IL-6R Inhibitors and Gastrointestinal Perforations: A Pharmacovigilance Study and a Predicting Nomogram
Abstract
:1. Introduction
2. Results
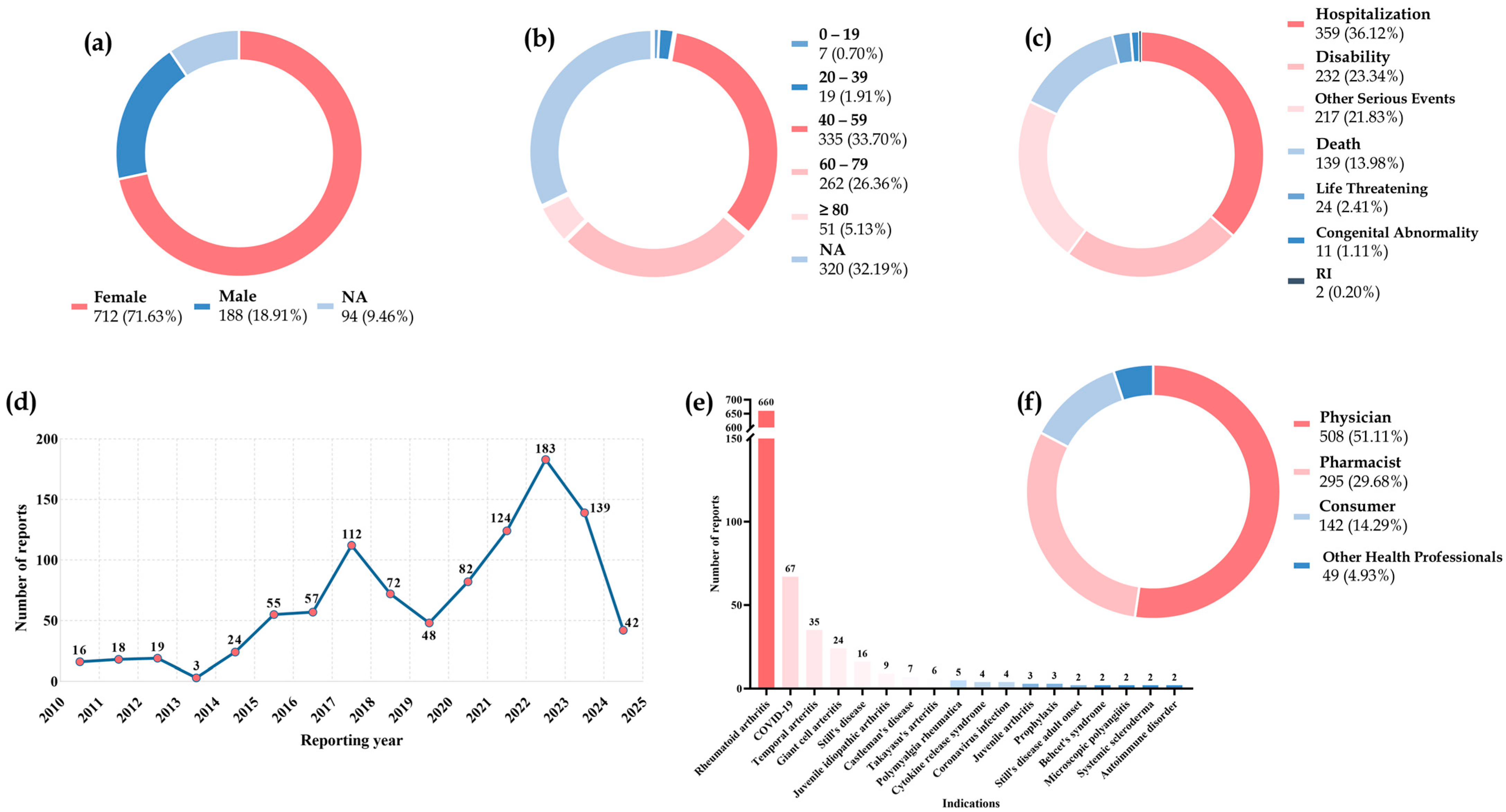
2.1. Descriptive Analysis
2.2. Perforation Location Descriptions
2.3. Disproportionality Analysis
2.4. Stratification Analysis
2.5. Time-to-Onset Analysis
2.6. Analysis of Risk Factors
2.7. A Predicting Nomogram and Validation
3. Discussion
4. Materials and Methods
4.1. Study Design and Data Mining
4.2. Time-to-Onset Analysis
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lipsky, P.E. Interleukin-6 and rheumatic diseases. Arthritis Res. Ther. 2006, 8 (Suppl. 2), S4. [Google Scholar] [CrossRef] [PubMed]
- Antonio, A.A.; Santos, R.N.; Abariga, S.A. Tocilizumab for giant cell arteritis. Cochrane Database Syst. Rev. 2022, 5, Cd013484. [Google Scholar] [CrossRef]
- Scott, L.J. Tocilizumab: A Review in Rheumatoid Arthritis. Drugs 2017, 77, 1865–1879. [Google Scholar] [CrossRef]
- Yao, X.; Huang, J.; Zhong, H.; Shen, N.; Faggioni, R.; Fung, M.; Yao, Y. Targeting interleukin-6 in inflammatory autoimmune diseases and cancers. Pharmacol. Ther. 2014, 141, 125–139. [Google Scholar] [CrossRef]
- Klopfenstein, T.; Gendrin, V.; Kadiane-Oussou, N.J.; Conrozier, T.; Zayet, S. Tocilizumab in COVID-19 pneumonia: Practical proposals based on a narrative review of randomised trials. Rev. Med. Virol. 2022, 32, e2239. [Google Scholar] [CrossRef]
- Mańdziuk, J.; Kuchar, E.; Okarska-Napierała, M. How international guidelines recommend treating children who have severe COVID-19 or risk disease progression. Acta Paediatr. (1992) 2024, 113, 2345–2453. [Google Scholar] [CrossRef]
- Barbulescu, A.; Delcoigne, B.; Askling, J.; Frisell, T. Gastrointestinal perforations in patients with rheumatoid arthritis treated with biological disease-modifying antirheumatic drugs in Sweden: A nationwide cohort study. RMD Open 2020, 6, e001201. [Google Scholar] [CrossRef]
- Giles, J.T.; Sattar, N.; Gabriel, S.; Ridker, P.M.; Gay, S.; Warne, C.; Musselman, D.; Brockwell, L.; Shittu, E.; Klearman, M.; et al. Cardiovascular Safety of Tocilizumab Versus Etanercept in Rheumatoid Arthritis: A Randomized Controlled Trial. Arthritis Rheumatol. 2020, 72, 31–40. [Google Scholar] [CrossRef]
- Rempenault, C.; Lukas, C.; Combe, B.; Herrero, A.; Pane, I.; Schaeverbeke, T.; Wendling, D.; Pham, T.; Gottenberg, J.E.; Mariette, X.; et al. Risk of diverticulitis and gastrointestinal perforation in rheumatoid arthritis treated with tocilizumab compared to rituximab or abatacept. Rheumatology 2022, 61, 953–962. [Google Scholar] [CrossRef]
- Toniati, P.; Piva, S.; Cattalini, M.; Garrafa, E.; Regola, F.; Castelli, F.; Franceschini, F.; Airò, P.; Bazzani, C.; Beindorf, E.A.; et al. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: A single center study of 100 patients in Brescia, Italy. Autoimmun. Rev. 2020, 19, 102568. [Google Scholar] [CrossRef]
- Ludwig, H.; Terpos, E.; van de Donk, N.; Mateos, M.V.; Moreau, P.; Dimopoulos, M.A.; Delforge, M.; Rodriguez-Otero, P.; San-Miguel, J.; Yong, K.; et al. Prevention and management of adverse events during treatment with bispecific antibodies and CAR T cells in multiple myeloma: A consensus report of the European Myeloma Network. Lancet. Oncol. 2023, 24, e255–e269. [Google Scholar] [CrossRef]
- Ozluk, A.A.; Gunenc, D.; Yildirim, S.S.; Karaca, B. Tocilizumab in the treatment of steroid refractory immune-related hepatotoxicity: A case series and review of the literature. Melanoma Res. 2024, 34, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Kastrati, K.; Aletaha, D.; Burmester, G.R.; Chwala, E.; Dejaco, C.; Dougados, M.; McInnes, I.B.; Ravelli, A.; Sattar, N.; Stamm, T.A.; et al. A systematic literature review informing the consensus statement on efficacy and safety of pharmacological treatment with interleukin-6 pathway inhibition with biological DMARDs in immune-mediated inflammatory diseases. RMD Open 2022, 8, e002359. [Google Scholar] [CrossRef] [PubMed]
- Strangfeld, A.; Richter, A.; Siegmund, B.; Herzer, P.; Rockwitz, K.; Demary, W.; Aringer, M.; Meißner, Y.; Zink, A.; Listing, J. Risk for lower intestinal perforations in patients with rheumatoid arthritis treated with tocilizumab in comparison to treatment with other biologic or conventional synthetic DMARDs. Ann. Rheum. Dis. 2017, 76, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Gout, T.; Ostör, A.J.; Nisar, M.K. Lower gastrointestinal perforation in rheumatoid arthritis patients treated with conventional DMARDs or tocilizumab: A systematic literature review. Clin. Rheumatol. 2011, 30, 1471–1474. [Google Scholar] [CrossRef]
- Morris, C.R.; Harvey, I.M.; Stebbings, W.S.; Speakman, C.T.; Kennedy, H.J.; Hart, A.R. Anti-inflammatory drugs, analgesics and the risk of perforated colonic diverticular disease. Br. J. Surg. 2003, 90, 1267–1272. [Google Scholar] [CrossRef]
- Svenningsen, P.; Manoharan, T.; Foss, N.B.; Lauritsen, M.L.; Bay-Nielsen, M. Increased mortality in the elderly after emergency abdominal surgery. Dan. Med. J. 2014, 61, A4876. [Google Scholar] [PubMed]
- Hernández-Díaz, S.; Rodríguez, L.A. Steroids and risk of upper gastrointestinal complications. Am. J. Epidemiol. 2001, 153, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, K.A.; Manieri, N.A.; Liu, T.C.; Stappenbeck, T.S. IL-6 stimulates intestinal epithelial proliferation and repair after injury. PLoS ONE 2014, 9, e114195. [Google Scholar] [CrossRef]
- Nakahara, H.; Song, J.; Sugimoto, M.; Hagihara, K.; Kishimoto, T.; Yoshizaki, K.; Nishimoto, N. Anti-interleukin-6 receptor antibody therapy reduces vascular endothelial growth factor production in rheumatoid arthritis. Arthritis Rheum. 2003, 48, 1521–1529. [Google Scholar] [CrossRef] [PubMed]
- Wichelmann, T.A.; Abdulmujeeb, S.; Ehrenpreis, E.D. Bevacizumab and gastrointestinal perforations: A review from the FDA Adverse Event Reporting System (FAERS) database. Aliment. Pharmacol. Ther. 2021, 54, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.P.; Yang, H.Y.; Ouyang, M.L.; Cheng, Q.; Shi, X.; Sun, M.H. A disproportionality analysis of adverse events associated to pertuzumab in the FDA Adverse Event Reporting System (FAERS). BMC Pharmacol. Toxicol. 2023, 24, 62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Mao, W.; Li, X.; Wang, X.; Liu, J.; Hu, S.; Hu, J. Analysis of acute pancreatitis associated with SGLT-2 inhibitors and predictive factors of the death risk: Based on food and drug administration adverse event report system database. Front. Pharmacol. 2022, 13, 977582. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Sun, Y.; Zuo, X.; Zou, Y. Assessment of adverse events related to anti-interleukin-6 receptor monoclonal antibodies using the FDA adverse event reporting system:a real-world pharmacovigilance study. Expert. Opin. Drug Saf. 2024, 2024. 23, 1327–1339. [Google Scholar] [CrossRef]
- Yamamoto, K.; Goto, H.; Hirao, K.; Nakajima, A.; Origasa, H.; Tanaka, K.; Tomobe, M.; Totsuka, K. Longterm Safety of Tocilizumab: Results from 3 Years of Followup Postmarketing Surveillance of 5573 Patients with Rheumatoid Arthritis in Japan. J. Rheumatol. 2015, 42, 1368–1375. [Google Scholar] [CrossRef]
- Fleischmann, R.; Genovese, M.C.; Lin, Y.; St John, G.; van der Heijde, D.; Wang, S.; Gomez-Reino, J.J.; Maldonado-Cocco, J.A.; Stanislav, M.; Kivitz, A.J.; et al. Long-term safety of sarilumab in rheumatoid arthritis: An integrated analysis with up to 7 years’ follow-up. Rheumatology 2020, 59, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Burmester, G.R.; Strand, V.; Kivitz, A.J.; Hu, C.C.; Wang, S.; van Hoogstraten, H.; Klier, G.L.; Fleischmann, R. Long-term safety and efficacy of sarilumab with or without background csDMARDs in rheumatoid arthritis. Rheumatology 2023, 62, 3268–3279. [Google Scholar] [CrossRef]
- Agnes, A.; La Greca, A.; Tirelli, F.; Papa, V. Duodenal perforation in a SARS-CoV-2-positive patient with negative PCR results for SARS-CoV-2 in the peritoneal fluid. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 12516–12521. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Matsumoto, H.; Temmoku, J.; Shakespear, N.; Kiko, Y.; Kikuchi, K.; Sumichika, Y.; Saito, K.; Fujita, Y.; Matsuoka, N.; et al. Case report: Rapid development of amyloid A amyloidosis in temporal arteritis with SAA1.3 allele; An unusual case of intestinal amyloidosis secondary to temporal arteritis. Front. Immunol. 2023, 14, 1144397. [Google Scholar] [CrossRef]
- Curtis, J.R.; Lanas, A.; John, A.; Johnson, D.A.; Schulman, K.L. Factors associated with gastrointestinal perforation in a cohort of patients with rheumatoid arthritis. Arthritis Care Res. 2012, 64, 1819–1828. [Google Scholar] [CrossRef]
- Xie, F.; Yun, H.; Bernatsky, S.; Curtis, J.R. Brief Report: Risk of Gastrointestinal Perforation Among Rheumatoid Arthritis Patients Receiving Tofacitinib, Tocilizumab, or Other Biologic Treatments. Arthritis Rheumatol. 2016, 68, 2612–2617. [Google Scholar] [CrossRef]
- Siegmund, B.; Sennello, J.A.; Jones-Carson, J.; Gamboni-Robertson, F.; Lehr, H.A.; Batra, A.; Fedke, I.; Zeitz, M.; Fantuzzi, G. Leptin receptor expression on T lymphocytes modulates chronic intestinal inflammation in mice. Gut 2004, 53, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Peyrin-Biroulet, L.; Gonzalez, F.; Dubuquoy, L.; Rousseaux, C.; Dubuquoy, C.; Decourcelle, C.; Saudemont, A.; Tachon, M.; Béclin, E.; Odou, M.F.; et al. Mesenteric fat as a source of C reactive protein and as a target for bacterial translocation in Crohn’s disease. Gut 2012, 61, 78–85. [Google Scholar] [CrossRef]
- Conde Cardona, G.; Quintana Pájaro, L.D.; Quintero Marzola, I.D.; Ramos Villegas, Y.; Moscote Salazar, L.R. Neurotropism of SARS-CoV 2: Mechanisms and manifestations. J. Neurol. Sci. 2020, 412, 116824. [Google Scholar] [CrossRef]
- Chaugale, S.B.; Singhal, V.; Kapoor, D.; Singh, A. Gastrointestinal complications (gangrene or perforation) after corona virus disease 2019—A series of ten patients. Indian J. Gastroenterol. Off. J. Indian Soc. Gastroenterol. 2022, 41, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, H.; Li, L.; Hu, M.; Chen, L.; Xu, B.; Song, Q. A nomogram for predicting overall survival in patients with low-grade endometrial stromal sarcoma: A population-based analysis. Cancer Commun. 2020, 40, 301–312. [Google Scholar] [CrossRef]
- Zou, S.P.; Yang, H.Y.; Ouyang, M.; Cheng, Q.; Shi, X.; Sun, M.H. Post-marketing safety of anti-IL-5 monoclonal antibodies (mAbs): An analysis of the FDA Adverse Event Reporting System (FAERS). Expert. Opin. Drug Saf. 2024, 23, 353–362. [Google Scholar] [CrossRef]
- Kinoshita, S.; Hosomi, K.; Yokoyama, S.; Takada, M. Time-to-onset analysis of amiodarone-associated thyroid dysfunction. J. Clin. Pharm. Ther. 2020, 45, 65–71. [Google Scholar] [CrossRef]
- Jiang, J.J.; Zhao, B.; Li, J. Does eltrombopag lead to thrombotic events? A pharmacovigilance study of the FDA adverse event reporting system. J. Clin. Pharm. Ther. 2022, 47, 1556–1562. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Shu, Y.; Chen, G.; Li, J.; Li, F. A real-world pharmacovigilance study of FDA adverse event reporting system (FAERS) events for niraparib. Sci. Rep. 2022, 12, 20601. [Google Scholar] [CrossRef] [PubMed]
- Rothman, K.J.; Lanes, S.; Sacks, S.T. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol. Drug Saf. 2004, 13, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Ye, X.; Hu, F.; Xu, J.; Guo, X.; Zhuang, Y.; He, J. Endocrine toxicity of immune checkpoint inhibitors: A real-world study leveraging US Food and Drug Administration adverse events reporting system. J. Immunother. Cancer 2019, 7, 286. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Wang, L.; Ding, Y.; Zhang, Q. Disproportionality Analysis of Abemaciclib in the FDA Adverse Event Reporting System: A Real-World Post-Marketing Pharmacovigilance Assessment. Drug Saf. 2023, 46, 881–895. [Google Scholar] [CrossRef] [PubMed]
- Whiles, B.B.; Reich, D.A.; Green, J.; Yu, F.; Bird, V.G. Evaluation of fear, willingness to seek care, and healthcare delivery preferences for patients with nephrolithiasis during the COVID-19 pandemic. Transl. Androl. Urol. 2024, 13, 962–969. [Google Scholar] [CrossRef]
- Riley, R.D.; Ensor, J.; Snell, K.I.E.; Harrell, F.E., Jr.; Martin, G.P.; Reitsma, J.B.; Moons, K.G.M.; Collins, G.; van Smeden, M. Calculating the sample size required for developing a clinical prediction model. BMJ (Clin. Res. Ed.) 2020, 368, m441. [Google Scholar] [CrossRef] [PubMed]
- van Smeden, M.; Moons, K.G.; de Groot, J.A.; Collins, G.S.; Altman, D.G.; Eijkemans, M.J.; Reitsma, J.B. Sample size for binary logistic prediction models: Beyond events per variable criteria. Stat. Methods Med. Res. 2019, 28, 2455–2474. [Google Scholar] [CrossRef]



| Preferred Terms (PTs) | Tocilizumab | Sarilumab | p | ||||
|---|---|---|---|---|---|---|---|
| Cases | ROR | IC (IC025) | Cases | ROR | IC (IC025) | ||
| Duodenal perforation | 312 * | 19.45 (17.33–21.83) | 4.18 (3.72 a) | 14 * | 9.57 (5.66–16.17) | 3.25 (1.92 b) | 0.18 |
| Intestinal perforation | 176 * | 4.63 (3.99–5.37) | 2.19 (1.89 b) | 2 | - | 0.86 (0.02) | - |
| Gastrointestinal perforation | 150 * | 10.76 (9.14–12.68) | 3.37 (2.86 b) | 2 | - | 2.21 (0.18) | - |
| Large intestine perforation | 136 * | 3.95 (3.33–4.68) | 1.96 (1.66 b) | 7 * | 4.49 (2.14–9.43) | 2.16 (1.03) | 0.62 |
| Diverticular perforation | 101 * | 12.72 (10.41–15.54) | 3.60 (2.95 b) | 3 * | 7.63 (2.46–23.71) | 2.93 (0.94) | 0.23 |
| Small intestinal perforation | 42 * | 4.03 (2.97–5.47) | 1.99 (1.47) | 1 | - | - | - |
| Gastric perforation | 29 * | 2.12 (1.47–3.05) | 1.08 (0.75) | 1 | - | - | - |
| Perforation | 9 | - | 0.79 (0.41) | - | - | - | - |
| Upper gastrointestinal perforation | 3 * | 22.98 (7.04–75.05) | 4.40 (1.35) | - | - | - | - |
| Procedural intestinal perforation | 2 | - | 1.14 (0.28) | 1 | - | - | - |
| Lower gastrointestinal perforation | 3 * | 16.71 (5.19–53.83) | 3.97 (1.23) | - | - | - | - |
| Total | 963 * | 6.86 (6.43–7.31) | 2.74 (2.57 b) | 31 * | 4.03 (2.83–5.73) | 2.00 (1.41) | <0.001 |
| Variables | Univariate Logistic | Multivariate Logistic | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | S.E | Z | p | OR (95%CI) | β | S.E | Z | p | OR (95%CI) | |
| Sex | ||||||||||
| Male | 1.00 (Reference) | 1.00 (Reference) | ||||||||
| Female | 0.37 | 0.13 | 2.79 | 0.005 | 1.45 (1.12–1.88) | 0.42 | 0.14 | 3.08 | 0.002 | 1.52 (1.16–1.98) |
| Age, years | ||||||||||
| 0–19 | 1.00 (Reference) | 1.00 (Reference) | ||||||||
| 20–39 | 0.58 | 0.68 | 0.85 | 0.394 | 1.78 (0.47–6.75) | 0.31 | 0.68 | 0.46 | 0.647 | 1.37 (0.36–5.23) |
| ≥40 | 2.03 | 0.58 | 3.49 | <0.001 | 7.59 (2.43–23.72) | 1.73 | 0.59 | 2.94 | 0.003 | 5.63 (1.78–17.78) |
| Weight, kg | 0.01 | 0.00 | 4.38 | <0.001 | 1.01 (1.01–1.01) | 0.01 | 0.00 | 3.67 | <0.001 | 1.01 (1.01–1.01) |
| Glucocorticoids | ||||||||||
| No | 1.00 (Reference) | 1.00 (Reference) | ||||||||
| Yes | 0.55 | 0.11 | 5.09 | <0.001 | 1.74 (1.40–2.15) | 0.32 | 0.11 | 2.81 | 0.005 | 1.37 (1.10–1.72) |
| NSAIDs | ||||||||||
| No | 1.00 (Reference) | 1.00 (Reference) | ||||||||
| Yes | 1.31 | 0.11 | 12.03 | <0.001 | 3.70 (2.99–4.57) | 1.24 | 0.11 | 10.96 | <0.001 | 3.46 (2.77–4.32) |
| Methotrexate | ||||||||||
| No | 1.00 (Reference) | 1.00 (Reference) | ||||||||
| Yes | 0.48 | 0.11 | 4.37 | <0.001 | 1.62 (1.31–2.02) | 0.12 | 0.12 | 0.98 | 0.326 | 1.12 (0.89–1.42) |
| IL-6R inhibitor | ||||||||||
| Sarilumab | 1.00 (Reference) | 1.00 (Reference) | ||||||||
| Tocilizumab | 0.67 | 0.34 | 1.96 | 0.050 | 1.95 (1.01–3.81) | 1.08 | 0.35 | 3.13 | 0.002 | 2.94 (1.50–5.79) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, S.; Ouyang, M.; Cheng, Q.; Shi, X.; Zhao, Y.; Sun, M. IL-6R Inhibitors and Gastrointestinal Perforations: A Pharmacovigilance Study and a Predicting Nomogram. Biomedicines 2024, 12, 2860. https://doi.org/10.3390/biomedicines12122860
Zou S, Ouyang M, Cheng Q, Shi X, Zhao Y, Sun M. IL-6R Inhibitors and Gastrointestinal Perforations: A Pharmacovigilance Study and a Predicting Nomogram. Biomedicines. 2024; 12(12):2860. https://doi.org/10.3390/biomedicines12122860
Chicago/Turabian StyleZou, Shupeng, Mengling Ouyang, Qian Cheng, Xuan Shi, Yazheng Zhao, and Minghui Sun. 2024. "IL-6R Inhibitors and Gastrointestinal Perforations: A Pharmacovigilance Study and a Predicting Nomogram" Biomedicines 12, no. 12: 2860. https://doi.org/10.3390/biomedicines12122860
APA StyleZou, S., Ouyang, M., Cheng, Q., Shi, X., Zhao, Y., & Sun, M. (2024). IL-6R Inhibitors and Gastrointestinal Perforations: A Pharmacovigilance Study and a Predicting Nomogram. Biomedicines, 12(12), 2860. https://doi.org/10.3390/biomedicines12122860






