Decoding the Genes Orchestrating Egg and Sperm Fusion Reactions and Their Roles in Fertility
Abstract
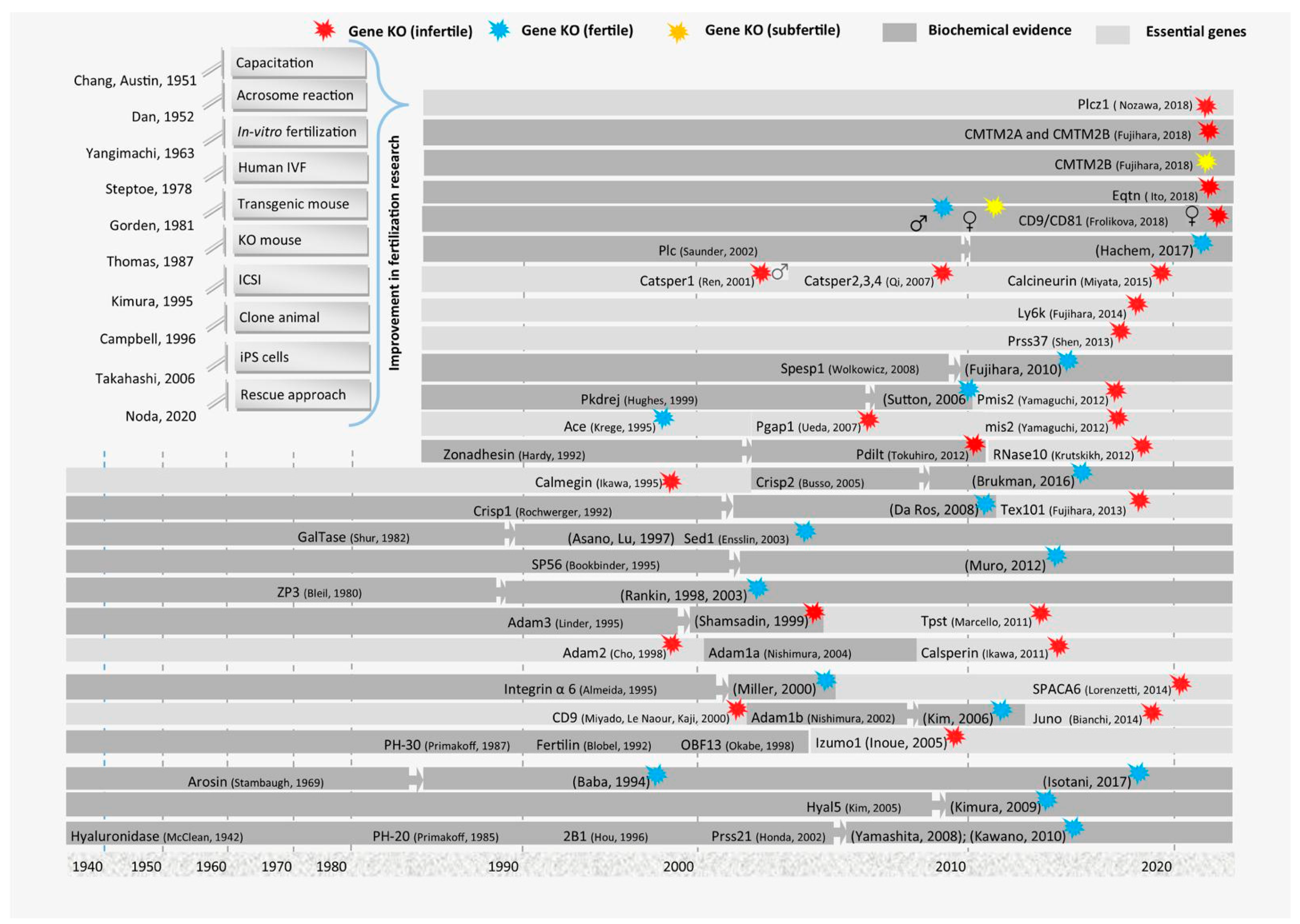
1. Introduction
2. Capacitation and the Acrosome Reaction
2.1. The Acrosome Reaction and Technological Advances
2.2. Zona Pellucida Penetration
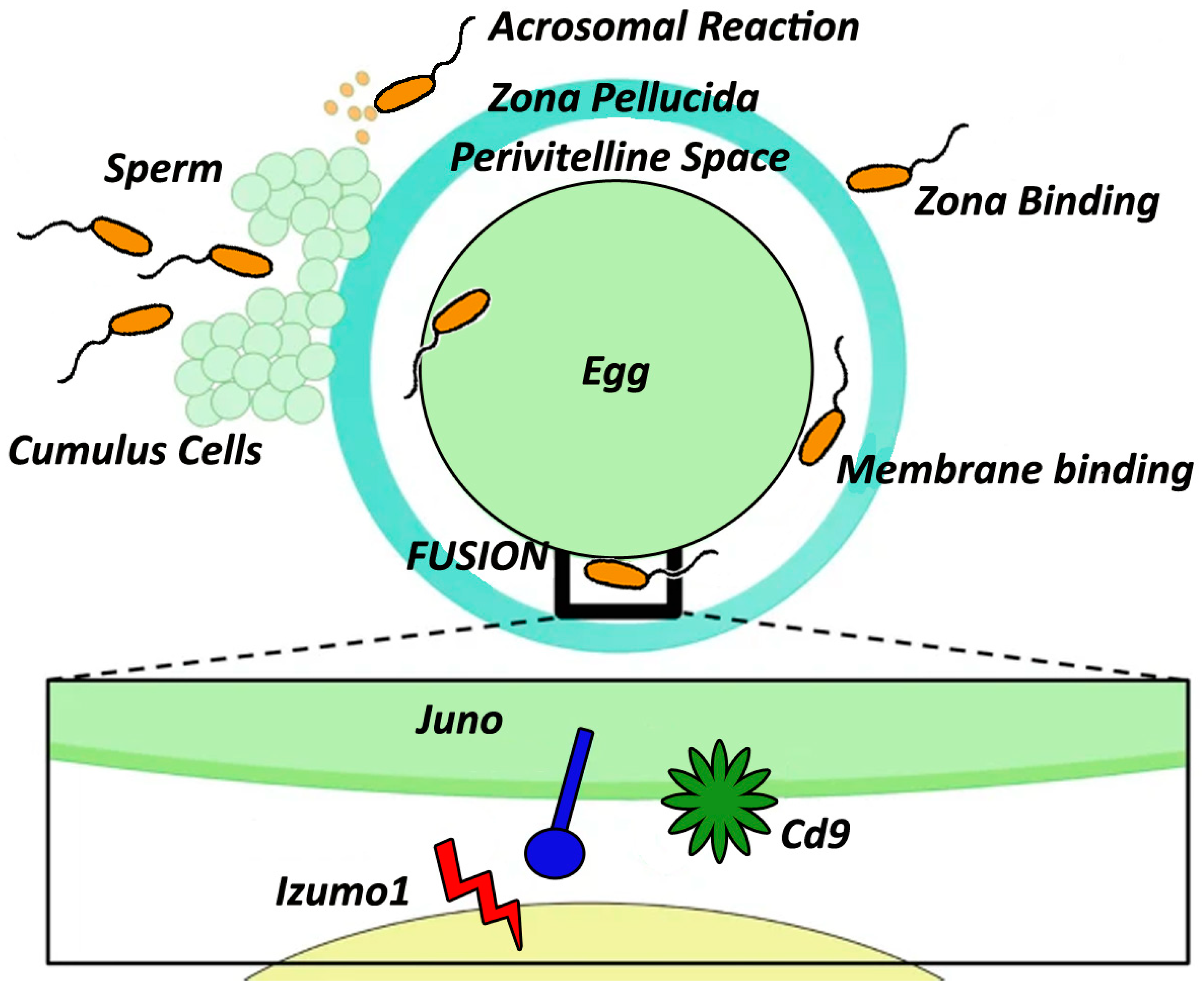
2.3. Sperm–Egg Attachment
2.4. Membrane Fusion
2.5. Egg Activation and Pronuclear Formation
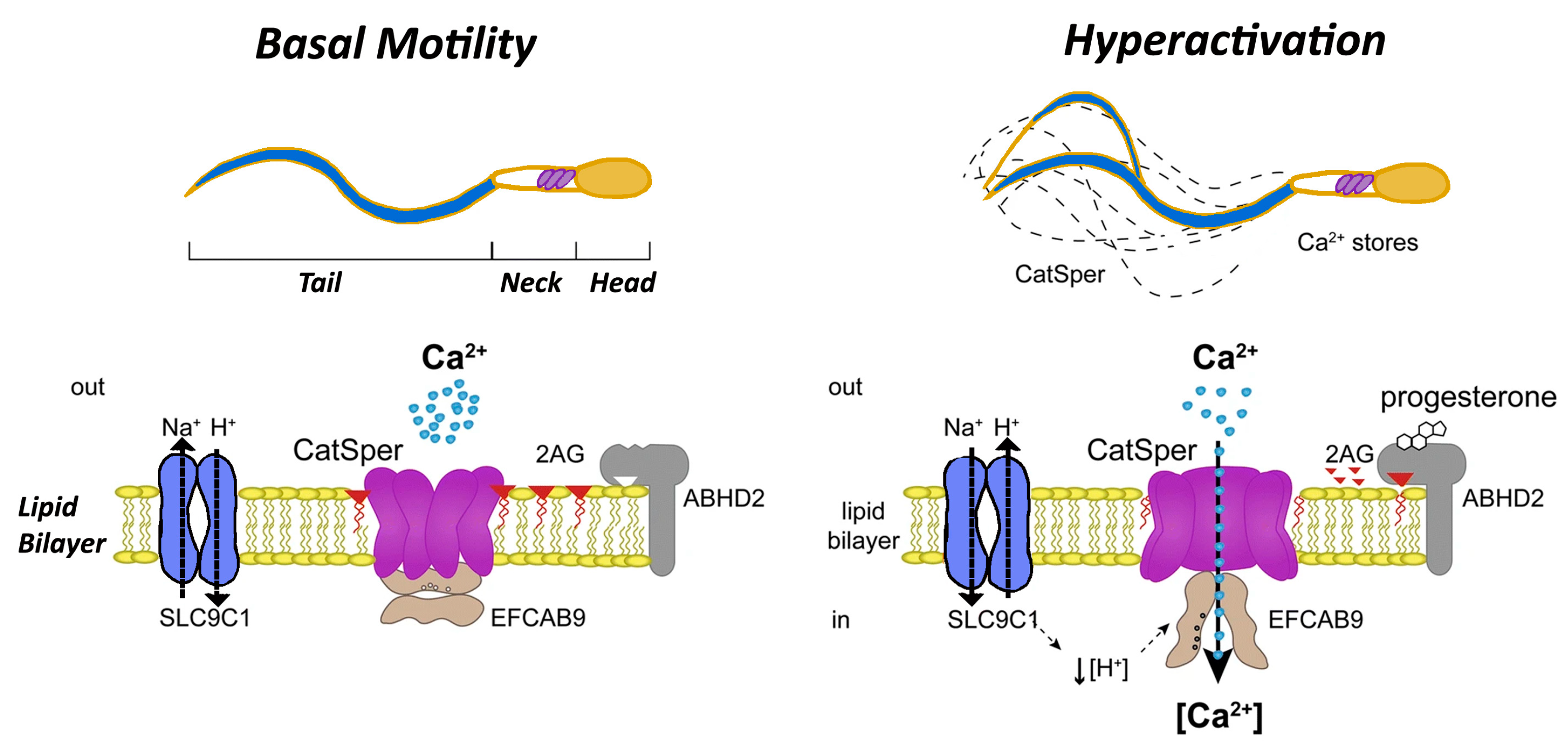
2.6. Mechanisms Underlying Capacitation
2.7. Acrosome Reaction: Key Triggers and Insights
3. Major Genes Involved in the Fusion of Gametes
3.1. IZUMO1 and Its Associated Proteins
3.2. Role of CD9 and CD81 in Mediating the Sperm–Egg Interaction
3.3. EQUATORIN (EQTN) Is Necessary for Gamete Adhesion
3.4. FIMP Is Indispensable for Fertilization
3.5. SOF1, TMEM95, and SPACA6 Are Required for Sperm–Oocyte Interactions
3.6. SLLP1 (Sperm Lyzozyme-Like Acrosomal Protein) and Its Role in Egg–Sperm Fusion
3.7. Other Accessory Proteins That Participate in the Fertilization Process
3.7.1. Testis-Specific ADAMs and Their Associated Proteins Support the Fusion Process
3.7.2. Sperm-Borne Phospholipase C Zeta-1 Is Essential for the Induction of Ca2+ Changes During Fertilization
3.7.3. Role of DC-STAMP Domain-Containing Protein 1/2 (DCST1 and DCST2) During Fertilization
4. Membrane Lipid Remodeling During Cell Fusion
5. Current Challenges and Future Directions
5.1. Ethical, Data Scarcity, and Practical Limitations
5.2. Model Organisms’ Specificity
5.2.1. Species-Specific Pathways
5.2.2. Incomplete Representation of Human Fertility Issues
6. Future Directions
6.1. CRISPR-Cas9 for Functional Gene Studies
6.2. Humanized Model Systems
6.3. High-Resolution Imaging for Molecular-Level Understanding
6.4. Integration of Omics Technologies
6.5. Advancing Fertility Treatments
6.6. Personalized Medicine
6.7. Broader Implications for Reproductive Health
7. Summary
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Noda, T.; Lu, Y.; Fujihara, Y.; Oura, S.; Koyano, T.; Kobayashi, S.; Matzuk, M.M.; Ikawa, M. Sperm proteins SOF1, TMEM95, and SPACA6 are required for sperm–oocyte fusion in mice. Proc. Natl. Acad. Sci. USA 2020, 117, 11493–11502. [Google Scholar] [CrossRef] [PubMed]
- Aydin, H.; Sultana, A.; Li, S.; Thavalingam, A.; Lee, J.E. Molecular architecture of the human sperm IZUMO1 and egg JUNO fertilization complex. Nature 2016, 534, 562–565. [Google Scholar] [CrossRef]
- Okabe, M. Sperm–egg interaction and fertilization: Past, present, and future. Biol. Reprod. 2018, 99, 134–146. [Google Scholar] [CrossRef]
- Bianchi, E.; Doe, B.; Goulding, D.; Wright, G.J. Juno is the egg Izumo receptor and is essential for mammalian fertilization. Nature 2014, 508, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.C. Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature 1951, 168, 697–698. [Google Scholar] [CrossRef] [PubMed]
- Austin, C.R. Observations on the penetration of the sperm in the mammalian egg. Aust. J. Sci. Res. B 1951, 4, 581–596. [Google Scholar] [CrossRef] [PubMed]
- Niijima, L.; Dan, J. The Acrosome Reaction in Mytilus Edulis. I. Fine Structure of the Intact Acrosome. J. Cell Biol. 1965, 25, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Austin, C.R.; Bishop, M.W. Role of the rodent acrosome and perforatorium in fertilization. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1958, 149, 241–248. [Google Scholar]
- Wassarman, P.M. Zona pellucida glycoproteins. J. Biol. Chem. 2008, 283, 24285–24289. [Google Scholar] [CrossRef]
- Miyado, K.; Yoshida, K.; Yamagata, K.; Sakakibara, K.; Okabe, M.; Wang, X.; Miyamoto, K.; Akutsu, H.; Kondo, T.; Takahashi, Y.; et al. The fusing ability of sperm is bestowed by CD9-containing vesicles released from eggs in mice. Proc. Natl. Acad. Sci. USA 2008, 105, 12921–12926. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.L.; Williams, C.J. Calcium signaling in mammalian egg activation and embryo development: The influence of subcellular localization. Mol. Reprod. Dev. 2012, 79, 742–756. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.C. The meaning of sperm capacitation. A historical perspective. J. Androl. 1984, 5, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Bedford, J.M. Significance of the need for sperm capacitation before fertilization in eutherian mammals. Biol. Reprod. 1983, 28, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.K. Influence of serum albumin on the fertilizing ability in vitro of rat spermatozoa. Proc. Soc. Exp. Biol. Med. 1976, 151, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.K.; Byrne, R.; Hungund, B. Studies on the mechanism of capacitation. II. Evidence for lipid transfer between plasma membrane of rat sperm and serum albumin during capacitation in vitro. Biochim. Biophys. Acta 1979, 558, 257–266. [Google Scholar] [CrossRef]
- Choi, Y.H.; Toyoda, Y. Cyclodextrin removes cholesterol from mouse sperm and induces capacitation in a protein-free medium. Biol. Reprod. 1998, 59, 1328–1333. [Google Scholar] [CrossRef]
- Tateno, H.; Krapf, D.; Hino, T.; Sánchez-Cárdenas, C.; Darszon, A.; Yanagimachi, R.; Visconti, P.E. Ca2+ ionophore A23187 can make mouse spermatozoa capable of fertilizing in vitro without activation of cAMP-dependent phosphorylation pathways. Proc. Natl. Acad. Sci. USA 2013, 110, 18543–18548. [Google Scholar] [CrossRef] [PubMed]
- Bedford, J.M. Site of the mammalian sperm physiological acrosome reaction. Proc. Natl. Acad. Sci. USA 2011, 108, 4703–4704. [Google Scholar] [CrossRef]
- Gupta, S.K. Human Zona Pellucida Glycoproteins: Binding Characteristics With Human Spermatozoa and Induction of Acrosome Reaction. Front. Cell Dev. Biol. 2021, 9, 619868. [Google Scholar] [CrossRef] [PubMed]
- Hoodbhoy, T.; Dean, J. Insights into the molecular basis of sperm-egg recognition in mammals. Reproduction 2004, 127, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Rankin, T.L.; Tong, Z.B.; Castle, P.E.; Lee, E.; Gore-Langton, R.; Nelson, L.M.; Dean, J. Human ZP3 restores fertility in Zp3 null mice without affecting order-specific sperm binding. Development 1998, 125, 2415–2424. [Google Scholar] [CrossRef] [PubMed]
- Gahlay, G.; Gauthier, L.; Baibakov, B.; Epifano, O.; Dean, J. Gamete recognition in mice depends on the cleavage status of an egg’s zona pellucida protein. Science 2010, 329, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Burkart, A.D.; Xiong, B.; Baibakov, B.; Jiménez-Movilla, M.; Dean, J. Ovastacin, a cortical granule protease, cleaves ZP2 in the zona pellucida to prevent polyspermy. J. Cell Biol. 2012, 197, 37–44. [Google Scholar] [CrossRef]
- Baibakov, B.; Gauthier, L.; Talbot, P.; Rankin, T.L.; Dean, J. Sperm binding to the zona pellucida is not sufficient to induce acrosome exocytosis. Development 2007, 134, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Fujiwara, E.; Kakiuchi, Y.; Okabe, M.; Satouh, Y.; Baba, S.A.; Chiba, K.; Hirohashi, N. Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization. Proc. Natl. Acad. Sci. USA 2011, 108, 4892–4896. [Google Scholar] [CrossRef] [PubMed]
- Romarowski, A.; Luque, G.M.; La Spina, F.A.; Krapf, D.; Buffone, M.G. Role of Actin Cytoskeleton During Mammalian Sperm Acrosomal Exocytosis. Adv. Anat. Embryol. Cell Biol. 2016, 220, 129–144. [Google Scholar]
- Hug, C.B.; Vaquerizas, J.M. The Birth of the 3D Genome during Early Embryonic Development. Trends Genet. 2018, 34, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.R.; Yin, T.L.; Zhou, L.Q. CRISPR/Cas9 technology: Applications in oocytes and early embryos. J. Transl. Med. 2023, 21, 746. [Google Scholar] [CrossRef] [PubMed]
- Jacinto, F.V.; Link, W.; Ferreira, B.I. CRISPR/Cas9-mediated genome editing: From basic research to translational medicine. J. Cell. Mol. Med. 2020, 24, 3766–3778. [Google Scholar] [CrossRef] [PubMed]
- Inoue, N.; Ikawa, M.; Okabe, M. The mechanism of sperm–egg interaction and the involvement of IZUMO1 in fusion. Asian J. Androl. 2011, 13, 81. [Google Scholar] [CrossRef] [PubMed]
- Inoue, N.; Hamada, D.; Kamikubo, H.; Hirata, K.; Kataoka, M.; Yamamoto, M.; Ikawa, M.; Okabe, M.; Hagihara, Y. Molecular dissection of IZUMO1, a sperm protein essential for sperm-egg fusion. Development 2013, 140, 3221–3229. [Google Scholar] [CrossRef]
- Inoue, N.; Ikawa, M.; Isotani, A.; Okabe, M. The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature 2005, 434, 234–238. [Google Scholar] [CrossRef]
- Inoue, N.; Kasahara, T.; Ikawa, M.; Okabe, M. Identification and disruption of sperm-specific angiotensin converting enzyme-3 (ACE3) in mouse. PLoS ONE 2010, 5, e10301. [Google Scholar] [CrossRef] [PubMed]
- Yamatoya, K.; Yoshida, K.; Ito, C.; Maekawa, M.; Yanagida, M.; Takamori, K.; Ogawa, H.; Araki, Y.; Miyado, K.; Toyama, Y.; et al. Equatorin: Identification and characterization of the epitope of the MN9 antibody in the mouse. Biol. Reprod. 2009, 81, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Klinovska, K.; Sebkova, N.; Dvorakova-Hortova, K. Sperm-egg fusion: A molecular enigma of mammalian reproduction. Int. J. Mol. Sci. 2014, 15, 10652–10668. [Google Scholar] [CrossRef] [PubMed]
- Stipp, C.S.; Orlicky, D.; Hemler, M.E. FPRP, a major, highly stoichiometric, highly specific CD81-and CD9-associated protein. J. Biol. Chem. 2001, 276, 4853–4862. [Google Scholar] [CrossRef]
- Boucheix, C.; Rubinstein, E. Tetraspanins. Cell. Mol. Life Sci. CMLS 2001, 58, 1189–1205. [Google Scholar] [CrossRef] [PubMed]
- Charrin, S.; Le Naour, F.; Silvie, O.; Milhiet, P.-E.; Boucheix, C.; Rubinstein, E. Lateral organization of membrane proteins: Tetraspanins spin their web. Biochem. J. 2009, 420, 133–154. [Google Scholar] [CrossRef] [PubMed]
- Hemler, M.E. Tetraspanin proteins mediate cellular penetration, invasion, and fusion events and define a novel type of membrane microdomain. Annu. Rev. Cell Dev. Biol. 2003, 19, 397–422. [Google Scholar] [CrossRef] [PubMed]
- Bassani, S.; Cingolani, L.A. Tetraspanins: Interactions and interplay with integrins. Int. J. Biochem. Cell 2012, 44, 703–708. [Google Scholar] [CrossRef]
- Frolikova, M.; Sebkova, N.; Ded, L.; Dvorakova-Hortova, K. Characterization of CD46 and β1 integrin dynamics during sperm acrosome reaction. Sci. Rep. 2016, 6, 33714. [Google Scholar] [CrossRef]
- Ito, C.; Yamatoya, K.; Yoshida, K.; Maekawa, M.; Miyado, K.; Toshimori, K. Tetraspanin family protein CD9 in the mouse sperm: Unique localization, appearance, behavior and fate during fertilization. Cell Tissue Res. 2010, 340, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Frolikova, M.; Manaskova-Postlerova, P.; Cerny, J.; Jankovicova, J.; Simonik, O.; Pohlova, A.; Secova, P.; Antalikova, J.; Dvorakova-Hortova, K. CD9 and CD81 interactions and their structural modelling in sperm prior to fertilization. Int. J. Mol. Sci. 2018, 19, 1236. [Google Scholar] [CrossRef] [PubMed]
- Anifandis, G.; Messini, C.; Dafopoulos, K.; Sotiriou, S.; Messinis, I. Molecular and cellular mechanisms of sperm-oocyte interactions opinions relative to in vitro fertilization (IVF). Int. J. Mol. Sci. 2014, 15, 12972–12997. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, E.; Ziyyat, A.; Prenant, M.; Wrobel, E.; Wolf, J.P.; Levy, S.; Le Naour, F.; Boucheix, C. Reduced fertility of female mice lacking CD81. Dev. Biol. 2006, 290, 351–358. [Google Scholar] [CrossRef]
- Berditchevski, F.; Odintsova, E.; Sawada, S.; Gilbert, E. Expression of the palmitoylation-deficient CD151 weakens the association of α3β1 integrin with the tetraspanin-enriched microdomains and affects integrin-dependent signaling. J. Biol. Chem. 2002, 277, 36991–37000. [Google Scholar] [CrossRef] [PubMed]
- Le Naour, F.; Rubinstein, E.; Jasmin, C.; Prenant, M.; Boucheix, C. Severely reduced female fertility in CD9-deficient mice. Science 2000, 287, 319–321. [Google Scholar] [CrossRef]
- Miller, B.J.; Georges-Labouesse, E.; Primakoff, P.; Myles, D.G. Normal fertilization occurs with eggs lacking the integrin alpha6beta1 and is CD9-dependent. J. Cell Biol. 2000, 149, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Jégou, A.; Ziyyat, A.; Barraud-Lange, V.; Perez, E.; Wolf, J.P.; Pincet, F.; Gourier, C. CD9 tetraspanin generates fusion competent sites on the egg membrane for mammalian fertilization. Proc. Natl. Acad. Sci. USA 2011, 108, 10946–10951. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.P. Sperm-egg interaction. Annu. Rev. Physiol. 2012, 74, 477–502. [Google Scholar] [CrossRef]
- Ravaux, B.; Favier, S.; Perez, E.; Gourier, C. Egg CD9 protein tides correlated with sperm oscillations tune the gamete fusion ability in mammal. J. Mol. Cell Biol. 2018, 10, 494–502. [Google Scholar] [CrossRef]
- Okabe, M. The cell biology of mammalian fertilization. Development 2013, 140, 4471–4479. [Google Scholar] [CrossRef] [PubMed]
- Wassarman, P.M. Reproductive biology: Sperm protein finds its mate. Nature 2014, 508, 466–467. [Google Scholar] [CrossRef]
- Kaji, K.; Oda, S.; Shikano, T.; Ohnuki, T.; Uematsu, Y.; Sakagami, J.; Tada, N.; Miyazaki, S.; Kudo, A. The gamete fusion process is defective in eggs of Cd9-deficient mice. Nat. Genet. 2000, 24, 279–282. [Google Scholar] [CrossRef]
- Ito, C.; Yamatoya, K.; Yoshida, K.; Fujimura, L.; Hatano, M.; Miyado, K.; Toshimori, K. Integration of the mouse sperm fertilization-related protein equatorin into the acrosome during spermatogenesis as revealed by super-resolution and immunoelectron microscopy. Cell Tissue Res. 2013, 352, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Ito, C.; Yamatoya, K.; Maekawa, M.; Toyama, Y.; Suzuki-Toyota, F.; Toshimori, K. A model of the acrosome reaction progression via the acrosomal membrane-anchored protein equatorin. Reproduction 2009, 139, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, K.; Saxena, D.; Oh-Oka, T.; Tanii, I.; Toshimori, K. Inhibition of mouse fertilization in vivo by intra-oviductal injection of an anti-equatorin monoclonal antibody. Reproduction 2001, 122, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Chen, M.; Ji, S.; Wang, X.; Wang, Y.; Huang, X.; Yang, L.; Wang, Y.; Cui, X.; Lv, L.; et al. Equatorin is not essential for acrosome biogenesis but is required for the acrosome reaction. Biochem. Biophys. Res. Commun. 2014, 444, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Ito, C.; Yamatoya, K.; Yoshida, K.; Fujimura, L.; Sugiyama, H.; Suganami, A.; Tamura, Y.; Hatano, M.; Miyado, K.; Toshimori, K. Deletion of Eqtn in mice reduces male fertility and sperm–egg adhesion. Reproduction 2018, 156, 579–590. [Google Scholar] [CrossRef]
- Fujihara, Y.; Lu, Y.; Noda, T.; Oji, A.; Larasati, T.; Kojima-Kita, K.; Yu, Z.; Matzuk, R.M.; Matzuk, M.M.; Ikawa, M. Spermatozoa lacking Fertilization Influencing Membrane Protein (FIMP) fail to fuse with oocytes in mice. Proc. Natl. Acad. Sci. USA 2020, 117, 9393–9400. [Google Scholar] [CrossRef] [PubMed]
- Pausch, H.; Kölle, S.; Wurmser, C.; Schwarzenbacher, H.; Emmerling, R.; Jansen, S.; Trottmann, M.; Fuerst, C.; Götz, K.-U.; Fries, R. A nonsense mutation in TMEM95 encoding a nondescript transmembrane protein causes idiopathic male subfertility in cattle. PLoS Genet. 2014, 10, e1004044. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Fuertes, B.; Laguna-Barraza, R.; Fernandez-Gonzalez, R.; Gutierrez-Adan, A.; Blanco-Fernandez, A.; O’Doherty, A.M.; Di Fenza, M.; Kelly, A.K.; Kölle, S.; Lonergan, P. Subfertility in bulls carrying a nonsense mutation in transmembrane protein 95 is due to failure to interact with the oocyte vestments. Biol. Reprod. 2017, 97, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Lorenzetti, D.; Poirier, C.; Zhao, M.; Overbeek, P.A.; Harrison, W.; Bishop, C.E. A transgenic insertion on mouse chromosome 17 inactivates a novel immunoglobulin superfamily gene potentially involved in sperm–egg fusion. Mamm. Genome 2014, 25, 141–148. [Google Scholar] [CrossRef]
- Zheng, H.; Mandal, A.; Shumilin, I.A.; Chordia, M.D.; Panneerdoss, S.; Herr, J.C.; Minor, W. Sperm Lysozyme-Like Protein 1 (SLLP1), an intra-acrosomal oolemmal-binding sperm protein, reveals filamentous organization in protein crystal form. Andrology 2015, 3, 756–771. [Google Scholar] [CrossRef]
- Seals, D.F.; Courtneidge, S.A. The ADAMs family of metalloproteases: Multidomain proteins with multiple functions. Genes Dev. 2003, 17, 7–30. [Google Scholar] [CrossRef]
- Blobel, C.P.; Wolfsberg, T.G.; Turck, C.W.; Myles, D.G.; Primakoff, P.; White, J.M. A potential fusion peptide and an integrin ligand domain in a protein active in sperm-egg fusion. Nature 1992, 356, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.; Bunch, D.O.; Faure, J.E.; Goulding, E.H.; Eddy, E.M.; Primakoff, P.; Myles, D.G. Fertilization defects in sperm from mice lacking fertilin beta. Science 1998, 281, 1857–1859. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Yamashita, M.; Nakanishi, T.; Park, K.E.; Kimura, M.; Kashiwabara, S.; Baba, T. Mouse sperm lacking ADAM1b/ADAM2 fertilin can fuse with the egg plasma membrane and effect fertilization. J. Biol. Chem. 2006, 281, 5634–5639. [Google Scholar] [CrossRef]
- Fujihara, Y.; Miyata, H.; Ikawa, M. Factors controlling sperm migration through the oviduct revealed by gene-modified mouse models. Exp. Anim. 2018, 67, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Griffith, J.W.; Sokol, C.L.; Luster, A.D. Chemokines and chemokine receptors: Positioning cells for host defense and immunity. Annu. Rev. Immunol. 2014, 32, 659–702. [Google Scholar] [CrossRef]
- Han, W.; Ding, P.; Xu, M.; Wang, L.; Rui, M.; Shi, S.; Liu, Y.; Zheng, Y.; Chen, Y.; Yang, T.; et al. Identification of eight genes encoding chemokine-like factor superfamily members 1–8 (CKLFSF1–8) by in silico cloning and experimental validation. Genomics 2003, 81, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, C.; Zheng, Y.; Qiu, X.; Wang, L.; Fan, H.; Han, W.; Lv, B.; Wang, Y.; Zhu, X.; et al. Molecular cloning and characterization of chemokine-like factor super family member 1 (CKLFSF1), a novel human gene with at least 23 alternative splicing isoforms in testis tissue. Int. J. Biochem. Cell Biol. 2004, 36, 1492–1501. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Rui, M.; Han, W.; Wang, Y.; Qiu, X.; Ding, P.; Han, W.; Lv, B.; Wang, Y.; Zhu, X.; et al. CKLFSF2 is highly expressed in testis and can be secreted into the seminiferous tubules. Int. J. Biochem. Cell Biol. 2005, 37, 1633–1640. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Xin, Z.C.; Chen, L.; Tian, L.; Yuan, Y.M.; Song, W.D.; Jiang, X.J.; Guo, Y.L.; Giwercman, A. Expression and localization of CKLFSF2 in human spermatogenesis. Asian J. Androl. 2007, 9, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, Y.; Oji, A.; Kojima-Kita, K.; Larasati, T.; Ikawa, M. Co-expression of sperm membrane proteins CMTM2A and CMTM2B is essential for ADAM3 localization and male fertility in mice. J. Cell Sci. 2018, 131. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.; Kashir, J.; Thanassoulas, A.; Safieh-Garabedian, B.; Lai, F.A.; Nomikos, M. Essential role of sperm-specific PLC-zeta in egg activation and male factor infertility: An update. Front. Cell Dev. Biol. 2020, 8, 28. [Google Scholar] [CrossRef]
- Wu, A.T.; Sutovsky, P.; Manandhar, G.; Xu, W.; Katayama, M.; Day, B.N.; Park, K.-W.; Yi, Y.-J.; Xi, Y.W.; Prather, R.S.; et al. PAWP, a sperm-specific WW domain-binding protein, promotes meiotic resumption and pronuclear development during fertilization. J. Biol. Chem. 2007, 282, 12164–12175. [Google Scholar] [CrossRef]
- Kouchi, Z.; Fukami, K.; Shikano, T.; Oda, S.; Nakamura, Y.; Takenawa, T.; Miyazaki, S. Recombinant phospholipase Cζ has high Ca2+ sensitivity and induces Ca2+ oscillations in mouse eggs. J. Biol. Chem. 2004, 279, 10408–10412. [Google Scholar] [CrossRef]
- Putney, J.W.; Tomita, T. Phospholipase C signaling and calcium influx. Adv. Biol. Regul. 2012, 52, 152. [Google Scholar] [CrossRef]
- Hachem, A.; Godwin, J.; Ruas, M.; Lee, H.C.; Ferrer Buitrago, M.; Ardestani, G.; Bassett, A.; Fox, S.; Navarrete, F.; de Sutter, P.; et al. PLCζ is the physiological trigger of the Ca(2+) oscillations that induce embryogenesis in mammals but conception can occur in its absence. Development 2017, 144, 2914–2924. [Google Scholar] [CrossRef] [PubMed]
- Nozawa, K.; Satouh, Y.; Fujimoto, T.; Oji, A.; Ikawa, M. Sperm-borne phospholipase C zeta-1 ensures monospermic fertilization in mice. Sci. Rep. 2018, 8, 1315. [Google Scholar] [CrossRef] [PubMed]
- Noda, T.; Blaha, A.; Fujihara, Y.; Gert, K.R.; Emori, C.; Deneke, V.E.; Oura, S.; Panser, K.; Lu, Y.; Berent, S.; et al. Sperm membrane proteins DCST1 and DCST2 are required for sperm-egg interaction in mice and fish. Commun. Biol. 2022, 5, 332. [Google Scholar] [CrossRef] [PubMed]
- Inoue, N.; Hagihara, Y.; Wada, I. Evolutionarily conserved sperm factors, DCST1 and DCST2, are required for gamete fusion. eLife 2021, 10, e66313. [Google Scholar] [CrossRef] [PubMed]
- Walters, J.L.H.; Gadella, B.M.; Sutherland, J.M.; Nixon, B.; Bromfield, E.G. Male Infertility: Shining a Light on Lipids and Lipid-Modulating Enzymes in the Male Germline. J. Clin. Med. 2020, 9, 327. [Google Scholar] [CrossRef] [PubMed]
- Azimi, F.C.; Dean, T.T.; Minari, K.; Basso, L.G.M.; Vance, T.D.R.; Serrão, V.H.B. A Frame-by-Frame Glance at Membrane Fusion Mechanisms: From Viral Infections to Fertilization. Biomolecules 2023, 13, 1130. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.J.; Bianchi, E. The challenges involved in elucidating the molecular basis of sperm-egg recognition in mammals and approaches to overcome them. Cell Tissue Res. 2016, 363, 227–235. [Google Scholar] [CrossRef]
- Harper, J.C.; Geraedts, J.; Borry, P.; Cornel, M.C.; Dondorp, W.; Gianaroli, L.; Harton, G.; Milachich, T.; Kääriäinen, H.; Liebaers, I.; et al. Current issues in medically assisted reproduction and genetics in Europe: Research, clinical practice, ethics, legal issues and policy. Eur. J. Hum. Genet. 2013, 21 (Suppl. S2), S1–S21. [Google Scholar] [CrossRef]
- Cescon, M.; Chianese, R.; Tavares, R.S. Environmental Impact on Male (In)Fertility via Epigenetic Route. J. Clin. Med. 2020, 9, 2520. [Google Scholar] [CrossRef] [PubMed]
- Umair, M.; Waqas, A. Undiagnosed Rare Genetic Disorders: Importance of Functional Characterization of Variants. Genes 2023, 14, 1469. [Google Scholar] [CrossRef]
- Tavakoli, K.; Pour-Aboughadareh, A.; Kianersi, F.; Poczai, P.; Etminan, A.; Shooshtari, L. Applications of CRISPR-Cas9 as an Advanced Genome Editing System in Life Sciences. BioTech 2021, 10, 14. [Google Scholar] [CrossRef]
- Nishizono, H.; Yasuda, R.; Laviv, T. Methodologies and Challenges for CRISPR/Cas9 Mediated Genome Editing of the Mammalian Brain. Front. Genome Ed. 2020, 2, 602970. [Google Scholar] [CrossRef] [PubMed]
- Umair, M. Rare genetic disorders: Beyond whole-exome sequencing. J. Gene Med. 2023, 25, e3503. [Google Scholar] [CrossRef] [PubMed]
- Alyafee, Y.; Al Tuwaijri, A.; Umair, M.; Alharbi, M.; Haddad, S.; Ballow, M.; Alayyar, L.; Alam, Q.; Althenayyan, S.; Al Ghilan, N.; et al. Non-invasive prenatal testing for autosomal recessive disorders: A new promising approach. Front. Genet. 2022, 13, 1047474. [Google Scholar] [CrossRef] [PubMed]
- Neri, Q.V.; Lee, B.; Rosenwaks, Z.; Machaca, K.; Palermo, G.D. Understanding fertilization through intracytoplasmic sperm injection (ICSI). Cell Calcium 2014, 55, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Nixon, B.; Aitken, R.J.; McLaughlin, E.A. New insights into the molecular mechanisms of sperm-egg interaction. Cell. Mol. Life Sci. 2007, 64, 1805–1823. [Google Scholar] [CrossRef] [PubMed]
| Protein | Key Findings in Knockout Models | Fertility Outcome |
|---|---|---|
| IZUMO1 | Zumo1-deficient male mice produced normal sperm and formed vaginal plugs, but sperm failed to fuse with eggs [27]. | Males were sterile; direct injection of sperm into the egg cytoplasm restored fertility. |
| ACE3 | Interacts with IZUMO1 in sperm but disappears post-acrosome reaction. Deficiency did not impair fertilization [28]. | Ace3 deletion had no effect on fertility in vivo or in vitro. |
| JUNO | Juno-deficient female mice produced eggs that failed to fuse with wild-type sperm [30]. | Females were infertile; JUNO is critical for binding and polyspermy prevention. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, R.; Azhar, M.; Umair, M. Decoding the Genes Orchestrating Egg and Sperm Fusion Reactions and Their Roles in Fertility. Biomedicines 2024, 12, 2850. https://doi.org/10.3390/biomedicines12122850
Khan R, Azhar M, Umair M. Decoding the Genes Orchestrating Egg and Sperm Fusion Reactions and Their Roles in Fertility. Biomedicines. 2024; 12(12):2850. https://doi.org/10.3390/biomedicines12122850
Chicago/Turabian StyleKhan, Ranjha, Muhammad Azhar, and Muhammad Umair. 2024. "Decoding the Genes Orchestrating Egg and Sperm Fusion Reactions and Their Roles in Fertility" Biomedicines 12, no. 12: 2850. https://doi.org/10.3390/biomedicines12122850
APA StyleKhan, R., Azhar, M., & Umair, M. (2024). Decoding the Genes Orchestrating Egg and Sperm Fusion Reactions and Their Roles in Fertility. Biomedicines, 12(12), 2850. https://doi.org/10.3390/biomedicines12122850





