Sclerostin and Cardiovascular Risk: Evaluating the Cardiovascular Safety of Romosozumab in Osteoporosis Treatment
Abstract
1. Introduction
2. Literature Search Method for Review
- studies that specifically addressed the effects of sclerostin or its inhibitor, romosozumab, on cardiovascular health;
- studies that employed either observational or interventional designs and were published in peer-reviewed journals within the past 5 years;
- high-quality systemic reviews and meta-analyses that synthesized relevant primary research and contributed to understanding broader trends or confirming key findings;
- commentaries on other articles that introduced novel perspectives or offered unique interpretations relevant to the cardiovascular implications of sclerostin inhibitors;
- studies were restricted to those published in English to ensure consistency and feasibility in the analysis.
- did not directly address the effects of sclerostin or romosozumab on cardiovascular health;
- were commentaries on other articles without introducing novel perspectives;
- featured author responses to comments that did not offer new insights.
3. Sclerostin and Cardiovascular Risk Factors
4. Sclerostin and Cardioprotective Role
5. Sclerostin Inhibitors and Cardiovascular Events
6. Efficacy and Safety of Romosozumab in Men with Osteoporosis
7. Pretreatment Assessments for Romosozumab Prescription
7.1. Cardiovascular History and Risk Factors
7.2. Baseline Cardiovascular Assessments
- electrocardiogram (ECG) and echocardiogram to detect any abnormalities in heart rhythm or structure;
- blood pressure measurements and lipid profile assessments to obtain insights into cardiovascular health;
7.3. Monitoring During Treatment
- ongoing blood pressure monitoring to detect any signs of emerging hypertension;
- regular monitoring of glucose and lipid levels, particularly for patients with diabetes or metabolic conditions;
7.4. Lifestyle Modifications and Education
7.5. Personalized Risk–Benefit Analysis
8. Limitations and Future Directions
8.1. Study Design and Duration
8.2. Inclusion of Diverse Patient Populations
8.3. Cardiovascular Risk Assessment
8.4. Publication Bias and Language Bias
8.5. Exploring Alternative Therapeutic Targets
8.6. Individualized Risk Stratification
8.7. Future Research Directions
- Long-term cardiovascular safety of romosozumab in diverse populations, particularly those with preexisting cardiovascular conditions. The current body of evidence on romosozumab is limited by short follow-up durations in clinical trials, typically 1–2 years. This timeframe may be insufficient to fully assess long-term cardiovascular outcomes, such as major adverse cardiovascular events (MACE), heart failure, or progressive vascular calcification. Future randomized controlled trials (RCTs) should involve extended follow-up periods of 5–10 years to capture chronic cardiovascular risks;
- Mechanistic studies to elucidate the impact of sclerostin inhibition on the Wnt/β-catenin pathway in both bone and vascular tissues. Advanced mechanistic studies using preclinical models, such as genetically modified animals, organ-on-chip systems, and three-dimensional vascular tissue cultures, could elucidate these molecular interactions. Techniques like CRISPR-Cas9 genome editing and transcriptomic profiling could identify the specific signaling nodes linking sclerostin inhibition to both beneficial and adverse cardiovascular effects;
- Development of novel therapies that retain the bone-building benefits of romosozumab while minimizing the associated cardiovascular risks. Given the cardiovascular concerns linked to romosozumab due to its inhibition of sclerostin, future research should focus on selectively targeting specific structural domains of sclerostin. The study by Yu et al. [41] highlights that sclerostin’s loop 3 plays a central role in inhibiting bone formation while being non-essential for cardiovascular protection [41]. Genetic truncation and pharmacological inhibition of loop3 maintained sclerostin’s cardiovascular protective effects while promoting bone formation;
- Creation of personalized risk assessment models that integrate genetic, metabolic, and cardiovascular markers to optimize treatment outcomes. Future research should involve patient-centered models that incorporate quality-of-life assessments and long-term treatment satisfaction. Real-world data from patient-reported outcomes could inform shared decision-making processes, balancing osteoporosis treatment benefits with potential cardiovascular risks.
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McClung, M.R.; Grauer, A.; Boonen, S.; Bolognese, M.A.; Brown, J.P.; Diez-Perez, A.; Langdahl, B.L.; Reginster, J.Y.; Zanchetta, J.R.; Wasserman, S.M.; et al. Romosozumab in postmenopausal women with low bone mineral density. N. Engl. J. Med. 2014, 370, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Cummings, S.R.; Melton, L.J. Epidemiology and outcomes of osteoporotic fractures. Lancet 2002, 359, 1761–1767. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.J.; Abrahamsen, B. Cardiovascular Safety of Antifracture Medications in Patients With Osteoporosis: A Narrative Review of Evidence From Randomized Studies. JBMR Plus 2021, 5, e10522. [Google Scholar] [CrossRef] [PubMed]
- Laroche, M.; Pécourneau, V.; Blain, H.; Breuil, V.; Chapurlat, R.; Cortet, B.; Sutter, B.; Degboe, Y.; GRIO scientific committee. Osteoporosis and ischemic cardiovascular disease. Jt. Bone Spine 2017, 84, 427–432. [Google Scholar] [CrossRef]
- Lello, S.; Capozzi, A.; Scambia, G. Osteoporosis and cardiovascular disease: An update. Gynecol. Endocrinol. 2015, 31, 590–594. [Google Scholar] [CrossRef]
- Sardu, C.; Paolisso, G.; Marfella, R. Inflammatory Related Cardiovascular Diseases: From Molecular Mechanisms to Therapeutic Targets. Curr. Pharm. Des. 2020, 26, 2565–2573. [Google Scholar] [CrossRef]
- Lian, X.L.; Zhang, Y.P.; Li, X.; Jing, L.D.; Cairang, Z.M.; Gou, J.Q. Exploration on the relationship between the elderly osteoporosis and cardiovascular disease risk factors. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 4386–4390. [Google Scholar]
- Aditya, S.; Rattan, A. Sclerostin Inhibition: A Novel Target for the Treatment of Postmenopausal Osteoporosis. J. Mid-Life Health 2021, 12, 267–275. [Google Scholar] [CrossRef]
- Cosman, F.; Crittenden, D.B.; Adachi, J.D.; Binkley, N.; Czerwinski, E.; Ferrari, S.; Hofbauer, L.C.; Lau, E.; Lewiecki, E.M.; Miyauchi, A.; et al. Romosozumab Treatment in Postmenopausal Women with Osteoporosis. N. Engl. J. Med. 2016, 375, 1532–1543. [Google Scholar] [CrossRef]
- Saag, K.G.; Petersen, J.; Brandi, M.L.; Karaplis, A.C.; Lorentzon, M.; Thomas, T.; Maddox, J.; Fan, M.; Meisner, P.D.; Grauer, A. Romosozumab or Alendronate for Fracture Prevention in Women with Osteoporosis. N. Engl. J. Med. 2017, 377, 1417–1427. [Google Scholar] [CrossRef]
- Langdahl, B.L.; Hofbauer, L.C.; Forfar, J.C. Cardiovascular Safety and Sclerostin Inhibition. J. Clin. Endocrinol. Metab. 2021, 106, 1845–1853. [Google Scholar] [CrossRef]
- Katsanos, S.; Mavrogenis, A.F.; Kafkas, N.; Sardu, C.; Kamperidis, V.; Katsanou, P.; Farmakis, D.; Parissis, J. Cardiac Biomarkers Predict 1-Year Mortality in Elderly Patients Undergoing Hip Fracture Surgery. Orthopedics 2017, 40, e417–e424. [Google Scholar] [CrossRef]
- Zou, Y.; Yang, M.; Wang, J.; Cui, L.; Jiang, Z.; Ding, J.; Li, M.; Zhou, H. Association of sclerostin with cardiovascular events and mortality in dialysis patients. Ren. Fail. 2020, 42, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Kalousová, M.; Dusilová-Sulková, S.; Kuběna, A.A.; Zakiyanov, O.; Tesař, V.; Zima, T. Sclerostin levels predict cardiovascular mortality in long-term hemodialysis patients: A prospective observational cohort study. Physiol. Res. 2019, 68, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Wu, B.; Yu, X.; Wang, N.; Xu, X.; Zeng, M.; Zhang, B.; Mao, H.; Xing, C. Association of Serum Sclerostin Level, Coronary Artery Calcification, and Patient Outcomes in Maintenance Dialysis Patients. Blood Purif. 2022, 51, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Stavrinou, E.; Sarafidis, P.A.; Loutradis, C.; Memmos, E.; Faitatzidou, D.; Giamalis, P.; Koumaras, C.; Karagiannis, A.; Papagianni, A. Associations of serum sclerostin and Dickkopf-related protein-1 proteins with future cardiovascular events and mortality in haemodialysis patients: A prospective cohort study. Clin. Kidney J. 2020, 14, 1165–1172. [Google Scholar] [CrossRef]
- Takashi, Y.; Kawanami, D. The Role of Bone-Derived Hormones in Glucose Metabolism, Diabetic Kidney Disease, and Cardiovascular Disorders. Int. J. Mol. Sci. 2022, 23, 2376. [Google Scholar] [CrossRef]
- He, J.; Pan, M.; Xu, M.; Chen, R. Circulating miRNA-29b and Sclerostin Levels Correlate with Coronary Artery Calcification and Cardiovascular Events in Maintenance Hemodialysis Patients. Cardiol. Res. Pract. 2021, 2021, 9208634. [Google Scholar] [CrossRef] [PubMed]
- Frysz, M.; Gergei, I.; Scharnagl, H.; Smith, G.D.; Zheng, J.; Lawlor, D.A.; Herrmann, M.; Maerz, W.; Tobias, J.H. Circulating Sclerostin Levels Are Positively Related to Coronary Artery Disease Severity and Related Risk Factors. J. Bone Miner. Res. 2022, 37, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Yüksel, Y.; Yıldız, C.; Rakıcı, I.T. Relationship Between Sclerostin Levels and Coronary Artery Calcification and Plaque Composition. Turk. Kardiyol. Dern. Ars. 2023, 51, 266–273. [Google Scholar] [CrossRef]
- Leto, G.; Tartaglione, L.; Rotondi, S.; Pasquali, M.; Maddaloni, E.; Mignogna, C.; D’Onofrio, L.; Zampetti, S.; Carlone, A.; Muci, M.L.; et al. Diastolic Pressure and ACR Are Modifiable Risk Factors of Arterial Stiffness in T2DM Without Cardiovascular Disease. J. Clin. Endocrinol. Metab. 2022, 107, e3857–e3865. [Google Scholar] [CrossRef] [PubMed]
- González-Salvatierra, S.; García-Martín, A.; García-Fontana, B.; Martínez-Heredia, L.; García-Fontana, C.; Muñoz-Torres, M. Bone proteins are associated with cardiovascular risk according to the SCORE2-Diabetes algorithm. Cardiovasc. Diabetol. 2024, 23, 311. [Google Scholar] [CrossRef] [PubMed]
- Morena-Carrere, M.; Jaussent, I.; Chenine, L.; Dupuy, A.M.; Bargnoux, A.S.; Leray-Moragues, H.; Klouche, K.; Vernhet, H.; Canaud, B.; Cristol, J.P. Severe coronary artery calcifications in chronic kidney disease patients, coupled with inflammation and bone mineral disease derangement, promote major adverse cardiovascular events (MACE) through vascular remodeling. Kidney Blood Press. Res. 2024, 1–20. [Google Scholar] [CrossRef]
- Garcia-de Los Ríos, C.; Medina-Casado, M.; Díaz-Chamorro, A.; Sierras-Jiménez, M.; Lardelli-Claret, P.; Cáliz-Cáliz, R.; Sabio, J.M. Sclerostin as a biomarker of cardiovascular risk in women with systemic lupus erythematosus. Sci. Rep. 2022, 12, 21621. [Google Scholar] [CrossRef]
- Lee, J.; Cho, D.H.; Min, H.J.; Son, Y.B.; Kim, T.B.; Oh, S.W.; Kim, M.G.; Cho, W.Y.; Jo, S.K.; Yang, J. Higher sclerostin is associated with pulmonary hypertension in pre-dialysis end-stage kidney disease patients: A cross-sectional prospective observational cohort study. BMC Pulm. Med. 2024, 24, 78. [Google Scholar] [CrossRef]
- Carrillo-López, N.; Martínez-Arias, L.; Fernández-Villabrille, S.; Ruiz-Torres, M.P.; Dusso, A.; Cannata-Andía, J.B.; Naves-Díaz, M.; Panizo, S.; European Renal Osteodystrophy (EUROD) Workgroup. Role of the RANK/RANKL/OPG and Wnt/β-Catenin Systems in CKD Bone and Cardiovascular Disorders. Calcif. Tissue Int. 2021, 108, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Ueland, T.; Abraityte, A.; Norum, H.; Varathalingam, S.; Gullestad, L.; Aukrust, P.; Andreassen, A.K. Circulating regulators of the wingless pathway in precapillary pulmonary hypertension. Respirology 2021, 26, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Kern, A.; Stompór, T.; Kiewisz, J.; Kraziński, B.E.; Kiezun, J.; Kiezun, M.; Górny, J.; Sienkiewicz, E.; Drozdowska, B.; Bil, J. Association of serum sclerostin levels with atherosclerosis severity in patients referred for invasive coronary angiography. Kardiol. Pol. 2020, 78, 1271–1273. [Google Scholar] [CrossRef]
- Sanabria-de la Torre, R.; González-Salvatierra, S.; García-Fontana, C.; Andújar-Vera, F.; García-Fontana, B.; Muñoz-Torres, M.; Riquelme-Gallego, B. Exploring the Role of Sclerostin as a Biomarker of Cardiovascular Disease and Mortality: A Scoping Review. Int. J. Environ. Res. Public Health 2022, 19, 15981. [Google Scholar] [CrossRef] [PubMed]
- Golledge, J.; Thanigaimani, S. Role of Sclerostin in Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2022, 42, e187–e202. [Google Scholar] [CrossRef] [PubMed]
- Tobias, J.H. Sclerostin and Cardiovascular Disease. Curr. Osteoporos. Rep. 2023, 21, 519–526. [Google Scholar] [CrossRef]
- Alcalde-Herraiz, M.; Xie, J.; Newby, D.; Prats, C.; Gill, D.; Gordillo-Marañón, M.; Prieto-Alhambra, D.; Català, M.; Prats-Uribe, A. Effect of genetically predicted sclerostin on cardiovascular biomarkers, risk factors, and disease outcomes. Nat. Commun. 2024, 15, 9832. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Chen, A.; Wang, H.; Cui, J.; Sun, Y.; Xu, L.; Mao, Y. The relationship between sclerostin and carotid artery atherosclerosis in patients with stage 3-5 chronic kidney disease. Int. Urol. Nephrol. 2020, 52, 1329–1336. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, L.; Maddaloni, E.; Buzzetti, R. Osteocalcin and sclerostin: Background characters or main actors in cardiovascular disease? Diabetes Metab. Res. Rev. 2020, 36, e3217. [Google Scholar] [CrossRef]
- Rroji, M.; Figurek, A.; Spasovski, G. Should We Consider the Cardiovascular System While Evaluating CKD-MBD? Toxins 2020, 12, 140. [Google Scholar] [CrossRef]
- Shalash, M.A.M.; Rohoma, K.H.; Kandil, N.S.; Abdel Mohsen, M.A.; Taha, A.A.F. Serum sclerostin level and its relation to subclinical atherosclerosis in subjects with type 2 diabetes. J. Diabetes Complicat. 2019, 33, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Onda, D.A.; Yang, C.H.; Lewis, J.R.; Levinger, I.; Loh, K. Roles of bone-derived hormones in type 2 diabetes and cardiovascular pathophysiology. Mol. Metab. 2020, 40, 101040. [Google Scholar] [CrossRef]
- Skrzypczyk, P.; Ofiara, A.; Szyszka, M.; Stelmaszczyk-Emmel, A.; Górska, E.; Pańczyk-Tomaszewska, M. Serum Sclerostin Is Associated with Peripheral and Central Systolic Blood Pressure in Pediatric Patients with Primary Hypertension. J. Clin. Med. 2021, 10, 3574. [Google Scholar] [CrossRef]
- Ibrahim, I.M.; Farag, E.M.; Tabl, M.A.E.; Abdelaziz, M. Relationship between sclerostin and coronary tortuosity in postmenopausal females with non-obstructive coronary artery disease. Int. J. Cardiol. 2021, 322, 29–33. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Li, C.; Chen, Q.; Xiang, T.; Wang, P.; Pang, J. Serum sclerostin and adverse outcomes in elderly patients with stable coronary artery disease undergoing percutaneous coronary intervention. Aging Clin. Exp. Res. 2020, 32, 2065–2072. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, L.; Ni, S.; Li, D.; Liu, J.; Chu, H.Y.; Zhang, N.; Sun, M.; Li, N.; Ren, Q.; et al. Targeting loop3 of sclerostin preserves its cardiovascular protective action and promotes bone formation. Nat. Commun. 2022, 13, 4241. [Google Scholar] [CrossRef]
- Takeuchi, Y. Romosozumab and cardiovascular safety in Japan. Osteoporos. Sarcopenia 2021, 7, 89–91. [Google Scholar] [CrossRef]
- Lv, F.; Cai, X.; Yang, W.; Gao, L.; Chen, L.; Wu, J.; Ji, L. Denosumab or romosozumab therapy and risk of cardiovascular events in patients with primary osteoporosis: Systematic review and meta- analysis. Bone 2020, 130, 115121. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard Kvist, A.; Faruque, J.; Vallejo-Yagüe, E.; Weiler, S.; Winter, E.M.; Burden, A.M. Cardiovascular Safety Profile of Romosozumab: A Pharmacovigilance Analysis of the US Food and Drug Administration Adverse Event Reporting System (FAERS). J. Clin. Med. 2021, 10, 1660. [Google Scholar] [CrossRef]
- Turk, J.R.; Deaton, A.M.; Yin, J.; Stolina, M.; Felx, M.; Boyd, G.; Bienvenu, J.G.; Varela, A.; Guillot, M.; Holdsworth, G.; et al. Nonclinical cardiovascular safety evaluation of romosozumab, an inhibitor of sclerostin for the treatment of osteoporosis in postmenopausal women at high risk of fracture. Regul. Toxicol. Pharmacol. 2020, 115, 104697. [Google Scholar] [CrossRef] [PubMed]
- Fixen, C.; Tunoa, J. Romosozumab: A Review of Efficacy, Safety, and Cardiovascular Risk. Curr. Osteoporos. Rep. 2021, 19, 15–22. [Google Scholar] [CrossRef]
- Asadipooya, K.; Weinstock, A. Cardiovascular Outcomes of Romosozumab and Protective Role of Alendronate. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, A.; Wada, K.; Okazaki, K.; Nishi, H.; Terayama, Y.; Kato, Y. Early clinical effects, safety, and predictors of the effects of romosozumab treatment in osteoporosis patients: One-year study. Osteoporos. Int. 2021, 32, 1999–2009. [Google Scholar] [CrossRef] [PubMed]
- Cejka, D. Cardiovascular Safety of Anti-Sclerostin Therapy in Chronic Kidney Disease. Metabolites 2021, 11, 770. [Google Scholar] [CrossRef]
- Kawaguchi, H. Global ‘Conditional’ Assurance of Romosozumab Safety: International Consensus on the Uniqueness of Adverse Cardiovascular Events in Japan. Calcif. Tissue Int. 2024, 115, 455–458. [Google Scholar] [CrossRef]
- Seeto, A.H.; Tadrous, M.; Gebre, A.K.; Lewis, J.R.; Fink, H.A.; Ebeling, P.R.; Rodríguez, A.J. Evidence for the cardiovascular effects of osteoporosis treatments in randomized trials of post-menopausal women: A systematic review and Bayesian network meta-analysis. Bone 2023, 167, 116610. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Gong, M.; Bao, D.; Sun, J.; Xiang, Z. Denosumab and romosozumab do not increase the risk of cardiovascular events in patients with primary osteoporosis: A reanalysis of the meta-analysis. Bone 2020, 134, 115270. [Google Scholar] [CrossRef]
- Jacob, J.J.; Paul, T.V. Romosozumab and cardiovascular safety-should we learn lessons from pioglitazone? Osteoporos. Int. 2022, 33, 1397–1399. [Google Scholar] [CrossRef] [PubMed]
- Lv, F.; Cai, X.; Ji, L. Response to “Denosumab and Romosozumab do not increase the risk of cardiovascular events in patients with primary osteoporosis: A reanalysis of the meta-analysis”. Bone 2020, 134, 115271. [Google Scholar] [CrossRef]
- Bovijn, J.; Krebs, K.; Chen, C.Y.; Boxall, R.; Censin, J.C.; Ferreira, T.; Pulit, S.L.; Glastonbury, C.A.; Laber, S.; Millwood, I.Y.; et al. Evaluating the cardiovascular safety of sclerostin inhibition using evidence from meta-analysis of clinical trials and human genetics. Sci. Transl. Med. 2020, 12, eaay6570. [Google Scholar] [CrossRef] [PubMed]
- Hólm, H.; Sulem, P.; Tragante, V.; Thorsteinsdottir, U.; Gudbjartsson, D.F.; Stefansson, K. Comment on “Evaluating the cardiovascular safety of sclerostin inhibition using evidence from meta-analysis of clinical trials and human genetics”. Sci. Transl. Med. 2021, 13, eabe8497. [Google Scholar] [CrossRef] [PubMed]
- Bovijn, J.; Krebs, K.; Chen, C.Y.; Boxall, R.; Censin, J.C.; Ferreira, T.; Pulit, S.L.; Glastonbury, C.A.; Laber, S.; Millwood, I.Y.; et al. Response to comment on “Evaluating the cardiovascular safety of sclerostin inhibition using evidence from meta-analysis of clinical trials and human genetics”. Sci. Transl. Med. 2021, 13, eabf4530. [Google Scholar] [CrossRef]
- Masuda, S.; Fukasawa, T.; Matsuda, S.; Yoshida, S.; Kawakami, K. Comparative effectiveness and cardiovascular safety of romosozumab versus teriparatide in patients with osteoporosis: A population-based cohort study. Osteoporos. Int. 2024, 35, 2165–2174. [Google Scholar] [CrossRef]
- Cheng, S.H.; Chu, W.; Chou, W.H.; Chu, W.C.; Kang, Y.N. Cardiovascular Safety of Romosozumab Compared to Commonly Used Anti-osteoporosis Medications in Postmenopausal Osteoporosis: A Systematic Review and Network Meta-analysis of Randomized Controlled Trials. Drug Saf. 2024. Epub ahead of print. [Google Scholar] [CrossRef]
- Stokar, J.; Szalat, A. Cardiovascular Safety of Romosozumab vs. PTH Analogs for Osteoporosis Treatment: A Propensity Score Matched Cohort Study. J. Clin. Endocrinol. Metab. 2024, dgae173. [Google Scholar] [CrossRef]
- Wong, R.M.Y.; Wong, P.Y.; Liu, C.; Wong, H.Y.; Fong, M.K.; Zhang, N.; Cheung, W.H.; Law, S.W. Treatment effects, adverse outcomes and cardiovascular safety of romosozumab–Existing worldwide data: A systematic review and meta-analysis. J. Orthop. Transl. 2024, 48, 107–122. [Google Scholar] [CrossRef]
- Macrae, F.; Clark, E.M.; Walsh, K.; Bailey, S.J.; Roy, M.; Hardcastle, S.; Cockill, C.; Tobias, J.H.; Faber, B.G. Cardiovascular risk assessment for osteoporosis patients considering Romosozumab. Bone 2025, 190, 117305. [Google Scholar] [CrossRef]
- Lewiecki, E.M.; Blicharski, T.; Goemaere, S.; Lippuner, K.; Meisner, P.D.; Miller, P.D.; Miyauchi, A.; Maddox, J.; Chen, L.; Horlait, S. A Phase III Randomized Placebo-Controlled Trial to Evaluate Efficacy and Safety of Romosozumab in Men With Osteoporosis. J. Clin. Endocrinol. Metab. 2018, 103, 3183–3193. [Google Scholar] [CrossRef] [PubMed]
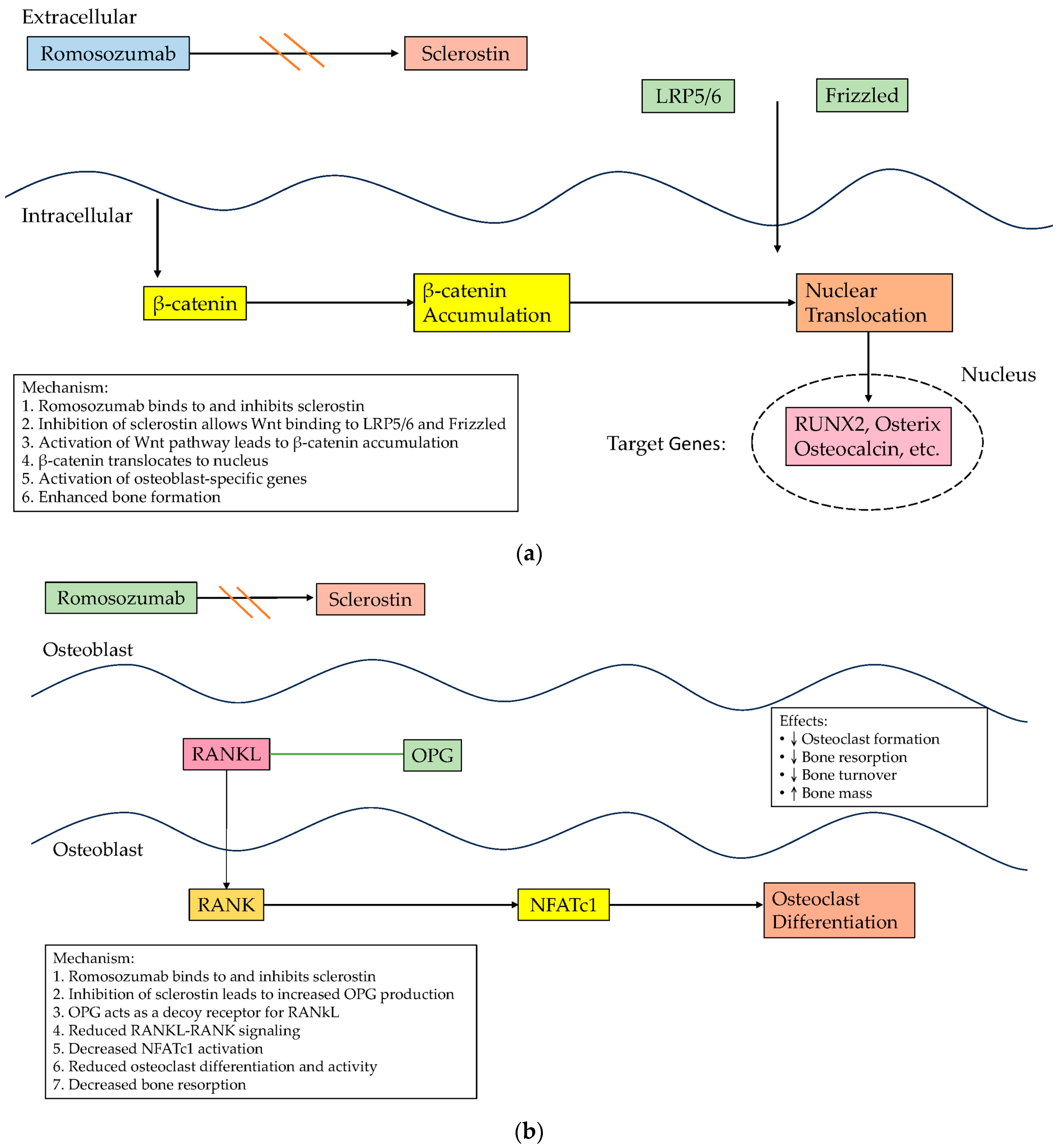
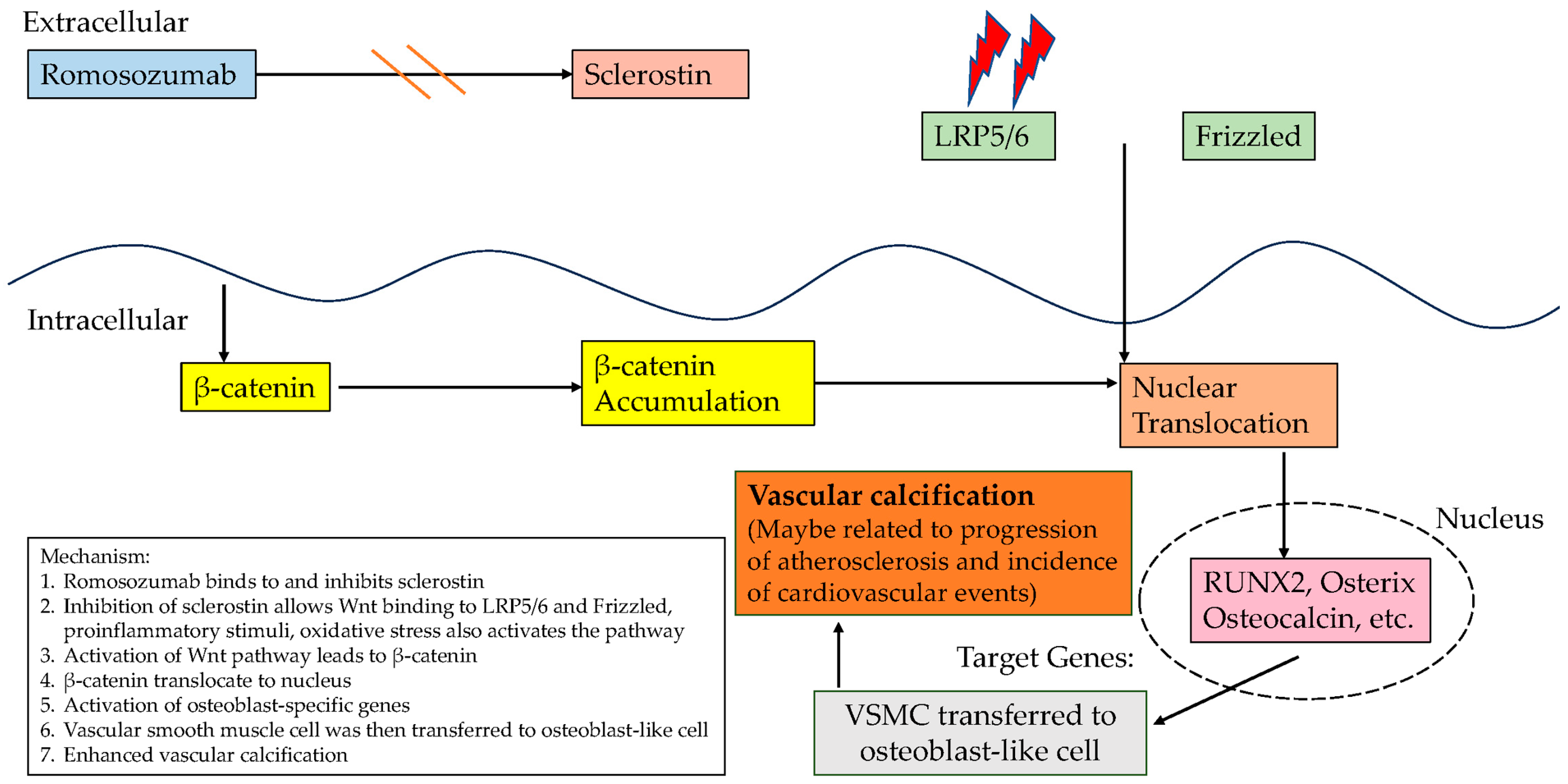
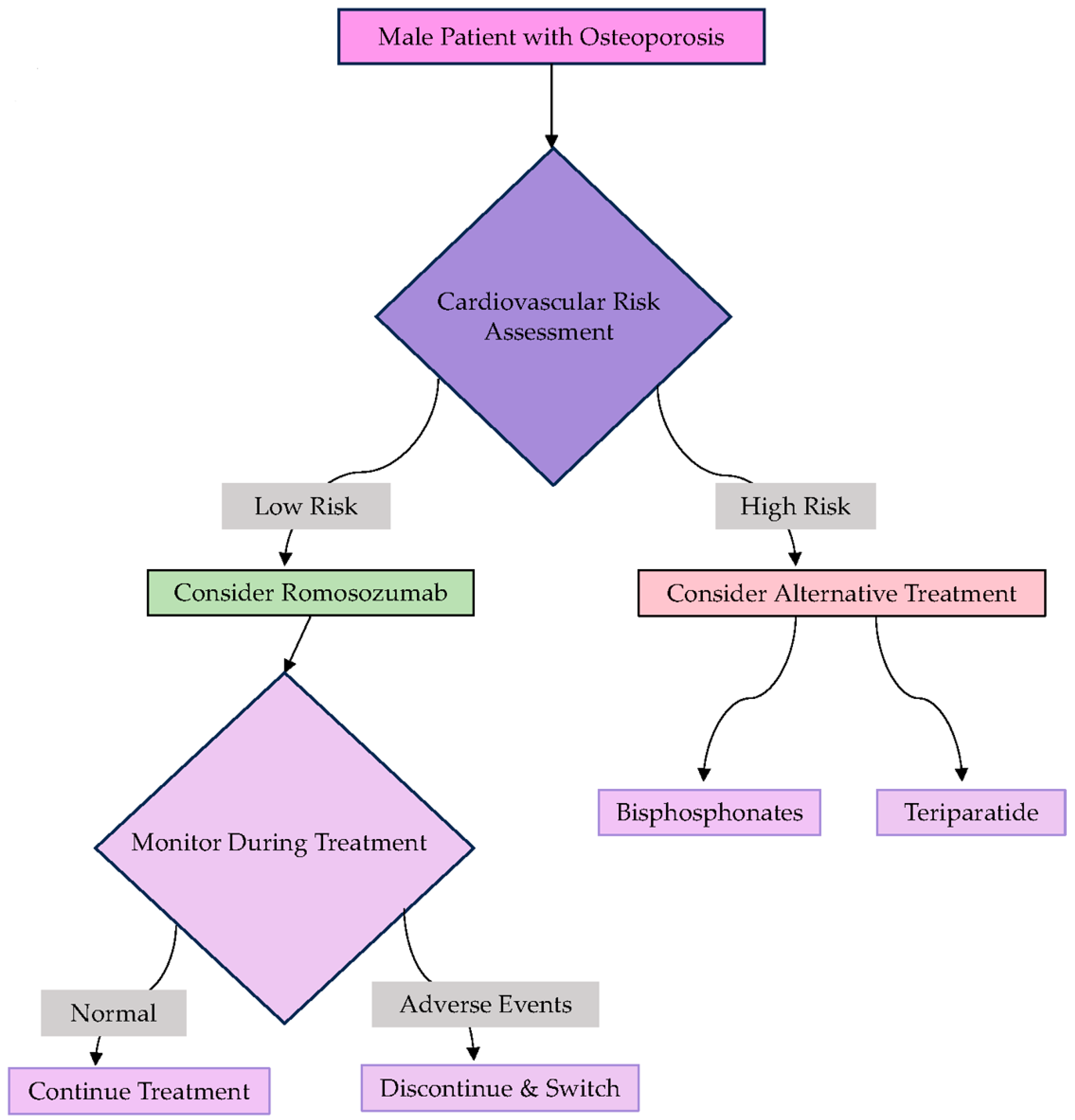
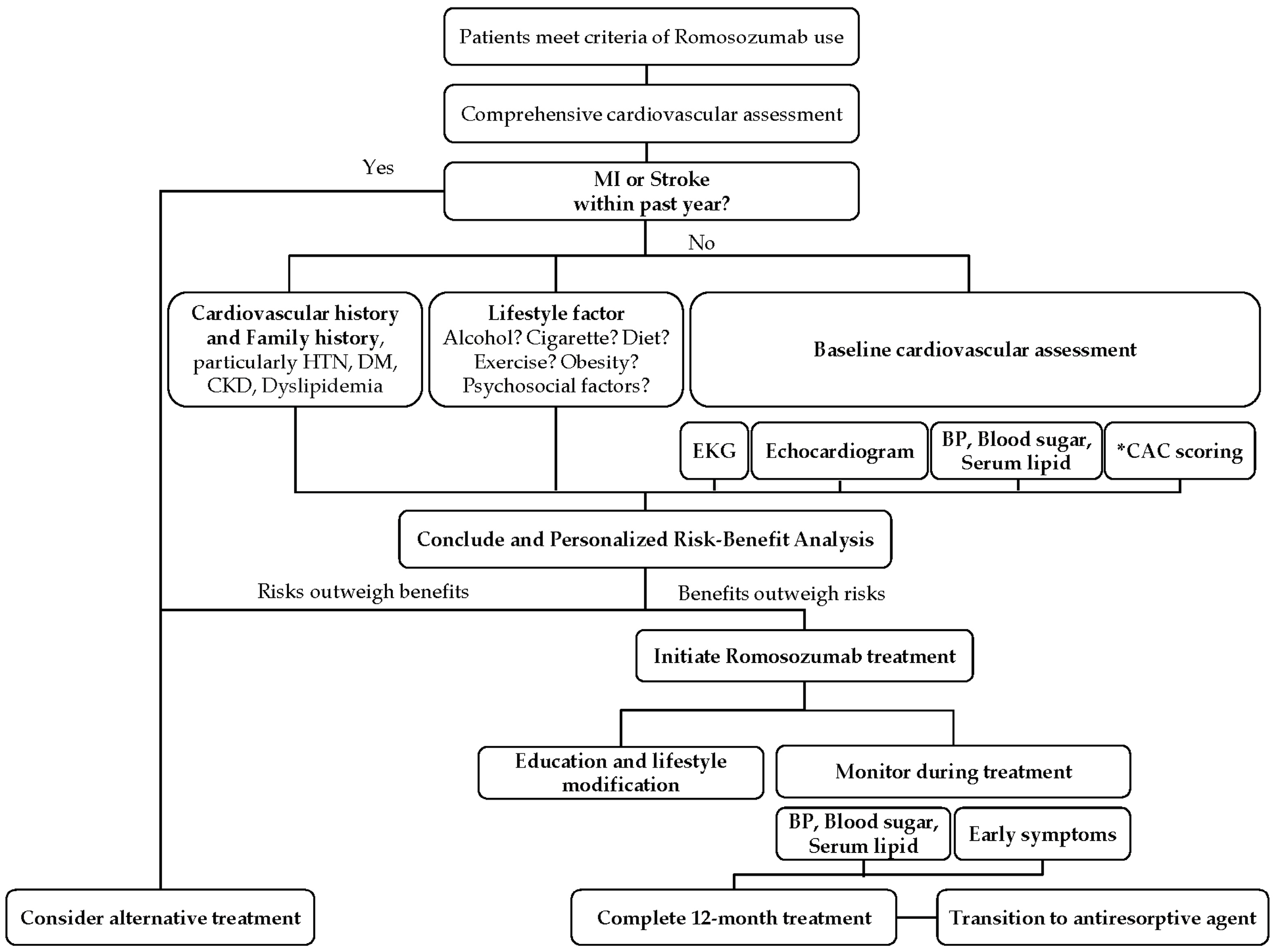
| Author (Year) | Methodology | Study Characteristics and Sample Size According to Gender | Key Finding |
|---|---|---|---|
| Zou et al., 2020 [13] | POCS | 165 dialysis patients (55.8% male, mean age: 56.5 years) | Positive association between sclerostin levels and cardiovascular events in peritoneal dialysis patients, not in hemodialysis patients. |
| Kalousová et al., 2019 [14] | POCS | 106 long-term hemodialysis patients (60.34% male, mean age: 61 years) | Sclerostin levels predict cardiovascular mortality in long-term hemodialysis patients. |
| Ge et al., 2022 [15] | POCS | 65 dialysis patients (HD: 26, PD: 39, 52.3% male, mean age: 48.4 years) | Although elevated sclerostin levels are associated with coronary artery calcification, they are not independently linked to a greater incidence of cardiovascular events and mortality. |
| Stavrinou et al., 2020 [16] | POCS | 80 hemodialysis patients (56.3% male, mean age: 60.9 years) | High sclerostin levels are linked to higher risks of cardiovascular events and mortality, but Dkk-1 is not. |
| Takashi and Kawanami, 2022 [17] | Review | Not applicable | Sclerostin implicated in insulin resistance and diabetic complications. |
| He et al., 2021 [18] | POCS | 66 hemodialysis patients (57.6% male, mean age: 56.3 years) | Higher serum sclerostin levels were significantly correlated with increased coronary artery calcification and cumulative risk of cardiovascular events, and sclerostin was identified as an independent risk factor for aortic calcification. |
| Frysz et al., 2022 [19] | POCS | 327 CAD patients (64.8% male, mean age: 64.1 years) | Sclerostin levels were associated with increased CAD severity and mortality, cardiovascular disease risk factors like DM, reduced eGFR, higher TGs, and lower HDL cholesterol. |
| Yüksel et al., 2023 [20] | POCS | 175 patients with coronary artery calcification and plaque composition (43.4% male, mean age: 58.4 years) | Sclerostin levels were positively associated with CAC scores and coronary plaque burden, and they predicted MACE over a one-year follow-up period. |
| Leto et al., 2022 [21] | POCS | 174 type 2 diabetes patients (57.5% male, mean age: 56 years) | Sclerostin levels were higher in patients with early vascular stiffness. |
| González-Salvatierra et al., 2024 [22] | POCS | 104 type 2 diabetes patients (60% male, mean age: 62 years) | Sclerostin levels positively correlated with cardiovascular risk based on SCORE2-Diabetes values. |
| Morena-Carrere et al., 2024 [23] | POCS | 425 non-dialysis CKD patients (50.5% male, mean age: 65 years) | Sclerostin contributes to vascular calcification and MACE in CKD patients, but its independent predictive value diminishes after adjusting for confounding factors like diabetes and cardiovascular history. |
| Garcia-de los Ríos et al., 2022 [24] | POCS | 68 women with systemic lupus erythematosus (0% male, mean age: 43.8 years) | A 10-unit rise in sclerostin levels was linked to a 44% higher likelihood of carotid plaque, even after adjusting for other cardiovascular risk factors. |
| Lee et al., 2024 [25] | Cross-sectional POCS | 44 pre-dialysis ESRD patients (58.1% male, median age: 54 years) | Higher sclerostin levels are associated with pulmonary hypertension in pre-dialysis ESRD patients. (estimated pulmonary artery systolic pressure >35 mmHg on echocardiography) |
| Carrillo-López et al., 2021 [26] | Review | Not applicable | Disrupted Wnt signaling with elevated sclerostin and RANKL/OPG imbalances contribute to vascular calcification and increased cardiovascular risk in CKD. |
| Ueland et al., 2021 [27] | POCS | 106 precapillary PH patients (30% male, mean age: 44 years) | Although Wnt-related proteins such as Wnt5a, DKK3, sFRP3, and WIF1 were associated with sclerostin as part of the broader Wnt signaling pathway, sclerostin itself did not show a direct correlation with increased pulmonary vascular resistance or poor prognosis in precapillary pulmonary hypertension. |
| Kern et al., 2020 [28] | POCS | 205 patients referred for invasive coronary angiography (70.2% male, mean age: 62.9 years) | Although no direct link was found between sclerostin levels and CAD severity, sclerostin levels showed significant correlations with higher hs-CRP, higher Klotho protein, higher mean BMI, lower estimated glomerular filtration rate and lower iPTH. |
| Sanabria-de la Torre et al., 2022 [29] | Scoping review | 11 studies, total 2786 patients (male: from 43.87% to 68%, mean age: from 52.5 to 83 years) | The review found mixed results regarding the relationship between elevated sclerostin levels and CVD and mortality, but there appears to be a positive trend in specific populations that suggests an increased risk of CVD and cardiovascular mortality with higher sclerostin levels. |
| Author (Year) | Methodology | Study Characteristics and Sample Size According to Gender | Key Finding |
|---|---|---|---|
| Golledge and Thanigaimani, 2022 [30] | Review article | Mixed (cross-sectional and prospective cohort studies, experimental studies, RCTs, MR studies) | Sclerostin’s role in cardiovascular disease remains controversial, with both protective and detrimental effects reported. |
| Tobias J.H., 2023 [31] | Review article | Mixed (preclinical studies, RCT, cross-sectional studies) | While experimental studies, clinical trials, and Mendelian randomization studies suggest that sclerostin may have a cardioprotective role, observational studies have shown conflicting results. |
| Alcalde-Herraiz et al., 2024 [32] | Mendelian Randomization | Meta-analysis (49,568 individuals, 55% male) | Lower genetically predicted sclerostin levels were linked to increased risk of coronary artery disease and type 2 diabetes, along with unfavorable cardiovascular biomarkers such as reduced HDL and elevated triglycerides. |
| Zhao et al., 2020 [33] | Cross-sectional study | 140 CKD patients (stage 3–5, 51.4% male, mean age: 64.0 years) | Serum sclerostin levels increase as renal function declines in CKD patients and are associated with carotid artery atherosclerosis, with a suggested feedback mechanism potentially protecting against atherosclerosis by inhibiting vascular calcification and inflammation. |
| D’Onofrio et al., 2020 [34] | Review article | Mixed (preclinical studies, RCTS, cross-sectional studies) | The contribution of osteocalcin and sclerostin to the development of cardiovascular disease is still debated, mainly because of the controversial association between their serum levels and clinically significant cardiovascular outcomes. |
| Rroji et al., 2020 [35] | Review article | Not applicable | Sclerostin exhibited a dual role by promoting vascular calcification while potentially offering protective cardiovascular effects through modulation of the Wnt/β-catenin signaling pathway. |
| Shalash et al., 2019 [36] | Cross-sectional study | 50 patients with diabetes (46% male, mean age: 52.4 years), 20 healthy controls (35% male, mean age: 50.3 years) | T2DM patients have higher serum sclerostin levels than controls, correlating with increased carotid intima-media thickness and subclinical atherosclerosis. This elevation may be a compensatory response to inhibit vascular calcification and maintain vascular homeostasis. |
| Lin et al., 2020 [37] | Review | Not applicable | Sclerostin has a dual role: promoting insulin resistance in type 2 diabetes and providing anti-calcification effects during cardiovascular disease. |
| Skrzypczyk et al., 2021 [38] | Cross-sectional study | 60 pediatric patients with hypertension (61.7% male, mean age: 15.0 years), 20 healthy controls (45.0% male, mean age: 14.4 years) | Sclerostin levels are not significantly different between hypertensive and normotensive children; inversely associated with blood pressure. |
| Ibrahim et al., 2021 [39] | Cross-sectional study | 273 postmenopausal females with non-obstructive CAD [no CT: 145, CT: 128, mean age: 57.3 (no CT), 61.3 (CT)] | Sclerostin exerts a protective role by reducing arterial stiffness, inhibiting vascular calcification, and potentially mitigating atherosclerosis, thereby influencing the development of coronary tortuosity in aging, hypertensive, and postmenopausal populations. |
| He et al., 2020 [40] | Prospective cohort study | 310 SCAD patients (43.9% male, mean age: 76.1 years) undergoing PCI | High serum sclerostin levels are associated with better MACCE-free survival and overall survival in elderly SCAD patients undergoing PCI. |
| Yu et al., 2022 [41] | Experimental study | Not applicable | Targeting sclerostin loop3 can maintain cardiovascular protection and promote bone formation. |
| Author (Year) | Methodology | Study Characteristics and Sample Size According to Gender | Key Finding |
|---|---|---|---|
| Takeuchi Y., 2021 [42] | Review | Mixed (RCTs, pharmacovigilance studies, observational studies…) | While romosozumab is effective in preventing osteoporotic fractures, concerns remain about its potential cardiovascular risks. |
| Lv et al., 2020 [43] | Systematic review and meta-analysis | 6 RCTs (1 exclusively included men, others predominantly included women) | Romosozumab may increase the risk of 4P MACE in elderly men and postmenopausal women. |
| Vestergaard Kvist et al., 2021 [44] | Pharmacovigilance analysis | Data from FAERS | Romosozumab exhibits a mixed cardiovascular safety profile, with increased reports of major adverse cardiovascular events (MACE) primarily in older, high-risk patients, while its precise cardiovascular impact remains unclear due to potential confounding factors and uncertain biological mechanisms. |
| Turk et al., 2020 [45] | Nonclinical safety evaluation | Animal studies | Preclinical studies demonstrated that romosozumab has a neutral impact on atherosclerosis, showing no promotion of vascular calcification, atheroprogression, or related inflammatory processes in animal models. |
| Fixen and Tunoa., 2021 [46] | Review | 6 RCTs (1 exclusively included men, others predominantly included women) | Romosozumab has shown increased cardiovascular adverse events in high-risk patients despite unclear biological mechanisms, while sclerostin’s elevated expression in vascular tissues suggests a potential protective role against vascular calcification. |
| Asadipooya and Weinstock., 2019 [47] | Review | Mixed (RCTs, pharmacovigilance studies, observational studies…) | Romosozumab shows efficacy in increasing bone mineral density and reducing fractures, but it may be associated with an increased risk of cardiovascular events. |
| Tominaga et al., 2021 [48] | POCS | 262 osteoporosis patients (13.36% men, mean age: 77.06 years, 76.72% primary osteoporosis, 23.28% secondary) | Romosozumab effectively increases bone mineral density but has a context-dependent cardiovascular risk profile, with no cardiovascular events reported in this study despite concerns from previous trials. |
| Cejka D., 2021 [49] | Review | Mixed (experimental studies, clinical trials, cohort studies, meta-analysis, genetic studies) | Romosozumab treatment in chronic kidney disease patients, particularly those on hemodialysis, is linked to manageable hypocalcemia without clear evidence of increased cardiovascular events, though its impact on vascular calcification remains under investigation. |
| Kawaguchi, 2024 [50] | Perspective and pharmacovigilance report | Post-marketing data from Japan | Japan reported higher rates of MACE linked to romosozumab due to expanded use in older patients with cardiovascular risks. |
| Seeto et al., 2023 [51] | Systematic review and Bayesian network meta-analysis | 136,940 postmenopausal women | Romosozumab ranked higher for cardiovascular adverse events compared to placebo and most osteoporosis treatments, with the exception of certain bisphosphonates and PTH analogues. |
| Li et al., 2020 [52] | Meta-analysis reanalysis | 6 RCTs (1 exclusively included men, others predominantly included women) | Romosozumab showed no increased risk of cardiovascular events when compared to placebo. |
| Jacob and Paul., 2022 [53] | Commentary | Not applicable | Romosozumab’s inhibition of sclerostin and activation of the WNT-β catenin pathway may increase cardiovascular risk by reducing adipogenesis and promoting free fatty acid deposition in visceral fat and arterial walls. |
| Lv et al., 2020 [54] | Response to a meta-analysis reanalysis | Not applicable | Further studies with longer-term follow-up are necessary to better understand romosozumab’s safety profiles. |
| Bovijn et al., 2020 [55] | Meta-analysis and MR analysis | Meta-analysis (4 RCT), MR analysis (biobank, cohort studies) | Romosozumab may increase cardiovascular risk; genetic analysis supports findings. |
| Hólm et al., 2021 [56] | Commentary | Not applicable | The genetic data weakly link SOST inhibition to cardiovascular risk, likely influenced by other genetic factors. |
| Bovijn et al., 2021 [57] | Response to commentary | Not applicable | Potential cardiovascular risks of romosozumab warrant further investigation. |
| Masuda et al., 2024 [58] | Population-based cohort Study | 49,104 patients (68% female, mean age: 73 years) | No significant difference in MACE between romosozumab and teriparatide. |
| Cheng et al., 2024 [59] | Systematic review and network meta-analysis of RCTs | 16,777 postmenopausal women | No increased cardiovascular mortality or Mace between romosozumab and placebo. |
| Stokar and Szalat, 2024 [60] | POCS | 11,220 patients (50% female, mean age: 72 years) | Romosozumab was associated with fewer 3PMACE compared to PTH analogs. |
| Wong et al., 2024 [61] | Systematic review and meta-analysis | 13,507 patients (95% female) | No difference in cardiovascular events of Asian subgroup with romosozumab versus placebo. |
| Macrae et al., 2025 [62] | POCS | 41 women (mean age: 71 years) | QRISK3 overestimated cardiovascular risk compared to ESC SCORE, leading to wider romosozumab use despite perceived higher CV risk. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, S.-H.; Wu, W.-T.; Yao, T.-K.; Peng, C.-H.; Yeh, K.-T. Sclerostin and Cardiovascular Risk: Evaluating the Cardiovascular Safety of Romosozumab in Osteoporosis Treatment. Biomedicines 2024, 12, 2880. https://doi.org/10.3390/biomedicines12122880
Chiu S-H, Wu W-T, Yao T-K, Peng C-H, Yeh K-T. Sclerostin and Cardiovascular Risk: Evaluating the Cardiovascular Safety of Romosozumab in Osteoporosis Treatment. Biomedicines. 2024; 12(12):2880. https://doi.org/10.3390/biomedicines12122880
Chicago/Turabian StyleChiu, Shi-Hsun, Wen-Tien Wu, Ting-Kuo Yao, Cheng-Huan Peng, and Kuang-Ting Yeh. 2024. "Sclerostin and Cardiovascular Risk: Evaluating the Cardiovascular Safety of Romosozumab in Osteoporosis Treatment" Biomedicines 12, no. 12: 2880. https://doi.org/10.3390/biomedicines12122880
APA StyleChiu, S.-H., Wu, W.-T., Yao, T.-K., Peng, C.-H., & Yeh, K.-T. (2024). Sclerostin and Cardiovascular Risk: Evaluating the Cardiovascular Safety of Romosozumab in Osteoporosis Treatment. Biomedicines, 12(12), 2880. https://doi.org/10.3390/biomedicines12122880








