Effects of Hydrogen Sulfide at Normal Body Temperature and in the Cold on Isolated Tail and Carotid Arteries from Rats and TRPA1 Knockout and Wild-Type Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Their Housing
2.2. Vessel Isolation and Preparation
2.3. Experimental Procedures
2.4. Drugs and Substances
2.5. Statistics
3. Results
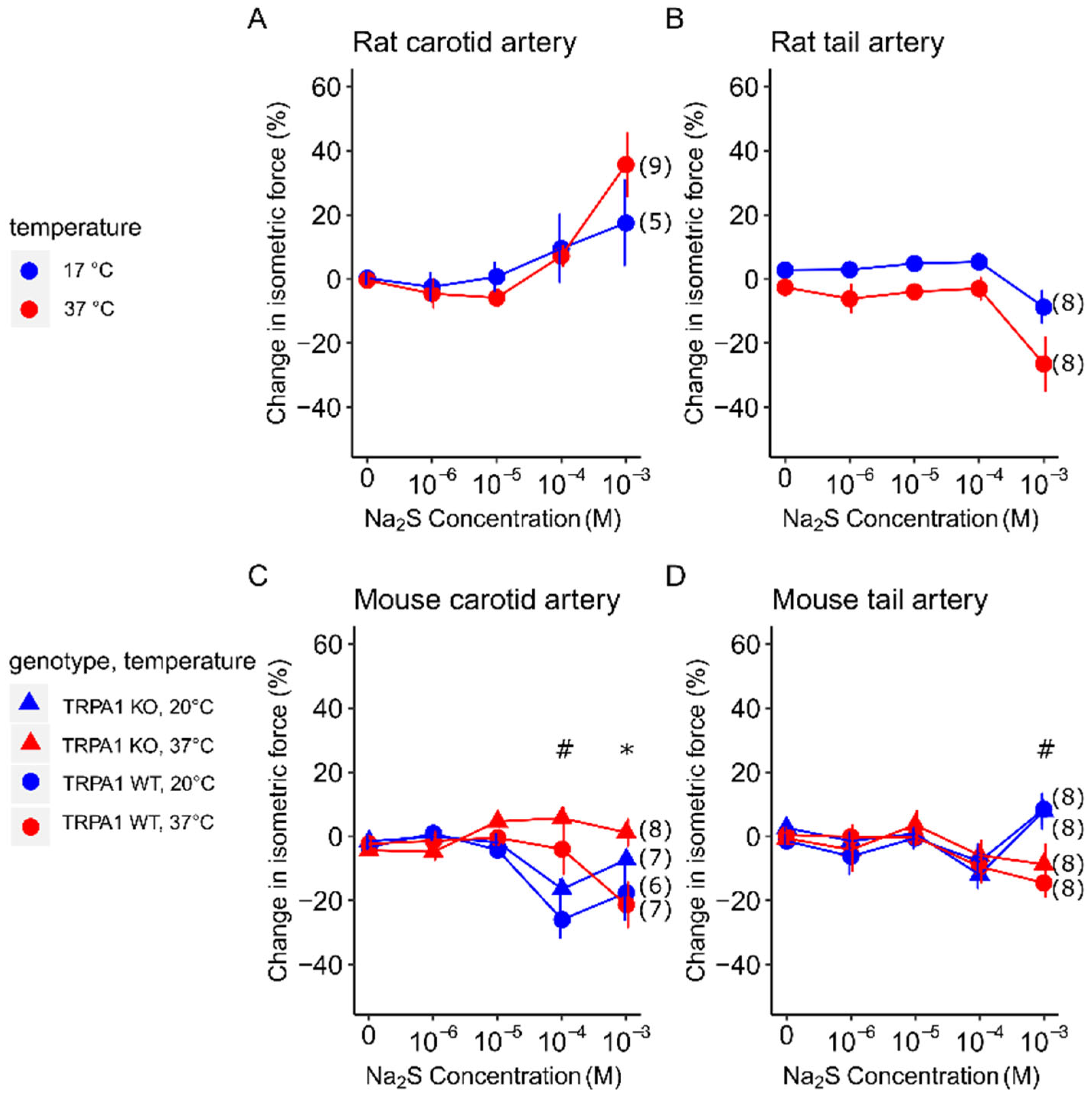
3.1. Vasomotor Response to Na2S in Carotid and Tail Arteries of Rats and Mice at Different Temperatures
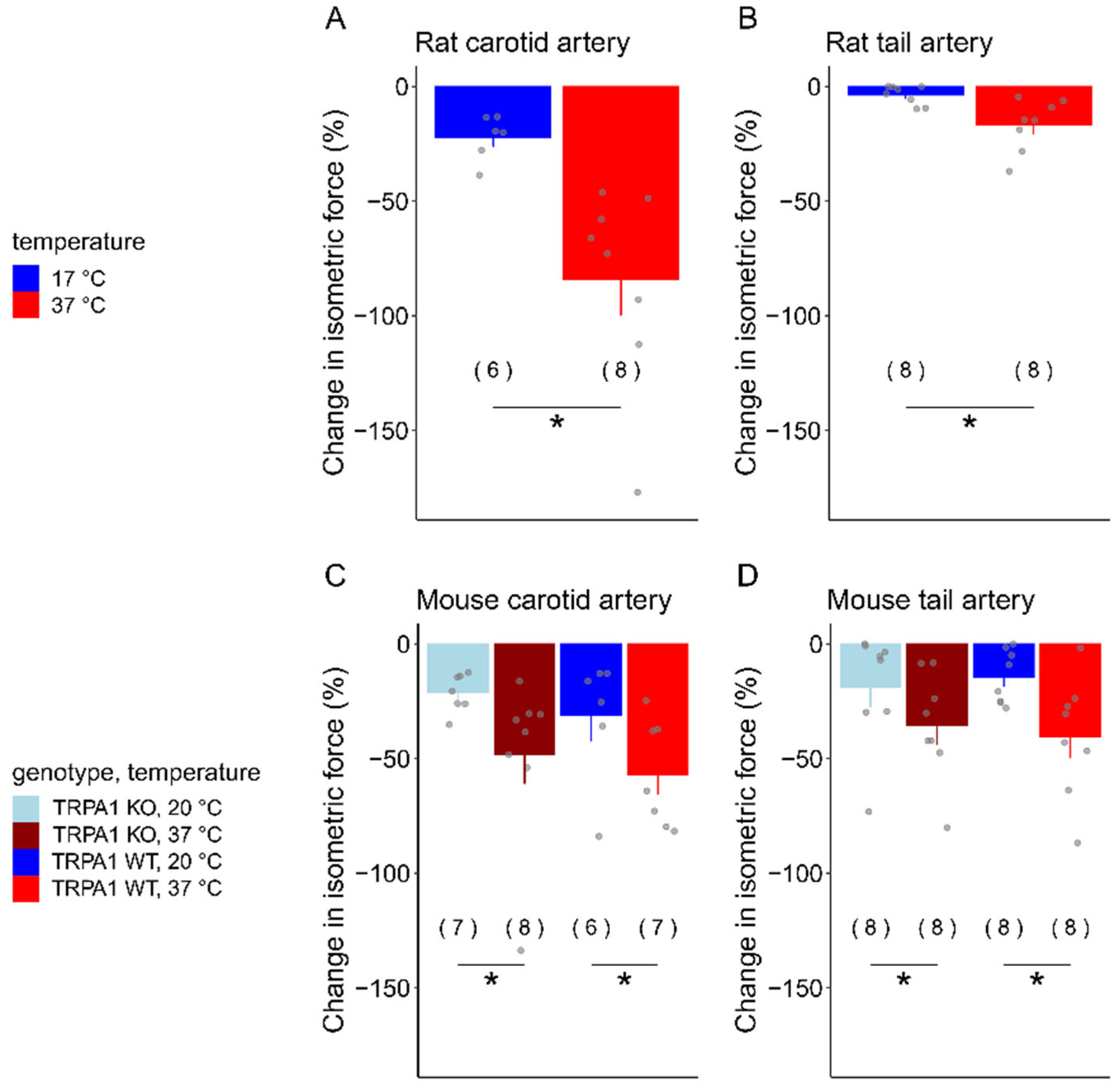
3.2. Acetylcholine-Induced Vasomotor Responses in Carotid and Tail Arteries of Rats and Mice at Different Temperatures
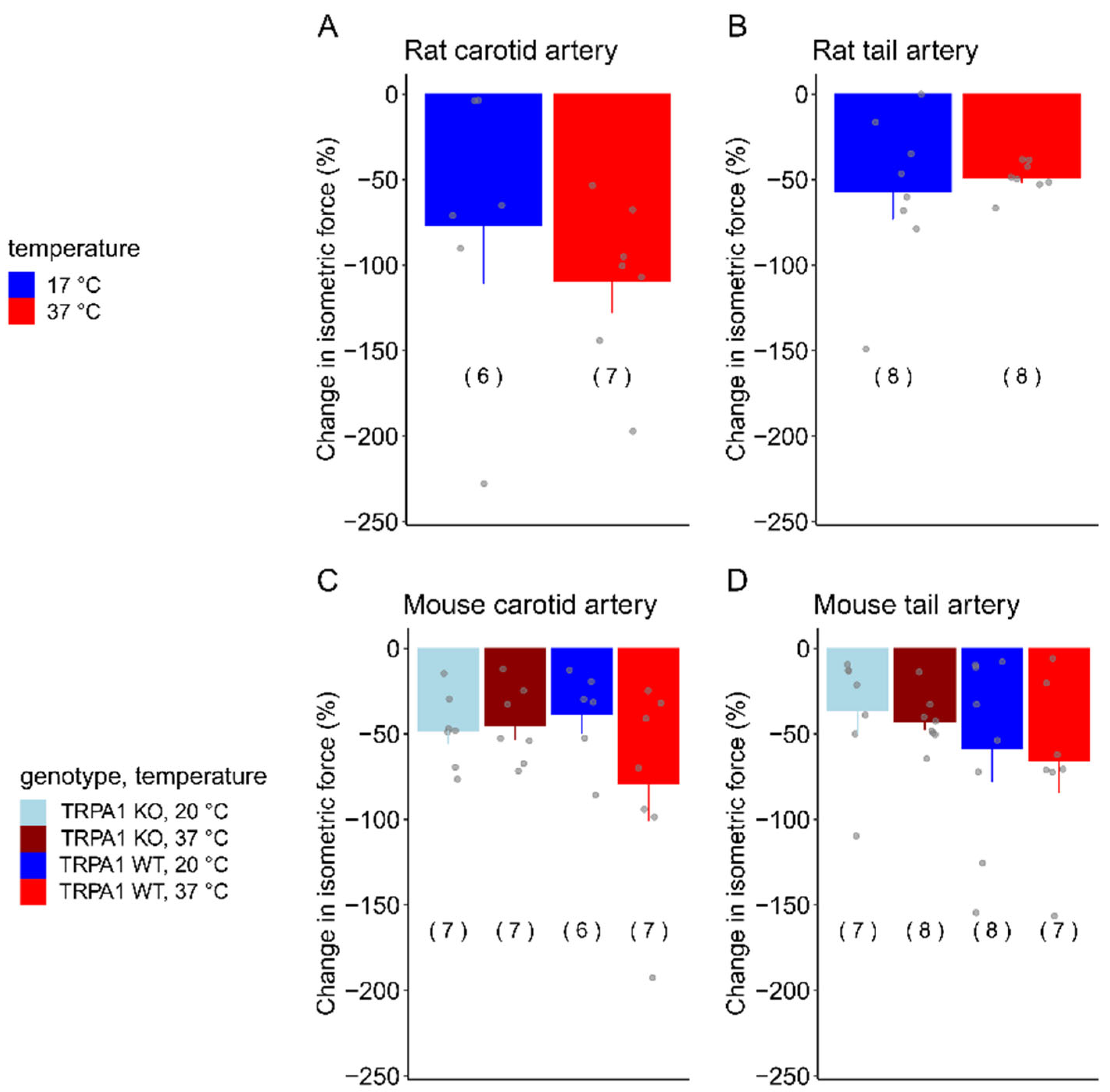
3.3. SNP-Induced Vasomotor Responses in Carotid and Tail Arteries of Rats and Mice at Different Temperatures
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olson, K.R. H2S and polysulfide metabolism: Conventional and unconventional pathways. Biochem. Pharmacol. 2018, 149, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Levitt, M.D.; Abdel-Rehim, M.S.; Furne, J. Free and acid-labile hydrogen sulfide concentrations in mouse tissues: Anomalously high free hydrogen sulfide in aortic tissue. Antioxid. Redox Signal. 2011, 15, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, V.; Genah, S.; Morbidelli, L. Endothelium as a Source and Target of H2S to Improve Its Trophism and Function. Antioxidants 2021, 10, 486. [Google Scholar] [CrossRef] [PubMed]
- Blackstone, E.; Morrison, M.; Roth, M.B. H2S induces a suspended animation-like state in mice. Science 2005, 308, 518. [Google Scholar] [CrossRef]
- Yoo, D.; Jupiter, R.C.; Pankey, E.A.; Reddy, V.G.; Edward, J.A.; Swan, K.W.; Peak, T.C.; Mostany, R. Analysis of cardiovascular responses to the H2S donors Na2S and NaHS in the rat. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H605–H614. [Google Scholar] [CrossRef]
- Meents, J.E.; Ciotu, C.I.; Fischer, M.J.M. TRPA1: A molecular view. J. Neurophysiol. 2019, 121, 427–443. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Du, Q.; Gu, J.; Wu, J.; Liu, Q.; Li, Z.; Zhang, T.; Xu, J.; Xie, R. Research progress on TRPA1 in diseases. J. Membr. Biol. 2023, 256, 301–316. [Google Scholar] [CrossRef]
- Thakore, P.; Ali, S.; Earley, S. Regulation of vascular tone by transient receptor potential ankyrin 1 channels. Curr. Top. Membr. 2020, 85, 119–150. [Google Scholar] [CrossRef]
- Story, G.M.; Peier, A.M.; Reeve, A.J.; Eid, S.R.; Mosbacher, J.; Hricik, T.R.; Earley, T.J.; Hergarden, A.C.; Andersson, D.A.; Hwang, S.W.; et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 2003, 112, 819–829. [Google Scholar] [CrossRef]
- Ogawa, H.; Takahashi, K.; Miura, S.; Imagawa, T.; Saito, S.; Tominaga, M.; Ohta, T. H2S functions as a nociceptive messenger through transient receptor potential ankyrin 1 (TRPA1) activation. Neuroscience 2012, 218, 335–343. [Google Scholar] [CrossRef]
- Talavera, K.; Startek, J.B.; Alvarez-Collazo, J.; Boonen, B.; Alpizar, Y.A.; Sanchez, A.; Naert, R.; Nilius, B. Mammalian transient receptor potential TRPA1 channels: From structure to disease. Physiol. Rev. 2020, 100, 725–803. [Google Scholar] [CrossRef] [PubMed]
- Olah, E.; Rumbus, Z.; Kormos, V.; Tekus, V.; Pakai, E.; Wilson, H.V.; Fekete, K.; Solymar, M.; Kelava, L.; Keringer, P.; et al. The hypothermic effect of hydrogen sulfide is mediated by the transient receptor potential ankyrin-1 channel in mice. Pharmaceuticals 2021, 14, 992. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.R.; Dombkowski, R.A.; Russell, M.J.; Doellman, M.M.; Head, S.K.; Whitfield, N.L.; Madden, J.A. Hydrogen sulfide as an oxygen sensor/transducer in vertebrate hypoxic vasoconstriction and hypoxic vasodilation. J. Exp. Biol. 2006, 209, 4011–4023. [Google Scholar] [CrossRef]
- Lv, B.; Chen, S.; Tang, C.; Jin, H.; Du, J.; Huang, Y. Hydrogen sulfide and vascular regulation—An update. J. Adv. Res. 2021, 27, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Garami, A.; Pakai, E.; McDonald, H.A.; Reilly, R.M.; Gomtsyan, A.; Corrigan, J.J.; Pinter, E.; Zhu, D.X.D.; Lehto, S.G.; Gavva, N.R.; et al. TRPV1 antagonists that cause hypothermia, instead of hyperthermia, in rodents: Compounds’ pharmacological profiles, in vivo targets, thermoeffectors recruited and implications for drug development. Acta Physiol. 2018, 223, e13038. [Google Scholar] [CrossRef] [PubMed]
- Nowack, J.; Turbill, C. Survivable hypothermia or torpor in a wild-living rat: Rare insights broaden our understanding of endothermic physiology. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2022, 192, 183–192. [Google Scholar] [CrossRef]
- Paal, P.; Brugger, H.; Strapazzon, G. Accidental hypothermia. Handb. Clin. Neurol. 2018, 157, 547–563. [Google Scholar] [CrossRef]
- Romanovsky, A.A. Skin temperature: Its role in thermoregulation. Acta Physiol. 2014, 210, 498–507. [Google Scholar] [CrossRef]
- Garami, A.; Steiner, A.A.; Romanovsky, A.A. Fever and hypothermia in systemic inflammation. Handb. Clin. Neurol. 2018, 157, 565–597. [Google Scholar] [CrossRef]
- Rumbus, Z.; Garami, A. Fever, hypothermia, and mortality in sepsis. Temperature 2019, 6, 101–103. [Google Scholar] [CrossRef]
- Fernandez, R.A.; Soriano, R.N.; Francescato, H.D.; Sabino, J.P.; Coimbra, T.M.; Branco, L.G. Cryogenic role of central endogenous hydrogen sulfide in the rat model of endotoxic shock. Brain Res. 2016, 1650, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Basto, F.M.; Lyden, P. Hypothermia in acute ischemic stroke therapy. Handb. Clin. Neurol. 2018, 157, 823–837. [Google Scholar] [CrossRef] [PubMed]
- Olah, E.; Poto, L.; Rumbus, Z.; Pakai, E.; Romanovsky, A.A.; Hegyi, P.; Garami, A. POLAR study revisited: Therapeutic hypothermia in severe brain trauma should not be abandoned. J. Neurotrauma 2021, 38, 2772–2776. [Google Scholar] [CrossRef] [PubMed]
- Olah, E.; Poto, L.; Hegyi, P.; Szabo, I.; Hartmann, P.; Solymar, M.; Petervari, E.; Balasko, M.; Habon, T.; Rumbus, Z.; et al. Therapeutic whole-body hypothermia reduces death in severe traumatic brain injury if the cooling index is sufficiently high: Meta-analyses of the effect of single cooling parameters and their integrated measure. J. Neurotrauma 2018, 35, 2407–2417. [Google Scholar] [CrossRef]
- Cai, S.; Li, Q.; Fan, J.; Zhong, H.; Cao, L.; Duan, M. Therapeutic hypothermia combined with hydrogen sulfide treatment attenuated early blood-brain barrier disruption and brain edema induced by cardiac arrest and resuscitation in rat model. Neurochem. Res. 2023, 48, 967–979. [Google Scholar] [CrossRef]
- Dai, H.B.; Ji, X.; Zhu, S.H.; Hu, Y.M.; Zhang, L.D.; Miao, X.L.; Ma, R.M.; Duan, M.L.; Li, W.Y. Hydrogen sulphide and mild hypothermia activate the CREB signaling pathway and prevent ischemia-reperfusion injury. BMC Anesthesiol. 2015, 15, 119. [Google Scholar] [CrossRef]
- Dai, H.B.; Xu, M.M.; Lv, J.; Ji, X.J.; Zhu, S.H.; Ma, R.M.; Miao, X.L.; Duan, M.L. Mild hypothermia combined with hydrogen sulfide treatment during resuscitation reduces hippocampal neuron apoptosis via NR2A, NR2B, and PI3K-Akt signaling in a rat model of cerebral ischemia-reperfusion injury. Mol. Neurobiol. 2016, 53, 4865–4873. [Google Scholar] [CrossRef]
- de Oliveira, C.; Garami, A.; Lehto, S.G.; Pakai, E.; Tekus, V.; Pohoczky, K.; Youngblood, B.D.; Wang, W.; Kort, M.E.; Kym, P.R. Transient receptor potential channel ankyrin-1 is not a cold sensor for autonomic thermoregulation in rodents. J. Neurosci. 2014, 34, 4445–4452. [Google Scholar] [CrossRef]
- Pozsgai, G.; Payrits, M.; Sághy, É.; Sebestyén-Bátai, R.; Steen, E.; Szőke, É.; Sándor, Z.; Solymár, M.; Garami, A.; Orvos, P. Analgesic effect of dimethyl trisulfide in mice is mediated by TRPA1 and sst4 receptors. Nitric Oxide 2017, 65, 10–21. [Google Scholar] [CrossRef]
- Ivic, I.; Solymar, M.; Pakai, E.; Rumbus, Z.; Pinter, E.; Koller, A.; Garami, A. Transient receptor potential vanilloid-1 channels contribute to the regulation of acid-and base-induced vasomotor responses. J. Vasc. Res. 2017, 53, 279–290. [Google Scholar] [CrossRef]
- Kelava, L.; Ivić, I.; Pakai, E.; Fekete, K.; Maroti, P. Stereolithography 3D printing of a heat exchanger for advanced temperature control in wire myography. Polymers 2022, 14, 471. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhang, J.; Lu, Y.; Wang, R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J. 2001, 20, 6008–6016. [Google Scholar] [CrossRef] [PubMed]
- DeLeon, E.R.; Stoy, G.F.; Olson, K.R. Passive loss of hydrogen sulfide in biological experiments. Anal. Biochem. 2012, 421, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Flavahan, S.; Flavahan, N.A. Cooling-induced dilatation of cutaneous arteries is mediated by increased myoendothelial communication. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H123–H132. [Google Scholar] [CrossRef]
- Marjanovic, M.; Willis, J.S. ATP dependence of Na+-K+ pump of cold-sensitive and cold-tolerant mammalian red blood cells. J. Physiol. 1992, 456, 575–590. [Google Scholar] [CrossRef]
- Taddei, S.; Mattei, P.; Virdis, A.; Sudano, I.; Ghiadoni, L.; Salvetti, A. Effect of potassium on vasodilation to acetylcholine in essential hypertension. Hypertension 1994, 23, 485–490. [Google Scholar] [CrossRef]
- Lincoln, T.M.; Komalavilas, P.; Cornwell, T.L. Pleiotropic regulation of vascular smooth muscle tone by cyclic GMP-dependent protein kinase. Hypertension 1994, 23, 1141–1147. [Google Scholar] [CrossRef]
- Cogolludo, A.L.; Pérez-Vizcaíno, F.; Zaragozá-Arnáez, F.; Ibarra, M.; López-López, G.; López-Miranda, V.; Tamargo, J. Mechanisms involved in SNP-induced relaxation and [Ca2+] i reduction in piglet pulmonary and systemic arteries. Br. J. Pharmacol. 2001, 132, 959–967. [Google Scholar] [CrossRef]
- Hansted, A.K.; Jensen, L.J.; Olesen, J.; Jansen-Olesen, I. Localization of TRPA1 channels and characterization of TRPA1 mediated responses in dural and pial arteries in vivo after intracarotid infusion of Na2S. Cephalalgia 2020, 40, 1310–1320. [Google Scholar] [CrossRef]
- Pozsgai, G.; Hajna, Z.; Bagoly, T.; Boros, M.; Kemény, Á.; Materazzi, S.; Nassini, R.; Helyes, Z.; Szolcsányi, J.; Pintér, E. The role of transient receptor potential ankyrin 1 (TRPA1) receptor activation in hydrogen-sulphide-induced CGRP-release and vasodilation. Eur. J. Pharmacol. 2012, 689, 56–64. [Google Scholar] [CrossRef]
- Aubdool, A.A.; Graepel, R.; Kodji, X.; Alawi, K.M.; Bodkin, J.V.; Srivastava, S.; Gentry, C.; Heads, R.; Grant, A.D.; Fernandes, E.S.; et al. TRPA1 is essential for the vascular response to environmental cold exposure. Nat. Commun. 2014, 5, 5732. [Google Scholar] [CrossRef]
- Earley, S. TRPA1 channels in the vasculature. Br. J. Pharmacol. 2012, 167, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bukoski, R.D. Distribution of the perivascular nerve Ca2+ receptor in rat arteries. Br. J. Pharmacol. 1998, 125, 1397–1404. [Google Scholar] [CrossRef]
- Yang, X.-P.; Chiba, S. Effects of prolonged cold storage on purinergic and adrenergic components of sympathetic co-transmission in isolated canine splenic arteries. Jpn. J. Pharmacol. 1999, 81, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Schubert, R.; Gaynullina, D.; Shvetsova, A.; Tarasova, O.S. Myography of isolated blood vessels: Considerations for experimental design and combination with supplementary techniques. Front. Physiol. 2023, 14, 1176748. [Google Scholar] [CrossRef] [PubMed]
- Centeno, J.M.; López-Morales, M.A.; Aliena-Valero, A.; Jover-Mengual, T.; Burguete, M.C.; Castelló-Ruiz, M.; Miranda, F.J. Potassium channels contribute to the increased sensitivity of the rabbit carotid artery to hydrogen sulfide in diabetes. Eur. J. Pharmacol. 2019, 853, 33–40. [Google Scholar] [CrossRef]
- Hildebrandt, T.M.; Grieshaber, M.K. Three enzymatic activities catalyze the oxidation of sulfide to thiosulfate in mammalian and invertebrate mitochondria. FEBS J. 2008, 275, 3352–3361. [Google Scholar] [CrossRef]
- Swoap, S.J.; Overton, J.M.; Garber, G. Effect of ambient temperature on cardiovascular parameters in rats and mice: A comparative approach. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R391–R396. [Google Scholar] [CrossRef]
- Swoap, S.J.; Gutilla, M.J. Cardiovascular changes during daily torpor in the laboratory mouse. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R769–R774. [Google Scholar] [CrossRef]
- Jensen, B.S.; Fago, A. Sulfide metabolism and the mechanism of torpor. J. Exp. Biol. 2021, 224, jeb215764. [Google Scholar] [CrossRef]
- del Camino, D.; Murphy, S.; Heiry, M.; Barrett, L.B.; Earley, T.J.; Cook, C.A.; Petrus, M.J.; Zhao, M.; D’Amours, M.; Deering, N.; et al. TRPA1 contributes to cold hypersensitivity. J. Neurosci. 2010, 30, 15165–15174. [Google Scholar] [CrossRef] [PubMed]
- Karashima, Y.; Talavera, K.; Everaerts, W.; Janssens, A.; Kwan, K.Y.; Vennekens, R.; Nilius, B.; Voets, T. TRPA1 acts as a cold sensor in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2009, 106, 1273–1278. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, T.; Kistner, K.; Miermeister, F.; Winkelmann, R.; Wittmann, J.; Fischer, M.J.; Weidner, C.; Reeh, P.W. TRPA1 and TRPV1 are differentially involved in heat nociception of mice. Eur. J. Pain 2013, 17, 1472–1482. [Google Scholar] [CrossRef]
- Moparthi, L.; Kichko, T.I.; Eberhardt, M.; Hogestatt, E.D.; Kjellbom, P.; Johanson, U.; Reeh, P.W.; Leffler, A.; Filipovic, M.R.; Zygmunt, P.M. Human TRPA1 is a heat sensor displaying intrinsic U-shaped thermosensitivity. Sci. Rep. 2016, 6, 28763. [Google Scholar] [CrossRef]
- Vandewauw, I.; De Clercq, K.; Mulier, M.; Held, K.; Pinto, S.; Van Ranst, N.; Segal, A.; Voet, T.; Vennekens, R.; Zimmermann, K.; et al. A TRP channel trio mediates acute noxious heat sensing. Nature 2018, 555, 662–666. [Google Scholar] [CrossRef]
- Mustafa, S.; Thulesius, O. Cooling-induced carotid artery dilatation: An experimental study in isolated vessels. Stroke 2002, 33, 256–260. [Google Scholar] [CrossRef]
- Mustafa, S. The effect of temperature on vascular smooth muscle: Cooling-induced vasodilation in deep arteries and veins. Pflug. Arch. Eur. J. Physiol. 2023, 475, 1089–1095. [Google Scholar] [CrossRef]
- Sullivan, M.N.; Gonzales, A.L.; Pires, P.W.; Bruhl, A.; Leo, M.D.; Li, W.; Oulidi, A.; Boop, F.A.; Feng, Y.; Jaggar, J.H.; et al. Localized TRPA1 channel Ca2+ signals stimulated by reactive oxygen species promote cerebral artery dilation. Sci. Signal. 2015, 8, ra2. [Google Scholar] [CrossRef]
- Martinez-Lemus, L.A. The dynamic structure of arterioles. Basic Clin. Pharmacol. Toxicol. 2012, 110, 5–11. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kelava, L.; Pakai, E.; Ogasawara, K.; Fekete, K.; Pozsgai, G.; Pinter, E.; Garami, A. Effects of Hydrogen Sulfide at Normal Body Temperature and in the Cold on Isolated Tail and Carotid Arteries from Rats and TRPA1 Knockout and Wild-Type Mice. Biomedicines 2024, 12, 2874. https://doi.org/10.3390/biomedicines12122874
Kelava L, Pakai E, Ogasawara K, Fekete K, Pozsgai G, Pinter E, Garami A. Effects of Hydrogen Sulfide at Normal Body Temperature and in the Cold on Isolated Tail and Carotid Arteries from Rats and TRPA1 Knockout and Wild-Type Mice. Biomedicines. 2024; 12(12):2874. https://doi.org/10.3390/biomedicines12122874
Chicago/Turabian StyleKelava, Leonardo, Eszter Pakai, Kazushi Ogasawara, Kata Fekete, Gabor Pozsgai, Erika Pinter, and Andras Garami. 2024. "Effects of Hydrogen Sulfide at Normal Body Temperature and in the Cold on Isolated Tail and Carotid Arteries from Rats and TRPA1 Knockout and Wild-Type Mice" Biomedicines 12, no. 12: 2874. https://doi.org/10.3390/biomedicines12122874
APA StyleKelava, L., Pakai, E., Ogasawara, K., Fekete, K., Pozsgai, G., Pinter, E., & Garami, A. (2024). Effects of Hydrogen Sulfide at Normal Body Temperature and in the Cold on Isolated Tail and Carotid Arteries from Rats and TRPA1 Knockout and Wild-Type Mice. Biomedicines, 12(12), 2874. https://doi.org/10.3390/biomedicines12122874






