Prediction of Bacterial Etiology in Pediatric Patients with Acute Epididymitis: A Comparison of C-Reactive Protein and Urinalysis in Terms of Diagnostic Accuracy †
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Clinical Data
2.2. Statistical Analysis
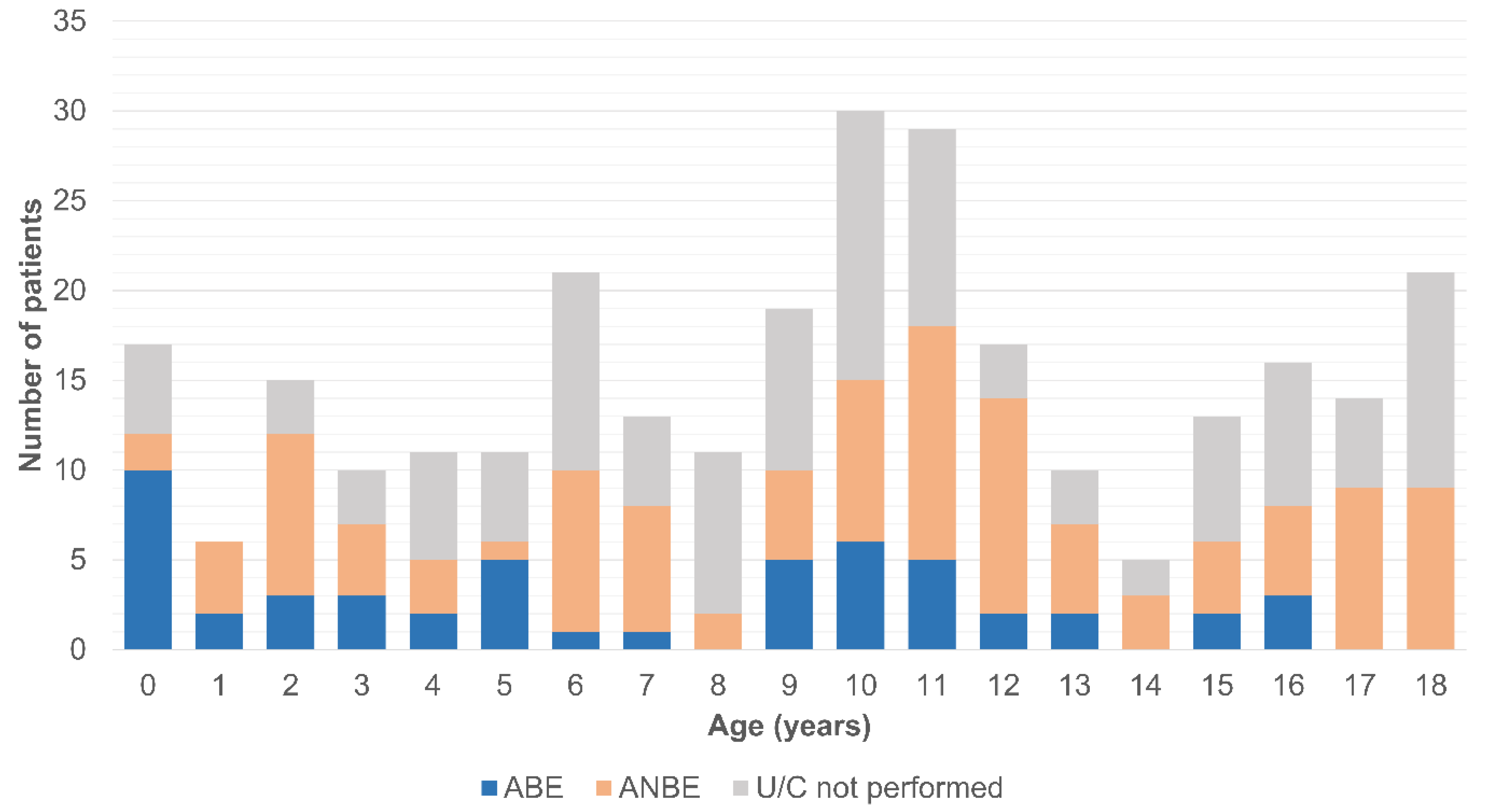
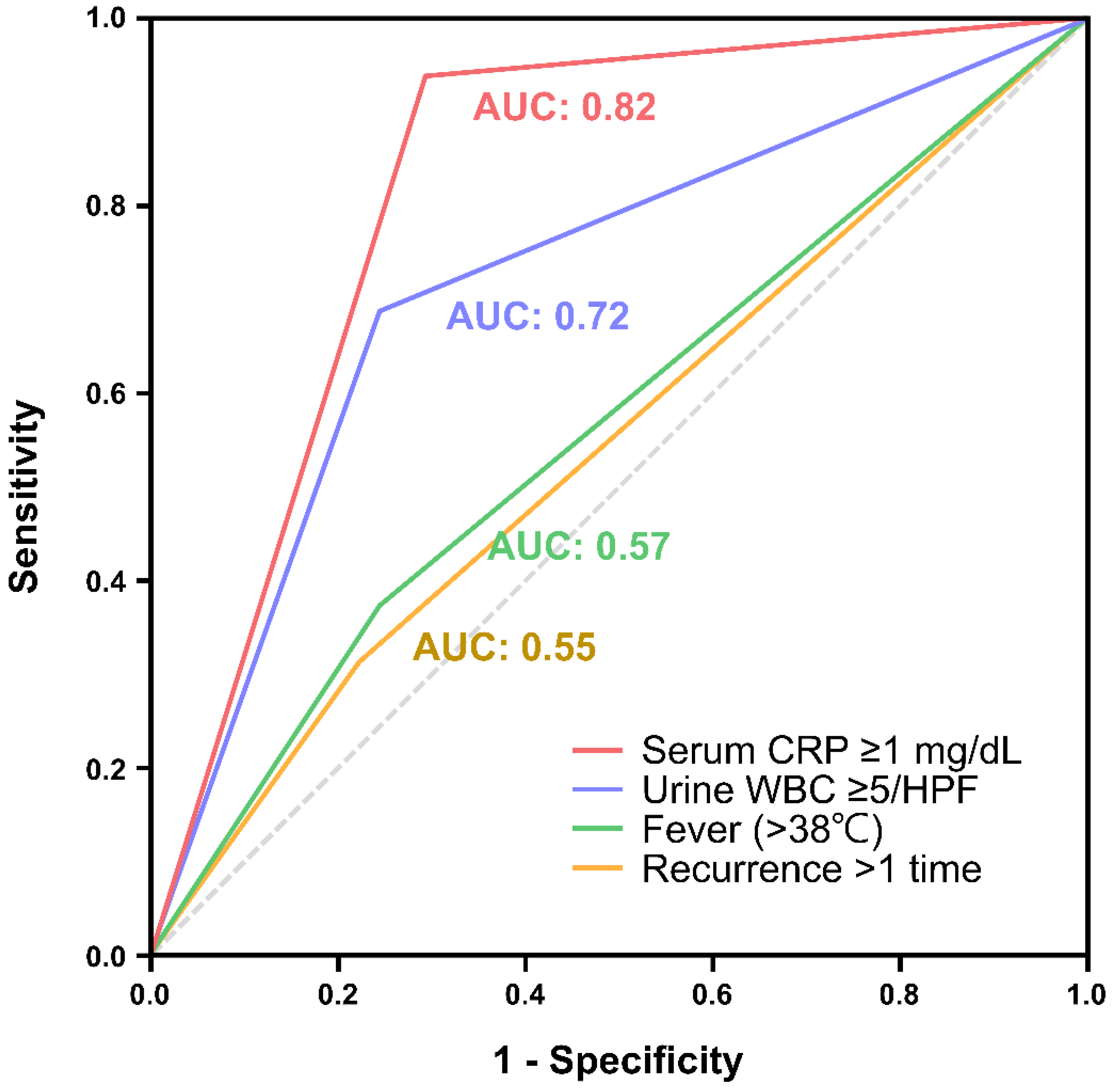
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Somekh, E.; Gorenstein, A.; Serour, F. Acute Epididymitis in Boys: Evidence of a Post-Infectious Etiology. J. Urol. 2004, 171, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, L.; Rogel, R.; Sanchez-Gonzalez, J.V.; Perez-Ardavin, J.; Moreno, E.; Lujan, S.; Broseta, E.; Boronat, F. Evaluation of adult acute scrotum in the emergency room: Clinical characteristics, diagnosis, management, and costs. Urology 2016, 94, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Ogasawara, Y.; Nikai, K.; Yamada, S.; Fujiwara, K.; Okazaki, T. Acute scrotum and testicular torsion in children: A retrospective study in a single institution. J. Pediatr. Urol. 2020, 16, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Gislason, T.; Noronha, R.F.; Gregory, J.G. Acute epididymitis in boys: A 5-year retrospective study. J. Urol. 1980, 124, 533–534. [Google Scholar] [CrossRef] [PubMed]
- Siegel, A.; Snyder, H.; Duckett, J.W. Epididymitis in infants and boys: Underlying urogenital anomalies and efficacy of imaging modalities. J. Urol. 1987, 138, 1100–1103. [Google Scholar] [CrossRef]
- Kranz, J.; Bartoletti, R.; Bruyère, F.; Cai, T.; Geerlings, S.; Köves, B.; Schubert, S.; Pilatz, A.; Veeratterapillay, R.; Wagenlehner, F.M. European Association of Urology Guidelines on Urological Infections: Summary of the 2024 Guidelines. Eur. Urol. 2024, 86, 27–41. [Google Scholar] [CrossRef]
- Sintim-Damoa, A.; Cohen, H.L. Pearls and pitfalls of pediatric scrotal imaging. Semin. Ultrasound CT MRI 2022, 43, 115–129. [Google Scholar] [CrossRef]
- Sakellaris, G.S.; Charissis, G.C. Acute epididymitis in Greek children: A 3-year retrospective study. Eur. J. Pediatr. 2008, 167, 765–769. [Google Scholar] [CrossRef]
- Graumann, L.A.; Dietz, H.G.; Stehr, M. Urinalysis in children with epididymitis. Eur. J. Pediatr. Surg. 2010, 20, 247–249. [Google Scholar] [CrossRef]
- Joo, J.M.; Yang, S.H.; Kang, T.W.; Jung, J.H.; Kim, S.J.; Kim, K.J. Acute epididymitis in children: The role of the urine test. Korean J. Urol. 2013, 54, 135–138. [Google Scholar] [CrossRef]
- Cristoforo, T.A. Evaluating the necessity of antibiotics in the treatment of acute epididymitis in pediatric patients: A literature review of retrospective studies and data analysis. Pediatr. Emerg. Care 2021, 37, e1675–e1680. [Google Scholar] [CrossRef] [PubMed]
- Redshaw, J.D.; Tran, T.L.; Wallis, M.C.; deVries, C.R. Epididymitis: A 21-year retrospective review of presentations to an outpatient urology clinic. J. Urol. 2014, 192, 1203–1207. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.-S.; Wong, S.-M.; Kuo, M.-C.; Chiang, I.-N.; Huang, K.-H.; Huang, C.-Y.; Pu, Y.-S.; Chang, H.-C.; Lu, Y.-C. MP53-06 Bacterial or Nonbacterial Epididymitis in Boys: A Preliminary Study Comparing C-Reactive Protein and Urinalysis in Diagnostic Accuracy. J. Urol. 2019, 201, e776–e777. [Google Scholar] [CrossRef]
- Tracy, C.R.; Costabile, R.A. The evaluation and treatment of acute epididymitis in a large university based population: Are CDC guidelines being followed? World J. Urol. 2009, 27, 259–263. [Google Scholar] [CrossRef]
- Pilatz, A.; Hossain, H.; Kaiser, R.; Mankertz, A.; Schuettler, C.G.; Domann, E.; Schuppe, H.-C.; Chakraborty, T.; Weidner, W.; Wagenlehner, F. Acute epididymitis revisited: Impact of molecular diagnostics on etiology and contemporary guideline recommendations. Eur. Urol. 2015, 68, 428–435. [Google Scholar] [CrossRef]
- Berger, R.E.; Kessler, D.; Holmes, K.K. Etiology and manifestations of epididymitis in young men: Correlations with sexual orientation. J. Infect. Dis. 1987, 155, 1341–1343. [Google Scholar] [CrossRef]
- Melekos, M.D.; Asbach, H.W. Epididymitis: Aspects concerning etiology and treatment. J. Urol. 1987, 138, 83–86. [Google Scholar] [CrossRef]
- Haecker, F.M.; Hauri-Hohl, A.; von Schweinitz, D. Acute epididymitis in children: A 4-year retrospective study. Eur. J. Pediatr. Surg. 2005, 15, 180–186. [Google Scholar] [CrossRef]
- Carignan, A.; Roussy, J.-F.; Lapointe, V.; Valiquette, L.; Sabbagh, R.; Pépin, J. Increasing risk of infectious complications after transrectal ultrasound–guided prostate biopsies: Time to reassess antimicrobial prophylaxis? Eur. Urol. 2012, 62, 453–459. [Google Scholar] [CrossRef]
- Wagenlehner, F.; Bartoletti, R.; Cek, M.; Grabe, M.; Kahlmeter, G.; Pickard, R.; Bjerklund-Johansen, T.E. Antibiotic stewardship: A call for action by the urologic community. Eur. Urol. 2013, 64, 358–360. [Google Scholar] [CrossRef]
- Wagenlehner, F.M.; van Oostrum, E.; Tenke, P.; Tandogdu, Z.; Cek, M.; Grabe, M.; Wullt, B.; Pickard, R.; Naber, K.G.; Pilatz, A. Infective complications after prostate biopsy: Outcome of the Global Prevalence Study of Infections in Urology (GPIU) 2010 and 2011, a prospective multinational multicentre prostate biopsy study. Eur. Urol. 2013, 63, 521–527. [Google Scholar] [CrossRef] [PubMed]
- McAndrew, H.; Pemberton, R.; Kikiros, C.; Gollow, I. The incidence and investigation of acute scrotal problems in children. Pediatr. Surg. Int. 2002, 18, 435–437. [Google Scholar] [CrossRef] [PubMed]
- Santillanes, G.; Gausche-Hill, M.; Lewis, R.J. Are antibiotics necessary for pediatric epididymitis? Pediatr. Emerg. Care 2011, 27, 174–178. [Google Scholar] [CrossRef]
- Thompson, D.; Pepys, M.B.; Wood, S.P. The physiological structure of human C-reactive protein and its complex with phosphocholine. Structure 1999, 7, 169–177. [Google Scholar] [CrossRef]
- Sproston, N.R.; Ashworth, J.J. Role of C-reactive protein at sites of inflammation and infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef]
- Meštrović, J.; Biočić, M.; Pogorelić, Z.; Furlan, D.; Družijanić, N.; Todorić, D.; Čapkun, V. Differentiation of inflammatory from non-inflammatory causes of acute scrotum using relatively simple laboratory tests: Prospective study. J. Pediatr. Urol. 2013, 9, 313–317. [Google Scholar] [CrossRef]
- Paul, S.K.; Ghosh, P.K.; Islam, R.; Biswas, P.K.; Ali, M.A.; Rashid, M.A. Role of C-reactive protein for Assessment for Exploration of Acute Scrotum in Children: A Study was done in a Tertiary Care Hospital. Sch. J. App. Med. Sci. 2022, 12, 2139–2145. [Google Scholar] [CrossRef]
- Shi, J.; Zhan, Z.-S.; Zheng, Z.-S.; Zhu, X.-X.; Zhou, X.-Y.; Zhang, S.-Y. Correlation of procalcitonin and c-reactive protein levels with pathogen distribution and infection localization in urinary tract infections. Sci. Rep. 2023, 13, 17164. [Google Scholar] [CrossRef]
- Xu, R.-Y.; Liu, H.-W.; Liu, J.-L.; Dong, J.-H. Procalcitonin and C-reactive protein in urinary tract infection diagnosis. BMC Urol. 2014, 14, 45. [Google Scholar] [CrossRef]
- Zunder, S.; Vollaard, A.; van Nieuwkoop, C.; Stalenhoef, J.; Delfos, N.; Spelt, I.; Blom, J.; Leyten, E.; Koster, T.; Ablij, H. Prognostic value of pro-adrenomedullin, procalcitonin and C-reactive protein in predicting outcome of febrile urinary tract infection. Clin. Microbiol. Infect. 2014, 20, 1048–1054. [Google Scholar] [CrossRef]
- Kathiresan, S.; Larson, M.G.; Vasan, R.S.; Guo, C.-Y.; Gona, P.; Keaney, J.F., Jr.; Wilson, P.W.; Newton-Cheh, C.; Musone, S.L.; Camargo, A.L. Contribution of clinical correlates and 13 C-reactive protein gene polymorphisms to interindividual variability in serum C-reactive protein level. Circulation 2006, 113, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
| All Patients (n = 289) | ANBE (n = 115) | ABE (n = 52) | p Value | ||
|---|---|---|---|---|---|
| Age, years | 9.5 ± 5.2 | 9.3 ± 5.5 | 6.8 ± 5.2 | 0.02 * | |
| Laterality (n = 247) | 0.46 | ||||
| Right | 117 (47.4) | 49 (48.5) | 23 (45.1) | ||
| Left | 114 (46.2) | 46 (45.5) | 22 (43.1) | ||
| Bilateral | 16 (6.5) | 6 (5.9) | 6 (11.8) | ||
| Concomitant diseases | 0.69 | ||||
| Urological | 57 (19.7) | 25 (21.7) | 13 (25.0) | ||
| Non-urological | 30 (10.4) | 13 (11.3) | 8 (15.4) | ||
| Recurrence Symptoms/signs | 34 (11.8) | 18 (15.7) | 10 (19.2) | 0.36 | |
| LUT syndrome | 5 (1.7) | 3 (2.6) | 2 (3.8) | 0.53 | |
| Scrotal swelling | 198 (68.5) | 84 (73.0) | 41 (78.8) | 0.37 | |
| Scrotal erythema | 142 (49.1) | 67 (58.3) | 27 (51.9) | 0.09 | |
| Tenderness of epididymis | 222 (76.8) | 97 (84.3) | 43 (82.7) | 0.02 | |
| Body temperature, °C (n = 117) | 36.8 (36.5–37.2) | 36.8 (36.6–37.2) | 36.8 (36.5–37.3) | 0.81 * | |
| Fever (>38 °C) (n = 117) | 17 (14.5) | 11 (15.5) | 6 (13.0) | 0.54 | |
| Antibiotic therapy | 216 (74.7) | 97 (84.3) | 45 (86.5) | 0.58 | |
| Serum WBC count, 103/μL (n = 87) | 10.56 (8.09–14.65) | 10.42 (7.57–14.09) | 10.96 (8.33–15.99) | 0.23 * | |
| CRP, mg/dL (n = 76) | 0.80 (0.09–3.54) | 0.25 (0.05–1.49) | 3.68 (1.56–8.45) | <0.001 * | |
| RBC ≥ 5/HPF on urine analysis (n = 211) | 32 (17.5) | 14 (14.7) | 16 (38.1) | 0.003 | |
| WBC ≥ 5/HPF on urine analysis (n = 210) | 53 (25.2) | 25 (23.1) | 21 (41.2) | 0.005 | |
| Univariable Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Age | 0.89 (0.83–0.94) | 0.001 | 0.88 (0.78–1.01) | 0.07 |
| Laterality | ||||
| Right | Reference | |||
| Left | 1.16 (0.58–2.31) | 0.67 | ||
| Bilateral | 2.52 (0.67–9.54) | 0.16 | ||
| Recurrence | 1.24 (0.55–3.01) | 0.43 | ||
| Fever (>38 °C) | 1.11 (0.38–3.25) | 0.57 | ||
| WBC ≥ 5/HPF on urinalysis | 2.32 (1.14–4.75) | 0.02 | 2.09 (0.47–9.29) | 0.33 |
| Serum CRP ≥ 1mg/dL | 43.77 (5.52-379.36) | <0.001 | 61.96 (6.10-629.01) | <0.001 |
| Diagnostic Method | Advantages | Disadvantages |
|---|---|---|
| CRP |
|
|
| Urinalysis |
|
|
| ||
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, K.; Tseng, C.-S.; Wong, S.-M.; Huang, K.-H.; Chiang, I.-N.; Huang, C.-Y.; Chiang, C.-H. Prediction of Bacterial Etiology in Pediatric Patients with Acute Epididymitis: A Comparison of C-Reactive Protein and Urinalysis in Terms of Diagnostic Accuracy. Biomedicines 2024, 12, 2866. https://doi.org/10.3390/biomedicines12122866
Liu K, Tseng C-S, Wong S-M, Huang K-H, Chiang I-N, Huang C-Y, Chiang C-H. Prediction of Bacterial Etiology in Pediatric Patients with Acute Epididymitis: A Comparison of C-Reactive Protein and Urinalysis in Terms of Diagnostic Accuracy. Biomedicines. 2024; 12(12):2866. https://doi.org/10.3390/biomedicines12122866
Chicago/Turabian StyleLiu, Kang, Chi-Shin Tseng, Shin-Mei Wong, Kuo-How Huang, I-Ni Chiang, Chao-Yuan Huang, and Chih-Hung Chiang. 2024. "Prediction of Bacterial Etiology in Pediatric Patients with Acute Epididymitis: A Comparison of C-Reactive Protein and Urinalysis in Terms of Diagnostic Accuracy" Biomedicines 12, no. 12: 2866. https://doi.org/10.3390/biomedicines12122866
APA StyleLiu, K., Tseng, C.-S., Wong, S.-M., Huang, K.-H., Chiang, I.-N., Huang, C.-Y., & Chiang, C.-H. (2024). Prediction of Bacterial Etiology in Pediatric Patients with Acute Epididymitis: A Comparison of C-Reactive Protein and Urinalysis in Terms of Diagnostic Accuracy. Biomedicines, 12(12), 2866. https://doi.org/10.3390/biomedicines12122866






