GABALAGEN Alleviates Stress-Induced Sleep Disorders in Rats
Abstract
1. Introduction
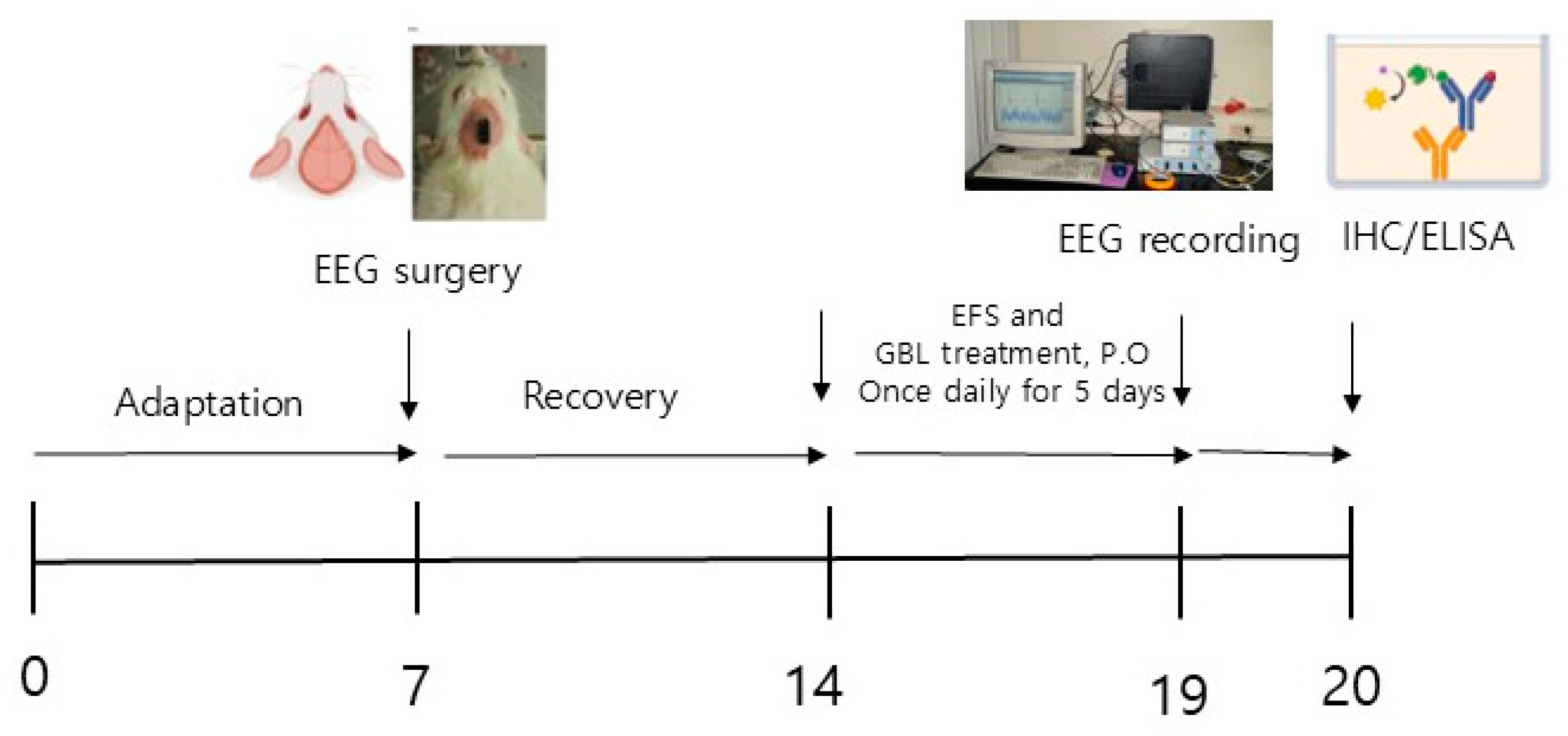
2. Materials and Methods
2.1. Preparation of GBL
2.2. GABAA Receptor Binding Assay
2.3. Animals
2.4. Surgery
2.5. EEG Recording
2.6. Immunohistochemistry
2.7. ELISA (Enzyme-Linked Immunosorbent Assay)
2.8. Statistical Analysis
3. Results
3.1. GABAA and 5-HT2c Receptor Binding Assay
3.2. Effect of GBL on EEG Sleep Architecture
3.3. The Effect of GBL on the Number of GABAA Receptor Positive Cells in the Ventrolateral Preoptic Nucleus (VLPO)
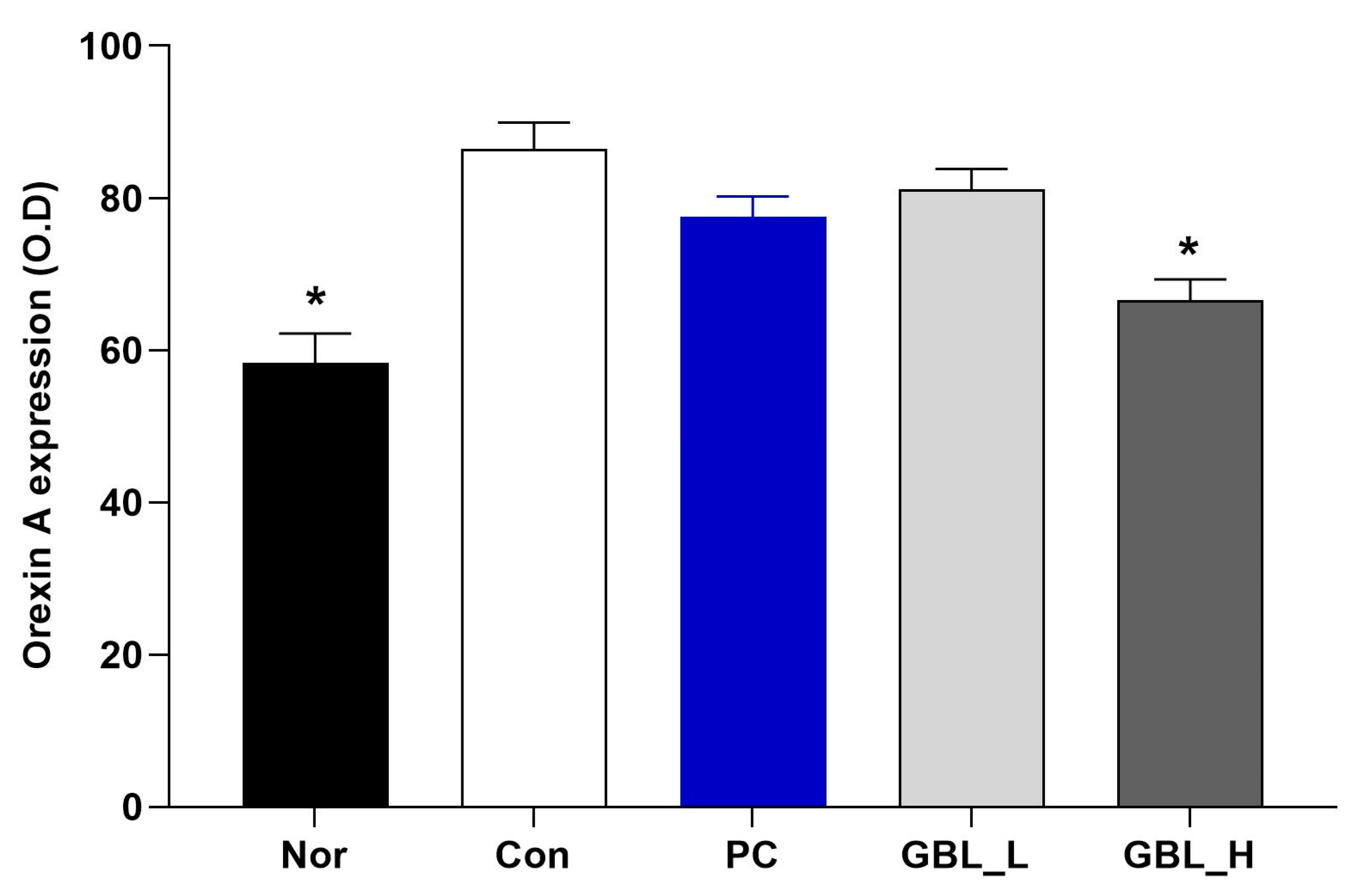
3.4. The Effect of GBL on the Orexin Level in the Lateral Hypothalamus (LH)
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arrigoni, E.; Fuller, P.M. The Sleep-Promoting Ventrolateral Preoptic Nucleus: What Have We Learned over the Past 25 Years? Int. J. Mol. Sci. 2022, 23, 2905. [Google Scholar] [CrossRef] [PubMed]
- Wakizono, T.; Sawamura, T.; Shimizu, K.; Nibuya, M.; Suzuki, G.; Toda, H.; Hirano, J.; Kikuchi, A.; Takahashi, Y.; Nomura, S. Stress vulnerabilities in an animal model of post-traumatic stress disorder. Physiol. Behav. 2007, 90, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.M.; Zhou, W.W.; Ji, Y.J.; Li, Y.; Zhao, N.; Chen, H.X.; Xue, R.; Mei, X.G.; Zhang, Y.Z.; Wang, H.L.; et al. Anxiolytic effects of ketamine in animal models of posttraumatic stress disorder. Psychopharmacology 2015, 232, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Cohen, Z.L.; Eigenberger, P.M.; Sharkey, K.M.; Conroy, M.L.; Wilkins, K.M. Insomnia and Other Sleep Disorders in Older Adults. Psychiatr. Clin. N. Am. 2022, 45, 717–734. [Google Scholar] [CrossRef]
- Dopheide, J.A. Insomnia overview: Epidemiology, pathophysiology, diagnosis and monitoring, and nonpharmacologic therapy. Am. J. Manag. Care 2020, 26, S76–S84. [Google Scholar] [CrossRef]
- Pawlyk, A.C.; Morrison, A.R.; Ross, R.J.; Brennan, F.X. Stress-induced changes in sleep in rodents: Models and mechanisms. Neurosci. Biobehav. Rev. 2008, 32, 99–117. [Google Scholar] [CrossRef]
- DaSilva, J.K.; Lei, Y.; Madan, V.; Mann, G.L.; Ross, R.J.; Tejani-Butt, S.; Morrison, A.R. Fear conditioning fragments REM sleep in stress-sensitive Wistar-Kyoto, but not Wistar, rats. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 67–73. [Google Scholar] [CrossRef]
- Polta, S.A.; Fenzl, T.; Jakubcakova, V.; Kimura, M.; Yassouridis, A.; Wotjak, C.T. Prognostic and symptomatic aspects of rapid eye movement sleep in a mouse model of posttraumatic stress disorder. Front. Behav. Neurosci. 2013, 7, 60. [Google Scholar] [CrossRef]
- Vazquez-Palacios, G.; Velazquez-Moctezuma, J. Effect of electric foot shocks, immobilization, and corticosterone administration on the sleep-wake pattern in the rat. Physiol. Behav. 2000, 71, 23–28. [Google Scholar] [CrossRef]
- El-Khoury, F.; Ben Ghezala, I.; Hatem, G.; Jaffal, Z.; Soares, A.; Yacini, L.; Duchesne, S.; Dommergues, M.; Bretelle, F.; Eudeline, S.; et al. IROND-L: Study protocol for a French prospective, quasi-experimental, multicentre trial to examine the impact of a coordinated multidisciplinary approach for women victims of violence. BMJ Open 2024, 14, e086143. [Google Scholar] [CrossRef]
- Bassetti, C.L.A.; Adamantidis, A.; Burdakov, D.; Han, F.; Gay, S.; Kallweit, U.; Khatami, R.; Koning, F.; Kornum, B.R.; Lammers, G.J.; et al. Narcolepsy—Clinical spectrum, aetiopathophysiology, diagnosis and treatment. Nat. Rev. Neurol. 2019, 15, 519–539. [Google Scholar] [CrossRef] [PubMed]
- Blattner, M.; Maski, K. Narcolepsy and Idiopathic Hypersomnia. Sleep Med. Clin. 2023, 18, 183–199. [Google Scholar] [CrossRef] [PubMed]
- Chemelli, R.M.; Willie, J.T.; Sinton, C.M.; Elmquist, J.K.; Scammell, T.; Lee, C.; Richardson, J.A.; Williams, S.C.; Xiong, Y.; Kisanuki, Y.; et al. Narcolepsy in orexin knockout mice: Molecular genetics of sleep regulation. Cell 1999, 98, 437–451. [Google Scholar] [CrossRef] [PubMed]
- Kornum, B.R.; Knudsen, S.; Ollila, H.M.; Pizza, F.; Jennum, P.J.; Dauvilliers, Y.; Overeem, S. Narcolepsy. Nat. Rev. Dis. Primers 2017, 3, 16100. [Google Scholar] [CrossRef]
- Lin, J.; Luo, Z.; Fan, M.; Liu, Y.; Shi, X.; Cai, Y.; Yang, Z.; Chen, L.; Pan, J. Abnormal hypothalamic functional connectivity and serum arousal-promoting neurotransmitters in insomnia disorder patients: A pilot study. PeerJ 2024, 12, e18540. [Google Scholar] [CrossRef]
- Toossi, H.; Del Cid-Pellitero, E.; Jones, B.E. GABA Receptors on Orexin and Melanin-Concentrating Hormone Neurons Are Differentially Homeostatically Regulated Following Sleep Deprivation. eNeuro 2016, 3, 1–11. [Google Scholar] [CrossRef]
- Eggermann, E.; Bayer, L.; Serafin, M.; Saint-Mleux, B.; Bernheim, L.; Machard, D.; Jones, B.E.; Mühlethaler, M. The wake-promoting hypocretin-orexin neurons are in an intrinsic state of membrane depolarization. J. Neurosci. Off. J. Soc. Neurosci. 2003, 23, 1557–1562. [Google Scholar] [CrossRef]
- Kilman, V.; van Rossum, M.C.; Turrigiano, G.G. Activity deprivation reduces miniature IPSC amplitude by decreasing the number of postsynaptic GABA(A) receptors clustered at neocortical synapses. J. Neurosci. Off. J. Soc. Neurosci. 2002, 22, 1328–1337. [Google Scholar] [CrossRef]
- Xie, X.; Crowder, T.L.; Yamanaka, A.; Morairty, S.R.; Lewinter, R.D.; Sakurai, T.; Kilduff, T.S. GABA(B) receptor-mediated modulation of hypocretin/orexin neurones in mouse hypothalamus. J. Physiol. 2006, 574, 399–414. [Google Scholar] [CrossRef]
- Parker, D.A.; Marino, V.; Ong, J. Pharmacological actions of thymol and an analogue at GABAB autoreceptors. Clin. Exp. Pharmacol. Physiol. 2014, 41, 623–627. [Google Scholar] [CrossRef]
- Premoli, I.; Rivolta, D.; Espenhahn, S.; Castellanos, N.; Belardinelli, P.; Ziemann, U.; Müller-Dahlhaus, F. Characterization of GABAB-receptor mediated neurotransmission in the human cortex by paired-pulse TMS-EEG. NeuroImage 2014, 103, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Bollu, P.C.; Kaur, H. Sleep Medicine: Insomnia and Sleep. Mo. Med. 2019, 116, 68–75. [Google Scholar] [PubMed]
- Gottesmann, C. GABA mechanisms and sleep. Neuroscience 2002, 111, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jo, K.; Hong, K.B.; Han, S.H.; Suh, H.J. GABA and l-theanine mixture decreases sleep latency and improves NREM sleep. Pharm. Biol. 2019, 57, 65–73. [Google Scholar] [CrossRef]
- Campo-Soria, C.; Chang, Y.; Weiss, D.S. Mechanism of action of benzodiazepines on GABAA receptors. Br. J. Pharmacol. 2006, 148, 984–990. [Google Scholar] [CrossRef]
- Yamatsu, A.; Yamashita, Y.; Maru, I.; Yang, J.; Tatsuzaki, J.; Kim, M. The Improvement of Sleep by Oral Intake of GABA and Apocynum venetum Leaf Extract. J. Nutr. Sci. Vitaminol. 2015, 61, 182–187. [Google Scholar] [CrossRef]
- Casertano, M.; Fryganas, C.; Valentino, V.; Troise, A.D.; Vitaglione, P.; Fogliano, V.; Ercolini, D. Gut production of GABA by a probiotic formula: An in vitro study. Benef. Microbes 2024, 15, 67–81. [Google Scholar] [CrossRef]
- Cataldo, P.G.; Urquiza Martínez, M.P.; Villena, J.; Kitazawa, H.; Saavedra, L.; Hebert, E.M. Comprehensive characterization of γ-aminobutyric acid (GABA) production by Levilactobacillus brevis CRL 2013: Insights from physiology, genomics, and proteomics. Front. Microbiol. 2024, 15, 1408624. [Google Scholar] [CrossRef]
- Chen, M.; Xia, H.; Zuo, X.; Tang, D.; Zhou, H.; Huang, Z.; Guo, A.; Lv, J. Screening and characterization of lactic acid bacteria and fermentation of gamma-aminobutyric acid-enriched bamboo shoots. Front. Microbiol. 2024, 15, 1333538. [Google Scholar] [CrossRef]
- Qadi, W.S.M.; Mediani, A.; Kasim, Z.M.; Misnan, N.M.; Sani, N.A.; Jamar, N.H. Biological Characterization and Metabolic Variations among Cell-Free Supernatants Produced by Selected Plant-Based Lactic Acid Bacteria. Metabolites 2023, 13, 849. [Google Scholar] [CrossRef]
- Kang, Y.M.; Lee, B.J.; Kim, J.I.; Nam, B.H.; Cha, J.Y.; Kim, Y.M.; Ahn, C.B.; Choi, J.S.; Choi, I.S.; Je, J.Y. Antioxidant effects of fermented sea tangle (Laminaria japonica) by Lactobacillus brevis BJ20 in individuals with high level of γ-GT: A randomized, double-blind, and placebo-controlled clinical study. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2012, 50, 1166–1169. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, H.; Suh, H.J.; Choi, H.S. Lactobacillus brevis-Fermented Gamma-Aminobutyric Acid Ameliorates Depression- and Anxiety-Like Behaviors by Activating the Brain-Derived Neurotrophic Factor-Tropomyosin Receptor Kinase B Signaling Pathway in BALB/C Mice. J. Agric. Food Chem. 2024, 72, 2977–2988. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, H.; Chang, Y.B.; Han, K.; Choi, H.S.; Han, S.H.; Suh, H.J. Lactobacillus brevis M2-Fermented Whey Protein Hydrolysate Increases Slow-Wave Sleep via GABA(A) Receptors in Rodent Models. Foods 2024, 13, 2049. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C.; Pennisi, M.; Topple, A. Bregma, lambda and the interaural midpoint in stereotaxic surgery with rats of different sex, strain and weight. J. Neurosci. Methods 1985, 13, 139–143. [Google Scholar] [CrossRef]
- Norouzi, E.; Naseri, A.; Rezaie, L.; Bender, A.M.; Salari, N.; Khazaie, H. Combined mindfulness-based stress reduction and physical activity improved psychological factors and sleep quality in patients with MDD: A randomized controlled trial study. Arch. Psychiatr. Nurs. 2024, 53, 215–223. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, H.; Li, J.; Lv, Z.; Tian, Y.; Lei, X. Gene expression is associated with brain function of insomnia disorder, rather than brain structure. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2024, 136, 111209. [Google Scholar] [CrossRef]
- Reffi, A.N.; Kalmbach, D.A.; Cheng, P.; Drake, C.L. The sleep response to stress: How sleep reactivity can help us prevent insomnia and promote resilience to trauma. J. Sleep Res. 2023, 32, e13892. [Google Scholar] [CrossRef]
- Brown, B.; Jones, E.C.; Clark, K.P.; Jefferson, F. Sleep disturbances and post-traumatic stress disorder in women. Neuro Endocrinol. Lett. 2014, 35, 560–566. [Google Scholar]
- Horenstein, A.; Morrison, A.S.; Goldin, P.; Ten Brink, M.; Gross, J.J.; Heimberg, R.G. Sleep quality and treatment of social anxiety disorder. Anxiety Stress Coping 2019, 32, 387–398. [Google Scholar] [CrossRef]
- Meinhausen, C.; Prather, A.A.; Sumner, J.A. Posttraumatic stress disorder (PTSD), sleep, and cardiovascular disease risk: A mechanism-focused narrative review. Health Psychol. Off. J. Div. Health Psychol. Am. Psychol. Assoc. 2022, 41, 663–673. [Google Scholar] [CrossRef]
- Miller, K.E.; Brownlow, J.A.; Woodward, S.; Gehrman, P.R. Sleep and Dreaming in Posttraumatic Stress Disorder. Curr. Psychiatry Rep. 2017, 19, 71. [Google Scholar] [CrossRef] [PubMed]
- Moore, B.A.; Brock, M.S.; Brager, A.; Collen, J.; LoPresti, M.; Mysliwiec, V. Posttraumatic Stress Disorder, Traumatic Brain Injury, Sleep, and Performance in Military Personnel. Sleep Med. Clin. 2020, 15, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liu, B.; Liu, S.; Wu, D.J.H.; Wang, J.; Qian, Y.; Ye, D.; Mao, Y. Sleep disturbance and psychiatric disorders: A bidirectional Mendelian randomisation study. Epidemiol. Psychiatr. Sci. 2022, 31, e26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ren, R.; Yang, L.H.; Tang, X.D. A Review of the Posttraumatic Stress Disorder and Sleep Disturbances. Sichuan Da Xue Xue Bao. Yi Xue Ban = J. Sichuan University. Med. Sci. Ed. 2021, 52, 28–32. [Google Scholar] [CrossRef]
- Venner, A.; Anaclet, C.; Broadhurst, R.Y.; Saper, C.B.; Fuller, P.M. A Novel Population of Wake-Promoting GABAergic Neurons in the Ventral Lateral Hypothalamus. Curr. Biol. CB 2016, 26, 2137–2143. [Google Scholar] [CrossRef]
- Huo, C.; Lombardi, F.; Blanco-Centurion, C.; Shiromani, P.J.; Ivanov, P.C. Role of the Locus Coeruleus Arousal Promoting Neurons in Maintaining Brain Criticality across the Sleep-Wake Cycle. J. Neurosci. Off. J. Soc. Neurosci. 2024, 44, 1–10. [Google Scholar] [CrossRef]
- Venner, A.; De Luca, R.; Sohn, L.T.; Bandaru, S.S.; Verstegen, A.M.J.; Arrigoni, E.; Fuller, P.M. An Inhibitory Lateral Hypothalamic-Preoptic Circuit Mediates Rapid Arousals from Sleep. Curr. Biol. CB 2019, 29, 4155–4168.e5. [Google Scholar] [CrossRef]
- Cao, Q.; Jiang, Y.; Cui, S.Y.; Tu, P.F.; Chen, Y.M.; Ma, X.L.; Cui, X.Y.; Huang, Y.L.; Ding, H.; Song, J.Z.; et al. Tenuifolin, a saponin derived from Radix Polygalae, exhibits sleep-enhancing effects in mice. Phytomedicine Int. J. Phytother. Phytopharm. 2016, 23, 1797–1805. [Google Scholar] [CrossRef]
- Boi, L.; Johansson, Y.; Tonini, R.; Moratalla, R.; Fisone, G.; Silberberg, G. Serotonergic and dopaminergic neurons in the dorsal raphe are differentially altered in a mouse model for parkinsonism. eLife 2024, 12, 1–20. [Google Scholar] [CrossRef]
- Mendiguren, A.; Aostri, E.; Pineda, J. Modulation of Noradrenergic and Serotonergic Systems by Cannabinoids: Electrophysiological, Neurochemical and Behavioral Evidence. Adv. Exp. Med. Biol. 2021, 1297, 111–132. [Google Scholar] [CrossRef]
- Oikonomou, G.; Altermatt, M.; Zhang, R.W.; Coughlin, G.M.; Montz, C.; Gradinaru, V.; Prober, D.A. The Serotonergic Raphe Promote Sleep in Zebrafish and Mice. Neuron 2019, 103, 686–701.e8. [Google Scholar] [CrossRef] [PubMed]
- Rancillac, A. Serotonin and sleep-promoting neurons. Oncotarget 2016, 7, 78222–78223. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.C.; Maejima, T.; Nishitani, M.; Hasegawa, E.; Yanagawa, Y.; Mieda, M.; Sakurai, T. Monoamines Inhibit GABAergic Neurons in Ventrolateral Preoptic Area That Make Direct Synaptic Connections to Hypothalamic Arousal Neurons. J. Neurosci. Off. J. Soc. Neurosci. 2018, 38, 6366–6378. [Google Scholar] [CrossRef] [PubMed]
- Wisden, W.; Yu, X.; Franks, N.P. GABA Receptors and the Pharmacology of Sleep. Handb. Exp. Pharmacol. 2019, 253, 279–304. [Google Scholar] [CrossRef]
- Bruni, O.; Ferini-Strambi, L.; Giacomoni, E.; Pellegrino, P. Herbal Remedies and Their Possible Effect on the GABAergic System and Sleep. Nutrients 2021, 13, 530. [Google Scholar] [CrossRef]
- Brown, R.E.; Spratt, T.J.; Kaplan, G.B. Translational approaches to influence sleep and arousal. Brain Res. Bull. 2022, 185, 140–161. [Google Scholar] [CrossRef]
- Min, B.; Ahn, Y.; Cho, H.J.; Kwak, W.K.; Jo, K.; Suh, H.J. Chemical compositions and sleep-promoting activities of hop (Humulus lupulus L.) varieties. J. Food Sci. 2023, 88, 2217–2228. [Google Scholar] [CrossRef]
- Mabunga, D.F.; Gonzales, E.L.; Kim, H.J.; Choung, S.Y. Treatment of GABA from Fermented Rice Germ Ameliorates Caffeine-Induced Sleep Disturbance in Mice. Biomol. Ther. 2015, 23, 268–274. [Google Scholar] [CrossRef]
- Byun, J.I.; Shin, Y.Y.; Chung, S.E.; Shin, W.C. Safety and Efficacy of Gamma-Aminobutyric Acid from Fermented Rice Germ in Patients with Insomnia Symptoms: A Randomized, Double-Blind Trial. J. Clin. Neurol. 2018, 14, 291–295. [Google Scholar] [CrossRef]
- Jones, B.E. Arousal and sleep circuits. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2020, 45, 6–20. [Google Scholar] [CrossRef]
- Mogavero, M.P.; Godos, J.; Grosso, G.; Caraci, F.; Ferri, R. Rethinking the Role of Orexin in the Regulation of REM Sleep and Appetite. Nutrients 2023, 15, 3679. [Google Scholar] [CrossRef] [PubMed]
- Scammell, T.E.; Arrigoni, E.; Lipton, J.O. Neural Circuitry of Wakefulness and Sleep. Neuron 2017, 93, 747–765. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.D.; Kilduff, T.S. The Neurobiology of Sleep and Wakefulness. Psychiatr. Clin. N. Am. 2015, 38, 615–644. [Google Scholar] [CrossRef] [PubMed]
- Falup-Pecurariu, C.; Diaconu, Ș.; Țînț, D.; Falup-Pecurariu, O. Neurobiology of sleep (Review). Exp. Ther. Med. 2021, 21, 272. [Google Scholar] [CrossRef]
- Koob, G.F.; Colrain, I.M. Alcohol use disorder and sleep disturbances: A feed-forward allostatic framework. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2020, 45, 141–165. [Google Scholar] [CrossRef]
- Luppi, P.H.; Fort, P. Sleep-wake physiology. Handb. Clin. Neurol. 2019, 160, 359–370. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.-J.; Rhie, S.J.; Jeong, W.; Kim, K.-R.; Rheu, K.-M.; Lee, B.-J.; Shim, I. GABALAGEN Alleviates Stress-Induced Sleep Disorders in Rats. Biomedicines 2024, 12, 2905. https://doi.org/10.3390/biomedicines12122905
Park H-J, Rhie SJ, Jeong W, Kim K-R, Rheu K-M, Lee B-J, Shim I. GABALAGEN Alleviates Stress-Induced Sleep Disorders in Rats. Biomedicines. 2024; 12(12):2905. https://doi.org/10.3390/biomedicines12122905
Chicago/Turabian StylePark, Hyun-Jung, Sung Ja Rhie, Woojin Jeong, Kyu-Ri Kim, Kyoung-Min Rheu, Bae-Jin Lee, and Insop Shim. 2024. "GABALAGEN Alleviates Stress-Induced Sleep Disorders in Rats" Biomedicines 12, no. 12: 2905. https://doi.org/10.3390/biomedicines12122905
APA StylePark, H.-J., Rhie, S. J., Jeong, W., Kim, K.-R., Rheu, K.-M., Lee, B.-J., & Shim, I. (2024). GABALAGEN Alleviates Stress-Induced Sleep Disorders in Rats. Biomedicines, 12(12), 2905. https://doi.org/10.3390/biomedicines12122905





