Hydroxyproline in Urine Microvesicles as a Biomarker of Fibrosis in the Renal Transplant Patient
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Design
2.2. Processing of Urine Samples
2.3. Analytical Procedures
3. Results
3.1. Demographic Study
3.2. Comparison of Transplant Patients with Control Group
3.3. Transplanted Patients Study
3.3.1. Comparison of Markers in Relation to Clinical Characteristics
3.3.2. Comparison of Urinary Markers Relative to Histological Characteristics
3.3.3. Correlation Study of Urinary Markers, Serum Creatinine, and Parameters of Bone Mineral Metabolism
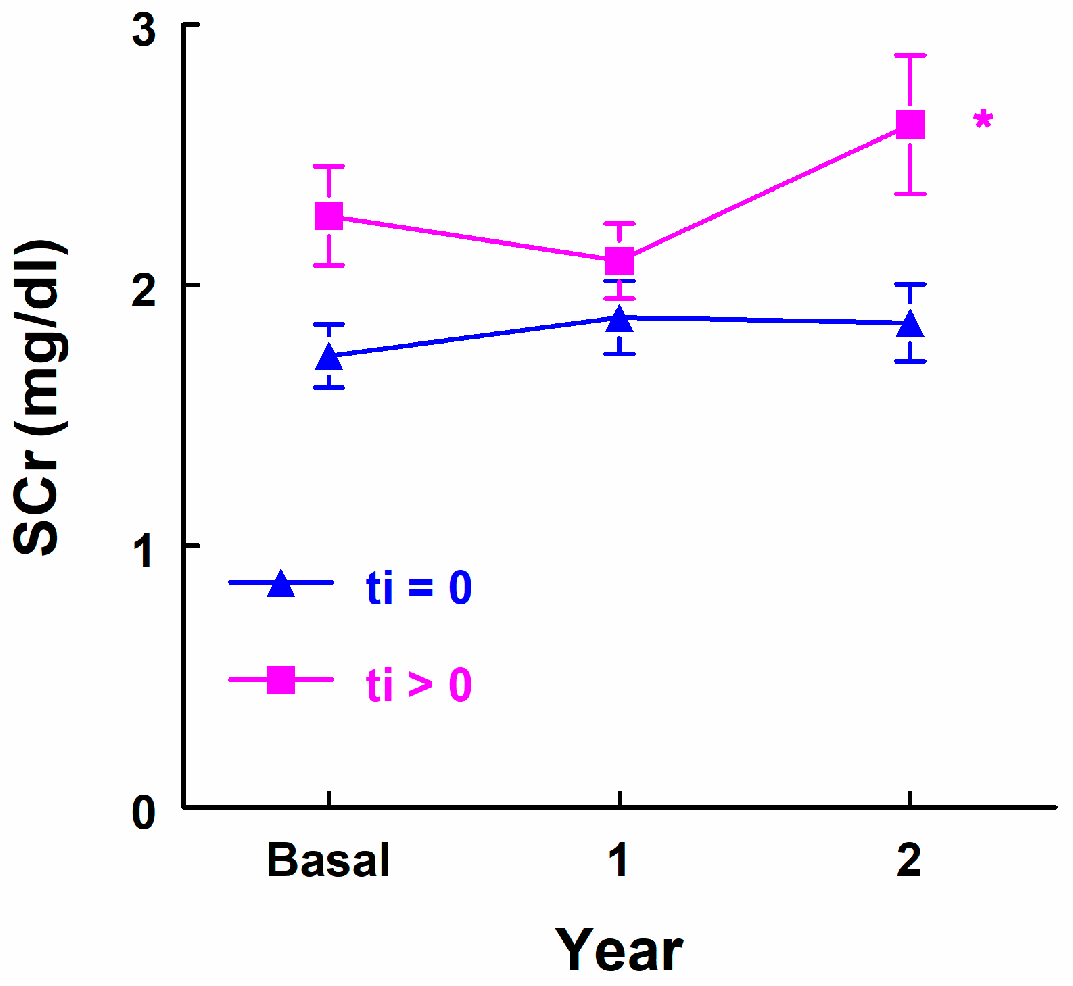
3.3.4. Renal Function Evolution Study Following Urinary Marker Determination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lamb, K.E.; Lodhi, S.; Meier-Kriesche, H.-U. Long-term renal allograft survival in the United States: A critical reappraisal. Am. J. Transplant. 2011, 11, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Cieślik, A.; Burban, A.; Gniewkiewicz, M.; Gozdowska, J.; Dęborska-Materkowska, D.; Perkowska-Ptasinska, A.; Kosieradzki, M.; Durlik, M. The Importance of 1-Year Protocol Biopsy in the Long-Term Prognosis of Kidney Transplants—5-Years Follow-Up. Transplant. Proc. 2023, 55, 2053–2057. [Google Scholar] [CrossRef]
- Miura, Y.; Satoh, S.; Saito, M.; Numakura, K.; Inoue, T.; Obara, T.; Tsuruta, H.; Narita, S.; Horikawa, Y.; Tsuchiya, N.; et al. Factors increasing quantitative interstitial fibrosis from 0 hr to 1 year in living kidney transplant patients receiving tacrolimus. Transplantation 2011, 91, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Berchtold, L.; Ponte, B.; Moll, S.; Hadaya, K.; Seyde, O.; Bachtler, M.; Vallée, J.-P.; Martin, P.-Y.; Pasch, A.; de Seigneux, S. Phosphocalcic Markers and Calcification Propensity for Assessment of Interstitial Fibrosis and Vascular Lesions in Kidney Allograft Recipients. PLoS ONE 2016, 11, e0167929. [Google Scholar] [CrossRef] [PubMed]
- Bohle, A.; Mackensen-Haen, S.; Gise, H.V. Significance of tubulointerstitial changes in the renal cortex for the excretory function and concentration ability of the kidney: A morphometric contribution. Am. J. Nephrol. 1987, 7, 421–433. [Google Scholar] [CrossRef]
- Roufosse, C.; Simmonds, N.; Groningen, M.C.-V.; Haas, M.; Henriksen, K.J.; Horsfield, C.; Loupy, A.; Mengel, M.; Perkowska-Ptasińska, A.; Rabant, M.; et al. A 2018 Reference Guide to the Banff Classification of Renal Allograft Pathology. Transplantation 2018, 102, 1795–1814, Erratum in Transplantation 2018, 102, e497; Erratum in Transplantation 2022, 106, e528. [Google Scholar] [CrossRef]
- Heilman, R.L.; Smith, M.L.; Kurian, S.M.; Huskey, J.; Batra, R.K.; Chakkera, H.A.; Katariya, N.N.; Khamash, H.; Moss, A.; Salomon, D.R.; et al. Transplanting Kidneys from Deceased Donors with Severe Acute Kidney Injury. Am. J. Transplant. 2015, 15, 2143–2151. [Google Scholar] [CrossRef]
- Loupy, A.; Vernerey, D.; Tinel, C.; Aubert, O.; Duong van Huyen, J.P.; Rabant, M.; Verine, J.; Nochy, D.; Empana, J.P.; Martinez, F.; et al. Subclinical Rejection Phenotypes at 1 Year Post-Transplant and Outcome of Kidney Allo-grafts. J. Am. Soc. Nephrol. 2015, 26, 1721–1731. [Google Scholar] [CrossRef]
- Nankivell, B.J.; Borrows, R.J.; Fung, C.L.-S.; O’Connell, P.J.; Allen, R.D.; Chapman, J.R. The natural history of chronic allograft nephropathy. N. Engl. J. Med. 2003, 349, 2326–2333. [Google Scholar] [CrossRef]
- Naesens, M.; Kuypers, D.R.; De Vusser, K.; Evenepoel, P.; Claes, K.; Bammens, B.; Meijers, B.; Sprangers, B.; Pirenne, J.; Monbaliu, D.; et al. The histology of kidney transplant failure: A long-term follow-up study. Transplantation 2014, 98, 427–435. [Google Scholar] [CrossRef]
- Mannon, R.B.; Matas, A.J.; Grande, J.; LeDuc, R.; Connett, J.; Kasiske, B.; Cecka, J.M.; Gaston, R.S.; Cosio, F.; Gourishankar, S.; et al. Inflammation in areas of tubular atrophy in kidney allograft biopsies: A potent predictor of allograft failure. Am. J. Transplant. 2010, 10, 2066–2073. [Google Scholar] [CrossRef]
- Park, W.D.; Griffin, M.D.; Cornell, L.D.; Cosio, F.G.; Stegall, M.D. Fibrosis with inflammation at one year predicts transplant functional decline. J. Am. Soc. Nephrol. 2010, 21, 1987–1997. [Google Scholar] [CrossRef] [PubMed]
- Serón, D.; Moreso, F.; Ramón, J.M.; Hueso, M.; Condom, E.; Fulladosa, X.; Bover, J.; Gil-Vernet, S.; Castelao, A.M.; Alsina, J.; et al. Protocol renal allograft biopsies and the design of clinical trials aimed to prevent or treat chronic allograft nephropathy1. Transplantation 2000, 69, 1849–1855. [Google Scholar] [CrossRef] [PubMed]
- Colvin, R.B.; Chang, A. Diagnostic Pathology: Kidney Diseases; Amirsys: Salt Lake City, UT, USA, 2011. [Google Scholar]
- Mehta, R.; Sood, P.; Hariharan, S. Subclinical Rejection in Renal Transplantation: Reappraised. Transplantation 2016, 100, 1610–1618. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Farkash, E. Protocol Biopsies: Utility and Limitations. Adv. Chronic Kidney Dis. 2016, 23, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Hofman, K.; Hall, B.; Cleaver, H.; Marshall, S. High-throughput quantification of hydroxyproline for determination of collagen. Anal. Biochem. 2011, 417, 289–291. [Google Scholar] [CrossRef]
- Saritas, T.; Kramann, R. Kidney Allograft Fibrosis: Diagnostic and Therapeutic Strategies. Transplantation 2021, 105, e114–e130. [Google Scholar] [CrossRef]
- Halloran, P.F.; Famulski, K.S.; Reeve, J. Molecular assessment of disease states in kidney transplant biopsy samples. Nat. Rev. Nephrol. 2016, 12, 534–548. [Google Scholar] [CrossRef]
- Schwarz, C.; Lawitschka, A.; Böhmig, G.A.; Dauber, E.M.; Greinix, H.; Kozakowski, N.; Mühlbacher, F.; Berlakovich, G.A.; Wekerle, T. Kidney Transplantation With Corticosteroids Alone After Haploidentical HSCT From The Same Donor. Transplantation 2016, 100, 2219–2221. [Google Scholar] [CrossRef]
- Banff Foundation for Allograft Pathology. Reference Guide to the Banff Classification. Banff Classification for Renal Allograft Pathology. 2022. Available online: https://banfffoundation.org/central-repository-for-banff-2019-resources-3/ (accessed on 7 November 2024).
- Poggio, E.D. Imaging as a Noninvasive Tool for Evaluating Interstitial Fibrosis in Kidney Allografts. Clin. J. Am. Soc. Nephrol. 2019, 14, 1286–1287. [Google Scholar] [CrossRef]
- Belostotsky, R.; Frishberg, Y. Catabolism of Hydroxyproline in Vertebrates: Physiology, Evolution, Genetic Diseases and New siRNA Approach for Treatment. Int. J. Mol. Sci. 2022, 23, 1005. [Google Scholar] [CrossRef] [PubMed]
- Schnackenberg, L.K.; Sun, J.; Pence, L.M.; Bhattacharyya, S.; da Costa, G.G.; Beger, R.D. Metabolomics evaluation of hydroxyproline as a potential marker of melamine and cyanuric acid nephrotoxicity in male and female Fischer F344 rats. Food Chem. Toxicol. 2012, 50, 3978–3983. [Google Scholar] [CrossRef] [PubMed]
- Montoro-Molina, S.; López-Carmona, A.; Quesada, A.; O’valle, F.; Martín-Morales, N.; Osuna, A.; Vargas, F.; Wangensteen, R. Klotho and Aminopeptidases as Early Biomarkers of Renal Injury in Zucker Obese Rats. Front. Physiol. 2018, 9, 1599. [Google Scholar] [CrossRef] [PubMed]
- Can, N.; Alagöz, A. The Relationship Among Bone Mineral Density, Bone Biomarkers and Vitamin D Levels in Patients with Parkinson’s Disease. Clin. Lab. 2020, 66, 1439–1444. [Google Scholar] [CrossRef]
- Uguru, C.C.; Onwuka, C.I.; Obiechina, A.E. Evaluation of urinary hydroxyproline and creatinine level in patients with benign mandibular odontogenic tumor. Clin. Exp. Dent. Res. 2021, 7, 934–940. [Google Scholar] [CrossRef]
- Mavrogeorgis, E.; Mischak, H.; Latosinska, A.; Vlahou, A.; Schanstra, J.P.; Siwy, J.; Jankowski, V.; Beige, J.; Jankowski, J. Collagen-Derived Peptides in CKD: A Link to Fibrosis. Toxins 2021, 14, 10. [Google Scholar] [CrossRef]
- Haddad, R.G.; Couranz, S.; Aviolp, L.V. Nondialyzable Urinary Hydroxyproline as an Index of Bone Collagen Formation. J. Clin. Endocrinol. Metab. 1970, 30, 282–287. [Google Scholar] [CrossRef]
- Mengel, M.; Reeve, J.; Bunnag, S.; Einecke, G.; Jhangri, G.S.; Sis, B.; Famulski, K.; Guembes-Hidalgo, L.; Halloran, P.F. Scoring total inflammation is superior to the current banff inflammation score in predicting outcome and the degree of molecular disturbance in renal allografts. Am. J. Transplant. 2009, 9, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- Marx, D.; Anglicheau, D.; Caillard, S.; Moulin, B.; Kochman, A.; Mischak, H.; Latosinska, A.; Bienaimé, F.; Prié, D.; Marquet, P.; et al. Urinary collagen peptides: Source of markers for bone metabolic processes in kidney transplant recipients. Proteom. Clin. Appl. 2023, 17, e2200118. [Google Scholar] [CrossRef]
- Zhou, H.; Yuen, P.; Pisitkun, T.; Gonzales, P.; Yasuda, H.; Dear, J.; Gross, P.; Knepper, M.; Star, R. Collection, storage, preservation, and normalization of human urinary exosomes for biomarker discovery. Kidney Int. 2006, 69, 1471–1476. [Google Scholar] [CrossRef]
- Jayachandran, M.; Lugo, G.; Heiling, H.; Miller, V.M.; Rule, A.D.; Lieske, J.C. Extracellular vesicles in urine of women with but not without kidney stones manifest patterns similar to men: A case control study. Biol. Sex Differ. 2015, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- van Balkom, B.W.; Pisitkun, T.; Verhaar, M.C.; Knepper, M.A. Exosomes and the kidney: Prospects for diagnosis and therapy of renal diseases. Kidney Int. 2011, 80, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Quesada, A.; Segarra, A.B.; Montoro-Molina, S.; Gracia, M.d.C.d.; Osuna, A.; O’valle, F.; Gómez-Guzmán, M.; Vargas, F.; Wangensteen, R. Glutamyl aminopeptidase in microvesicular and exosomal fractions of urine is related with renal dysfunction in cisplatin-treated rats. PLoS ONE 2017, 12, e0175462. [Google Scholar] [CrossRef] [PubMed]


| Qualitative Demographic Data | ||||
|---|---|---|---|---|
| Receptor Sex | Male | Female | ||
| 71.74% (33) * | 28.26% (13) | |||
| Etiology | Glomerular | Unknown | Interstitial | |
| 30.43% (14) | 19.57% (9) | 17.39% (8) | ||
| Donation process | Live donor | Brain death | Cardiac death | |
| 6.52% (3) | 56.52% (26) | 34.78% (16) | ||
| Induction treatment | No | Basiliximab | Thymoglobulin | |
| 23.91% (11) | 23.91% (11) | 50% (23) | ||
| Yes | No | |||
| Diabetes mellitus preTx | 8.7% (4) | 91.3% (42) | ||
| Hypertension preTx | 86.96% (40) | 13.04% (6) | ||
| IS change | 21.74% (10) | 78.26% (36) | ||
| Diabetes mellitus post-Tx | 13.04% (6) | 86.96% (40) | ||
| ACE inhibitors/ARA II | 34.78% (16) | 65.22% (30) | ||
| Antialdosteronic therapy | 10.87% (5) | 89.13% (41) | ||
| Cell active rejection | 6.52% (3) | 93.48% (43) | ||
| AMR | 19.57% (9) | 80.43% (37) | ||
| CMV infection | 45.65% (21) | 54.35% (25) | ||
| BK infection | 15.22% (39) | 84.78% (39) | ||
| Urinary infections | 39.13% (18) | 60.87% (28) | ||
| Graft failure | 4.35% (2) | 95.65% (44) | ||
| Quantitative demographic data | ||||
| Receptor age | 45.61 ± 14.88 ** | |||
| Donor age | 47.65 ± 15.12 | |||
| Cold ischemia time | 13 ± 5.59 | |||
| Histological Feature | Score * | |||
|---|---|---|---|---|
| Interstitial Inflammation (i) | i0 | i1 | i2 | i3 |
| 63.04% (29) | 19.57% (9) | 0% | 2.17% (1) | |
| Tubulitis (t) | t0 | t1 | t2 | t3 |
| 39.13% (18) | 23.91% (11) | 8.7% (4) | 0% | |
| Arteritis (v) | v0 | v1 | v2 | v3 |
| 54.35% (1) | 2.17% (1) | 2.17% (1) | 0% | |
| Glomerulitis (g) | g0 | g1 | g2 | g3 |
| 43.48% (20) | 17.39% (8) | 10.87% (5) | 13.04% (6) | |
| Peritubular Capillaritis (ptc) | ptc0 | ptc1 | ptc2 | ptc3 |
| 58.70% (27) | 8.7% (4) | 13.04% (6) | 2.17% (1) | |
| Total Inflammation (ti) | ti0 | ti1 | ti2 | ti3 |
| 39.13% (18) | 30.43% (14) | 2.17%(1) | 0% | |
| Interstitial fibrosis and tubular atrophy (IFTA) | IFTA 0 | IFTA 1 | IFTA 2 | IFTA 3 |
| 30.43% (14) | 26.09% (12) | 36.96% (17) | 2.17% (1) | |
| C4d | C4d0 | C4d1 | C4d2 | C4d3 |
| 76.09% (35) | 13.04% (6) | 6.52% (3) | 0% | |
| Glomerular Basement Membrane Double Contours (cg) | No (cg0) | Yes (cg > 0) | ||
| 89.13% (41) | 10.87% (5) | |||
| Marker | Case (n = 45) | Control (n = 18) | p-Value |
|---|---|---|---|
| Microvesicular hydroxyproline in urine (ng/mL) | 28.024 (5.53) * | 2.51 (1.16) | p < 0.001 |
| 25.200 ± 0.99 ** | 2.61 ± 0.897 | ||
| Hydroxyproline/microvesicular protein (ng/mg) | 1136.818 (1136.82) * | 84.595 (104.14) | p < 0.001 |
| 1845.031 ± 2052 ** | 118.83 ± 150.85 | ||
| Hydroxyproline/Creatinine in urine (ng/mg) | 34.72 (36.54) * | 3.19 (3.69) | p < 0.001 |
| 45.38 ± 30.54 ** | 4.25 ± 3.09 |
| Marker | ti | Mean ± SD * | Median (IQR) ** | p-Value |
|---|---|---|---|---|
| Microvesicular hydroxyproline in urine (ng/mL) | No (18) | 22.72 ± 8.697 | 27.12 (7.04) | p = 0.034 |
| Yes (13) | 29.91 ± 2.797 | 31.298 (3.84) | ||
| Hydroxyproline/microvesicular protein (ng/mg) | No (18) | 2301.29 ± 4243.14 | 883.64 (986.13) | p = 0.522 |
| Yes (13) | 1725.06 ± 1657.08 | 1642.18 (1922.24) | ||
| Hydroxyproline/Creatinine in urine (ng/mg) | No (18) | 37.71 ± 22.49 | 30.03 (36.03) | p = 0.275 |
| Yes (13) | 46.47 ± 21.03 | 45.83 (37.02) |
| Marker | COV | PPV (%) | NPV (%) | Sens (%) | Spec (%) |
|---|---|---|---|---|---|
| Hydroxyproline (ng/mL) | 29.83 | 100% | 72.5% | 60.71% | 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres Sánchez, M.J.; Ruiz Fuentes, M.C.; Clavero García, E.; Rísquez Chica, N.; Espinoza Muñoz, K.; Espigares Huete, M.J.; Caba Molina, M.; Osuna, A.; Wangensteen, R. Hydroxyproline in Urine Microvesicles as a Biomarker of Fibrosis in the Renal Transplant Patient. Biomedicines 2024, 12, 2836. https://doi.org/10.3390/biomedicines12122836
Torres Sánchez MJ, Ruiz Fuentes MC, Clavero García E, Rísquez Chica N, Espinoza Muñoz K, Espigares Huete MJ, Caba Molina M, Osuna A, Wangensteen R. Hydroxyproline in Urine Microvesicles as a Biomarker of Fibrosis in the Renal Transplant Patient. Biomedicines. 2024; 12(12):2836. https://doi.org/10.3390/biomedicines12122836
Chicago/Turabian StyleTorres Sánchez, María José, María Carmen Ruiz Fuentes, Elena Clavero García, Noelia Rísquez Chica, Karla Espinoza Muñoz, María José Espigares Huete, Mercedes Caba Molina, Antonio Osuna, and Rosemary Wangensteen. 2024. "Hydroxyproline in Urine Microvesicles as a Biomarker of Fibrosis in the Renal Transplant Patient" Biomedicines 12, no. 12: 2836. https://doi.org/10.3390/biomedicines12122836
APA StyleTorres Sánchez, M. J., Ruiz Fuentes, M. C., Clavero García, E., Rísquez Chica, N., Espinoza Muñoz, K., Espigares Huete, M. J., Caba Molina, M., Osuna, A., & Wangensteen, R. (2024). Hydroxyproline in Urine Microvesicles as a Biomarker of Fibrosis in the Renal Transplant Patient. Biomedicines, 12(12), 2836. https://doi.org/10.3390/biomedicines12122836








