Peptide Lv and Angiogenesis: A Newly Discovered Angiogenic Peptide
Abstract
1. Introduction
2. Discovery of Peptide Lv and Its Bioactivities
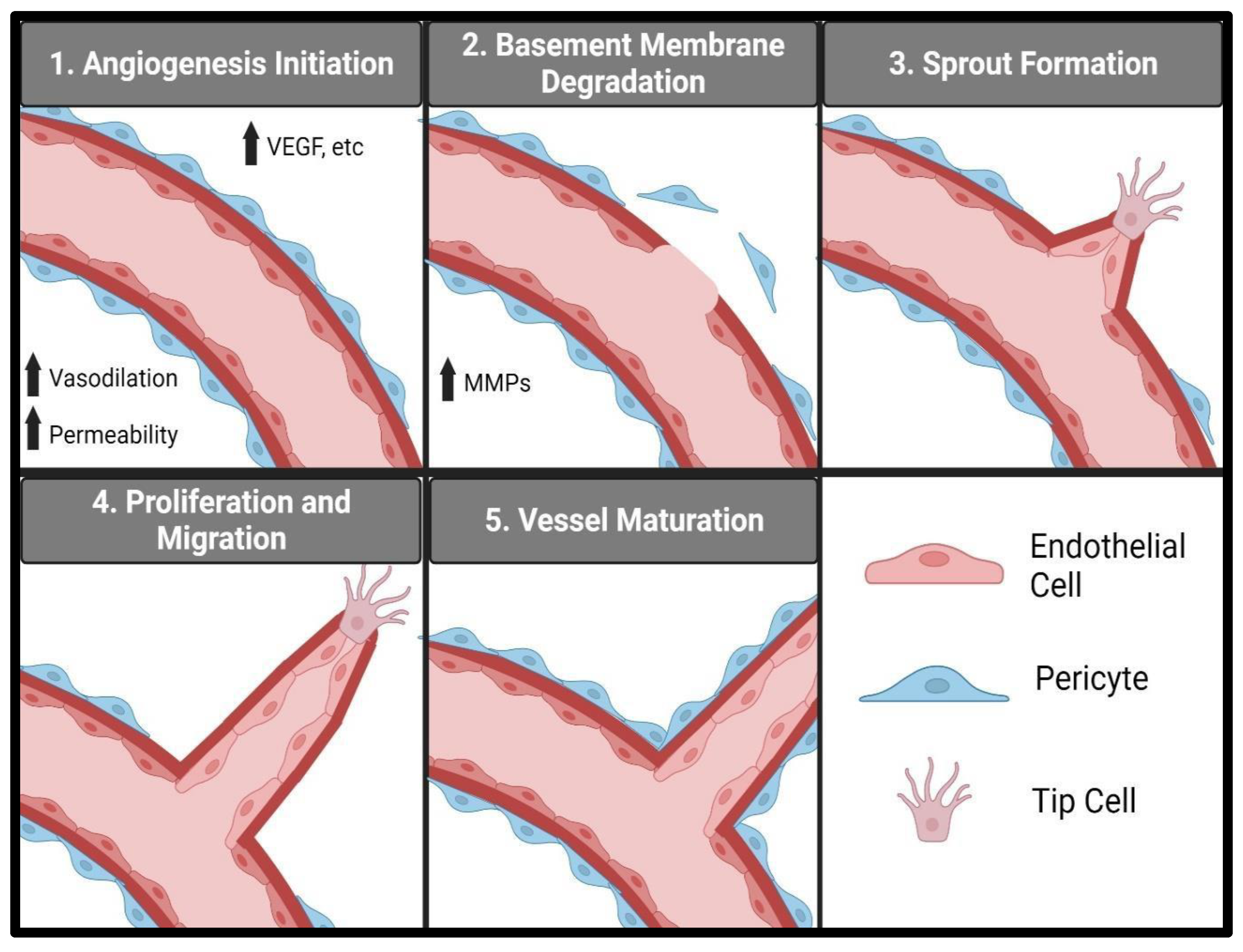
3. Vascular Endothelial Cells and Angiogenesis
4. Peptide Lv and Angiogenesis
5. Peptide Lv and Pathological Angiogenesis
6. VEGF, Endothelial Cells, and Vasodilation
7. Peptide Lv and Vasodilation
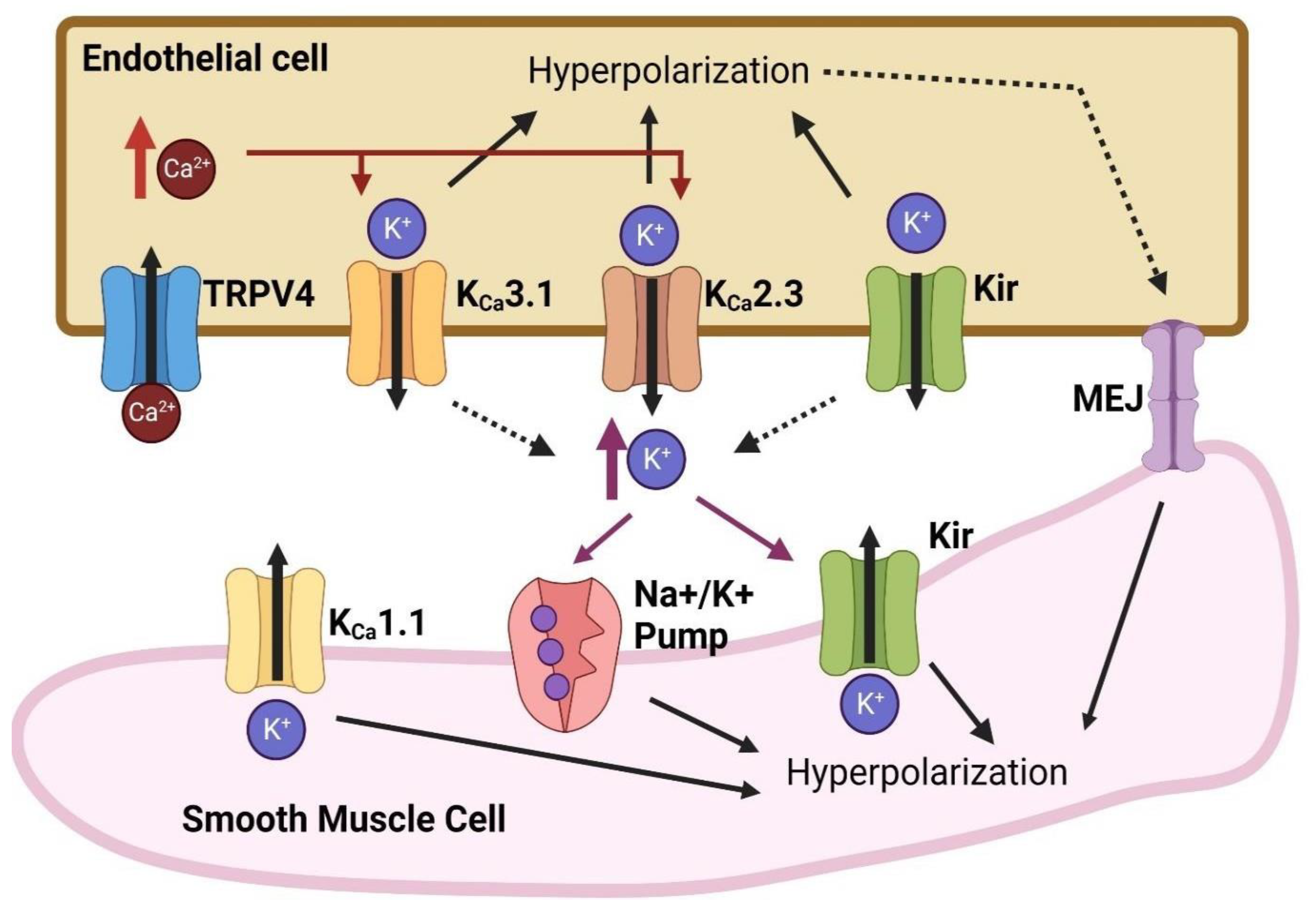
8. Vasodilation and Angiogenesis: Involvement of Endothelial Ion Channels
8.1. Calcium-Dependent Potassium (KCa) Channels
8.2. Inward-Rectifying Potassium (Kir) Channels
8.3. ATP-Sensitive Potassium (KATP) Channels
8.4. Transient Receptor Potential Cation (TRP) Channels
9. Peptide Lv Augments Endothelial KCa3.1 Channels
10. Scientific and Clinical Relevance of Peptide Lv
11. Potential Therapeutics Targeting Peptide Lv
Funding
Conflicts of Interest
References
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Adair, T.H.; Montani, J.P. Angiogenesis; Morgan & Claypool Life Sciences: San Rafael, CA, USA, 2010. [Google Scholar]
- Yehya, A.H.S.; Asif, M.; Petersen, S.H.; Subramaniam, A.V.; Kono, K.; Majid, A.; Oon, C.E. Angiogenesis: Managing the culprits behind tumorigenesis and metastasis. Medicina 2018, 54, 8. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P. Vegf as a key mediator of angiogenesis in cancer. Oncology 2005, 69 (Suppl. 3), 4–10. [Google Scholar] [CrossRef] [PubMed]
- Sedding, D.G.; Boyle, E.C.; Demandt, J.A.F.; Sluimer, J.C.; Dutzmann, J.; Haverich, A.; Bauersachs, J. Vasa vasorum angiogenesis: Key player in the initiation and progression of atherosclerosis and potential target for the treatment ofcardiovascular disease. Front. Immunol. 2018, 9, 706. [Google Scholar] [CrossRef] [PubMed]
- Elshabrawy, H.A.; Chen, Z.; Volin, M.V.; Ravella, S.; Virupannavar, S.; Shahrara, S. The pathogenic role of angiogenesis in rheumatoid arthritis. Angiogenesis 2015, 18, 433–448. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; McGinnis, J.F. Diabetic retinopathy: Animal models, therapies, and perspectives. J. Diabetes Res. 2016, 2016, 3789217. [Google Scholar] [CrossRef]
- Campochiaro, P.A. Ocular neovascularization. J. Mol. Med. 2013, 91, 311–321. [Google Scholar] [CrossRef]
- Aiello, L.P.; Avery, R.L.; Arrigg, P.G.; Keyt, B.A.; Jampel, H.D.; Shah, S.T.; Pasquale, L.R.; Thieme, H.; Iwamoto, M.A.; Park, J.E.; et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N. Engl. J. Med. 1994, 331, 1480–1487. [Google Scholar] [CrossRef]
- Pe’er, J.; Folberg, R.; Itin, A.; Gnessin, H.; Hemo, I.; Keshet, E. Upregulated expression of vascular endothelial growth factor in proliferative diabetic retinopathy. Br. J. Ophthalmol. 1996, 80, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Lux, A.; Llacer, H.; Heussen, F.M.; Joussen, A.M. Non-responders to bevacizumab (avastin) therapy of choroidal neovascular lesions. Br. J. Ophthalmol. 2007, 91, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Tranos, P.; Vacalis, A.; Asteriadis, S.; Koukoula, S.; Vachtsevanos, A.; Perganta, G.; Georgalas, I. Resistance to antivascular endothelial growth factor treatment in age-related macular degeneration. Drug Des. Dev. Ther. 2013, 7, 485–490. [Google Scholar]
- Binder, S. Loss of reactivity in intravitreal anti-vegf therapy: Tachyphylaxis or tolerance? Br. J. Ophthalmol. 2012, 96, 1–2. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pham, D.L.; Niemi, A.; Ko, M.L.; Ko, G.Y.P. Peptide lv augments intermediate-conductance calcium-dependent potassium channels (kca3.1) in endothelial cells to promote angiogenesis. PLoS ONE 2022, 17, e0276744. [Google Scholar] [CrossRef]
- Shi, L.; Ko, M.L.; Abbott, L.C.; Ko, G.Y. Identification of peptide lv, a novel putative neuropeptide that regulates the expression of l-type voltage-gated calcium channels in photoreceptors. PLoS ONE 2012, 7, e43091. [Google Scholar] [CrossRef] [PubMed]
- Striessnig, J.; Koschak, A.; Sinnegger-Brauns, M.J.; Hetzenauer, A.; Nguyen, N.K.; Busquet, P.; Pelster, G.; Singewald, N. Role of voltage-gated l-type ca2+ channel isoforms for brain function. Biochem. Soc. Trans. 2006, 34, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Chang, J.Y.; Yu, F.; Ko, M.L.; Ko, G.Y. The contribution of l-type ca (v)1.3 channels to retinal light responses. Front. Mol. Neurosci. 2017, 10, 394. [Google Scholar] [PubMed]
- Barnes, S.; Kelly, M.E. Calcium channels at the photoreceptor synapse. Adv. Exp. Med. Biol. 2002, 514, 465–476. [Google Scholar]
- Shi, L.; Ko, S.; Ko, M.L.; Kim, A.J.; Ko, G.Y. Peptide lv augments l-type voltage-gated calcium channels through vascular endothelial growth factor receptor 2 (vegfr2) signaling. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2015, 1853, 1154–1164. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.L.; Liu, Y.; Dryer, S.E.; Ko, G.Y. The expression of l-type voltage-gated calcium channels in retinal photoreceptors is under circadian control. J. Neurochem. 2007, 103, 784–792. [Google Scholar] [CrossRef]
- Gao, T.; Yatani, A.; Dell’Acqua, M.L.; Sako, H.; Green, S.A.; Dascal, N.; Scott, J.D.; Hosey, M.M. Camp-dependent regulation of cardiac l-type ca2+ channels requires membrane targeting of pka and phosphorylation of channel subunits. Neuron 1997, 19, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Olsson, A.K.; Dimberg, A.; Kreuger, J.; Claesson-Welsh, L. Vegf receptor signalling-In control of vascular function. Nat. Rev. Mol. Cell Biol. 2006, 7, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Chikaev, N.A.; Bykova, E.A.; Najakshin, A.M.; Mechetina, L.V.; Volkova, O.Y.; Peklo, M.M.; Shevelev, A.Y.; Vlasik, T.N.; Roesch, A.; Vogt, T.; et al. Cloning and characterization of the human fcrl2 gene. Genomics 2005, 85, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, I.; Greer, C.A.; Mok, M.Y.; Mombaerts, P. A putative pheromone receptor gene expressed in human olfactory mucosa. Nat. Genet. 2000, 26, 18–19. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.H.; Alitalo, K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat. Rev. Mol. Cell Biol. 2007, 8, 464–478. [Google Scholar] [CrossRef]
- Bayless, K.J.; Kwak, H.I.; Su, S.C. Investigating endothelial invasion and sprouting behavior in three-dimensional collagen matrices. Nat. Protoc. 2009, 4, 1888–1898. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Conway, E.M.; Collen, D.; Carmeliet, P. Molecular mechanisms of blood vessel growth. Cardiovasc. Res. 2001, 49, 507–521. [Google Scholar] [CrossRef]
- Lamalice, L.; Le Boeuf, F.; Huot, J. Endothelial cell migration during angiogenesis. Circ. Res. 2007, 100, 782–794. [Google Scholar] [CrossRef]
- Risau, W. Mechanisms of angiogenesis. Nature 1997, 386, 671–674. [Google Scholar] [CrossRef]
- Sainson, R.C.; Aoto, J.; Nakatsu, M.N.; Holderfield, M.; Conn, E.; Koller, E.; Hughes, C.C. Cell-autonomous notch signaling regulates endothelial cell branching and proliferation during vascular tubulogenesis. FASEB J. 2005, 19, 1027–1029. [Google Scholar] [CrossRef]
- Gerhardt, H.; Golding, M.; Fruttiger, M.; Ruhrberg, C.; Lundkvist, A.; Abramsson, A.; Jeltsch, M.; Mitchell, C.; Alitalo, K.; Shima, D.; et al. Vegf guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 2003, 161, 1163–1177. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, H.; Betsholtz, C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res. 2003, 314, 15–23. [Google Scholar] [CrossRef] [PubMed]
- You, D.; Waeckel, L.; Ebrahimian, T.G.; Blanc-Brude, O.; Foubert, P.; Barateau, V.; Duriez, M.; Lericousse-Roussanne, S.; Vilar, J.; Dejana, E.; et al. Increase in vascular permeability and vasodilation are critical for proangiogenic effects of stem cell therapy. Circulation 2006, 114, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, H.F.; Brown, L.F.; Detmar, M.; Dvorak, A.M. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am. J. Pathol. 1995, 146, 1029–1039. [Google Scholar] [PubMed]
- Ashina, K.; Tsubosaka, Y.; Kobayashi, K.; Omori, K.; Murata, T. Vegf-induced blood flow increase causes vascular hyper-permeability in vivo. Biochem. Biophys. Res. Commun. 2015, 464, 590–595. [Google Scholar] [CrossRef] [PubMed]
- McDonald, D.M. Angiogenesis and remodeling of airway vasculature in chronic inflammation. Am. J. Respir. Crit. Care Med. 2001, 164, S39–S45. [Google Scholar] [CrossRef] [PubMed]
- Feletou, M.; Bonnardel, E.; Canet, E. Bradykinin and changes in microvascular permeability in the hamster cheek pouch: Role of nitric oxide. Br. J. Pharmacol. 1996, 118, 1371–1376. [Google Scholar] [CrossRef] [PubMed][Green Version]
- van Hinsbergh, V.W.; Engelse, M.A.; Quax, P.H. Pericellular proteases in angiogenesis and vasculogenesis. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 716–728. [Google Scholar] [CrossRef]
- Norton, K.A.; Popel, A.S. Effects of endothelial cell proliferation and migration rates in a computational model of sprouting angiogenesis. Sci. Rep. 2016, 6, 36992. [Google Scholar] [CrossRef]
- Luo, Y.; Radice, G.L. N-cadherin acts upstream of ve-cadherin in controlling vascular morphogenesis. J. Cell Biol. 2005, 169, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Lampugnani, M.G.; Orsenigo, F.; Rudini, N.; Maddaluno, L.; Boulday, G.; Chapon, F.; Dejana, E. Ccm1 regulates vascular-lumen organization by inducing endothelial polarity. J. Cell Sci. 2010, 123, 1073–1080. [Google Scholar] [CrossRef]
- Jain, R.K. Molecular regulation of vessel maturation. Nat. Med. 2003, 9, 685–693. [Google Scholar] [CrossRef]
- Campochiaro, P.A. Retinal and choroidal neovascularization. J. Cell Physiol. 2000, 184, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, H.; Baluk, P.; Morikawa, S.; McLean, J.W.; Thurston, G.; Roberge, S.; Jain, R.K.; McDonald, D.M. Openings between defective endothelial cells explain tumor vessel leakiness. Am. J. Pathol. 2000, 156, 1363–1380. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N. Vegf as a therapeutic target in cancer. Oncology 2005, 69 (Suppl. 3), 11–16. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Shibuya, M. The vascular endothelial growth factor (vegf)/vegf receptor system and its role under physiological and pathological conditions. Clin. Sci. 2005, 109, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Henry, T.D.; Annex, B.H.; McKendall, G.R.; Azrin, M.A.; Lopez, J.J.; Giordano, F.J.; Shah, P.K.; Willerson, J.T.; Benza, R.L.; Berman, D.S.; et al. The viva trial: Vascular endothelial growth factor in ischemia for vascular angiogenesis. Circulation 2003, 107, 1359–1365. [Google Scholar] [CrossRef] [PubMed]
- Jaszai, J.; Schmidt, M.H.H. Trends and challenges in tumor anti-angiogenic therapies. Cells 2019, 8, 1102–1137. [Google Scholar] [CrossRef]
- Itatani, Y.; Kawada, K.; Yamamoto, T.; Sakai, Y. Resistance to anti-angiogenic therapy in cancer-alterations to anti-vegf pathway. Int. J. Mol. Sci. 2018, 19, 1232. [Google Scholar] [CrossRef]
- Shi, L.; Zhao, M.; Abbey, C.A.; Tsai, S.H.; Xie, W.; Pham, D.; Chapman, S.; Bayless, K.J.; Hein, T.W.; Rosa Jr, R.H.; et al. Newly identified peptide, peptide lv, promotes pathological angiogenesis. J. Am. Heart Assoc. 2019, 8, e013673. [Google Scholar] [CrossRef]
- Liang, C.C.; Park, A.Y.; Guan, J.L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef]
- Ribatti, D.; Vacca, A.; Roncali, L.; Dammacco, F. The chick embryo chorioallantoic membrane as a model for in vivo research on angiogenesis. Int. J. Dev. Biol. 1996, 40, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Hendrick, A.M.; Gibson, M.V.; Kulshreshtha, A. Diabetic retinopathy. Prim. Care 2015, 42, 451–464. [Google Scholar] [CrossRef]
- Hellstrom, A.; Smith, L.E.; Dammann, O. Retinopathy of prematurity. Lancet 2013, 382, 1445–1457. [Google Scholar] [CrossRef]
- Stahl, A.; Connor, K.M.; Sapieha, P.; Chen, J.; Dennison, R.J.; Krah, N.M.; Seaward, M.R.; Willett, K.L.; Aderman, C.M.; Guerin, K.I.; et al. The mouse retina as an angiogenesis model. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2813–2826. [Google Scholar] [CrossRef] [PubMed]
- Connor, K.M.; Krah, N.M.; Dennison, R.J.; Aderman, C.M.; Chen, J.; Guerin, K.I.; Sapieha, P.; Stahl, A.; Willett, K.L.; Smith, L.E. Quantification of oxygen-induced retinopathy in the mouse: A model of vessel loss, vessel regrowth and pathological angiogenesis. Nat. Protoc. 2009, 4, 1565–1573. [Google Scholar] [CrossRef]
- Mezu-Ndubuisi, O.J.; Song, Y.S.; Macke, E.; Johnson, H.; Nwaba, G.; Ikeda, A.; Sheibani, N. Retinopathy of prematurity shows alterations in vegfa (164) isoform expression. Pediatr. Res. 2022, 91, 1677–1685. [Google Scholar] [CrossRef]
- Lambert, V.; Lecomte, J.; Hansen, S.; Blacher, S.; Gonzalez, M.L.; Struman, I.; Sounni, N.E.; Rozet, E.; de Tullio, P.; Foidart, J.M.; et al. Laser-induced choroidal neovascularization model to study age-related macular degeneration in mice. Nat. Protoc. 2013, 8, 2197–2211. [Google Scholar] [CrossRef]
- Fleckenstein, M.; Keenan, T.D.L.; Guymer, R.H.; Chakravarthy, U.; Schmitz-Valckenberg, S.; Klaver, C.C.; Wong, W.T.; Chew, E.Y. Age-related macular degeneration. Nat. Rev. Dis. Primers 2021, 7, 31. [Google Scholar] [CrossRef]
- Hein, T.W.; Rosa Jr, R.H.; Ren, Y.; Xu, W.; Kuo, L. Vegf receptor-2-linked pi3k/calpain/sirt1 activation mediates retinal arteriolar dilations to vegf and shear stress. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5381–5389. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Myers, C.E.; Klein, B.E. Vasodilators, blood pressure-lowering medications, and age-related macular degeneration: The beaver dam eye study. Ophthalmology 2014, 121, 1604–1611. [Google Scholar] [CrossRef]
- Kolluru, G.K.; Sinha, S.; Majumder, S.; Muley, A.; Siamwala, J.H.; Gupta, R.; Chatterjee, S. Shear stress promotes nitric oxide production in endothelial cells by sub-cellular delocalization of enos: A basis for shear stress mediated angiogenesis. Nitric Oxide 2010, 22, 304–315. [Google Scholar] [CrossRef]
- Stefansson, E.; Landers, M.B.; Wolbarsht, M.L. Oxygenation and vasodilatation in relation to diabetic and other proliferative retinopathies. Ophthalmic Surg. 1983, 14, 209–226. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; Biaggioni, I. Role of nitric oxide in adenosine-induced vasodilation in humans. Hypertension 1998, 31, 1061–1064. [Google Scholar] [CrossRef] [PubMed]
- Emerson, G.G.; Segal, S.S. Electrical coupling between endothelial cells and smooth muscle cells in hamster feed arteries: Role in vasomotor control. Circ. Res. 2000, 87, 474–479. [Google Scholar] [CrossRef]
- Brozovich, F.V.; Nicholson, C.J.; Degen, C.V.; Gao, Y.Z.; Aggarwal, M.; Morgan, K.G. Mechanisms of vascular smooth muscle contraction and the basis for pharmacologic treatment of smooth muscle disorders. Pharmacol. Rev. 2016, 68, 476–532. [Google Scholar] [CrossRef]
- Amberg, G.C.; Navedo, M.F. Calcium dynamics in vascular smooth muscle. Microcirculation 2013, 20, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Furchgott, R.F.; Zawadzki, J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980, 288, 373–376. [Google Scholar] [CrossRef]
- Chen, K.; Pittman, R.N.; Popel, A.S. Nitric oxide in the vasculature: Where does it come from and where does it go? A quantitative perspective. Antioxid. Redox Signal. 2008, 10, 1185–1198. [Google Scholar] [CrossRef] [PubMed]
- Archer, S.L.; Huang, J.M.; Hampl, V.; Nelson, D.P.; Shultz, P.J.; Weir, E.K. Nitric oxide and cgmp cause vasorelaxation by activation of a charybdotoxin-sensitive k channel by cgmp-dependent protein kinase. Proc. Natl. Acad. Sci. USA 1994, 91, 7583–7587. [Google Scholar] [CrossRef]
- Bunting, S.; Moncada, S.; Vane, J.R. The prostacyclin--thromboxane a2 balance: Pathophysiological and therapeutic implications. Br. Med. Bull. 1983, 39, 271–276. [Google Scholar] [CrossRef]
- Fetalvero, K.M.; Martin, K.A.; Hwa, J. Cardioprotective prostacyclin signaling in vascular smooth muscle. Prostaglandins Other Lipid Mediat. 2007, 82, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Michaelis, U.R.; Fleming, I. From endothelium-derived hyperpolarizing factor (edhf) to angiogenesis: Epoxyeicosatrienoic acids (eets) and cell signaling. Pharmacol. Ther. 2006, 111, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Waldron, G.J.; Dong, H.; Cole, W.C.; Triggle, C.R. Endothelium-dependent hyperpolarization of vascular smooth muscle: Role for a non-nitric oxide synthase product. Zhongguo Yao Li Xue Bao 1996, 17, 3–7. [Google Scholar]
- Coleman, H.A.; Tare, M.; Parkington, H.C. Endothelial potassium channels, endothelium-dependent hyperpolarization and the regulation of vascular tone in health and disease. Clin. Exp. Pharmacol. Physiol. 2004, 31, 641–649. [Google Scholar] [CrossRef]
- Brandes, R.P.; Schmitz-Winnenthal, F.H.; Feletou, M.; Godecke, A.; Huang, P.L.; Vanhoutte, P.M.; Fleming, I.; Busse, R. An endothelium-derived hyperpolarizing factor distinct from no and prostacyclin is a major endothelium-dependent vasodilator in resistance vessels of wild-type and endothelial no synthase knockout mice. Proc. Natl. Acad. Sci. USA 2000, 97, 9747–9752. [Google Scholar] [CrossRef]
- Edwards, G.; Feletou, M.; Weston, A.H. Endothelium-derived hyperpolarising factors and associated pathways: A synopsis. Pflügers Arch.-Eur. J. Physiol. 2010, 459, 863–879. [Google Scholar] [CrossRef] [PubMed]
- Adelstein, R.S.; Sellers, J.R. Effects of calcium on vascular smooth muscle contraction. Am. J. Cardiol. 1987, 59, B4–B10. [Google Scholar] [CrossRef]
- Isakson, B.E.; Duling, B.R. Heterocellular contact at the myoendothelial junction influences gap junction organization. Circ. Res. 2005, 97, 44–51. [Google Scholar] [CrossRef]
- Haddock, R.E.; Grayson, T.H.; Brackenbury, T.D.; Meaney, K.R.; Neylon, C.B.; Sandow, S.L.; Hill, C.E. Endothelial coordination of cerebral vasomotion via myoendothelial gap junctions containing connexins 37 and 40. Am. J. Physiol.-Heart Circ. Physiol. 2006, 291, H2047–H2056. [Google Scholar] [CrossRef]
- Sandow, S.L.; Tare, M.; Coleman, H.A.; Hill, C.E.; Parkington, H.C. Involvement of myoendothelial gap junctions in the actions of endothelium-derived hyperpolarizing factor. Circ. Res. 2002, 90, 1108–1113. [Google Scholar] [CrossRef]
- Ungvari, Z.; Csiszar, A.; Koller, A. Increases in endothelial ca (2+) activate k (ca) channels and elicit edhf-type arteriolar dilation via gap junctions. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H1760–H1767. [Google Scholar] [CrossRef]
- Sandow, S.L.; Hill, C.E. Incidence of myoendothelial gap junctions in the proximal and distal mesenteric arteries of the rat is suggestive of a role in endothelium-derived hyperpolarizing factor-mediated responses. Circ. Res. 2000, 86, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Yashiro, Y.; Duling, B.R. Integrated ca (2+) signaling between smooth muscle and endothelium of resistance vessels. Circ. Res. 2000, 87, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Edwards, G.; Dora, K.A.; Gardener, M.J.; Garland, C.J.; Weston, A.H. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature 1998, 396, 269–272. [Google Scholar] [CrossRef]
- Bondarenko, A.; Sagach, V. Na+-k+-atpase is involved in the sustained ach-induced hyperpolarization of endothelial cells from rat aorta. Br. J. Pharmacol. 2006, 149, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Hibino, H.; Inanobe, A.; Furutani, K.; Murakami, S.; Findlay, I.; Kurachi, Y. Inwardly rectifying potassium channels: Their structure, function, and physiological roles. Physiol. Rev. 2010, 90, 291–366. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z. Mechanism of rectification in inward-rectifier k+ channels. Annu. Rev. Physiol. 2004, 66, 103–129. [Google Scholar] [CrossRef]
- Haddy, F.J.; Vanhoutte, P.M.; Feletou, M. Role of potassium in regulating blood flow and blood pressure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R546–R552. [Google Scholar] [CrossRef]
- Aittoniemi, J.; Fotinou, C.; Craig, T.J.; de Wet, H.; Proks, P.; Ashcroft, F.M. Sur1: A unique atp-binding cassette protein that functions as an ion channel regulator. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 257–267. [Google Scholar] [CrossRef]
- Fisslthaler, B.; Popp, R.; Kiss, L.; Potente, M.; Harder, D.R.; Fleming, I.; Busse, R. Cytochrome p450 2c is an edhf synthase in coronary arteries. Nature 1999, 401, 493–497. [Google Scholar] [CrossRef]
- Miura, H.; Gutterman, D.D. Human coronary arteriolar dilation to arachidonic acid depends on cytochrome p-450 monooxygenase and ca2+-activated k+ channels. Circ. Res. 1998, 83, 501–507. [Google Scholar] [CrossRef]
- Archer, S.L.; Gragasin, F.S.; Wu, X.; Wang, S.; McMurtry, S.; Kim, D.H.; Platonov, M.; Koshal, A.; Hashimoto, K.; Campbell, W.B.; et al. Endothelium-derived hyperpolarizing factor in human internal mammary artery is 11,12-epoxyeicosatrienoic acid and causes relaxation by activating smooth muscle bk(ca) channels. Circulation 2003, 107, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Grgic, I.; Kaistha, B.P.; Hoyer, J.; Kohler, R. Endothelial ca+-activated k+ channels in normal and impaired edhf-dilator responses--relevance to cardiovascular pathologies and drug discovery. Br. J. Pharmacol. 2009, 157, 509–526. [Google Scholar] [CrossRef] [PubMed]
- Sforna, L.; Megaro, A.; Pessia, M.; Franciolini, F.; Catacuzzeno, L. Structure, gating and basic functions of the ca2+-activated k channel of intermediate conductance. Curr. Neuropharmacol. 2018, 16, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Vriens, J.; Prenen, J.; Droogmans, G.; Voets, T.; Nilius, B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate trpv4 channels. Nature 2003, 424, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Kuo, L.; Davis, M.J.; Chilian, W.M. Endothelium-dependent, flow-induced dilation of isolated coronary arterioles. Am. J. Physiol. 1990, 259, H1063–H1070. [Google Scholar] [CrossRef] [PubMed]
- Goto, K.; Ohtsubo, T.; Kitazono, T. Endothelium-dependent hyperpolarization (edh) in hypertension: The role of endothelial ion channels. Int. J. Mol. Sci. 2018, 19, 315. [Google Scholar] [CrossRef]
- Dora, K.A.; Gallagher, N.T.; McNeish, A.; Garland, C.J. Modulation of endothelial cell kca3.1 channels during endothelium-derived hyperpolarizing factor signaling in mesenteric resistance arteries. Circ. Res. 2008, 102, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Sonkusare, S.K. Endothelial trpv4 channels and vasodilator reactivity. Curr. Top. Membr. 2020, 85, 89–117. [Google Scholar] [PubMed]
- Nilius, B.; Droogmans, G. Ion channels and their functional role in vascular endothelium. Physiol. Rev. 2001, 81, 1415–1459. [Google Scholar] [CrossRef]
- Pedersen, S.F.; Owsianik, G.; Nilius, B. Trp channels: An overview. Cell Calcium 2005, 38, 233–252. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Garland, C.J. Recent developments in vascular endothelial cell transient receptor potential channels. Circ. Res. 2005, 97, 853–863. [Google Scholar] [CrossRef]
- Zhang, D.X.; Mendoza, S.A.; Bubolz, A.H.; Mizuno, A.; Ge, Z.D.; Li, R.; Warltier, D.C.; Suzuki, M.; Gutterman, D.D. Transient receptor potential vanilloid type 4-deficient mice exhibit impaired endothelium-dependent relaxation induced by acetylcholine in vitro and in vivo. Hypertension 2009, 53, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Fleming, I.; Rueben, A.; Popp, R.; Fisslthaler, B.; Schrodt, S.; Sander, A.; Haendeler, J.; Falck, J.R.; Morisseau, C.; Hammock, B.D.; et al. Epoxyeicosatrienoic acids regulate trp channel dependent ca2+ signaling and hyperpolarization in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2612–2618. [Google Scholar] [CrossRef] [PubMed]
- Bussemaker, E.; Wallner, C.; Fisslthaler, B.; Fleming, I. The na-k-atpase is a target for an edhf displaying characteristics similar to potassium ions in the porcine renal interlobar artery. Br. J. Pharmacol. 2002, 137, 647–654. [Google Scholar] [CrossRef] [PubMed][Green Version]
- King, B.; Rizwan, A.P.; Asmara, H.; Heath, N.C.; Engbers, J.D.; Dykstra, S.; Bartoletti, T.M.; Hameed, S.; Zamponi, G.W.; Turner, R.W. Ikca channels are a critical determinant of the slow ahp in ca1 pyramidal neurons. Cell Rep. 2015, 11, 175–182. [Google Scholar] [CrossRef]
- Turner, R.W.; Asmara, H.; Engbers, J.D.; Miclat, J.; Rizwan, A.P.; Sahu, G.; Zamponi, G.W. Assessing the role of ikca channels in generating the sahp of ca1 hippocampal pyramidal cells. Channels 2016, 10, 313–319. [Google Scholar] [CrossRef]
- Wei, A.D.; Gutman, G.A.; Aldrich, R.; Chandy, K.G.; Grissmer, S.; Wulff, H. International union of pharmacology. Lii. Nomenclature and molecular relationships of calcium-activated potassium channels. Pharmacol. Rev. 2005, 57, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Fanger, C.M.; Ghanshani, S.; Logsdon, N.J.; Rauer, H.; Kalman, K.; Zhou, J.; Beckingham, K.; Chandy, K.G.; Cahalan, M.D.; Aiyar, J. Calmodulin mediates calcium-dependent activation of the intermediate conductance kca channel, ikca1. J. Biol. Chem. 1999, 274, 5746–5754. [Google Scholar] [CrossRef] [PubMed]
- Zygmunt, P.M.; Hogestatt, E.D. Role of potassium channels in endothelium-dependent relaxation resistant to nitroarginine in the rat hepatic artery. Br. J. Pharmacol. 1996, 117, 1600–1606. [Google Scholar] [CrossRef] [PubMed]
- Kohler, R.; Eichler, I.; Schonfelder, H.; Grgic, I.; Heinau, P.; Si, H.; Hoyer, J. Impaired edhf-mediated vasodilation and function of endothelial ca-activated k channels in uremic rats. Kidney Int. 2005, 67, 2280–2287. [Google Scholar] [CrossRef] [PubMed]
- Brahler, S.; Kaistha, A.; Schmidt, V.J.; Wolfle, S.E.; Busch, C.; Kaistha, B.P.; Kacik, M.; Hasenau, A.L.; Grgic, I.; Si, H.; et al. Genetic deficit of sk3 and ik1 channels disrupts the endothelium-derived hyperpolarizing factor vasodilator pathway and causes hypertension. Circulation 2009, 119, 2323–2332. [Google Scholar] [CrossRef]
- Seki, T.; Goto, K.; Kiyohara, K.; Kansui, Y.; Murakami, N.; Haga, Y.; Ohtsubo, T.; Matsumura, K.; Kitazono, T. Downregulation of endothelial transient receptor potential vanilloid type 4 channel and small-conductance of ca2+-activated k+ channels underpins impaired endothelium-dependent hyperpolarization in hypertension. Hypertension 2017, 69, 143–153. [Google Scholar] [CrossRef]
- Dabisch, P.A.; Liles, J.T.; Taylor, J.T.; Sears, B.W.; Saenz, R.; Kadowitz, P.J. Role of potassium channels in the nitric oxide-independent vasodilator response to acetylcholine. Pharmacol. Res. 2004, 49, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Grgic, I.; Eichler, I.; Heinau, P.; Si, H.; Brakemeier, S.; Hoyer, J.; Kohler, R. Selective blockade of the intermediate-conductance ca2+-activated k+ channel suppresses proliferation of microvascular and macrovascular endothelial cells and angiogenesis in vivo. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 704–709. [Google Scholar] [CrossRef]
- Wiecha, J.; Munz, B.; Wu, Y.; Noll, T.; Tillmanns, H.; Waldecker, B. Blockade of ca2+-activated k+ channels inhibits proliferation of human endothelial cells induced by basic fibroblast growth factor. J. Vasc. Res. 1998, 35, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, X.; Ma, J.; Lv, X.; Zhao, S.; Lang, W.; Zhang, Y. Blockade of the intermediate-conductance ca(2+)-activated k+ channel inhibits the angiogenesis induced by epidermal growth factor in the treatment of corneal alkali burn. Exp. Eye Res. 2013, 110, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Lopatin, A.N.; Makhina, E.N.; Nichols, C.G. Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature 1994, 372, 366–369. [Google Scholar] [CrossRef]
- Dunn, K.M.; Nelson, M.T. Potassium channels and neurovascular coupling. Circ. J. 2010, 74, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.L.; Feng, S.; Hilgemann, D.W. Direct activation of inward rectifier potassium channels by pip2 and its stabilization by gbetagamma. Nature 1998, 391, 803–806. [Google Scholar] [CrossRef]
- Zaritsky, J.J.; Eckman, D.M.; Wellman, G.C.; Nelson, M.T.; Schwarz, T.L. Targeted disruption of kir2.1 and kir2.2 genes reveals the essential role of the inwardly rectifying k (+) current in k (+)-mediated vasodilation. Circ. Res. 2000, 87, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Clement, J.P.t.; Kunjilwar, K.; Gonzalez, G.; Schwanstecher, M.; Panten, U.; Aguilar-Bryan, L.; Bryan, J. Association and stoichiometry of k (atp) channel subunits. Neuron 1997, 18, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Tucker, S.J.; Gribble, F.M.; Proks, P.; Trapp, S.; Ryder, T.J.; Haug, T.; Reimann, F.; Ashcroft, F.M. Molecular determinants of katp channel inhibition by atp. EMBO J. 1998, 17, 3290–3296. [Google Scholar] [CrossRef] [PubMed]
- Proks, P.; de Wet, H.; Ashcroft, F.M. Activation of the k (atp) channel by mg-nucleotide interaction with sur1. J. Gen. Physiol. 2010, 136, 389–405. [Google Scholar] [CrossRef] [PubMed]
- Figura, M.; Chilton, L.; Liacini, A.; Viskovic, M.M.; Phan, V.; Knight, D.; Millar, T.M.; Patel, K.; Kubes, P.; Giles, W.R.; et al. Blockade of k (atp) channels reduces endothelial hyperpolarization and leukocyte recruitment upon reperfusion after hypoxia. Am. J. Transplant. 2009, 9, 687–696. [Google Scholar] [CrossRef]
- Aziz, Q.; Thomas, A.M.; Gomes, J.; Ang, R.; Sones, W.R.; Li, Y.; Ng, K.E.; Gee, L.; Tinker, A. The atp-sensitive potassium channel subunit, kir6.1, in vascular smooth muscle plays a major role in blood pressure control. Hypertension 2014, 64, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Aziz, Q.; Anderson, N.; Ojake, L.; Tinker, A. Endothelial atp-sensitive potassium channel protects against the development of hypertension and atherosclerosis. Hypertension 2020, 76, 776–784. [Google Scholar] [CrossRef]
- Umaru, B.; Pyriochou, A.; Kotsikoris, V.; Papapetropoulos, A.; Topouzis, S. Atp-sensitive potassium channel activation induces angiogenesis in vitro and in vivo. J. Pharmacol. Exp. Ther. 2015, 354, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Samanta, A.; Hughes, T.E.T.; Moiseenkova-Bell, V.Y. Transient receptor potential (trp) channels. Subcell. Biochem. 2018, 87, 141–165. [Google Scholar]
- Venkatachalam, K.; Montell, C. Trp channels. Annu. Rev. Biochem. 2007, 76, 387–417. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Talavera, K.; Owsianik, G.; Prenen, J.; Droogmans, G.; Voets, T. Gating of trp channels: A voltage connection? J. Physiol. 2005, 567, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Montell, C. Forcing open trp channels: Mechanical gating as a unifying activation mechanism. Biochem. Biophys. Res. Commun. 2015, 460, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Baratchi, S.; Knoerzer, M.; Khoshmanesh, K.; Mitchell, A.; McIntyre, P. Shear stress regulates trpv4 channel clustering and translocation from adherens junctions to the basal membrane. Sci. Rep. 2017, 7, 15942. [Google Scholar] [CrossRef]
- Earley, S.; Pauyo, T.; Drapp, R.; Tavares, M.J.; Liedtke, W.; Brayden, J.E. Trpv4-dependent dilation of peripheral resistance arteries influences arterial pressure. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1096–H1102. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, S.A.; Fang, J.; Gutterman, D.D.; Wilcox, D.A.; Bubolz, A.H.; Li, R.; Suzuki, M.; Zhang, D.X. Trpv4-mediated endothelial ca2+ influx and vasodilation in response to shear stress. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H466–H476. [Google Scholar] [CrossRef] [PubMed]
- Sonkusare, S.K.; Bonev, A.D.; Ledoux, J.; Liedtke, W.; Kotlikoff, M.I.; Heppner, T.J.; Hill-Eubanks, D.C.; Nelson, M.T. Elementary ca2+ signals through endothelial trpv4 channels regulate vascular function. Science 2012, 336, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Antigny, F.; Girardin, N.; Frieden, M. Transient receptor potential canonical channels are required for in vitro endothelial tube formation. J. Biol. Chem. 2012, 287, 5917–5927. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.C.; Gu, S.Y.; Bu, J.W.; Du, J.L. Trpc1 is essential for in vivo angiogenesis in zebrafish. Circ. Res. 2010, 106, 1221–1232. [Google Scholar] [CrossRef] [PubMed]
- Thodeti, C.K.; Matthews, B.; Ravi, A.; Mammoto, A.; Ghosh, K.; Bracha, A.L.; Ingber, D.E. Trpv4 channels mediate cyclic strain-induced endothelial cell reorientation through integrin-to-integrin signaling. Circ. Res. 2009, 104, 1123–1130. [Google Scholar] [CrossRef]
- Hatano, N.; Suzuki, H.; Itoh, Y.; Muraki, K. Trpv4 partially participates in proliferation of human brain capillary endothelial cells. Life Sci. 2013, 92, 317–324. [Google Scholar] [CrossRef]
- Pham, D.L.; Niemi, A.; Blank, R.; Lomenzo, G.; Tham, J.; Ko, M.L.; Ko, G.Y. Peptide lv promotes trafficking and membrane insertion of k(ca)3.1 through the mek1-erk and pi3k-akt signaling pathways. Cells 2023, 12, 1651. [Google Scholar] [CrossRef]
- Mukai, M.; Uchida, K.; Okubo, T.; Takano, S.; Matsumoto, T.; Satoh, M.; Inoue, G.; Takaso, M. Regulation of tumor necrosis factor-alpha by peptide lv in bone marrow macrophages and synovium. Front. Med. 2021, 8, 702126. [Google Scholar] [CrossRef] [PubMed]
- Goedert, M.; Spillantini, M.G. A century of alzheimer’s disease. Science 2006, 314, 777–781. [Google Scholar] [CrossRef]
- Squire, L.R. Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychol. Rev. 1992, 99, 195–231. [Google Scholar] [CrossRef] [PubMed]


| Cell/Tissue Types | Bioactivities | References |
|---|---|---|
| Photoreceptors (chicken embryos) |
| Shi, et al., 2012. [15] PMID: 22912796 |
| Cardiomyocytes (chicken embryos) |
| Shi, et al., 2015. [19] PMID: 25698653 |
| Human umbilical vein endothelial cells (HUVECs) |
| Shi, et al., 2015. [19] PMID: 25698653 Shi, et al., 2019. [51] PMID: 31698979 Pham, et al., 2022. [14] PMID: 36282858 Pham, et al., 2023. [143] PMID: 37371121 |
| Human retinal microvascular endothelial cells (HRMECs) |
| Shi, et al., 2019. [19] PMID: 31698979 Pham, et al., 2022. [14] PMID: 36282858 Pham, et al., 2023. [143] PMID: 37371121 |
| Pig coronary and retinal arterioles (ex vivo) | Elicits dose-dependent vasodilation. | Shi, et al., 2019. [19] PMID: 31698979 |
| Chicken chorioallantoic membrane (ex ovo) | Promotes angiogenesis. | Shi, et al., 2019. [19] PMID: 31698979 |
| Mouse retina (in vivo) | Promotes angiogenesis and pathological neovascularization. | Shi, et al., 2019. [19] PMID: 31698979 |
| Mice (whole animals) | Dampens lipopolysaccharide-induced inflammatory responses. | Mukai et al., 2021. [144] PMID: 34386509 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pham, D.L.; Cox, K.; Ko, M.L.; Ko, G.Y.-P. Peptide Lv and Angiogenesis: A Newly Discovered Angiogenic Peptide. Biomedicines 2024, 12, 2851. https://doi.org/10.3390/biomedicines12122851
Pham DL, Cox K, Ko ML, Ko GY-P. Peptide Lv and Angiogenesis: A Newly Discovered Angiogenic Peptide. Biomedicines. 2024; 12(12):2851. https://doi.org/10.3390/biomedicines12122851
Chicago/Turabian StylePham, Dylan L., Kelsey Cox, Michael L. Ko, and Gladys Y.-P. Ko. 2024. "Peptide Lv and Angiogenesis: A Newly Discovered Angiogenic Peptide" Biomedicines 12, no. 12: 2851. https://doi.org/10.3390/biomedicines12122851
APA StylePham, D. L., Cox, K., Ko, M. L., & Ko, G. Y.-P. (2024). Peptide Lv and Angiogenesis: A Newly Discovered Angiogenic Peptide. Biomedicines, 12(12), 2851. https://doi.org/10.3390/biomedicines12122851





