasing Intestinal Permeability. Int. J. Mol. Sci. 2022, 23, 3832. [Google Scholar] [CrossRef]
- Hu, J.; Huang, H.; Che, Y.; Ding, C.; Zhang, L.; Wang, Y.; Hao, H.; Shen, H.; Cao, L. Qingchang Huashi Formula attenuates DSS-induced colitis in mice by restoring gut microbiota-metabolism homeostasis and goblet cell function. J. Ethnopharmacol. 2021, 266, 113394. [Google Scholar] [CrossRef]
- Pi, Y.; Zhang, X.; Wu, Y.; Wang, Z.; Bai, Y.; Liu, X.; Han, D.; Zhao, J.; Tobin, I.; Zhao, J.; et al. Alginate Alleviates Dextran Sulfate Sodium-Induced Colitis by Promoting Bifidobacterium animalis and Intestinal Hyodeoxycholic Acid Synthesis in Mice. Microbiol. Spectr. 2022, 10, e0297922. [Google Scholar] [CrossRef]
- Liu, F.; Wang, X.; Li, D.; Cui, Y.; Li, X. Apple polyphenols extract alleviated dextran sulfate sodium-induced ulcerative colitis in C57BL/6 male mice by restoring bile acid metabolism disorder and gut microbiota dysbiosis. Phytother. Res. 2021, 35, 1468–1485. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, Y.; Zheng, Y.; Zhang, T.; Wu, Y.; Yan, Y.; Lei, Y.; Cao, X.; Wang, X.; Yan, F.; et al. Bifidobacterium pseudolongum-Derived Bile Acid from Dietary Carvacrol and Thymol Supplementation Attenuates Colitis via cGMP-PKG-mTORC1 Pathway. Adv. Sci. 2024, 11, e2406917. [Google Scholar] [CrossRef]
- Zhang, M.; Mo, R.; Wang, H.; Liu, T.; Zhang, G.; Wu, Y. Grape seed proanthocyanidin improves intestinal inflammation in canine through regulating gut microbiota and bile acid compositions. FASEB J. 2023, 37, e23285. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, X.; Deng, Z.; Bai, T.; Gao, B.; Xu, C.; Fu, J.; Zhao, Y.; Zhang, Y.; Zhang, M.; et al. Orally biomimetic metal-phenolic nanozyme with quadruple safeguards for intestinal homeostasis to ameliorate ulcerative colitis. J. Nanobiotechnol. 2024, 22, 545. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Xue, Q.; Liu, Y.; Xu, Y.; Xiong, C.; Lu, J.; Yang, H.; Zhang, Q.; Huang, Y. Analysis of Intestinal Microflora and Metabolites from Mice with DSS-Induced IBD Treated with Schistosoma Soluble Egg Antigen. Front. Cell Dev. Biol. 2021, 9, 777218. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.Q.; Yuan, Z.W.; Zhang, X.S.; Dong, J.Q.; Liu, X.N.; Peng, X.T.; Yao, W.L.; Ji, P.; Wei, Y.M.; Hua, Y.L. Total alkaloids of Sophora alopecuroides L. ameliorated murine colitis by regulating bile acid metabolism and gut microbiota. J. Ethnopharmacol. 2020, 255, 112775. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Liu, A.X.; Liu, K.H.; Zhang, S.; Gong, Z.H.; Xiao, W.J. l-Theanine Alleviates Ulcerative Colitis by Regulating Colon Immunity via the Gut Microbiota in an MHC-II-Dependent Manner. J. Agric. Food Chem. 2024, 72, 19852–19868. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Z.; Liu, X.; Xiao, H.; Liu, Y.; Wang, J.; Chen, C.; Wang, X.; Liu, W.; Xiang, Z.; et al. Therapeutic effect of Yiyi Fuzi Baijiang formula on TNBS-induced ulcerative colitis via metabolism and Th17/Treg cell balance. J. Ethnopharmacol. 2023, 309, 116301. [Google Scholar] [CrossRef]
- Yu, Z.; Li, D.; Sun, H. Herba Origani alleviated DSS-induced ulcerative colitis in mice through remolding gut microbiota to regulate bile acid and short-chain fatty acid metabolisms. Biomed. Pharmacother. 2023, 161, 114409. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.; Wei, J.; Zhang, Y.; Xu, Y.; Yue, T.; Yuan, Y. Protective Mechanism of Eurotium amstelodami from Fuzhuan Brick Tea against Colitis and Gut-Derived Liver Injury Induced by Dextran Sulfate Sodium in C57BL/6 Mice. Nutrients 2024, 16, 1178. [Google Scholar] [CrossRef]
- Shen, J.C.; Qi, Q.; Han, D.; Lu, Y.; Huang, R.; Zhu, Y.; Zhang, L.S.; Qin, X.D.; Zhang, F.; Wu, H.G.; et al. Moxibustion improves experimental colitis in rats with Crohn’s disease by regulating bile acid enterohepatic circulation and intestinal farnesoid X receptor. J. Integr. Med. 2023, 21, 194–204. [Google Scholar] [CrossRef]
- Liu, F.; Yao, Y.; Wang, Q.; Zhang, F.; Wang, M.; Zhu, C.; Lin, C. Nigakinone alleviates DSS-induced experimental colitis via regulating bile acid profile and FXR/NLRP3 signaling pathways. Phytother. Res. 2023, 37, 15–34. [Google Scholar] [CrossRef]
- Li, S.; Zhuge, A.; Chen, H.; Han, S.; Shen, J.; Wang, K.; Xia, J.; Xia, H.; Jiang, S.; Wu, Y.; et al. Sedanolide alleviates DSS-induced colitis by modulating the intestinal FXR-SMPD3 pathway in mice. J. Adv. Res. 2024. [Google Scholar] [CrossRef]
- Li, X.H.; Liu, L.; Wu, W.Z. Trans-Anethole Alleviates DSS-Induced Ulcerative Colitis by Remodeling the Intestinal Flora to Regulate Immunity and Bile Acid Metabolism. Mediat. Inflamm. 2023, 2023, 4188510. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Zhu, M.; Wang, K.; Zhao, X.; Hu, L.; Jing, W.; Lu, H.; Wang, S. Dihydromyricetin improves DSS-induced colitis in mice via modulation of fecal-bacteria-related bile acid metabolism. Pharmacol. Res. 2021, 171, 105767. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Li, W.; Zhang, B.; Yin, J.; Liuqi, S.; Wang, J.; Peng, B.; Wang, S. Fucoidan Ameliorated Dextran Sulfate Sodium-Induced Ulcerative Colitis by Modulating Gut Microbiota and Bile Acid Metabolism. J. Agric. Food Chem. 2022, 70, 14864–14876. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.L.; Jia, Y.Q.; Zhang, X.S.; Yuan, Z.W.; Ji, P.; Hu, J.J.; Wei, Y.M. Baitouweng Tang ameliorates DSS-induced ulcerative colitis through the regulation of the gut microbiota and bile acids via pathways involving FXR and TGR5. Biomed. Pharmacother. 2021, 137, 111320. [Google Scholar] [CrossRef]
- Zhai, Z.; Niu, K.M.; Liu, Y.; Lin, C.; Wu, X. The Gut Microbiota-Bile Acids-TGR5 Axis Mediates Eucommia ulmoides Leaf Extract Alleviation of Injury to Colonic Epithelium Integrity. Front. Microbiol. 2021, 12, 727681. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Ma, X.; Xu, B.; Chen, L.; Chen, C.; Liu, W.; Liu, Y.; Xiang, Z. Therapeutic effect of Patrinia villosa on TNBS-induced ulcerative colitis via metabolism, vitamin D receptor and NF-kappaB signaling pathways. J. Ethnopharmacol. 2022, 288, 114989. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, J.; Du, H.; Zhou, Z.; Han, Y.; Luo, M.; Guo, X.; Gu, M.; Yang, H.; Xiao, H. The Anti-inflammatory Potential of a Strain of Probiotic Bifidobacterium pseudocatenulatum G7: In Vitro and In Vivo Evidence. J. Agric. Food Chem. 2024, 72, 10355–10365. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, Y.; Jiang, J.; Yu, L.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W.; Zhai, Q. Identification of the key characteristics of Bifidobacterium longum strains for the alleviation of ulcerative colitis. Food Funct. 2021, 12, 3476–3492. [Google Scholar] [CrossRef]
- Lv, W.; Zhang, D.; He, T.; Liu, Y.; Shao, L.; Lv, Z.; Pu, X.; Wang, Y.; Liu, L. Combination of Lactobacillus plantarum improves the effects of tacrolimus on colitis in a mouse model. Front. Cell Infect. Microbiol. 2023, 13, 1130820. [Google Scholar] [CrossRef]
- Wong, W.Y.; Chan, B.D.; Sham, T.T.; Lee, M.M.; Chan, C.O.; Chau, C.T.; Mok, D.K.; Kwan, Y.W.; Tai, W.C. Lactobacillus casei Strain Shirota Ameliorates Dextran Sulfate Sodium-Induced Colitis in Mice by Increasing Taurine-Conjugated Bile Acids and Inhibiting NF-kappaB Signaling via Stabilization of IkappaBalpha. Front. Nutr. 2022, 9, 816836. [Google Scholar] [CrossRef]
- Yan, Y.; Lei, Y.; Qu, Y.; Fan, Z.; Zhang, T.; Xu, Y.; Du, Q.; Brugger, D.; Chen, Y.; Zhang, K.; et al. Bacteroides uniformis-induced perturbations in colonic microbiota and bile acid levels inhibit TH17 differentiation and ameliorate colitis developments. NPJ Biofilms Microbiomes 2023, 9, 56. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wang, Y.; Li, C.; Xie, Z.; Dai, L. Amelioration of Colitis by a Gut Bacterial Consortium Producing Anti-Inflammatory Secondary Bile Acids. Microbiol. Spectr. 2023, 11, e0333022. [Google Scholar] [CrossRef] [PubMed]
- Van der Lelie, D.; Oka, A.; Taghavi, S.; Umeno, J.; Fan, T.J.; Merrell, K.E.; Watson, S.D.; Ouellette, L.; Liu, B.; Awoniyi, M.; et al. Rationally designed bacterial consortia to treat chronic immune-mediated colitis and restore intestinal homeostasis. Nat. Commun. 2021, 12, 3105. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, H. Probiotics alleviate inflammatory bowel disease in mice by regulating intestinal microorganisms-bile acid-NLRP3 inflammasome pathway. Acta Biochim. Pol. 2021, 68, 687–693. [Google Scholar] [CrossRef]
- Xiao, F.; Dong, F.; Li, X.; Li, Y.; Yu, G.; Liu, Z.; Wang, Y.; Zhang, T. Bifidobacterium longum CECT 7894 Improves the Efficacy of Infliximab for DSS-Induced Colitis via Regulating the Gut Microbiota and Bile Acid Metabolism. Front. Pharmacol. 2022, 13, 902337. [Google Scholar] [CrossRef]
- He, Q.; Wu, J.; Ke, J.; Zhang, Q.; Zeng, W.; Luo, Z.; Gong, J.; Chen, Y.; He, Z.; Lan, P. Therapeutic role of ursodeoxycholic acid in colitis-associated cancer via gut microbiota modulation. Mol. Ther. 2023, 31, 585–598. [Google Scholar] [CrossRef]
- Gao, R.Y.; Shearn, C.T.; Orlicky, D.J.; Battista, K.D.; Alexeev, E.E.; Cartwright, I.M.; Lanis, J.M.; Kostelecky, R.E.; Ju, C.; Colgan, S.P.; et al. Bile acids modulate colonic MAdCAM-1 expression in a murine model of combined cholestasis and colitis. Mucosal Immunol. 2021, 14, 479–490. [Google Scholar] [CrossRef]
- Yu, J.; Sheng, S.; Zou, X.; Shen, Z. Dihydroartemisinin-ursodeoxycholic acid conjugate is a potential treatment agent for inflammatory bowel disease. Int. Immunopharmacol. 2023, 117, 109918. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, J.; Chen, Z.; Xie, L.; Wang, W. Clinical effects of ursodeoxycholic acid on patients with ulcerative colitis may improve via the regulation of IL-23-IL-17 axis and the changes of the proportion of intestinal microflora. Saudi J. Gastroenterol. 2021, 27, 149–157. [Google Scholar] [CrossRef]
- Kubota, H.; Ishizawa, M.; Kodama, M.; Nagase, Y.; Kato, S.; Makishima, M.; Sakurai, K. Vitamin D Receptor Mediates Attenuating Effect of Lithocholic Acid on Dextran Sulfate Sodium Induced Colitis in Mice. Int. J. Mol. Sci. 2023, 24, 3517. [Google Scholar] [CrossRef]
- Lajczak-McGinley, N.K.; Porru, E.; Fallon, C.M.; Smyth, J.; Curley, C.; McCarron, P.A.; Tambuwala, M.M.; Roda, A.; Keely, S.J. The secondary bile acids, ursodeoxycholic acid and lithocholic acid, protect against intestinal inflammation by inhibition of epithelial apoptosis. Physiol. Rep. 2020, 8, e14456. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Chen, Z.; Fujita, K.; Nishikawa, M.; Ueda, H.; Iguchi, Y.; Une, M.; Nishida, T.; Imura, J. Hyodeoxycholic Acid (HDCA) Prevents Development of Dextran Sulfate Sodium (DSS)-Induced Colitis in Mice: Possible Role of Synergism between DSS and HDCA in Increasing Fecal Bile Acid Levels. Biol. Pharm. Bull. 2022, 45, 1503–1509. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, J.; Ren, X.; Zhang, Y.; Ke, Z.; Zhou, J.; Wang, Y.; Zhang, Y.; Liu, Y. Cholecystectomy-induced secondary bile acids accumulation ameliorates colitis through inhibiting monocyte/macrophage recruitment. Gut Microbes 2022, 14, 2107387. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.S.; Zimmermann, E.M.; Chuang, B.M.; Song, B.; Nwokoye, A.; Wilkinson, J.E.; Eaton, K.A.; Kaufman, R.J. The unfolded protein response and chemical chaperones reduce protein misfolding and colitis in mice. Gastroenterology 2013, 144, 989–1000.e6. [Google Scholar] [CrossRef]
- Zhao, J.; Hao, S.; Chen, Y.; Ye, X.; Fang, P.; Hu, H. Tauroursodeoxycholic acid liposome alleviates DSS-induced ulcerative colitis through restoring intestinal barrier and gut microbiota. Colloids Surf. B Biointerfaces 2024, 236, 113798. [Google Scholar] [CrossRef]
- Huang, K.; Deng, R.S.; Liu, T.C.; Wang, M.; Gremida, A.K.; Deepak, P.; Chen, C.H.; Davidson, N.O.; Kaufman, R.J.; Ciorba, M.A. A Translational Phase I Study of Tauroursodeoxycholic Acid (Tudca) to Reduce Symptoms and Er Stress in Active Ulcerative Colitis. Gastroenterology 2021, 160, S707–S708. [Google Scholar] [CrossRef]
- Lv, L.; Chen, Z.; Bai, W.; Hao, J.; Heng, Z.; Meng, C.; Wang, L.; Luo, X.; Wang, X.; Cao, Y.; et al. Taurohyodeoxycholic acid alleviates trinitrobenzene sulfonic acid induced ulcerative colitis via regulating Th1/Th2 and Th17/Treg cells balance. Life Sci. 2023, 318, 121501. [Google Scholar] [CrossRef]
- Li, W.; Hang, S.; Fang, Y.; Bae, S.; Zhang, Y.; Zhang, M.; Wang, G.; McCurry, M.D.; Bae, M.; Paik, D.; et al. A bacterial bile acid metabolite modulates T(reg) activity through the nuclear hormone receptor NR4A1. Cell Host Microbe 2021, 29, 1366–1377.e9. [Google Scholar] [CrossRef]
- Campbell, C.; McKenney, P.T.; Konstantinovsky, D.; Isaeva, O.I.; Schizas, M.; Verter, J.; Mai, C.; Jin, W.B.; Guo, C.J.; Violante, S.; et al. Bacterial metabolism of bile acids promotes generation of peripheral regulatory T cells. Nature 2020, 581, 475–479. [Google Scholar] [CrossRef]
- Xu, M.; Cen, M.; Shen, Y.; Zhu, Y.; Cheng, F.; Tang, L.; Hu, W.; Dai, N. Deoxycholic Acid-Induced Gut Dysbiosis Disrupts Bile Acid Enterohepatic Circulation and Promotes Intestinal Inflammation. Dig. Dis. Sci. 2021, 66, 568–576. [Google Scholar] [CrossRef]
- Li, D.; Zhou, J.; Wang, L.; Gong, Z.; Le, H.; Huang, Y.; Xu, C.; Tian, C.; Cai, W.; Wu, J. Gut microbial metabolite deoxycholic acid facilitates Th17 differentiation through modulating cholesterol biosynthesis and participates in high-fat diet-associated colonic inflammation. Cell Biosci. 2023, 13, 186. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Zhang, C.; Yang, J.; Yang, Q.; Yin, P.; Sun, X. Deoxycholic acid exacerbates intestinal inflammation by modulating interleukin-1beta expression and tuft cell proportion in dextran sulfate sodium-induced murine colitis. PeerJ 2023, 11, e14842. [Google Scholar] [CrossRef] [PubMed]
- Nakhi, A.; Wong, H.L.; Weldy, M.; Khoruts, A.; Sadowsky, M.J.; Dosa, P.I. Structural modifications that increase gut restriction of bile acid derivatives. RSC Med. Chem. 2021, 12, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Sugaya, T.; Inui, A.; Yoshihara, S. Effectiveness of Ursodeoxycholic Acid in the Treatment of Primary Sclerosing Cholangitis with Ulcerative Colitis: A Pediatric Case. Tohoku J. Exp. Med. 2021, 253, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Qi, M.; Cai, C.; Zhu, Y.; Li, Y.; Coulter, S.; Sun, F.; Liddle, C.; Uboha, N.V.; Halberg, R.; et al. Farnesoid X receptor mediates macrophage-intrinsic responses to suppress colitis-induced colon cancer progression. JCI Insight 2024, 9, 170428. [Google Scholar] [CrossRef]
- Xu, P.; Xi, Y.; Zhu, J.; Zhang, M.; Luka, Z.; Stolz, D.B.; Cai, X.; Xie, Y.; Xu, M.; Ren, S.; et al. Intestinal Sulfation Is Essential to Protect Against Colitis and Colonic Carcinogenesis. Gastroenterology 2021, 161, 271–286.e11. [Google Scholar] [CrossRef]
- Fathima, A.; Jamma, T. UDCA ameliorates inflammation driven EMT by inducing TGR5 dependent SOCS1 expression in mouse macrophages. Sci. Rep. 2024, 14, 24285. [Google Scholar] [CrossRef]
- Kempinska-Podhorodecka, A.; Adamowicz, M.; Ostrycharz, E.; Chmielarz, M.; Wojcicki, M.; Milkiewicz, P.; Milkiewicz, M. Role of miR-506 in ulcerative colitis associated with primary sclerosing cholangitis. Sci. Rep. 2021, 11, 10134. [Google Scholar] [CrossRef]
- Kempinska-Podhorodecka, A.; Blatkiewicz, M.; Wunsch, E.; Krupa, L.; Gutkowski, K.; Milkiewicz, P.; Milkiewicz, M. Oncomir MicroRNA-346 Is Upregulated in Colons of Patients with Primary Sclerosing Cholangitis. Clin. Transl. Gastroenterol. 2020, 11, e00112. [Google Scholar] [CrossRef]
- Lavelle, A.; Nancey, S.; Reimund, J.M.; Laharie, D.; Marteau, P.; Treton, X.; Allez, M.; Roblin, X.; Malamut, G.; Oeuvray, C.; et al. Fecal microbiota and bile acids in IBD patients undergoing screening for colorectal cancer. Gut Microbes 2022, 14, 2078620. [Google Scholar] [CrossRef]
- Rotondo-Trivette, S.; Wang, B.; Gayer, C.; Parsana, R.; Luan, Y.; Sun, F.; Michail, S. Decreased secondary faecal bile acids in children with ulcerative colitis and Clostridioides difficile infection. Aliment. Pharmacol. Ther. 2021, 54, 792–804. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.B.; Li, T.T.; Huo, D.; Qu, S.; Li, X.V.; Arifuzzaman, M.; Lima, S.F.; Shi, H.Q.; Wang, A.; Putzel, G.G.; et al. Genetic manipulation of gut microbes enables single-gene interrogation in a complex microbiome. Cell 2022, 185, 547–562.e22. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.L.; Huang, X.; Wang, H.; Hegner, C.; Liu, Y.; Shang, J.; Eliason, A.; Diao, H.; Park, H.; Frey, B.; et al. CAR directs T cell adaptation to bile acids in the small intestine. Nature 2021, 593, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Shen, Y.; Cen, M.; Zhu, Y.; Cheng, F.; Tang, L.; Zheng, X.; Kim, J.J.; Dai, N.; Hu, W. Modulation of the Gut Microbiota-farnesoid X Receptor Axis Improves Deoxycholic Acid-induced Intestinal Inflammation in Mice. J. Crohns Colitis 2021, 15, 1197–1210. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Shirakami, Y.; Mizutani, T.; Maruta, A.; Ideta, T.; Kubota, M.; Sakai, H.; Ibuka, T.; Genovese, S.; Fiorito, S.; et al. Novel FXR agonist nelumal A suppresses colitis and inflammation-related colorectal carcinogenesis. Sci. Rep. 2021, 11, 492. [Google Scholar] [CrossRef]
- Chen, L.; Jiao, T.; Liu, W.; Luo, Y.; Wang, J.; Guo, X.; Tong, X.; Lin, Z.; Sun, C.; Wang, K.; et al. Hepatic cytochrome P450 8B1 and cholic acid potentiate intestinal epithelial injury in colitis by suppressing intestinal stem cell renewal. Cell Stem Cell 2022, 29, 1366–1381.e9. [Google Scholar] [CrossRef]
- Xin, Z.; Zhai, Z.; Long, H.; Zhang, F.; Ni, X.; Deng, J.; Yi, L.; Deng, B. Metabolic Profiling by UPLC-Orbitrap-MS/MS of Liver from C57BL/6 Mice with DSS-Induced Inflammatory Bowel Disease. Mediat. Inflamm. 2020, 2020, 6020247. [Google Scholar] [CrossRef]
- Hernandez-Rocha, C.; Borowski, K.; Turpin, W.; Filice, M.; Nayeri, S.; Raygoza Garay, J.A.; Stempak, J.M.; Silverberg, M.S. Integrative Analysis of Colonic Biopsies from Inflammatory Bowel Disease Patients Identifies an Interaction Between Microbial Bile Acid-inducible Gene Abundance and Human Angiopoietin-like 4 Gene Expression. J. Crohns Colitis 2021, 15, 2078–2087. [Google Scholar] [CrossRef]
- Yan, S.; Du, R.; Yao, W.; Zhang, H.; Xue, Y.; Teligun; Li, Y.; Bao, H.; Zhao, Y.; Cao, S.; et al. Host-microbe interaction-mediated resistance to DSS-induced inflammatory enteritis in sheep. Microbiome 2024, 12, 208. [Google Scholar] [CrossRef]
- Gadaleta, R.M.; Garcia-Irigoyen, O.; Cariello, M.; Scialpi, N.; Peres, C.; Vetrano, S.; Fiorino, G.; Danese, S.; Ko, B.; Luo, J.; et al. Fibroblast Growth Factor 19 modulates intestinal microbiota and inflammation in presence of Farnesoid X Receptor. eBioMedicine 2020, 54, 102719. [Google Scholar] [CrossRef]
- He, S.; Li, J.; Yao, Z.; Gao, Z.; Jiang, Y.; Chen, X.; Peng, L. Insulin alleviates murine colitis through microbiome alterations and bile acid metabolism. J. Transl. Med. 2023, 21, 498. [Google Scholar] [CrossRef]
- Foley, S.E.; Tuohy, C.; Dunford, M.; Grey, M.J.; De Luca, H.; Cawley, C.; Szabady, R.L.; Maldonado-Contreras, A.; Houghton, J.M.; Ward, D.V.; et al. Gut microbiota regulation of P-glycoprotein in the intestinal epithelium in maintenance of homeostasis. Microbiome 2021, 9, 183. [Google Scholar] [CrossRef]
- Zhao, Y.; Xiang, Z.; Pan, H.; Huang, X.; Chen, W.; Huang, Z. FGL2 improves experimental colitis related to gut microbiota structure and bile acid metabolism by regulating macrophage autophagy and apoptosis. Heliyon 2024, 10, e34349. [Google Scholar] [CrossRef]
- Peters, D.E.; Norris, L.D.; Tenora, L.; Snajdr, I.; Ponti, A.K.; Zhu, X.; Sakamoto, S.; Veeravalli, V.; Pradhan, M.; Alt, J.; et al. A gut-restricted glutamate carboxypeptidase II inhibitor reduces monocytic inflammation and improves preclinical colitis. Sci. Transl. Med. 2023, 15, eabn7491. [Google Scholar] [CrossRef]
- Gonzalez, C.G.; Mills, R.H.; Kordahi, M.C.; Carrillo-Terrazas, M.; Secaira-Morocho, H.; Widjaja, C.E.; Tsai, M.S.; Mittal, Y.; Yee, B.A.; Vargas, F.; et al. The Host-Microbiome Response to Hyperbaric Oxygen Therapy in Ulcerative Colitis Patients. Cell Mol. Gastroenterol. Hepatol. 2022, 14, 35–53. [Google Scholar] [CrossRef]
- Liu, A.; Li, C.; Wang, C.; Liang, X.; Zhang, X. Impact of Mesenchymal Stem Cells on the Gut Microbiota and Microbiota Associated Functions in Inflammatory Bowel Disease: A Systematic Review of Preclinical Evidence on Animal Models. Curr. Stem Cell Res. Ther. 2024, 19, 981–992. [Google Scholar] [CrossRef]
- Yang, F.; Ni, B.; Liu, Q.; He, F.; Li, L.; Zhong, X.; Zheng, X.; Lu, J.; Chen, X.; Lin, H.; et al. Human umbilical cord-derived mesenchymal stem cells ameliorate experimental colitis by normalizing the gut microbiota. Stem Cell Res. Ther. 2022, 13, 475. [Google Scholar] [CrossRef]
- Ocansey, D.K.W.; Zhang, Z.; Xu, X.; Liu, L.; Amoah, S.; Chen, X.; Wang, B.; Zhang, X.; Mao, F. Mesenchymal stem cell-derived exosome mitigates colitis via the modulation of the gut metagenomics-metabolomics-farnesoid X receptor axis. Biomater. Sci. 2022, 10, 4822–4836. [Google Scholar] [CrossRef]
- Mohanty, I.; Mannochio-Russo, H.; Schweer, J.V.; El Abiead, Y.; Bittremieux, W.; Xing, S.; Schmid, R.; Zuffa, S.; Vasquez, F.; Muti, V.B.; et al. The underappreciated diversity of bile acid modifications. Cell 2024, 187, 1801–1818.e20. [Google Scholar] [CrossRef]
- Vich Vila, A.; Zhang, J.; Liu, M.; Faber, K.N.; Weersma, R.K. Untargeted faecal metabolomics for the discovery of biomarkers and treatment targets for inflammatory bowel diseases. Gut 2024, 73, 1909–1920. [Google Scholar] [CrossRef]
| Bile Acids | Pooled IBD Samples | Crohn’s Disease | Ulcerative Colitis |
|---|---|---|---|
| Primary Bile Acids | |||
| CA | ↑ [44,45] | ↑ [55,56] | |
| CDCA | ↑ [44] ↓ [38] | ↑ [56] ↓ [38,64] | |
| GCA | ↑ [37] | ↑ [46,47] | ↑ [56] ↓ [64] |
| GCDCA | ↓ [46] | ↑ [55,57] ↓ [64] | |
| GHDCA | ↓ [50] | ||
| TCA | ↓ [46] | ↑ [55,56] | |
| TCDCA | ↑ [55,57] | ||
| Secondary Bile Acids | |||
| DCA | ↓ [38] | ↑ [47,51] ↓ [48,49] | ↓ [50,55,56] |
| GDCA | ↓ [38] | ↑ [46,47] | ↓ [55] |
| GLCA | ↓ [38] | ↓ [55] | |
| HDCA | ↓ [56] | ||
| isoLCA | ↓ [39] | ↑ [47] | ↓ [56] |
| 12-KLCA | ↓ [56] | ||
| LCA | ↓ [38] | ↓ [46,48,49] | ↓ [50,55,56,64] |
| 3-oxoLCA | ↓ [39] | ||
| TDCA | ↓ [38] | ↑ [47] | |
| TLCA | ↓ [38] | ↓ [55] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, S.H.; Chandnani, A.; Cao, S. Bile Acids in Inflammatory Bowel Disease: From Pathophysiology to Treatment. Biomedicines 2024, 12, 2910. https://doi.org/10.3390/biomedicines12122910
Bai SH, Chandnani A, Cao S. Bile Acids in Inflammatory Bowel Disease: From Pathophysiology to Treatment. Biomedicines. 2024; 12(12):2910. https://doi.org/10.3390/biomedicines12122910
Chicago/Turabian StyleBai, Samantha H., Arun Chandnani, and Siyan Cao. 2024. "Bile Acids in Inflammatory Bowel Disease: From Pathophysiology to Treatment" Biomedicines 12, no. 12: 2910. https://doi.org/10.3390/biomedicines12122910
APA StyleBai, S. H., Chandnani, A., & Cao, S. (2024). Bile Acids in Inflammatory Bowel Disease: From Pathophysiology to Treatment. Biomedicines, 12(12), 2910. https://doi.org/10.3390/biomedicines12122910





