Combined Utility of Speckle Tracking Echocardiography and Cardiac Biomarkers for Early Detection of Anthracycline-Induced Cardiotoxicity in Pediatric Oncology Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Legal and Ethical Considerations
2.2. Inclusion and Exclusion Criteria
2.3. Measurements and Definitions
2.4. Data Collection and Management
2.5. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Baseline GLS and Biomarker Levels
3.3. Correlation and Regression Analysis
4. Discussion
4.1. Analysis of Findings
4.2. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zahnreich, S.; Schmidberger, H. Childhood Cancer: Occurrence, Treatment and Risk of Second Primary Malignancies. Cancers 2021, 13, 2607. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Williams, A.M.; Liu, Q.; Bhakta, N.; Krull, K.R.; Hudson, M.M.; Robison, L.L.; Yasui, Y. Rethinking Success in Pediatric Oncology: Beyond 5-Year Survival. J. Clin. Oncol. 2021, 39, 2227–2231, Erratum in J. Clin. Oncol. 2022, 40, 2283. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mancilla, T.R.; Iskra, B.; Aune, G.J. Doxorubicin-Induced Cardiomyopathy in Children. Compr. Physiol. 2019, 9, 905–931. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hitawala, G.; Jain, E.; Castellanos, L.; Garimella, R.; Akku, R.; Chamavaliyathil, A.K.; Irfan, H.; Jaiswal, V.; Quinonez, J.; Dakroub, M.; et al. Pediatric Chemotherapy Drugs Associated with Cardiotoxicity. Cureus 2021, 13, e19658. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Harake, D.; Franco, V.I.; Henkel, J.M.; Miller, T.L.; Lipshultz, S.E. Cardiotoxicity in childhood cancer survivors: Strategies for prevention and management. Future Cardiol. 2012, 8, 647–670. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lipshultz, S.E.; Karnik, R.; Sambatakos, P.; Franco, V.I.; Ross, S.W.; Miller, T.L. Anthracycline-related cardiotoxicity in childhood cancer survivors. Curr. Opin. Cardiol. 2014, 29, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Armenian, S.H.; Hudson, M.M.; Chen, M.H.; Colan, S.D.; Lindenfeld, L.; Mills, G.; Siyahian, A.; Gelehrter, S.; Dang, H.; Hein, W.; et al. Rationale and design of the Children’s Oncology Group (COG) study ALTE1621: A randomized, placebo-controlled trial to determine if low-dose carvedilol can prevent anthracycline-related left ventricular remodeling in childhood cancer survivors at high risk for developing heart failure. BMC Cardiovasc. Disord. 2016, 16, 187. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cardinale, D.; Colombo, A.; Bacchiani, G.; Tedeschi, I.; Meroni, C.A.; Veglia, F.; Civelli, M.; Lamantia, G.; Colombo, N.; Curigliano, G.; et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 2015, 131, 1981–1988. [Google Scholar] [CrossRef] [PubMed]
- Volkova, M.; Russell, R., III. Anthracycline Cardiotoxicity: Prevalence, Pathogenesis and Treatment. Curr. Cardiol. Rev. 2011, 7, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, D.P.S.; Silva, J.B.M.; do Carmo Rassi, D.; Freitas, A.F., Jr.; Rassi, S. Echocardiographic strategy for early detection of cardiotoxicity of doxorubicin: A prospective observational study. Cardiooncology 2022, 8, 17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stoodley, P.W.; Richards, D.A.; Boyd, A.; Hui, R.; Harnett, P.R.; Meikle, S.R.; Clarke, J.L.; Thomas, L. Altered left ventricular longitudinal diastolic function correlates with reduced systolic function immediately after anthracycline chemotherapy. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Boyd, A.; Stoodley, P.; Richards, D.; Hui, R.; Harnett, P.; Vo, K.; Marwick, T.; Thomas, L. Anthracyclines induce early changes in left ventricular systolic and diastolic function: A single centre study. PLoS ONE 2017, 12, e0175544. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wilke, L.; Abellan Schneyder, F.E.; Roskopf, M.; Jenke, A.C.; Heusch, A.; Hensel, K.O. Speckle tracking stress echocardiography in children: Interobserver and intraobserver reproducibility and the impact of echocardiographic image quality. Sci. Rep. 2018, 8, 9185. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bansal, N.; Mercadante, A.; Rochelson, E.; Mahgerefteh, J.; Clark, B.C. Speckle Tracking Echocardiography in Pediatric Patients with Premature Ventricular Contractions. Pediatr. Cardiol. 2020, 41, 1587–1593. [Google Scholar] [CrossRef] [PubMed]
- Rolfs, N.; Huber, C.; Opgen-Rhein, B.; Altmann, I.; Anderheiden, F.; Hecht, T.; Fischer, M.; Wiegand, G.; Reineker, K.; Voges, I.; et al. Prognostic Value of Speckle Tracking Echocardiography-Derived Strain in Unmasking Risk for Arrhythmias in Children with Myocarditis. Biomedicines 2024, 12, 2369. [Google Scholar] [CrossRef]
- Tanaka, H.; Tatsumi, K.; Matsumoto, K.; Kawai, H.; Hirata, K. Emerging role of three-dimensional speckle tracking strain for accurate quantification of left ventricular dyssynchrony. Echocardiography 2013, 30, E292–E295. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, D.; Szwoch, M.; Kwiatkowska, J.; Raczak, G.; Daniłowicz-Szymanowicz, L. Global longitudinal strain can predict heart failure exacerbation in stable outpatients with ischemic left ventricular systolic dysfunction. PLoS ONE 2019, 14, e0225829. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hegde, S.; Shnoda, M.; Alkhadra, Y.; Bhattacharya, A.; Nikolaeva, M.; Maysky, M. Prevalence of abnormal left ventricular global longitudinal strain by speckle tracking echocardiography and its prognostic value in patients with COVID-19. Open Heart 2024, 11, e002397. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tissot, C.; Singh, Y.; Sekarski, N. Echocardiographic Evaluation of Ventricular Function-For the Neonatologist and Pediatric Intensivist. Front. Pediatr. 2018, 6, 79. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fernandes, J.M.G.; de Oliveira Romão, B.; Rivera, I.R.; Mendonça, M.A.; Costa, F.A.; Lira Handro, M.S.; Campos, O.; De Paola, Â.A.V.; Moisés, V.A. Clinical value of myocardial performance index in patients with isolated diastolic dysfunction. Cardiovasc. Ultrasound 2019, 17, 17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cao, Z.; Jia, Y.; Zhu, B. BNP and NT-proBNP as Diagnostic Biomarkers for Cardiac Dysfunction in Both Clinical and Forensic Medicine. Int. J. Mol. Sci. 2019, 20, 1820. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- O’Donnell, C.; Ashland, M.D.; Vasti, E.C.; Lu, Y.; Chang, A.Y.; Wang, P.; Daniels, L.B.; de Lemos, J.A.; Morrow, D.A.; Rodriguez, F.; et al. N-Terminal Pro-B-Type Natriuretic Peptide as a Biomarker for the Severity and Outcomes With COVID-19 in a Nationwide Hospitalized Cohort. J. Am. Heart Assoc. 2021, 10, e022913. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, J.R.; Chen, I.C.; Dai, Z.K.; Hung, J.F.; Hsu, J.H. Early Elevated B-Type Natriuretic Peptide Levels are Associated with Cardiac Dysfunction and Poor Clinical Outcome in Pediatric Septic Patients. Acta Cardiol. Sin. 2015, 31, 485–493. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jefferies, J.L.; Mazur, W.M.; Howell, C.R.; Plana, J.C.; Ness, K.K.; Li, Z.; Joshi, V.M.; Green, D.M.; Mulrooney, D.A.; Towbin, J.A.; et al. Cardiac remodeling after anthracycline and radiotherapy exposure in adult survivors of childhood cancer: A report from the St Jude Lifetime Cohort Study. Cancer 2021, 127, 4646–4655. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ardelean, A.M.; Olariu, I.C.; Isac, R.; Jurac, R.; Stolojanu, C.; Murariu, M.; Toma, A.O.; Braescu, L.; Mavrea, A.; Doros, G. Correlation of Speckle-Tracking Echocardiography with Traditional Biomarkers in Predicting Cardiotoxicity among Pediatric Hemato-Oncology Patients: A Comprehensive Evaluation of Anthracycline Dosages and Treatment Protocols. Children 2023, 10, 1479. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kang, Y.; Xu, X.; Cheng, L.; Li, L.; Sun, M.; Chen, H.; Pan, C.; Shu, X. Two-dimensional speckle tracking echocardiography combined with high-sensitive cardiac troponin T in early detection and prediction of cardiotoxicity during epirubicine-based chemotherapy. Eur. J. Heart Fail. 2014, 16, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Mulrooney, D.A.; Armstrong, G.T.; Huang, S.; Ness, K.K.; Ehrhardt, M.J.; Joshi, V.M.; Plana, J.C.; Soliman, E.Z.; Green, D.M.; Srivastava, D.; et al. Cardiac Outcomes in Adult Survivors of Childhood Cancer Exposed to Cardiotoxic Therapy: A Cross-sectional Study. Ann. Intern. Med. 2016, 164, 93–101. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Avilès, A.; Nambo, M.J.; Huerta-Guzmàn, J.; Neri, N.; Cleto, S. Speckle-Tracking Echocardiography to Detect Cardiac Toxicity in Children Who Received Anthracyclines During Pregnancy. Clin. Lymphoma Myeloma Leuk. 2016, 16, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Amedro, P.; Vincenti, M.; Abassi, H.; Lanot, N.; De La Villeon, G.; Guillaumont, S.; Gamon, L.; Mura, T.; Lopez-Perrin, K.; Haouy, S.; et al. Use of speckle tracking echocardiography to detect late anthracycline-induced cardiotoxicity in childhood cancer: A prospective controlled cross-sectional study. Int. J. Cardiol. 2022, 354, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Arman Bilir, Ö.; Çetin, İ.İ.; Kaçar, D.; Aker, C.B.; Özbek, N.Y.; Yaralı, N. Evaluation of early-onset cardiotoxic effects of anthracyclines used during the treatment of childhood acute lymphoblastic leukemia by speckle-tracking echocardiography. Anatol. J. Cardiol. 2022, 26, 57–62. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, V.W.; So, E.K.; Wong, W.H.; Cheung, Y.F. Myocardial Deformation Imaging by Speckle-Tracking Echocardiography for Assessment of Cardiotoxicity in Children during and after Chemotherapy: A Systematic Review and Meta-Analysis. J. Am. Soc. Echocardiogr. 2022, 35, 629–656. [Google Scholar] [CrossRef] [PubMed]
- Levy, P.T.; Machefsky, A.; Sanchez, A.A.; Patel, M.D.; Rogal, S.; Fowler, S.; Yaeger, L.; Hardi, A.; Holland, M.R.; Hamvas, A.; et al. Reference Ranges of Left Ventricular Strain Measures by Two-Dimensional Speckle-Tracking Echocardiography in Children: A Systematic Review and Meta-Analysis. J. Am. Soc. Echocardiogr. 2016, 29, 209–225.e6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Çetin, S.; Babaoğlu, K.; Başar, E.Z.; Deveci, M.; Çorapçıoğlu, F. Subclinical anthracycline-induced cardiotoxicity in long-term follow-up of asymptomatic childhood cancer survivors: Assessment by speckle tracking echocardiography. Echocardiography 2018, 35, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Hasan, D.; Ismail, Y.; Al Tibi, A.; Al-Zeidaneen, S.A.; Odeh, M.; Burghel, G.J.; Natsheh, I.; Abdelnour, A. Serum Biomarkers for Chemotherapy Cardiotoxicity Risk Detection of Breast Cancer Patients. Asian Pac. J. Cancer Prev. 2021, 22, 3355–3363. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tong, D.C.; Whitbourn, R.; MacIsaac, A.; Wilson, A.; Burns, A.; Palmer, S.; Layland, J. High-Sensitivity C-Reactive Protein Is a Predictor of Coronary Microvascular Dysfunction in Patients with Ischemic Heart Disease. Front. Cardiovasc. Med. 2018, 4, 81. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, Z.; Kang, L.; Wang, Y.; Xiang, J.; Wu, Q.; Xu, C.; Zhou, Y.; Chen, S.; Fang, H.; Liu, J.; et al. Role of IL-37 in Cardiovascular Disease Inflammation. Can. J. Cardiol. 2019, 35, 923–930, Erratum in Can. J. Cardiol. 2020, 36, 588. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.-R.; Cheng, C.-H.; Liu, J.-C.; Chen, H.-Y.; Chen, J.-J.; Cheng, T.-H. Understanding Galectin-3’s Role in Diastolic Dysfunction: A Contemporary Perspective. Life 2024, 14, 906. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. Targeting galectin-3 in myocardial infarction: A unique opportunity for biomarker-guided therapy. Cardiovasc. Res. 2023, 119, 2495–2496. [Google Scholar] [CrossRef]
- Sygitowicz, G.; Maciejak-Jastrzębska, A.; Sitkiewicz, D. The Diagnostic and Therapeutic Potential of Galectin-3 in Cardiovascular Diseases. Biomolecules 2022, 12, 46. [Google Scholar] [CrossRef] [PubMed]

| Variables | Patients (n = 99) | Controls (n = 50) | p |
|---|---|---|---|
| Age (years, mean ± SD) | 10.6 ± 4.3 | 10.8 ± 3.8 | 0.78 |
| Age range (years) | 2–17 | 3–18 | |
| Sex (Male/Female) | 57/42 | 28/22 | 0.85 |
| BMI (kg/m2, mean ± SD) | 19.2 ± 3.7 | 19.5 ± 3.6 | 0.68 |
| Underweight (<5th %) | 5 (5.1%) | 2 (4.0%) | 0.75 |
| Normal weight (5th–85th %) | 80 (80.8%) | 41 (82.0%) | 0.88 |
| Overweight (>85th %) | 14 (14.1%) | 7 (14.0%) | 0.99 |
| Treatment | |||
| Anthracycline only | 21 (21.2%) | N/A | – |
| Anthracycline + Vincristine | 33 (33.3%) | N/A | – |
| Anthracycline + Methotrexate | 14 (14.1%) | N/A | – |
| Anthracycline + Cyclophosphamide | 27 (27.3%) | N/A | – |
| Multi-agent combination | 7 (7.1%) | N/A | – |
| Variables | Patients (n = 99) | Type of Anthracycline | Dose |
|---|---|---|---|
| Acute Lymphoblastic Leukemia | 31 (31.3%) | Doxorubicin | 282 ± 71 |
| Hodgkin Lymphoma | 14 (14.1%) | Doxorubicin | 319 ± 82 |
| Osteosarcoma | 12 (12.1%) | Doxorubicin | 448 ± 52 |
| Neuroblastoma | 10 (10.1%) | Doxorubicin | 301 ± 63 |
| Ewing Sarcoma | 9 (9.1%) | Doxorubicin | 352 ± 91 |
| Rhabdomyosarcoma | 8 (8.1%) | Epirubicin | 279 ± 66 |
| Wilms Tumor | 8 (8.1%) | Epirubicin | 263 ± 54 |
| Others | 7 (7.1%) | Doxorubicin/Epirubicin | 309 ± 74 |
| Parameters | Normal Range | Patients (n = 99) | Controls (n = 50) | p |
|---|---|---|---|---|
| GLS (% mean ± SD) | −18% to −22% | −18.5 ± 2.3 | −19.0 ± 2.5 | 0.12 |
| SMOD EF (% mean ± SD) | 55% to 70% | 60.5 ± 5.2 | 61.0 ± 5.1 | 0.48 |
| MPI (mean ± SD) | 0.25 to 0.45 | 0.33 ± 0.04 | 0.33 ± 0.04 | 0.85 |
| cTnI (ng/mL mean ± SD) | <0.01 ng/mL | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.06 |
| BNP (pg/mL mean ± SD) | <100 pg/mL | 35 ± 10 | 32 ± 9 | 0.14 |
| Time Point | GLS (% Mean ± SD) | SMOD EF (% Mean ± SD) | MPI (Mean ± SD) | p (Trend) |
|---|---|---|---|---|
| Baseline | −18.5 ± 2.3 | 60.5 ± 5.2 | 0.33 ± 0.04 | |
| 3 Months | −17.5 ± 2.6 | 58.0 ± 5.6 | 0.35 ± 0.04 | |
| 6 Months | −16.8 ± 2.9 | 56.2 ± 6.0 | 0.37 ± 0.05 | |
| 12 Months | −16.0 ± 3.1 | 54.8 ± 6.5 | 0.40 ± 0.05 | <0.001 |
| Time Point | cTnI (ng/mL Mean ± SD) | BNP (pg/mL Mean ± SD) | p (Trend) |
|---|---|---|---|
| Baseline | 0.02 ± 0.01 | 35 ± 10 | |
| 3 Months | 0.04 ± 0.02 | 50 ± 15 | |
| 6 Months | 0.06 ± 0.02 | 65 ± 20 | |
| 12 Months | 0.08 ± 0.03 | 85 ± 25 | <0.001 |
| Parameters | Cardiotoxicity (n = 28) | No Cardiotoxicity (n = 71) | p |
|---|---|---|---|
| GLS (% mean ± SD) | −14.0 ± 2.5 | −17.0 ± 2.7 | <0.001 |
| SMOD EF (% mean ± SD) | 50.5 ± 4.5 | 56.8 ± 5.2 | <0.001 |
| MPI (mean ± SD) | 0.43 ± 0.04 | 0.38 ± 0.04 | <0.001 |
| cTnI (ng/mL mean ± SD) | 0.12 ± 0.03 | 0.06 ± 0.02 | <0.001 |
| BNP (pg/mL mean ± SD) | 110 ± 20 | 70 ± 15 | <0.001 |
| Cumulative Anthracycline Dose (mg/m2) | Number of Patients (n = 99) | Number with Cardiotoxicity (n = 28) | Percentage with Cardiotoxicity (%) | Percentage with Clinical Heart Failure (%) |
|---|---|---|---|---|
| <300 | 36 | 5 | 13.90% | 2.78% |
| 300–400 | 30 | 8 | 26.70% | 8.57% |
| 401–500 | 16 | 6 | 37.50% | 14.29% |
| >500 | 17 | 9 | 52.90% | 17.86% |
| Total | 99 | 28 | 28.30% | 46.43% |
| Parameters | cTnI Correlation (r) | BNP Correlation (r) | p |
|---|---|---|---|
| GLS | 0.65 | 0.58 | <0.001 |
| SMOD EF | −0.60 | −0.55 | <0.001 |
| MPI | 0.62 | 0.57 | <0.001 |
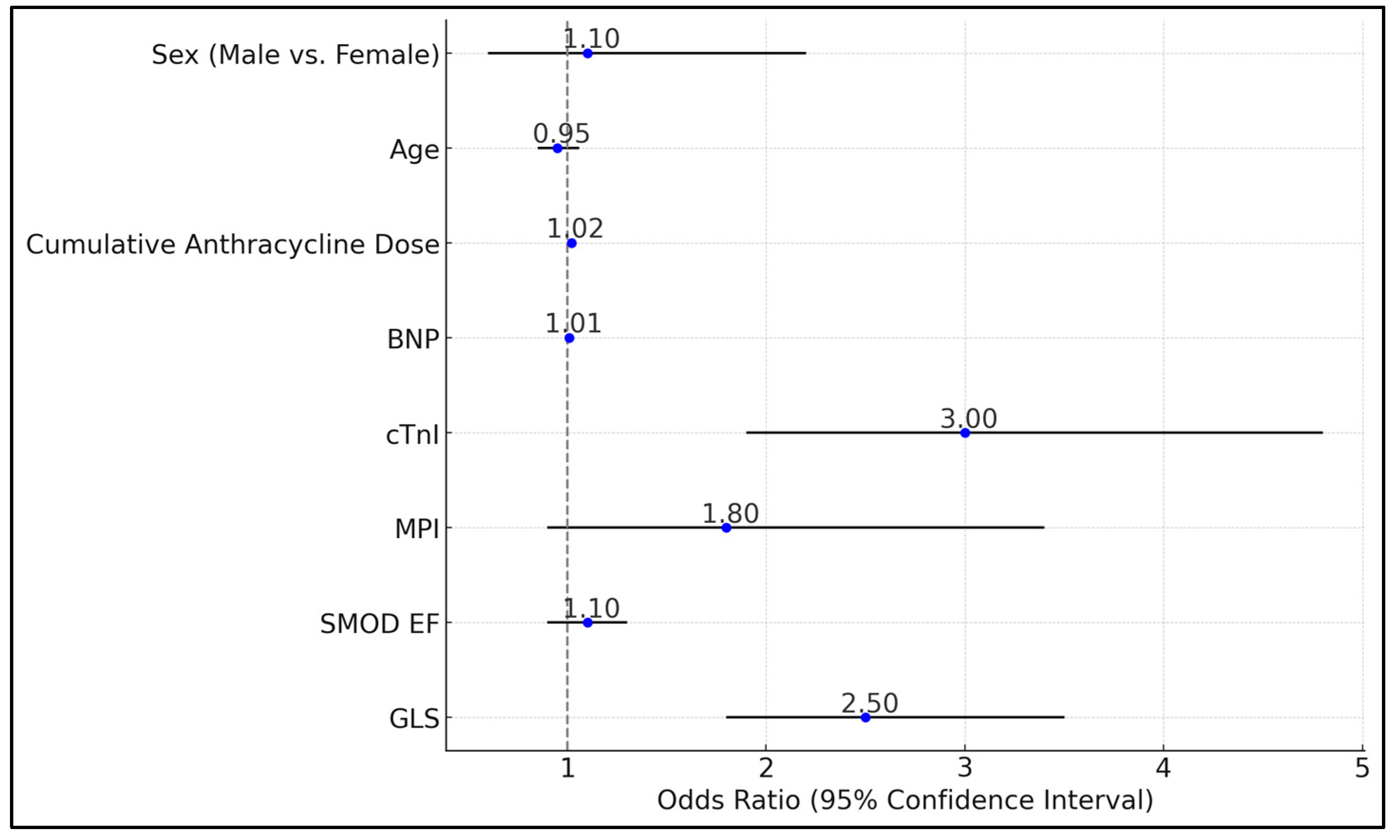
| Variables | Odds Ratio | 95% CI | p |
|---|---|---|---|
| GLS | 2.5 | 1.8–3.5 | <0.001 |
| SMOD EF | 1.1 | 0.9–1.3 | 0.2 |
| MPI | 1.8 | 0.9–3.4 | 0.08 |
| cTnI | 3 | 1.9–4.8 | <0.001 |
| BNP | 1.01 | 0.99–1.02 | 0.12 |
| Cumulative Anthracycline Dose (mg/m2) | 1.02 | 1.01–1.03 | 0.002 |
| Age | 0.95 | 0.85–1.06 | 0.35 |
| Sex (Male vs. Female) | 1.1 | 0.6–2.2 | 0.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stolojanu, C.; Steflea, R.; Micsescu-Olah, A.M.; Alexandra, I.; Popoiu, A.; Doros, G. Combined Utility of Speckle Tracking Echocardiography and Cardiac Biomarkers for Early Detection of Anthracycline-Induced Cardiotoxicity in Pediatric Oncology Patients. Biomedicines 2024, 12, 2849. https://doi.org/10.3390/biomedicines12122849
Stolojanu C, Steflea R, Micsescu-Olah AM, Alexandra I, Popoiu A, Doros G. Combined Utility of Speckle Tracking Echocardiography and Cardiac Biomarkers for Early Detection of Anthracycline-Induced Cardiotoxicity in Pediatric Oncology Patients. Biomedicines. 2024; 12(12):2849. https://doi.org/10.3390/biomedicines12122849
Chicago/Turabian StyleStolojanu, Cristiana, Ruxandra Steflea, Andrada Mara Micsescu-Olah, Ioana Alexandra, Anca Popoiu, and Gabriela Doros. 2024. "Combined Utility of Speckle Tracking Echocardiography and Cardiac Biomarkers for Early Detection of Anthracycline-Induced Cardiotoxicity in Pediatric Oncology Patients" Biomedicines 12, no. 12: 2849. https://doi.org/10.3390/biomedicines12122849
APA StyleStolojanu, C., Steflea, R., Micsescu-Olah, A. M., Alexandra, I., Popoiu, A., & Doros, G. (2024). Combined Utility of Speckle Tracking Echocardiography and Cardiac Biomarkers for Early Detection of Anthracycline-Induced Cardiotoxicity in Pediatric Oncology Patients. Biomedicines, 12(12), 2849. https://doi.org/10.3390/biomedicines12122849






