Current Challenges in Pancreas and Islet Transplantation: A Scoping Review
Abstract
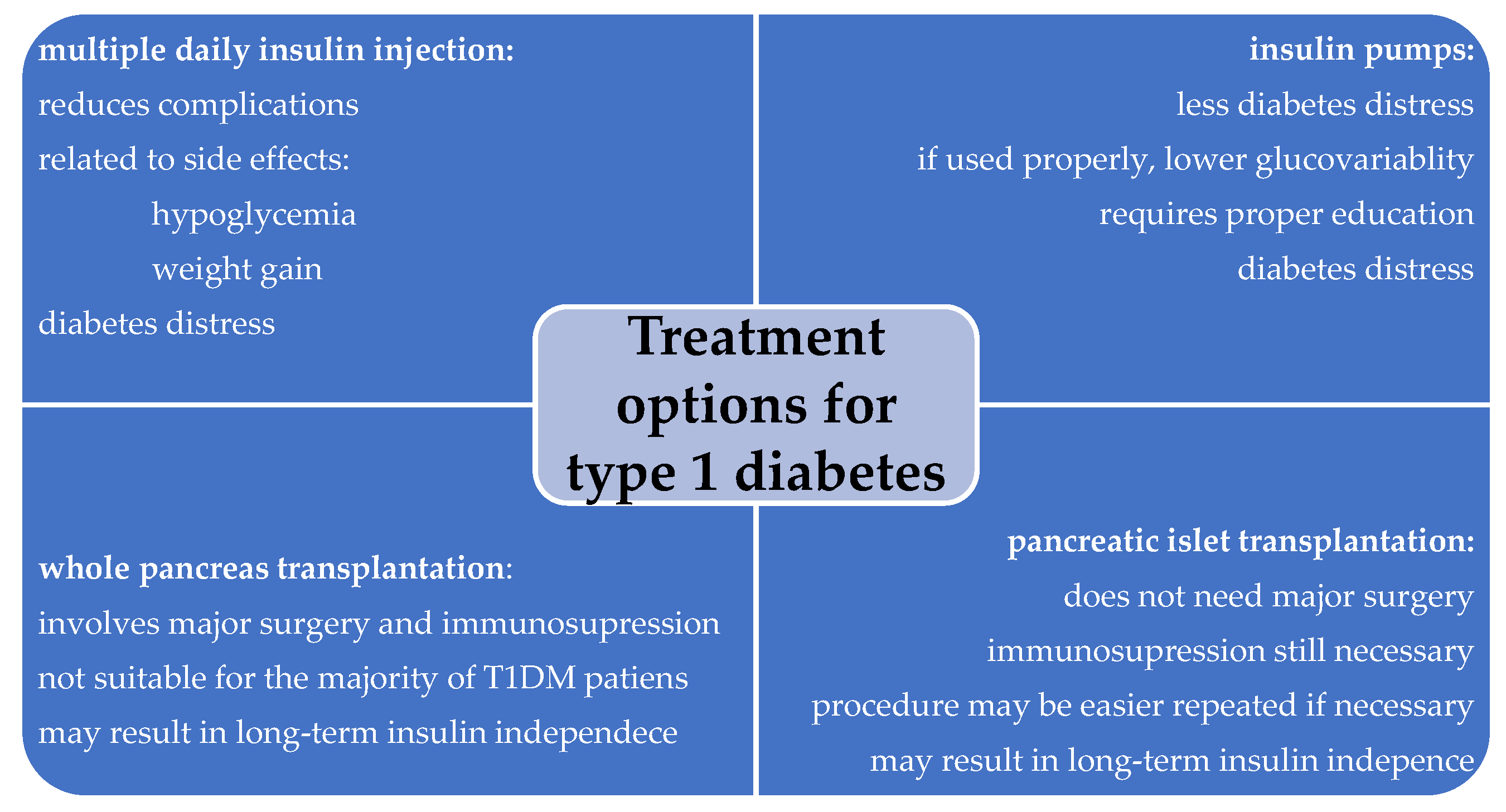
1. Introduction
2. Materials and Methods
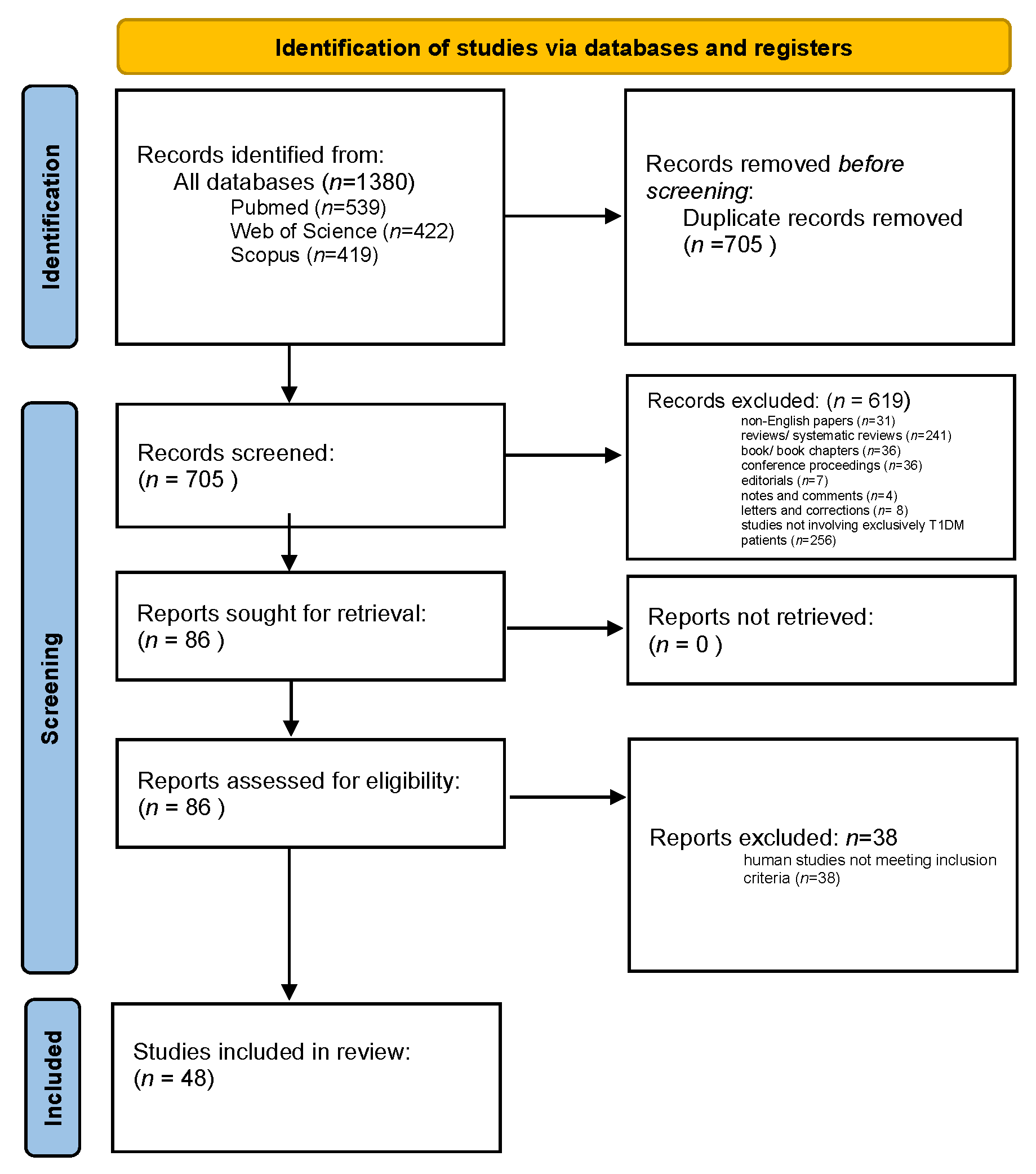
2.1. Methods, Procedures, and Screening Process
2.2. Inclusion Criteria
2.3. Exclusion Criteria
3. Results
Overall Search Results
4. Discussion
4.1. Pancreas Transplantation
4.2. Pancreatic Islet Transplantation
4.3. Islet Stem Cell Transplantation
5. Ethical Considerations in Pancreas and Pancreatic Islet Transplantation, and Stem Cell-Base Therapies
6. Key Limitations of Published Studies
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sacks, D.B.; Arnold, M.; Bakris, G.L.; Bruns, D.E.; Horvath, A.R.; Lernmark, Å.; Metzger, B.E.; Nathan, D.M.; Kirkman, M.S. Guidelines and Recommendations for Laboratory Analysis in the Diagnosis and Management of Diabetes Mellitus. Clin. Chem. 2023, 69, 808–868. [Google Scholar] [CrossRef] [PubMed]
- Diagnosis and Classification of Diabetes: Standards of Care in Diabetes—2024. Diabetes Care 2024, 47, S20–S42. [CrossRef] [PubMed]
- Available online: https://Www.Who.Int/News-Room/Fact-Sheets/Detail/Diabetes (accessed on 9 November 2024).
- Atkinson, M.A.; Eisenbarth, G.S.; Michels, A.W. Type 1 Diabetes. Lancet 2014, 383, 69–82. [Google Scholar] [CrossRef]
- Longendyke, R.; Grundman, J.B.; Majidi, S. Acute and Chronic Adverse Outcomes of Type 1 Diabetes. Endocrinol. Metab. Clin. 2024, 53, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Boscari, F.; Avogaro, A. Current Treatment Options and Challenges in Patients with Type 1 Diabetes: Pharmacological, Technical Advances and Future Perspectives. Rev. Endocr. Metab. Disord. 2021, 22, 217–240. [Google Scholar] [CrossRef]
- Larkin, H. New Drug Delays Type 1 Diabetes Onset. JAMA 2023, 329, 14. [Google Scholar] [CrossRef]
- Rodríguez-Muñoz, A.; Picón-César, M.J.; Tinahones, F.J.; Martínez-Montoro, J.I. Type 1 Diabetes-Related Distress: Current Implications in Care. Eur. J. Intern. Med. 2024, 125, 19–27. [Google Scholar] [CrossRef]
- Bellin, M.D.; Dunn, T.B. Transplant Strategies for Type 1 Diabetes: Whole Pancreas, Islet and Porcine Beta Cell Therapies. Diabetologia 2020, 63, 2049–2056. [Google Scholar] [CrossRef] [PubMed]
- Gruessner, A.C.; Gruessner, R.W. Pancreas Transplantation of US and Non-US Cases from 2005 to 2014 as Reported to the United Network for Organ Sharing (UNOS) and the International Pancreas Transplant Registry (IPTR). Rev. Diabet. Stud. RDS 2016, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://Casp-Uk.Net/Casp-Tools-Checklists/Cohort-Study-Checklist/ (accessed on 5 December 2024).
- Marfil-Garza, B.; Hefler, J.; Verhoeff, K.; Lam, A.; Dajani, K.; Anderson, B.; O’Gorman, D.; Kin, T.; Bello-Chavolla, O.; Grynoch, D.; et al. Pancreas and Islet Transplantation: Comparative Outcome Analysis of a Single-Centre Cohort Over 20-Years. Ann. Surg. 2023, 277, 672–680. [Google Scholar] [CrossRef]
- Chetboun, M.; Drumez, E.; Ballou, C.; Maanaoui, M.; Payne, E.; Barton, F.; Kerr-Conte, J.; Vantyghem, M.-C.; Piemonti, L.; Rickels, M.R.; et al. Association between Primary Graft Function and 5-Year Outcomes of Islet Allogeneic Transplantation in Type 1 Diabetes: A Retrospective, Multicentre, Observational Cohort Study in 1210 Patients from the Collaborative Islet Transplant Registry. Lancet Diabetes Endocrinol. 2023, 11, 391–401. [Google Scholar] [CrossRef]
- Hering, B.J.; Ballou, C.M.; Bellin, M.D.; Payne, E.H.; Kandeel, F.; Witkowski, P.; Alejandro, R.; Rickels, M.R.; Barton, F.B. Factors Associated with Favourable 5 Year Outcomes in Islet Transplant Alone Recipients with Type 1 Diabetes Complicated by Severe Hypoglycaemia in the Collaborative Islet Transplant Registry. Diabetologia 2023, 66, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Rickels, M.; Eggerman, T.; Bayman, L.; Qidwai, J.; Alejandro, R.; Bridges, N.; Hering, B.; Markmann, J.; Senior, P.; Hunsicker, L.; et al. Long-Term Outcomes with Islet-Alone and Islet-After-Kidney Transplantation for Type 1 Diabetes in the Clinical Islet Transplantation Consortium: The CIT-08 Study. Diabetes Care 2022, 45, 2967–2975. [Google Scholar] [CrossRef]
- Boggi, U.; Baronti, W.; Amorese, G.; Pilotti, S.; Occhipinti, M.; Perrone, V.; Marselli, L.; Barsotti, M.; Campani, D.; Gianetti, E.; et al. Treating Type 1 Diabetes by Pancreas Transplant Alone: A Cohort Study on Actual Long-Term (10 Years) Efficacy and Safety. Transplantation 2022, 106, 147–157. [Google Scholar] [CrossRef]
- Marfil-Garza, B.A.; Imes, S.; Verhoeff, K.; Hefler, J.; Lam, A.; Dajani, K.; Anderson, B.; O’Gorman, D.; Kin, T.; Bigam, D.; et al. Pancreatic Islet Transplantation in Type 1 Diabetes: 20-Year Experience from a Single-Centre Cohort in Canada. Lancet Diabetes Endocrinol. 2022, 10, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Lablanche, S.; Borot, S.; Wojtusciszyn, A.; Skaare, K.; Penfornis, A.; Malvezzi, P.; Badet, L.; Thivolet, C.; Morelon, E.; Buron, F.; et al. Ten-Year Outcomes of Islet Transplantation in Patients with Type 1 Diabetes: Data from the Swiss-French GRAGIL Network. Am. J. Transplant. 2021, 21, 3725–3733. [Google Scholar] [CrossRef] [PubMed]
- Anteby, R.; Lucander, A.; Bachul, P.; Pyda, J.; Grybowski, D.; Basto, L.; Generette, G.; Perea, L.; Golab, K.; Wang, L.; et al. Evaluating the Prognostic Value of Islet Autoantibody Monitoring in Islet Transplant Recipients with Long-Standing Type 1 Diabetes Mellitus. J. Clin. Med. 2021, 10, 2708. [Google Scholar] [CrossRef] [PubMed]
- Vantyghem, M.; Chetboun, M.; Gmyr, V.; Jannin, A.; Espiard, S.; Le Mapihan, K.; Raverdy, V.; Delalleau, N.; Machuron, F.; Hubert, T.; et al. Ten-Year Outcome of Islet Alone or Islet After Kidney Transplantation in Type 1 Diabetes: A Prospective Parallel-Arm Cohort Study. Diabetes Care 2019, 42, 2042–2049. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.; Miao, H.; Zhang, J.; Hu, J.; Zhou, W.; Gu, W.; Wang, W.; Ning, G. Clinical Benefits of Autologous Haematopoietic Stem Cell Transplantation in Type 1 Diabetes Patients. Diabetes Metab. 2018, 44, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Voglová, B.; Zahradnická, M.; Girman, P.; Kríž, J.; Berková, Z.; Koblas, T.; Vávrová, E.; Németová, L.; Kosinová, L.; Habart, D.; et al. Benefits of Islet Transplantation as an Alternative to Pancreas Transplantation: Retrospective Study of More Than 10 Ten Years of Experience in a Single Center. Rev. Diabet. Stud. RDS 2017, 14, 10–21. [Google Scholar] [CrossRef]
- Schive, S.; Foss, A.; Sahraoui, A.; Kloster-Jensen, K.; Hafsahl, G.; Kvalheim, G.; Lundgren, T.; von Zur-Mühlen, B.; Felldin, M.; Rafael, E.; et al. Cost and Clinical Outcome of Islet Transplantation in Norway 2010-2015. Clin. Transplant. 2017, 31, e12871. [Google Scholar] [CrossRef] [PubMed]
- Malmegrim, K.; de Azevedo, J.; Arruda, L.; Abreu, J.; Couri, C.; de Oliveira, G.; Palma, P.; Scortegagna, G.; Stracieri, A.; Moraes, D.; et al. Immunological Balance Is Associated with Clinical Outcome after Autologous Hematopoietic Stem Cell Transplantation in Type 1 Diabetes. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Delaune, V.; Toso, C.; Benhamou, P.; Wojtusciszyn, A.; Kessler, L.; Slits, F.; Demuylder-Mischler, S.; Pernin, N.; Lablanche, S.; Orci, L.; et al. Alloimmune Monitoring After Islet Transplantation: A Prospective Multicenter Assessment of 25 Recipients. Cell Transplant. 2016, 25, 2259–2268. [Google Scholar] [CrossRef] [PubMed]
- Moassesfar, S.; Masharani, U.; Frassetto, L.; Szot, G.; Tavakol, M.; Stock, P.; Posselt, A. A Comparative Analysis of the Safety, Efficacy, and Cost of Islet Versus Pancreas Transplantation in Nonuremic Patients with Type 1 Diabetes. Am. J. Transplant. 2016, 16, 518–526. [Google Scholar] [CrossRef]
- Hering, B.J.; Clarke, W.R.; Bridges, N.D.; Eggerman, T.L.; Alejandro, R.; Bellin, M.D.; Chaloner, K.; Czarniecki, C.W.; Goldstein, J.S.; Hunsicker, L.G.; et al. Phase 3 Trial of Transplantation of Human Islets in Type 1 Diabetes Complicated by Severe Hypoglycemia. Diabetes Care 2016, 39, 1230–1240. [Google Scholar] [CrossRef] [PubMed]
- Nijhoff, M.; Engelse, M.; Dubbeld, J.; Braat, A.; Ringers, J.; Roelen, D.; van Erkel, A.; Spijker, H.; Bouwsma, H.; van der Boog, P.; et al. Glycemic Stability Through Islet-After-Kidney Transplantation Using an Alemtuzumab-Based Induction Regimen and Long-Term Triple-Maintenance Immunosuppression. Am. J. Transplant. 2016, 16, 246–253. [Google Scholar] [CrossRef]
- Graciela Cantu-Rodriguez, O.; Lavalle-Gonzalez, F.; Angel Herrera-Rojas, M.; Carlos Jaime-Perez, J.; Angel Hawing-Zarate, J.; Homero Gutierrez-Aguirre, C.; Mancias-Guerra, C.; Gonzalez-Llano, O.; Zapata-Garrido, A.; Zacarias Villarreal-Perez, J.; et al. Long-Term Insulin Independence in Type 1 Diabetes Mellitus Using a Simplified Autologous Stem Cell Transplant. J. Clin. Endocrinol. Metab. 2016, 101, 2141–2148. [Google Scholar] [CrossRef]
- Lablanche, S.; Borot, S.; Wojtusciszyn, A.; Bayle, F.; Tetaz, R.; Badet, L.; Thivolet, C.; Morelon, E.; Frimat, L.; Penfomis, A.; et al. Five-Year Metabolic, Functional, and Safety Results of Patients with Type 1 Diabetes Transplanted with Allogenic Islets Within the Swiss-French GRAGIL Network. Diabetes Care 2015, 38, 1714–1722. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, R.; Graziano, J.; Brockmann, J.; Pfammatter, T.; Kron, P.; de Rougemont, O.; Mueller, T.; Zuellig, R.; Spinas, G.; Gerber, P. Glycemic Control in Simultaneous Islet-Kidney Versus Pancreas-Kidney Transplantation in Type 1 Diabetes: A Prospective 13-Year Follow-Up. Diabetes Care 2015, 38, 752–759. [Google Scholar] [CrossRef]
- Anazawa, T.; Saito, T.; Goto, M.; Kenmochi, T.; Uemoto, S.; Itoh, T.; Yasunami, Y.; Kenjo, A.; Kimura, T.; Ise, K.; et al. Long-Term Outcomes of Clinical Transplantation of Pancreatic Islets with Uncontrolled Donors After Cardiac Death: A Multicenter Experience in Japan. Transplant. Proc. 2014, 46, 1980–1984. [Google Scholar] [CrossRef] [PubMed]
- D’Addio, F.; Vasquez, A.; Ben Nasr, M.; Franek, E.; Zhu, D.; Li, L.; Ning, G.; Snarski, E.; Fiorina, P. Autologous Nonmyeloablative Hematopoietic Stem Cell Transplantation in New-Onset Type 1 Diabetes: A Multicenter Analysis. Diabetes 2014, 63, 3041–3046. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, P.; Holmes-Walker, D.; Goodman, D.; Hawthorne, W.; Loudovaris, T.; Gunton, J.; Thomas, H.; Grey, S.; Drogemuller, C.; Ward, G.; et al. Multicenter Australian Trial of Islet Transplantation: Improving Accessibility and Outcomes. Am. J. Transplant. 2013, 13, 1850–1858. [Google Scholar] [CrossRef] [PubMed]
- Danielson, K.; Hatipoglu, B.; Kinzer, K.; Kaplan, B.; Martellotto, J.; Qi, M.; Mele, A.; Benedetti, E.; Oberholzer, J. Reduction in Carotid Intima-Media Thickness After Pancreatic Islet Transplantation in Patients with Type 1 Diabetes. Diabetes Care 2013, 36, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Barton, F.; Rickels, M.; Alejandro, R.; Hering, B.; Wease, S.; Naziruddin, B.; Oberholzer, J.; Odorico, J.; Garfinkel, M.; Levy, M.; et al. Improvement in Outcomes of Clinical Islet Transplantation: 1999-2010. Diabetes Care 2012, 35, 1436–1445. [Google Scholar] [CrossRef]
- Hirsch, D.; Odorico, J.; Danobeitia, J.S.; Alejandro, R.; Rickels, M.R.; Hanson, M.; Radke, N.; Baidal, D.; Hullett, D.; Naji, A.; et al. Early Metabolic Markers That Anticipate Loss of Insulin Independence in Type 1 Diabetic Islet Allograft Recipients. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2012, 12, 1275–1289. [Google Scholar] [CrossRef] [PubMed]
- Bellin, M.; Barton, F.; Heitman, A.; Harmon, J.; Kandaswamy, R.; Balamurugan, A.; Sutherland, D.; Alejandro, R.; Hering, B. Potent Induction Immunotherapy Promotes Long-Term Insulin Independence After Islet Transplantation in Type 1 Diabetes. Am. J. Transplant. 2012, 12, 1576–1583. [Google Scholar] [CrossRef]
- Gu, W.; Hu, J.; Wang, W.; Li, L.; Tang, W.; Sun, S.; Cui, W.; Ye, L.; Zhang, Y.; Hong, J.; et al. Diabetic Ketoacidosis at Diagnosis Influences Complete Remission After Treatment with Hematopoietic Stem Cell Transplantation in Adolescents with Type 1 Diabetes. Diabetes Care 2012, 35, 1413–1419. [Google Scholar] [CrossRef] [PubMed]
- Borot, S.; Niclauss, N.; Wojtusciszyn, A.; Brault, C.; Demuylder-Mischler, S.; Müller, Y.; Giovannoni, L.; Parnaud, G.; Meier, R.; Badet, L.; et al. Impact of the Number of Infusions on 2-Year Results of Islet-After-Kidney Transplantation in the GRAGIL Network. Transplantation 2011, 92, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Socci, C.; Orsenigo, E.; Santagostino, I.; Caumo, A.; Caldara, R.; Parolini, D.; Aldrighetti, L.; Castoldi, R.; Frasson, M.; Carvello, M.; et al. Pancreata from Pediatric Donors Restore Insulin Independence in Adult Insulin-Dependent Diabetes Mellitus Recipients. Transplant. Proc. 2010, 42, 2068–2070. [Google Scholar] [CrossRef]
- Kave, B.; Yii, M.; Bell, R.; Kanellis, J.; Scott, D.; Saunder, A. Initial Australasian Experience with Portal-Enteric Drainage in Simultaneous Pancreas-Kidney Transplantation. ANZ J. Surg. 2010, 80, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Niclauss, N.; Bosco, D.; Morel, P.; Demuylder-Mischler, S.; Brault, C.; Milliat-Guittard, L.; Colin, C.; Parnaud, G.; Muller, Y.D.; Giovannoni, L.; et al. Influence of Donor Age on Islet Isolation and Transplantation Outcome. Transplantation 2011, 91, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Couri, C.; Oliveira, M.; Stracieri, A.; Moraes, D.; Pieroni, F.; Barros, G.; Madeira, M.; Malmegrim, K.; Foss-Freitas, M.; Simoes, B.; et al. C-Peptide Levels and Insulin Independence Following Autologous Nonmyeloablative Hematopoietic Stem Cell Transplantation in Newly Diagnosed Type 1 Diabetes Mellitus. JAMA-J. Am. Med. Assoc. 2009, 301, 1573–1579. [Google Scholar] [CrossRef]
- Barton, F.; CITR Research Group. 2007 Update on Allogeneic Islet Transplantation From the Collaborative Islet Transplant Registry (CITR). Cell Transplant. 2009, 18, 753–767. [Google Scholar] [CrossRef]
- Vantyghem, M.; Kerr-Conte, J.; Arnalsteen, L.; Sergent, G.; Defrance, F.; Gmyr, V.; Declerck, N.; Raverdy, V.; Vandewalle, B.; Pigny, P.; et al. Primary Graft Function, Metabolic Control, and Graft Survival After Islet Transplantation. Diabetes Care 2009, 32, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Gerber, P.; Pavlicek, V.; Zuellig, R.; Spinas, G.; Lehmann, R. Simultaneous Islet-Kidney vs Pancreas-Kidney Transplantation in Type 1 Diabetes Mellitus: A 5 Year Single Centre Follow-Up. Diabetologia 2008, 51, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Dieterle, C.D.; Arbogast, H.; Illner, W.-D.; Schmauss, S.; Landgraf, R. Metabolic Follow-up after Long-Term Pancreas Graft Survival. Eur. J. Endocrinol. 2007, 156, 603–610. [Google Scholar] [CrossRef]
- Voltarelli, J.; Couri, C.; Stracieri, A.; Oliveira, M.; Moraes, D.; Pieroni, F.; Coutinho, M.; Malmegrim, K.; Foss-Freitas, M.; Simoes, B.; et al. Autologous Nonmyeloablative Hematopoietic Stem Cell Transplantation in Newly Diagnosed Type 1 Diabetes Mellitus. JAMA-J. Am. Med. Assoc. 2007, 297, 1568–1576. [Google Scholar] [CrossRef] [PubMed]
- Keymeulen, B.; Gillard, P.; Mathieu, C.; Movahedi, B.; Maleux, G.; Delvaux, G.; Ysebaert, D.; Roep, B.; Vandemeulebroucke, E.; Marichal, M.; et al. Correlation between β Cell Mass and Glycemic Control in Type 1 Diabetic Recipients of Islet Cell Graft. Proc. Natl. Acad. Sci. USA 2006, 103, 17444–17449. [Google Scholar] [CrossRef]
- Shapiro, A.; Ricordi, C.; Hering, B.; Auchincloss, H.; Lindblad, R.; Robertson, P.; Secchi, A.; Brendel, M.; Berney, T.; Brennan, D.; et al. International Trial of the Edmonton Protocol for Islet Transplantation. N. Engl. J. Med. 2006, 355, 1318–1330. [Google Scholar] [CrossRef] [PubMed]
- Maffi, P.; Angeli, E.; Bertuzzi, F.; Paties, C.; Socci, C.; Fedeli, C.; De Taddeo, F.; Nano, R.; Di Carlo, V.; Del Maschio, A.; et al. Minimal Focal Steatosis of Liver after Islet Transplantation in Humans: A Long-Term Study. Cell Transplant. 2005, 14, 727–733. [Google Scholar] [CrossRef]
- Ryan, E.; Paty, B.; Senior, P.; Bigam, D.; Alfadhli, E.; Kneteman, N.; Lakey, J.; Shapir, A. Five-Year Follow-up after Clinical Islet Transplantation. Diabetes 2005, 54, 2060–2069. [Google Scholar] [CrossRef]
- Michalak, G.; Kwiatkowski, A.; Czerwinski, J.; Chmura, A.; Wszola, M.; Nosek, R.; Ostrowski, K.; Danielewicz, R.; Lisik, W.; Adadynski, L.; et al. Surgical Complications of Simultaneous Pancreas-Kidney Transplantation: A 16-Year-Experience at One Center. Transplant. Proc. 2005, 37, 3555–3557. [Google Scholar] [CrossRef] [PubMed]
- Boggi, U.; Mosca, F.; Vistoli, F.; Signori, S.; Del Chiaro, M.; Bartolo, T.V.; Amorese, G.; Coppelli, A.; Marchetti, P.; Mariotti, R.; et al. Ninety-Five Percent Insulin Independence Rate 3 Years after Pancreas Transplantation Alone with Portal-Enteric Drainage. Transplant. Proc. 2005, 37, 1274–1277. [Google Scholar] [CrossRef] [PubMed]
- Boggi, U.; Del Chiaro, M.; Signori, S.; Vistoli, F.; Amorese, G.; Croce, C.; Morelli, L.; Vanadia Bartolo, T.; Pietrabissa, A.; Barsotti, M.; et al. Pancreas Transplants from Donors Aged 45 Years or Older. Transplant. Proc. 2005, 37, 1265–1267. [Google Scholar] [CrossRef] [PubMed]
- Fiorina, P.; La Rocca, E.; Astorri, E.; Lucignani, G.; Rossetti, C.; Fazio, F.; Giudici, D.; di Carlo, V.; Cristallo, M.; Pozza, G.; et al. Reversal of Left Ventricular Diastolic Dysfunction after Kidney-Pancreas Transplantation in Type 1 Diabetic Uremic Patients. Diabetes Care 2000, 23, 1804–1810. [Google Scholar] [CrossRef]
- Secchi, A.; Socci, C.; Maffi, P.; Taglietti, M.V.; Falqui, L.; Bertuzzi, F.; De Nittis, P.; Piemonti, L.; Scopsi, L.; Di Carlo, V.; et al. Islet Transplantation in IDDM Patients. Diabetologia 1997, 40, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Kinkhabwala, M.; Wilkinson, A.; Danovitch, G.; Rosenthal, J.T.; Tooley, T.K.; Sanford, A.; Imagawa, D.; Rudich, S.; Seu, P.; Busuttil, R.W.; et al. The Role of Whole Organ Pancreas Transplantation in the Treatment of Type I Diabetes. Am. J. Surg. 1996, 171, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.; Alberti, P.; Demartines, N.; Phillips, M.; Casey, J.; Sutherland, A. Whole-Organ Pancreas and Islets Transplantations in UK: An Overview and Future Directions. J. Clin. Med. 2023, 12, 3245. [Google Scholar] [CrossRef]
- Fridell, J.A.; Stratta, R.J.; Gruessner, A.C. Pancreas Transplantation: Current Challenges, Considerations, and Controversies. J. Clin. Endocrinol. Metab. 2023, 108, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, C.; Perrone, V.G.; Amorese, G.; Vistoli, F.; Baronti, W.; Marchetti, P.; Boggi, U. Update on Pancreatic Transplantation in the Management of Diabetes. Minerva Med. 2017, 108, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Kandaswamy, R.; Stock, P.; Gustafson, S.; Skeans, M.; Urban, R.; Fox, A.; Israni, A.; Snyder, J.; Kasiske, B. OPTN/SRTR 2018 Annual Data Report: Pancreas. Am. J. Transplant. 2020, 20, 131–192. [Google Scholar] [CrossRef] [PubMed]
- Cerise, A.; Nagaraju, S.; Powelson, J.A.; Lutz, A.; Fridell, J.A. Pancreas Transplantation Following Total Pancreatectomy for Chronic Pancreatitis. Clin. Transplant. 2019, 33, e13731. [Google Scholar] [CrossRef]
- Esmeijer, K.; Hoogeveen, E.K.; van den Boog, P.J.; Konijn, C.; Mallat, M.J.; Baranski, A.G.; Dekkers, O.M.; de Fijter, J.W. Superior Long-Term Survival for Simultaneous Pancreas-Kidney Transplantation as Renal Replacement Therapy: 30-Year Follow-up of a Nationwide Cohort. Diabetes Care 2020, 43, 321–328. [Google Scholar] [CrossRef]
- Venstrom, J.M.; McBride, M.A.; Rother, K.I.; Hirshberg, B.; Orchard, T.J.; Harlan, D.M. Survival after Pancreas Transplantation in Patients with Diabetes and Preserved Kidney Function. JAMA 2003, 290, 2817–2823. [Google Scholar] [CrossRef] [PubMed]
- Fridell, J.A.; Niederhaus, S.; Curry, M.; Urban, R.; Fox, A.; Odorico, J. The Survival Advantage of Pancreas after Kidney Transplant. Am. J. Transplant. 2019, 19, 823–830. [Google Scholar] [CrossRef]
- Ibáñez, J.M.; Robledo, A.B.; López-Andujar, R. Late Complications of Pancreas Transplant. World J. Transplant. 2020, 10, 404. [Google Scholar] [CrossRef]
- Leguier, L.; Le Mapihan, K.; Defrance, F.; Lemaitre, M.; Jannin, A.; Chetboun, M.; Pattou, F.; Vantyghem, M.-C. 306.2: Comparison of Incidence of Solid Cancers After Islet or Combined Kidney-Whole Pancreas Transplantation. Transplantation 2021, 105, S18. [Google Scholar] [CrossRef]
- Gruessner, A.C.; Gruessner, R.W. The 2022 International Pancreas Transplant Registry Report—A Review. Transplant Proc. 2022, 54, 1918–1943. [Google Scholar] [CrossRef]
- Montagud-Marrahi, E.; Molina-Andújar, A.; Pané, A.; Ramírez-Bajo, M.J.; Amor, A.; Esmatjes, E.; Ferrer, J.; Musquera, M.; Diekmann, F.; Ventura-Aguiar, P. Outcomes of Pancreas Transplantation in Older Diabetic Patients. BMJ Open Diabetes Res. Care 2020, 8, e000916. [Google Scholar] [CrossRef] [PubMed]
- Kopp, W.; van Meel, M.; Putter, H.; Samuel, U.; Arbogast, H.; Schareck, W.; Ringers, J.; Braat, A. Center Volume Is Associated with Outcome after Pancreas Transplantation within the Eurotransplant Region. Transplantation 2017, 101, 1247–1253. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, Y.; Liu, L.; Zhao, X.; Sun, Y.; Mao, X.; Li, S. Pancreatic Islet Transplantation: Current Advances and Challenges. Front. Immunol. 2024, 15, 1391504. [Google Scholar] [CrossRef] [PubMed]
- Dean, P.G.; Kukla, A.; Stegall, M.D.; Kudva, Y.C. Pancreas Transplantation. BMJ 2017, 357. [Google Scholar] [CrossRef]
- Boggi, U.; Vistoli, F.; Andres, A.; Arbogast, H.P.; Badet, L.; Baronti, W.; Bartlett, S.T.; Benedetti, E.; Branchereau, J.; Burke 3rd, G.W.; et al. First World Consensus Conference on Pancreas Transplantation: Part II–Recommendations. Am. J. Transplant. 2021, 21, 17–59. [Google Scholar] [CrossRef]
- Venturini, M.; Sallemi, C.; Marra, P.; Palmisano, A.; Agostini, G.; Lanza, C.; Balzano, G.; Falconi, M.; Secchi, A.; Fiorina, P.; et al. Allo-and Auto-Percutaneous Intra-Portal Pancreatic Islet Transplantation (PIPIT) for Diabetes Cure and Prevention: The Role of Imaging and Interventional Radiology. Gland Surg. 2018, 7, 117. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.J.; Lakey, J.R.; Ryan, E.A.; Korbutt, G.S.; Toth, E.; Warnock, G.L.; Kneteman, N.M.; Rajotte, R.V. Islet Transplantation in Seven Patients with Type 1 Diabetes Mellitus Using a Glucocorticoid-Free Immunosuppressive Regimen. N. Engl. J. Med. 2000, 343, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Czarnecka, Z.; Dadheech, N.; Razavy, H.; Pawlick, R.; Shapiro, A.J. The Current Status of Allogenic Islet Cell Transplantation. Cells 2023, 12, 2423. [Google Scholar] [CrossRef] [PubMed]
- Forbes, S.; Oram, R.; Smith, A.; Lam, A.; Olateju, T.; Imes, S.; Malcolm, A.; Shapiro, A.; Senior, P. Validation of the BETA-2 Score: An Improved Tool to Estimate Beta Cell Function after Clinical Islet Transplantation Using a Single Fasting Blood Sample. Am. J. Transplant. 2016, 16, 2704–2713. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Ye, L.; Chen, Y.; He, S.; Zhang, C.; Mao, X.; Li, S. The Influence of Microenvironment on Survival of Intraportal Transplanted Islets. Front. Immunol. 2022, 13, 849580. [Google Scholar] [CrossRef]
- Maffi, P.; Nano, R.; Monti, P.; Melzi, R.; Sordi, V.; Mercalli, A.; Pellegrini, S.; Ponzoni, M.; Peccatori, J.; Messina, C.; et al. Islet Allotransplantation in the Bone Marrow of Patients with Type 1 Diabetes: A Pilot Randomized Trial. Transplantation 2019, 103, 839–851. [Google Scholar] [CrossRef]
- Baidal, D.A.; Ricordi, C.; Berman, D.M.; Alvarez, A.; Padilla, N.; Ciancio, G.; Linetsky, E.; Pileggi, A.; Alejandro, R. Bioengineering of an Intraabdominal Endocrine Pancreas. N. Engl. J. Med. 2017, 376, 1887–1889. [Google Scholar] [CrossRef]
- Shapiro, A.J.; Gallant, H.L.; Hao, E.G.; Lakey, J.R.; McCready, T.; Rajotte, R.V.; Yatscoff, R.W.; Kneteman, N.M. The Portal Immunosuppressive Storm: Relevance to Islet Transplantation? Ther. Drug Monit. 2005, 27, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Amorese, G.; Lombardo, C.; Tudisco, A.; Iacopi, S.; Menonna, F.; Marchetti, P.; Vistoli, F.; Boggi, U. Induction and Immunosuppressive Management of Pancreas Transplant Recipients. Curr. Pharm. Des. 2020, 26, 3425–3439. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Khan, S.J.; Lee, C.Y.F.; Zaidi, S.A.T.; Murtaza, S.F. Type 1 Diabetes Mellitus Management and Islet Cell Therapy: A New Chapter in Patient Care. Cureus 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://Clinicaltrials.Gov/Study/NCT00679042?Tab=results (accessed on 9 November 2024).
- Butler, A.; Huang, A.; Rao, P.; Bhushan, A.; Hogan, W.; Rizza, R.; Butler, P. Hematopoietic Stem Cells Derived from Adult Donors Are Not a Source of Pancreatic β-Cells in Adult Nondiabetic Humans. Diabetes 2007, 56, 1810–1816. [Google Scholar] [CrossRef] [PubMed]
- Pagliuca, F.W.; Millman, J.R.; Gürtler, M.; Segel, M.; Van Dervort, A.; Ryu, J.H.; Peterson, Q.P.; Greiner, D.; Melton, D.A. Generation of Functional Human Pancreatic β Cells in Vitro. Cell 2014, 159, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Pagliuca, F.W.; Melton, D.A. How to Make a Functional β-Cell. Development 2013, 140, 2472–2483. [Google Scholar] [CrossRef] [PubMed]
- Rezania, A.; Bruin, J.E.; Xu, J.; Narayan, K.; Fox, J.K.; O’Neil, J.J.; Kieffer, T.J. Enrichment of Human Embryonic Stem Cell-Derived NKX6. 1-Expressing Pancreatic Progenitor Cells Accelerates the Maturation of Insulin-Secreting Cells in Vivo. Stem Cells 2013, 31, 2432–2442. [Google Scholar] [CrossRef]
- Keymeulen, B.; De Groot, K.; Jacobs-Tulleneers-Thevissen, D.; Thompson, D.M.; Bellin, M.D.; Kroon, E.J.; Daniels, M.; Wang, R.; Jaiman, M.; Kieffer, T.J.; et al. Encapsulated Stem Cell–Derived β Cells Exert Glucose Control in Patients with Type 1 Diabetes. Nat. Biotechnol. 2023, 42, 1507–1514. [Google Scholar] [CrossRef]
- Henry, R.R.; Pettus, J.; Wilensky, J.; SHAPIRO, A.J.; Senior, P.A.; Roep, B.; Wang, R.; Kroon, E.J.; Scott, M.; D’AMOUR, K.; et al. Initial Clinical Evaluation of VC-01TM Combination Product—A Stem Cell–Derived Islet Replacement for Type 1 Diabetes (T1D). Diabetes 2018, 67. [Google Scholar] [CrossRef]
- Shapiro, A.J.; Thompson, D.; Donner, T.W.; Bellin, M.D.; Hsueh, W.; Pettus, J.; Wilensky, J.; Daniels, M.; Wang, R.M.; Brandon, E.P.; et al. Insulin Expression and C-Peptide in Type 1 Diabetes Subjects Implanted with Stem Cell-Derived Pancreatic Endoderm Cells in an Encapsulation Device. Cell Rep. Med. 2021, 2, 100466. [Google Scholar] [CrossRef] [PubMed]
- Ramzy, A.; Thompson, D.M.; Ward-Hartstonge, K.A.; Ivison, S.; Cook, L.; Garcia, R.V.; Loyal, J.; Kim, P.T.; Warnock, G.L.; Levings, M.K.; et al. Implanted Pluripotent Stem-Cell-Derived Pancreatic Endoderm Cells Secrete Glucose-Responsive C-Peptide in Patients with Type 1 Diabetes. Cell Stem Cell 2021, 28, 2047–2061. [Google Scholar] [CrossRef] [PubMed]
- Sackett, S.D.; Kaplan, S.J.; Mitchell, S.A.; Brown, M.E.; Burrack, A.L.; Grey, S.; Huangfu, D.; Odorico, J. Genetic Engineering of Immune Evasive Stem Cell-Derived Islets. Transpl. Int. 2022, 35, 10817. [Google Scholar] [CrossRef]
- Pietra, B.A.; Wiseman, A.; Bolwerk, A.; Rizeq, M.; Gill, R.G. CD4 T Cell–Mediated Cardiac Allograft Rejection Requires Donor but Not Host MHC Class II. J. Clin. Invest. 2000, 106, 1003–1010. [Google Scholar] [CrossRef]
- Reynoso, J.F.; Gruessner, C.E.; Sutherland, D.E.; Gruessner, R.W. Short-and Long-Term Outcome for Living Pancreas Donors. J. Hepato-Biliary-Pancreat. Sci. 2010, 17, 92–96. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, G.; Hilhorst, M.; Rings, E. Liver, Pancreas and Small Bowel Transplantation: Current Ethical Issues. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 281–292. [Google Scholar] [CrossRef]
- de Jongh, D.; Thom, R.L.; Cronin, A.J.; Bunnik, E.M.; Massey, E.K. Clinical Translation of Bio-Artificial Pancreas Therapies: Ethical, Legal and Psychosocial Interdisciplinary Considerations and Key Recommendations. Transpl. Int. 2023, 36, 11705. [Google Scholar] [CrossRef] [PubMed]
- Volarevic, V.; Markovic, B.S.; Gazdic, M.; Volarevic, A.; Jovicic, N.; Arsenijevic, N.; Armstrong, L.; Djonov, V.; Lako, M.; Stojkovic, M. Ethical and Safety Issues of Stem Cell-Based Therapy. Int. J. Med. Sci. 2018, 15, 36. [Google Scholar] [CrossRef]
- King, N.M.; Perrin, J. Ethical Issues in Stem Cell Research and Therapy. Stem Cell Res. Ther. 2014, 5, 85. [Google Scholar] [CrossRef] [PubMed]
| CASP Question: | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author/Year | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | Main Limitations: |
| Marfil Gaza, 2023 [12] | + | + | + | + | + | + | + | + | + | + | + | + | retrospective design |
| Chetboun, 2023 [13] | + | +/− | + | + | + | + | + | + | + | + | + | + | retrospective design data from voluntary registry T1DM duration not reported |
| Hering, 2023 [14] | + | +/− | + | + | + | + | + | + | + | + | + | + | data from voluntary registry |
| Rickels, 2022 [15] | + | + | + | + | + | +/− | + | + | + | +/− | +/− | +/− | substantial part of participants lost to follow up relatively small sample size |
| Boggi, 2022 [16] | + | + | + | + | + | + | + | + | + | + | + | + | single center study relatively small sample size |
| Marfil Gaza, 2022 [17] | + | + | + | + | + | + | + | + | + | + | + | + | single center study retrospective design |
| LaBlanche, 2021 [18] | + | + | + | + | + | +/− | + | + | + | + | + | + | retrospective design small sample size substantial part of participants lost to follow up |
| Anteby, 2021 [19] | + | + | + | + | + | + | + | + | + | + | + | + | small sample size |
| Vantyghem, 2019 [20] | + | + | + | + | + | + | + | + | + | + | + | + | small sample size |
| Gu, 2018 [21] | + | + | + | + | + | +/− | + | + | +/− | + | + | + | small sample size substantial part of participants lost to follow up |
| Voglova, 2017 [22] | + | + | + | + | + | + | + | + | + | + | + | + | relatively small sample size single center study |
| Schive, 2017 [23] | + | + | + | + | + | + | + | + | + | - | +/− | + | retrospective design small sample size |
| Malmegrim, 2017 [24] | + | + | + | + | + | + | + | + | + | + | + | + | small sample size |
| Delaune, 2016 [25] | + | +/− | + | + | +/− | + | + | + | +/− | + | + | + | small sample size T1DM duration not reported |
| Moassesfar, 2016 [26] | + | + | + | + | + | + | + | + | + | + | + | + | small sample size |
| Hering, 2016 [27] | + | + | + | + | + | + | + | + | + | + | + | + | small sample size |
| Nijhoff, 2016 [28] | + | + | + | + | + | + | + | + | + | + | + | + | small sample size |
| Cantu Rodriguez, 2016 [29] | + | +/− | +/− | + | + | + | + | + | +/− | + | + | + | small sample size variable T1DM duration no control group |
| LaBlanche, 2015 [30] | + | + | + | + | + | + | + | + | + | + | + | + | relatively small sample size retrospective design |
| Lehmann, 2015 [31] | + | + | + | + | + | + | + | + | + | + | + | + | relatively small study group |
| Anazawa, 2014 [32] | + | +/− | +/− | + | + | + | + | + | +/− | + | + | + | small sample size T1DM duration not reported |
| D’Addio, 2014 [33] | + | +/− | + | + | + | + | + | + | + | + | + | + | no control group relatively small sample size |
| O’Connell, 2013 [34] | + | +/− | + | + | + | + | + | + | + | + | + | + | small sample size |
| Danielson, 2013 [35] | + | + | + | + | + | + | + | + | + | + | + | + | small sample size |
| Barton, 2012 [36] | + | +/− | + | + | + | + | + | + | + | + | + | + | retrospective design |
| Hirsch, 2012 [37] | + | + | + | + | + | + | + | + | + | + | + | + | small sample size |
| Bellin, 2012 [38] | + | +/− | + | + | + | + | + | + | + | + | + | + | retrospective design data from voluntary registry T1DM duration not reported |
| Gu, 2012 [39] | + | +/− | + | + | + | + | + | + | + | + | + | + | small sample size no control group |
| Borot, 2011 [40] | + | + | + | + | + | + | + | + | + | + | + | + | small sample size |
| Socci, 2010 [41] | + | + | + | + | + | + | + | + | + | + | + | + | small sample size |
| Kave, 2010 [42] | + | + | + | + | + | + | + | + | + | + | + | + | relatively small sample size single center study |
| Niclauss, 2011 [43] | + | + | + | + | + | + | + | + | + | + | + | + | relatively small sample size |
| Couri, 2009 [44] | + | +/− | + | + | + | + | + | + | + | + | + | + | small sample size no control group |
| Barton, 2007 [45] | + | +/− | + | + | + | + | + | + | + | + | + | + | retrospective design data from voluntary registry |
| Vantyghem, 2009 [46] | + | + | + | + | + | + | + | + | + | + | + | + | small sample size |
| Gerber, 2008 [47] | + | + | + | + | + | + | + | + | + | + | + | + | small sample size |
| Dieterle, 2007 [48] | + | + | + | + | + | + | + | + | + | + | + | + | small sample size |
| Voltarelli, 2007 [49] | + | +/− | + | + | + | + | + | + | + | + | + | + | no control group small sample size |
| Keymeulen, 2006 [50] | + | +/− | + | + | + | + | + | + | + | + | + | + | small sample size T1DM duration not reported |
| Shapiro, 2006 [51] | + | + | + | + | + | + | + | + | + | + | + | + | small sample size |
| Maffi, 2005 [52] | + | + | + | + | + | + | + | + | + | + | + | + | small sample size |
| Ryan, 2005 [53] | + | + | + | + | + | + | + | + | + | + | + | + | relatively small sample size |
| Michalak, 2005 [54] | + | + | + | + | + | + | + | + | + | + | + | + | relatively small sample size |
| Boggi, 2005 [55] | + | + | + | + | + | + | + | + | + | + | + | + | small sample size |
| Boggi, 2005 [56] | + | +/− | + | + | + | + | + | + | + | + | + | + | small sample size |
| Fiorina, 2000 [57] | + | + | + | + | + | + | + | + | + | + | + | + | small sample size |
| Secchi, 1997 [58] | + | + | + | + | + | + | + | + | + | + | + | + | small sample size |
| Kinkhabwala, 1996 [59] | + | + | + | + | + | + | + | + | + | + | + | + | small sample size |
| Author/Year | Study Population/Follow Up Period | Major Findings on Insulin Independence |
|---|---|---|
| Marfil Gaza, 2023 [12] | 146 adults undergoing PTx median follow up period 7.4 years T1DM duration > 5 years | insulin independence occurred in 92.5% PTx recipients mean duration of insulin independence 6.7 (IQR 2.9–12.4) years |
| Chetboun, 2023 [13] | 209 adults undergoing PTx (SPK 172, PTA and PAK 37) 5 year follow up T1DM duration not reported | 186 (89%) patients were insulin independent after 5 years. |
| Boggi, 2022 [16] | 66 adults undergoing PTA follow up period 10 years T1DM duration > 3 years | insulin independence or minimal insulin requirement was observed in 57.4% and 3.2% of patients, respectively. |
| Voglova, 2017 [22] | 49 adults undergoing PTx (36 PTA,13 PAK) follow up period 5 years T1DM duration 16.5–31 years | insulin independence was observed in 73% of patients at one year, 68% at two years, and 55% at five years post-transplantation |
| Moassesfar, 2016 [26] | 15 adults undergoing received PTA follow up period 12–118 months T1DM duration 29.9 ± 8.12 years | insulin independence was reported in 93% of patients after 1 year, and in 64% after 3 years of follow up mean duration of insulin independence was 55 months |
| Lehmann, 2015 [31] | 94 adults undergoing SPK/PAK follow up period up to 13 years T1DM duration 32.1 ± 8.2 years | insulin independence was observed in 73.5% of patients five years post-transplantation |
| Bellin, 2012 [38] | 677 adults with T1DM undergoing PTA follow up period 5 years T1DM duration not reported | insulin independence was observed in 52% of patients |
| Socci, 2010 [41] | 17 adults with T1DM undergoing PTX (SPK 9, PTA 6, PAK 2). mean follow up period 37.1 months pediatric donors | insulin independence was observed in 82.35% of patients at 3 months, 69.68% at 6 months, and 63.35% at 1 year, respectively |
| Kave, 2010 [42] | 68 adults undergoing SPK, receiving either systemic-bladder (SB) or portal-enteric (PE) drainage follow up period 2 years T1DM duration: 24.0 ± 6.3 and 26.5 ± 5.5 years for the SB and PE group | insulin independence was 77.9 ± 6.9% for the SB group and 81.3 ± 7.5% for the PE group at 1 year insulin independence was 77.9 ± 6.4% for the SB group and 71.4 ± 9.3% for the PE group at 2 years |
| Gerber, 2008 [47] | 25 adults undergoing of SPK mean follow-up period 38 months T1DM duration: 30.3 ± 7.1 years | insulin independence was observed in 96% of patients after 1 year |
| Dieterle, 2007 [48] | 38 adults with insulin independence at least 10 years after SPK follow up period 10 years T1DM duration 25 ± 1 years at SPK | insulin independence was observed in 28% of patients |
| Michalak, 2005 [54] | 51 adults undergoing SPK follow up period 6–180 months T1DM 23 ± 4 years duration | insulin independence is observed in 68.5% of patients |
| Boggi, 2005 [55] | 40 adults undergoing PTA mean follow-up of 16.4 months T1DM duration 23.9 ± 10.5 years | insulin independence is observed in 94.9% of patients |
| Boggi, 2005 [56] | 16 adults undergoing PTA (6 SPK, 10 PTA) mean follow-up period of 26.6 months donor age > 45 years | insulin independence was observed in 81.2% and 67.7% after 1 and 5 years, respectively |
| Fiorina, 2000 [57] | 42 adults undergoing SPK follow up period of 42 months T1DM duration 24 ± 1 years | insulin independence was observed in 100% of patients |
| Kinkhabwala, 1996 [59] | 19 adults undergoing PTx (18 SPK, 1 PAK) mean follow-up period of 396 days mean T1DM duration 25 years | insulin independence was observed in 89% of patients |
| Author/Year | Study Population/Follow-Up Period | Major Findings on Insulin Independence |
|---|---|---|
| Marfil Gaza, 2023 [12] | 266 adults undergoing IT 20 year follow up duration of T1DM > 5 years | insulin independence occurred in 78.6% in IT recipients mean duration of insulin independence was 2.1 (IQR 0.8–4.6) for IT recipients |
| Chetboun, 2023 [13] | 1210 adults undergoing ITA and IAK 5 year follow up T1DM duration not reported | insulin independence reported in 23.5% after 5 years |
| Hering, 2023 [14] | 398 adults undergoing ITA follow up 5 years T1DM duration 30 ± 11 years | 53% of patients were insulin-independent 105 patients received a single IT, 196 2 IT, and 97 3 IT |
| Rickels, 2022 [15] | 72 adults undergoing ITx (48 ITA, 24 IAK) median follow up period 65.8 months (ITA) and 39.3 months (ITK) T1DM duration 31.5 ± 11.0 years (ITA) and 37.0 ± 10.0 years (IAK) | insulin independence was achieved by 74% of ITA and IAK transplantation recipients 57% of recipients maintained insulin independence during long-term follow-up 5 patients needed multiple procedures |
| Marfil Gaza, 2022 [17] | 255 adults undergoing IT median follow up 7.4 years T1DM duration > 5 years | insulin independence was reported in 79% of patients, with a Kaplan–Meier estimate of 61% (95% CI 54–67) at 1 year, 32% (25–39) at 5 years, 20% (14–27) at 10 years, 11% (6–18) at 15 years, and 8% (2–17) at 20 years |
| LaBlanche, 2021 [18] | 31 patients (13 IAK, 14 ITA, 4 lost to follow up) 10 year follow-up T1DM duration > 5 years | insulin independence was observed in 4.8% of patients patients usually received multiple infusions |
| Anteby, 2021 [19] | 12 adults undergoing ITA median follow up period 56 months median T1DM duration 31.5 years | insulin independence was observed in 33% of patients at last follow up 8 patients required 2 IT procedures |
| Vantyghem, 2019 [20] | 28 adults undergoing IT (14 ITA, 14 IAK) 10 year follow-up T1DM duration > 5 years | insulin independence was observed in 39% and 28% of patients 5 and 10 years after IT all patients received 2–3 islet infusions |
| Voglova, 2017 [22] | 30 adult patients undergoing IT (24 ITA, 4 IAK, 2 SIK) 2 year follow up median T1DM duration 27.5 | 17% of IT recipients were temporarily insulin-independent. 10 patients had a single ITX procedure, 9 had 2 IT procedures and 11 had 3 procedures |
| Schive, 2017 [23] | 18 adult patients undergoing ITA mean follow up 32.2 ±17.4 months mean T1DM duration 38, 26 and 47 years, respectively, in the single ITA, multiple ITA and ITA post PTX group | insulin independence was observed in 0% of patients after single ITA, in 20% after multiple ITA, and in 20% of patients after single ITA post PTx 3 patient had a single, IT, 10 patients multiple ITx procedures and 5 patients received ITx after PTx |
| Delaune, 2016 [25] | 25 adult patients (14 ITA, AIK 7, SIK 4) mean follow up period 30.7 months T1DM duration unreported | 76% of patients reached insulin independence at least at one point during follow up |
| Hering, 2016 [27] | 48 adults undergoing ITA follow up period for 1 year median T1DM duration 28.5 years | insulin independence was reported in 52.1% at day 365 22 subjects received one islet infusion, 25 subjects received two infusions, and 1 subject received three infusions. |
| Nijhoff, 2016 [28] | 13 adults undergoing IAK 2 year follow up T1DM duration 35 ± 9 years | the reported one- and 2-year insulin independence was 62% and 42%, respectively 13 patients received 22 IT in total |
| LaBlanche, 2015 [30] | 44 adult patients undergoing ITX (24 ITA and 20 IAK) follow up period 60 month mean T1DM duration 33 years | in the IAK group, 45%, 40%, 40%, 35%, and 31.5% met the criteria of insulin independence at 12, 24, 36, 48, and 60 months, respectively. in the IAT recipients, 37.5%, 45.8%, 37.5%, 25%, and 14% met the criteria of insulin independence at 12, 24, 36, 48, and 60 months, respectively. 32 patients received two infusions, and 4 patients received three infusions |
| Lehmann, 2015 [31] | 38 adults undergoing SIK/IAK transplantation follow up period up to 13 years T1DM duration 37.0 ± 11.0 years | the 5-year insulin independence rate was 9.3% |
| Anazawa, 2014 [32] | 18 patients undergoing ITA follow-up period 76.4 ± 3.3 months T1DM duration not reported | 3 patients achieved insulin independence for 14, 79 and 215 days 8, 4, and 6 recipients received 1, 2, and 3 islet infusions |
| O’Connell, 2013 [34] | 17 adults underwent ITA 1 year follow up T1DM duration not reported | 9 patients achieved insulin independence at study end 8 patients required 2–3 ITA procedures to become insulin independent |
| Danielson, 2013 [35] | 15 adults undergoing ITA 1–5 year follow up T1DM duration 28.7 years | reported insulin independence rate of 100% after 1–3 transplants |
| Barton, 2012 [36] | 677 adult undergoing ITA or IAK follow up 3 years T1DM duration 27.3, 29.6 and 31.4 years according to reported era | reported insulin independence at 3 years after IT were 27% in the early era (1999–2002 to 37% in the mid (2003–2006) and to 44% in the most recent era (2007–2010) 36% of the recipients received one infusion, 44% received two, 18% received three, 1.3% received four, and one person received six infusions |
| Hirsch, 2012 [37] | 30 adults undergoing ITx (24 ITA, 6 IAK) mean follow-up 59.9 ± 22 months T1DM duration > 9 years | 25 patients achieved insulin independence, 16 remained insulin independent > 1 year 1 patient received a single infusion, 19 patients 2 infusions, 8 patients 3 infusions, and 2 patients 4 infusions |
| Bellin, 2012 [38] | 263 adults undergoing IT with different immunosuppression protocols follow up 5 years | reported insulin independence rate was 50% in those receiving anti-CD3 antibodies alone or T cell depleting antibodies and TNF-a inhibition |
| Borot, 2011 [40] | 15 adults received IAK 2 year follow up mean T1DM duration 33 years | five of eight single-graft subjects (62.5%) became insulin independent versus five of seven in double-graft subjects (71.4%), five patients received a single infusion, others two |
| Niclauss, 2011 [43] | 56 adults receiving ITA 1 year follow up mean T1DM > 30 years | insulin independence rate of 40% observed after one year follow up some patients required up to 3 ITx procedures |
| Barton, 2007 [45] | 315 adults receiving ITx (285 IA, 30 IAK) 4 year follow up T1DM duration 18.7 ± 1.0 years | insulin independence was reported in 56% of patients 1 year after IT, and in 12% of patients 4 years after IT repetitive IT infusion boost the rate of insulin independence in the short term, with a decline afterwards to levels characteristic for single IT recipients |
| Vantyghem, 2009 [46] | 14 adults received ITA mean follow up 3.3 years T1DM duration > 5 years | eight patients (57%) remained insulin independent patients received up to three IT |
| Keymeulen, 2006 [50] | 22 adult patients receiving ITA 1 year follow up T1DM duration not reported | 10 patients insulin independent after 1 year, 5 of them with a single IT 13 patients received a second ITA after 2 months |
| Shapiro, 2006 [51] | 36 adults receiving ITA 1 year follow up T1DM duration > 5 years | insulin independence rate 44% afer 1 year 11 subjects receiving 1 infusion, 9 receiving 2 infusions, and 16 receiving 3 infusions |
| Maffi, 2005 [52] | 31 adults receiving IAK follow-up period 38 ± 4 months T1DM duration 27 ± 2 years | 9/31 of patients became insulin independent 8 patients received a second IAK |
| Ryan, 2005 [53] | 69 median follow-up period 35.5 months T1DM duration 27.1 ± 1.3 years | 44 patients were considered to have completed the islet transplant with insulin independence on the short term reported insulin independence rate of 10% after 5 years of follow up 52 patients had two transplants, and 11 subjects had three transplants |
| Secchi, 1997 [58] | 20 adults receiving ITA follow up period at least 12 months T1DM duration 25 ± 1 years | insulin independence was reported in seven patients for an interval of 21 ± 7 months, range 2–58 months) one patient received 2 ITA |
| Author | Study Population | Major Findings |
|---|---|---|
| Gu, 2018 [21] | 15 newly diagnosed T1DM patients received AHSC transplantation mean age: 18 ± 3.9 years 48-month follow-up period | 14/20 patients (70%) in the AHSC group experienced complete remission lasting 1.5–48 (median 20) months. three patients were insulin independent during 48 months single procedure |
| Malmegrim, 2017 [24] | 21 newly diagnosed T1DM patients were monitored after AHSC transplantation patients’ age range 12–35 years median follow-up of 78 (range 15–106) months | 21 patients became insulin-independent, but resuming insulin after median of 43 (range 6–100) months. single procedure |
| Cantu Rodriguez, 2016 [29] | 16 patients aged 8–17 years received AHSC median follow-up period 34 months T1DM duration: 2–92 months | insulin independence reported at 7 patients (44%) at study end single procedure |
| D’Addio, 2014 [33] | 65 patients with new-onset T1DM received AHSC follow up period 48 months reported patients’age: 20.4 ± 5.5 | 59% achieved insulin independence within the first 6 months, and 32% remained insulin independent to study end median duration of insulin independence: 12 months (range 12–40 months) single procedure |
| Gu, 2012 [39] | 28 patients with mean age 17.6± 3.7 years mean follow up period of 19.3 months newly diagnosed T1DM | insulin independence, was observed in 15 of 28 patients (53.6%) and lasted from 3 to 42 months (median 24 months) 8 (28.6%) maintained insulin independence to study end, mean duration of 23.8 ± 12.7 months (range 3–42 months) single treatment |
| Couri, 2009 [44] | 23 patients with newly diagnosed T1DM (aged 13–31 years, mean 18.4 years) mean follow-up period 29.8 months | insulin independence was reported in all patients (12 continuosly, 8 transiently) mean time of sustained insulin independence: 31 months, range,14–52months mean time of sustained insulin independence: 17.7 months, range 6–47months single procedure |
| Voltarelli, 2007 [49] | 15 patients with newly diagnosed type 1 DM without ketoacidosis age range 14–31 years mean follow up 18.8 months | 14 patients became insulin independent (1 patient for 35 months, 4 patients for at least 21 months, 7 patients for at least 6 months; and 2 patients with late response were insulin independent for 1 and 5 months, respectively) single procedure |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altabas, V.; Bulum, T. Current Challenges in Pancreas and Islet Transplantation: A Scoping Review. Biomedicines 2024, 12, 2853. https://doi.org/10.3390/biomedicines12122853
Altabas V, Bulum T. Current Challenges in Pancreas and Islet Transplantation: A Scoping Review. Biomedicines. 2024; 12(12):2853. https://doi.org/10.3390/biomedicines12122853
Chicago/Turabian StyleAltabas, Velimir, and Tomislav Bulum. 2024. "Current Challenges in Pancreas and Islet Transplantation: A Scoping Review" Biomedicines 12, no. 12: 2853. https://doi.org/10.3390/biomedicines12122853
APA StyleAltabas, V., & Bulum, T. (2024). Current Challenges in Pancreas and Islet Transplantation: A Scoping Review. Biomedicines, 12(12), 2853. https://doi.org/10.3390/biomedicines12122853







