Pathophysiological, Neuropsychological, and Psychosocial Influences on Neurological and Neuropsychiatric Symptoms of Post-Acute COVID-19 Syndrome: Impacts on Recovery and Symptom Persistence
Abstract
1. Introduction
2. Methods
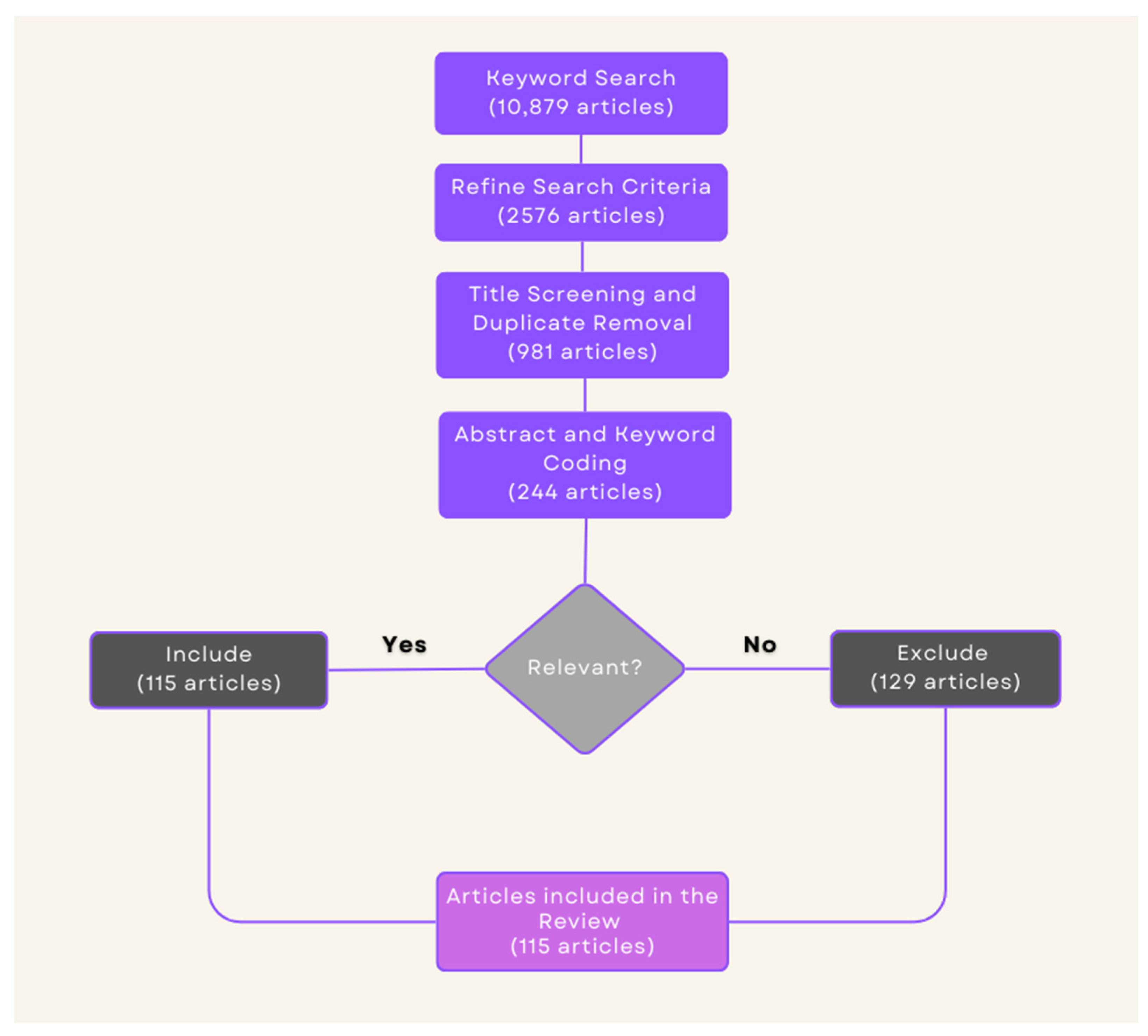
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Data Synthesis
3. Neurological, Neurocognitive, and Neuropsychiatric Symptomatology of PACS
3.1. Neurological Symptoms
3.2. Neurocognitive and Neuropsychological Symptoms
3.3. Neuropsychiatric Symptoms
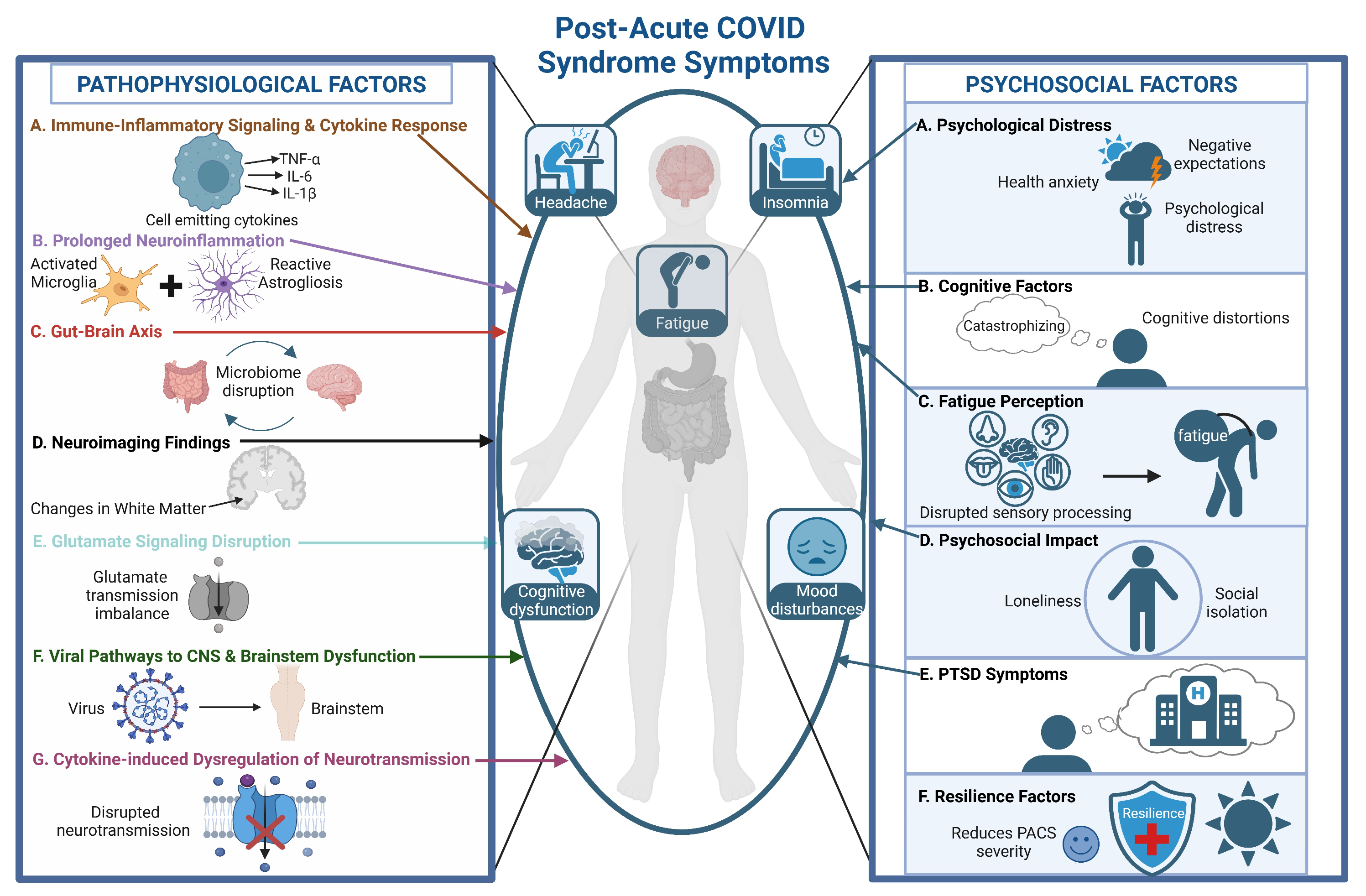
4. Pathophysiology
4.1. Immune–Inflammatory Signaling and Cytokine Response
4.2. Prolonged Neuroinflammation and Psychiatric and Neurocognitive Symptoms
4.3. Viral Pathways to the CNS and Brainstem Dysfunction
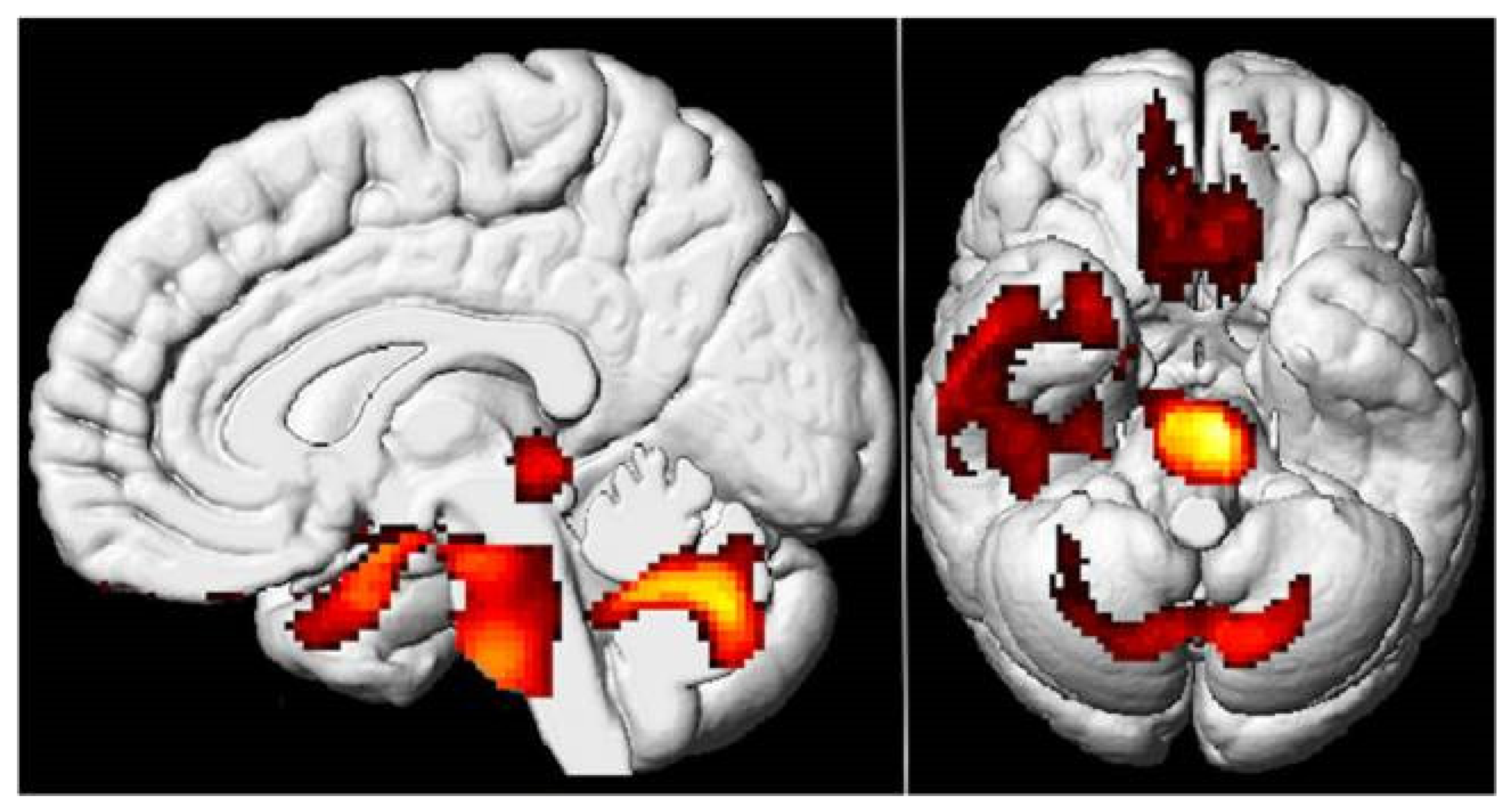
4.4. Neuroimaging Findings
4.5. Gut-Brain Axis
4.6. Impact of Viral Variants and Vaccination on PACS
4.7. Impact of Vaccination on PACS
5. The Psychological Dimensions of PACS
6. Future Directions
6.1. The Role of Neuropsychological Assessments and Standardized Protocols
6.2. Psychological Interventions
6.3. Multidisciplinary and Patient-Centered Approaches
6.4. The Role of AI in PACS Management
7. Discussion
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, X.; Laurent, S.; Onur, O.A.; Kleineberg, N.N.; Fink, G.R.; Schweitzer, F.; Warnke, C. A systematic review of neurological symptoms and complications of COVID-19. J. Neurol. 2020, 268, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Ezpeleta, D.; García-Azorín, D. Post–COVID-19 neurological symptoms. Neurol. Perspect. 2021, 1, S1–S3. [Google Scholar] [CrossRef] [PubMed]
- Al-Aly, Z.; Davis, H.; McCorkell, L.; Soares, L.; Wulf-Hanson, S.; Iwasaki, A.; Topol, E.J. Long COVID science, research and policy. Nat. Med. 2024, 30, 2148–2164. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Author Correction: Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 408. [Google Scholar] [CrossRef]
- Xu, E.; Xie, Y.; Al-Aly, Z. Long-term neurologic outcomes of COVID-19. Nat. Med. 2022, 28, 2406–2415. [Google Scholar] [CrossRef] [PubMed]
- Astin, R.; Banerjee, A.; Baker, M.R.; Dani, M.; Ford, E.; Hull, J.H.; Lim, P.B.; McNarry, M.; Morten, K.; O’Sullivan, O.; et al. Long COVID: Mechanisms, risk factors and recovery. Exp. Physiol. 2022, 108, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Van Kessel, S.A.M.; Hartman, T.C.O.; Lucassen, P.L.B.J.; Van Jaarsveld, C.H.M. Post-acute and long-COVID-19 symptoms in patients with mild diseases: A systematic review. Fam. Pract. 2021, 39, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 long-term effects of COVID-19: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 16144. [Google Scholar] [CrossRef] [PubMed]
- Woodrow, M.; Carey, C.; Ziauddeen, N.; Thomas, R.; Akrami, A.; Lutje, V.; Greenwood, D.C.; Alwan, N.A. Systematic Review of the Prevalence of Long COVID. Open Forum Infect. Dis. 2023, 10, ofad233. [Google Scholar] [CrossRef] [PubMed]
- Erlandson, K.M.; Geng, L.N.; Selvaggi, C.A.; Thaweethai, T.; Chen, P.; Erdmann, N.B.; Goldman, J.D.; Henrich, T.J.; Hornig, M.; Karlson, E.W.; et al. Differentiation of Prior SARS-CoV-2 Infection and Postacute Sequelae by Standard Clinical Laboratory Measurements in the RECOVER Cohort. Ann. Intern. Med. 2024, 177, 1209–1221. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Teodoro, T.; Chen, J.; Gelauff, J.; Edwards, M.J. Functional neurological disorder in people with long COVID: A systematic review. Eur. J. Neurol. 2023, 30, 1505–1514. [Google Scholar] [CrossRef]
- Vélez-Santamaría, R.; Fernández-Solana, J.; Méndez-López, F.; Domínguez-García, M.; González-Bernal, J.J.; Magallón-Botaya, R.; Oliván-Blázquez, B.; González-Santos, J.; Santamaría-Peláez, M. Functionality, physical activity, fatigue and quality of life in patients with acute COVID-19 and Long COVID infection. Sci. Rep. 2023, 13, 19907. [Google Scholar] [CrossRef]
- Thomas, B.; Pattinson, R.; Edwards, D.; Dale, C.; Jenkins, B.; Lande, H.; Bundy, C.; Davies, J.L. Definitions and measures of long COVID fatigue in adults: A scoping review protocol. JBI Evid. Synth. 2023, 22, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Tedjasukmana, R.; Budikayanti, A.; Islamiyah, W.R.; Witjaksono, A.M.A.L.; Hakim, M. Sleep disturbance in post COVID-19 conditions: Prevalence and quality of life. Front. Neurol. 2023, 13, 1095606. [Google Scholar] [CrossRef]
- Tan’ski, W.; Tomasiewicz, A.; Jankowska-Polańska, B. Sleep Disturbances as a Consequence of Long COVID-19: Insights from Actigraphy and Clinimetric Examinations—An Uncontrolled Prospective Observational Pilot Study. J. Clin. Med. 2024, 13, 839. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.N.; Dias, A.R.N.; Paranhos, A.C.M.; Silva, C.C.; Da Rocha Bastos, T.; De Brito, B.B.; Da Silva, N.M.; De Jesus Soares De Sousa, E.; Quaresma, J.A.S.; Falcão, L.F.M. Headache in long COVID as disabling condition: A clinical approach. Front. Neurol. 2023, 14, 1149294. [Google Scholar] [CrossRef] [PubMed]
- Rueb, M.; Ruzicka, M.; Fonseca, G.J.I.; Valdinoci, E.; Benesch, C.; Pernpruner, A.; Von Baum, M.; Remi, J.; Jebrini, T.; Schöberl, F.; et al. Headache severity in patients with post COVID-19 condition: A case-control study. Eur. Arch. Psychiatry Clin. Neurosci. 2024, 274, 1935–1943. [Google Scholar] [CrossRef]
- Seeley, M.-C.; Gallagher, C.; Ong, E.; Langdon, A.; Chieng, J.; Bailey, D.; Page, A.; Lim, H.S.; Lau, D.H. High Incidence of Autonomic Dysfunction and Postural Orthostatic Tachycardia Syndrome in Patients with Long COVID: Implications for Management and Health Care Planning. Am. J. Med. 2023. [Google Scholar] [CrossRef]
- Cantrell, C.; Reid, C.; Walker, C.S.; Tidd, S.J.S.; Zhang, R.; Wilson, R. Post-COVID postural orthostatic tachycardia syndrome (POTS): A new phenomenon. Front. Neurol. 2024, 15, 1297964. [Google Scholar] [CrossRef]
- Wong, T.L.; Weitzer, D.J. Long COVID and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS)—A Systemic Review and Comparison of Clinical Presentation and Symptomatology. Medicina 2021, 57, 418. [Google Scholar] [CrossRef] [PubMed]
- Riggott, C.; Ford, A.C.; Gracie, D.J. Review article: The role of the gut–brain axis in inflammatory bowel disease and its therapeutic implications. Aliment. Pharmacol. Ther. 2024, 60, 1200–1214. [Google Scholar] [CrossRef] [PubMed]
- Zamani, M.; Ebrahimtabar, F.; Alizadeh-Tabari, S.; Kasner, S.E.; Elkind, M.S.V.; Ananthakrishnan, A.N.; Choden, T.; Rubin, D.T.; Malekzadeh, R. Risk of Common Neurological Disorders in Adult Patients with Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. Inflamm. Bowel Dis. 2024, 30, 2195–2204. [Google Scholar] [CrossRef] [PubMed]
- Goudman, L.; Demuyser, T.; Pilitsis, J.G.; Billot, M.; Roulaud, M.; Rigoard, P.; Moens, M. Gut dysbiosis in patients with chronic pain: A systematic review and meta-analysis. Front. Immunol. 2024, 15, 1342833. [Google Scholar] [CrossRef] [PubMed]
- ZhZhang, D.; Zhou, Y.; Ma, Y.; Chen, P.; Tang, J.; Yang, B.; Li, H.; Liang, M.; Xue, Y.; Liu, Y.; et al. Gut Microbiota Dysbiosis Correlates With Long COVID-19 at One-Year After Discharge. J. Korean Med. Sci. 2023, 38, e120. [Google Scholar] [CrossRef]
- Liu, Q.; Mak, J.W.Y.; Su, Q.; Yeoh, Y.K.; Lui, G.C.-Y.; Ng, S.S.S.; Zhang, F.; Li, A.Y.L.; Lu, W.; Hui, D.S.-C.; et al. Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome. Gut 2022, 71, 544–552. [Google Scholar] [CrossRef]
- Vakili, K.; Fathi, M.; Yaghoobpoor, S.; Sayehmiri, F.; Nazerian, Y.; Nazerian, A.; Mohamadkhani, A.; Khodabakhsh, P.; Réus, G.Z.; Hajibeygi, R.; et al. The contribution of gut-brain axis to development of neurological symptoms in COVID-19 recovered patients: A hypothesis and review of literature. Front. Cell. Infect. Microbiol. 2022, 12, 983089. [Google Scholar] [CrossRef]
- Choudhury, A.; Tariq, R.; Jena, A.; Vesely, E.K.; Singh, S.; Khanna, S.; Sharma, V. Gastrointestinal manifestations of long COVID: A systematic review and meta-analysis. Ther. Adv. Gastroenterol. 2022, 15, 175628482211184. [Google Scholar] [CrossRef]
- Tavares-Júnior, J.W.L.; De Souza, A.C.C.; Borges, J.W.P.; Oliveira, D.N.; Siqueira-Neto, J.I.; Sobreira-Neto, M.A.; Braga-Neto, P. COVID-19 associated cognitive impairment: A systematic review. Cortex 2022, 152, 77–97. [Google Scholar] [CrossRef]
- Megari, K.; Thomaidou, E.; Chatzidimitriou, E. Highlighting the Neuropsychological Consequences of COVID-19: Evidence From a Narrative Review. INQUIRY J. Health Care Organ. Provis. Financ. 2024, 61. [Google Scholar] [CrossRef] [PubMed]
- Shariff, S.; Uwishema, O.; Mizero, J.; Thambi, V.D.; Nazir, A.; Mahmoud, A.; Kaushik, I.; Khayat, S.; Maigoro, A.Y.; Awde, S.; et al. Long-term cognitive dysfunction after the COVID-19 pandemic: A narrative review. Ann. Med. Surg. 2023, 85, 5504–5510. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Alonso, C.; Díez-Cirarda, M.; Pagán, J.; Pérez-Izquierdo, C.; Oliver-Mas, S.; Fernández-Romero, L.; Martínez-Petit, Á.; Valles-Salgado, M.; Gil-Moreno, M.J.; Yus, M.; et al. Unraveling brain fog in post-COVID syndrome: Relationship between subjective cognitive complaints and cognitive function, fatigue, and neuropsychiatric symptoms. Eur. J. Neurol. 2023, 32, e16084. [Google Scholar] [CrossRef] [PubMed]
- Daroische, R.; Hemminghyth, M.S.; Eilertsen, T.H.; Breitve, M.H.; Chwiszczuk, L.J. Cognitive Impairment After COVID-19—A Review on Objective Test Data. Front. Neurol. 2021, 12, 699582. [Google Scholar] [CrossRef]
- Baseler, H.A.; Aksoy, M.; Salawu, A.; Green, A.; Asghar, A.U.R. The negative impact of COVID-19 on working memory revealed using a rapid online quiz. PLoS ONE 2022, 17, e0269353. [Google Scholar] [CrossRef]
- Cui, R.; Gao, B.; Ge, R.; Li, M.; Li, M.; Lu, X.; Jiang, S. The effects of COVID-19 infection on working memory: A systematic review. Curr. Med. Res. Opin. 2023, 40, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Llana, T.; Zorzo, C.; Mendez-Lopez, M.; Mendez, M. Memory alterations after COVID-19 infection: A systematic review. Appl. Neuropsychol. Adult 2022, 31, 292–305. [Google Scholar] [CrossRef] [PubMed]
- Prabhakaran, D.; Day, G.S.; Munipalli, B.; Rush, B.K.; Pudalov, L.; Niazi, S.K.; Brennan, E.; Powers, H.R.; Durvasula, R.; Athreya, A.; et al. Neurophenotypes of COVID-19: Risk factors and recovery outcomes. Res. Sq. 2023, 30, 100648. [Google Scholar] [CrossRef]
- Kozik, V.; Reuken, P.; Utech, I.; Gramlich, J.; Stallmach, Z.; Demeyere, N.; Rakers, F.; Schwab, M.; Stallmach, A.; Finke, K. Characterization of neurocognitive deficits in patients with post-COVID-19 syndrome: Persistence, patients’ complaints, and clinical predictors. Front. Psychol. 2023, 14, 1233144. [Google Scholar] [CrossRef]
- Cummings, L. Long COVID: The impact on language and cognition. Lang. Health 2023, 1, 2–9. [Google Scholar] [CrossRef]
- McWhirter, L.; Smyth, H.; Hoeritzauer, I.; Couturier, A.; Stone, J.; Carson, A.J. What is brain fog? J. Neurol. Neurosurg. Psychiatry 2022, 94, 321–325. [Google Scholar] [CrossRef]
- Bulla, R.; Rossi, L.; Furlanis, G.; Agostinis, C.; Toffoli, M.; Balduit, A.; Mangogna, A.; Liccari, M.; Morosini, G.; Kishore, U.; et al. A likely association between low mannan-binding lectin level and brain fog onset in long COVID patients. Front. Immunol. 2023, 14, 1191083. [Google Scholar] [CrossRef] [PubMed]
- Jennings, G.; Monaghan, A.; Xue, F.; Duggan, E.; Romero-Ortuño, R. Comprehensive Clinical Characterisation of Brain Fog in Adults Reporting Long COVID Symptoms. J. Clin. Med. 2022, 11, 3440. [Google Scholar] [CrossRef]
- Sampogna, G.; Di Vincenzo, M.; Giallonardo, V.; Perris, F.; Volpicelli, A.; Del Vecchio, V.; Luciano, M.; Fiorillo, A. The Psychiatric Consequences of Long-COVID: A Scoping Review. J. Pers. Med. 2022, 12, 1767. [Google Scholar] [CrossRef] [PubMed]
- Zakia, H.; Pradana, K.; Iskandar, S. Risk factors for psychiatric symptoms in patients with long COVID: A systematic review. PLoS ONE 2023, 18, e0284075. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Xu, X.; Zhang, L.; Zheng, D.; Liu, Y.; Feng, B.; Hu, J.; Lin, Q.; Xi, X.; Wang, Q.; et al. Post-traumatic Stress Disorder Symptoms and Quality of Life of COVID-19 Survivors at 6-Month Follow-Up: A Cross-Sectional Observational Study. Front. Psychiatry 2022, 12, 782478. [Google Scholar] [CrossRef] [PubMed]
- Houben-Wilke, S.; Goërtz, Y.M.; Delbressine, J.M.; Vaes, A.W.; Meys, R.; Machado, F.V.; van Herck, M.; Burtin, C.; Posthuma, R.; Franssen, F.M.; et al. The Impact of Long COVID-19 on Mental Health: Observational 6-Month Follow-Up Study. JMIR Ment. Health 2022, 9, e33704. [Google Scholar] [CrossRef]
- Vo, H.T.; Dao, T.D.; Van Duong, T.; Nguyen, T.T.; Do, B.N.; Do, T.X.; Pham, K.M.; Vu, V.H.; Van Pham, L.; Nguyen, L.T.H.; et al. Impact of long COVID-19 on posttraumatic stress disorder as modified by health literacy: An observational study in Vietnam. Osong Public Health Res. Perspect. 2024, 15, 33–44. [Google Scholar] [CrossRef]
- Thye, A.Y.-K.; Law, J.W.-F.; Tan, L.T.-H.; Pusparajah, P.; Ser, H.-L.; Thurairajasingam, S.; Letchumanan, V.; Lee, L.-H. Psychological Symptoms in COVID-19 Patients: In-sights into Pathophysiology and Risk Factors of Long COVID-19. Biology 2022, 11, 61. [Google Scholar] [CrossRef]
- Mekhael, M.; Lim, C.H.; El Hajjar, A.H.; Noujaim, C.; Pottle, C.; Makan, N.; Dagher, L.; Zhang, Y.; Chouman, N.; Li, D.L.; et al. Studying the Effect of Long COVID-19 Infection on Sleep Quality Using Wearable Health Devices: Observational Study. J. Med. Internet Res. 2022, 24, e38000. [Google Scholar] [CrossRef]
- Sunada, N.; Nakano, Y.; Otsuka, Y.; Tokumasu, K.; Honda, H.; Sakurada, Y.; Matsuda, Y.; Hasegawa, T.; Omura, D.; Ochi, K.; et al. Characteristics of Sleep Disturbance in Patients with Long COVID: A Retrospective Observational Study in Japan. J. Clin. Med. 2022, 11, 7332. [Google Scholar] [CrossRef]
- Titze-De-Almeida, R.; Lacerda, P.H.A.; de Oliveira, E.P.; de Oliveira, M.E.F.; Vianna, Y.S.S.; Costa, A.M.; dos Santos, E.P.; Guérard, L.M.C.; Ferreira, M.A.d.M.; dos Santos, I.C.R.; et al. Sleep and memory complaints in long COVID: An insight into clustered psychological phenotypes. PeerJ 2024, 12, e16669. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.K.; Auger, R.R. Sleep and Long COVID—A Review and Exploration of Sleep Disturbances in Post Acute Sequelae of SARS-CoV-2 (PASC) and Therapeutic Possibilities. Curr. Sleep Med. Rep. 2024, 10, 169–180. [Google Scholar] [CrossRef]
- Schilling, C.; Nieters, A.; Schredl, M.; Peter, R.S.; Rothenbacher, D.; Brockmann, S.O.; Göpel, S.; Kindle, G.; Merle, U.; Steinacker, J.M.; et al. Pre-existing sleep problems as a predictor of post-acute sequelae of COVID-19. J. Sleep Res. 2023, 33, e13949. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.M.; Gilbert, E.B.; Riordan, P.A.; Helmke, N.; Von Isenburg, M.; Kincaid, B.R.; Shirey, K.G. COVID-19-associated psychosis: A systematic review of case reports. Gen. Hosp. Psychiatry 2021, 73, 84–100. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Pinto, I.; Branco, P. COVID-19 induced psychosis. Should we be concerned? Eur. Psychiatry 2022, 65 (Suppl. S1), S201–S202. [Google Scholar] [CrossRef]
- Păunescu, R.L.; Micluţia, I.V.; Verișezan, O.R.; Crecan-Suciu, B.D. Acute and long-term psychiatric symptoms associated with COVID-19 (Review). Biomed. Rep. 2022, 18, 4. [Google Scholar] [CrossRef] [PubMed]
- Ferrando, S.J.; Klepacz, L.; Lynch, S.; Tavakkoli, M.; Dornbush, R.; Baharani, R.; Smolin, Y.; Bartell, A. COVID-19 Psychosis: A Potential New Neuropsychiatric Condition Triggered by Novel Coronavirus Infection and the Inflammatory Response? Psychosomatics 2020, 61, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Gusev, E.; Sarapultsev, A. Exploring the Pathophysiology of Long COVID: The Central Role of Low-Grade Inflammation and Multisystem Involvement. Int. J. Mol. Sci. 2024, 25, 6389. [Google Scholar] [CrossRef] [PubMed]
- Bayat, A.-H.; Azimi, H.; Moghaddam, M.H.; Ebrahimi, V.; Fathi, M.; Vakili, K.; Mahmoudiasl, G.-R.; Forouzesh, M.; Boroujeni, M.E.; Nariman, Z.; et al. COVID-19 causes neuronal degeneration and reduces neurogenesis in human hippocampus. APOPTOSIS 2022, 27, 852–868. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.; Fatima, R.; Assaly, R. Elevated interleukin-6 and severe COVID-19: A meta-analysis. J. Med. Virol. 2020, 92, 2283–2285. [Google Scholar] [CrossRef]
- Zhu, J.; Pang, J.; Ji, P.; Zhong, Z.; Li, H.; Li, B.; Zhang, J. Elevated interleukin-6 is associated with severity of COVID-19: A meta-analysis. J. Med. Virol. 2020, 93, 35–37. [Google Scholar] [CrossRef]
- Kumar, S.; Veldhuis, A.; Malhotra, T. Neuropsychiatric and Cognitive Sequelae of COVID-19. Front. Psychol. 2021, 12, 577529. [Google Scholar] [CrossRef]
- Mazza, M.G.; Palladini, M.; De Lorenzo, R.; Magnaghi, C.; Poletti, S.; Furlan, R.; Ciceri, F.; Rovere-Querini, P.; Benedetti, F. Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: Effect of inflammatory biomarkers at three-month follow-up. Brain Behav. Immun. 2021, 94, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Saikarthik, J.; Saraswathi, I.; Alarifi, A.; Al-Atram, A.A.; Mickeymaray, S.; Paramasivam, A.; Shaikh, S.; Jeraud, M.; Alothaim, A.S. Role of neuroinflammation mediated potential alterations in adult neurogenesis as a factor for neuropsychiatric symptoms in Post-Acute COVID-19 syndrome—A narrative review. PeerJ 2022, 10, e14227. [Google Scholar] [CrossRef] [PubMed]
- VanElzakker, M.B.; Bues, H.F.; Brusaferri, L.; Kim, M.; Saadi, D.; Ratai, E.-M.; Dougherty, D.D.; Loggia, M.L. Neuroinflammation in post-acute sequelae of COVID-19 (PASC) as assessed by [11C]PBR28 PET correlates with vascular disease measures. Brain Behav. Immun. 2024, 119, 713–723. [Google Scholar] [CrossRef]
- Colizzi, M.; Peghin, M.; De Martino, M.; Bontempo, G.; Gerussi, V.; Palese, A.; Isola, M.; Tascini, C.; Balestrieri, M. Mental health symptoms one year after acute COVID-19 infection: Prevalence and risk factors. Rev. Psiquiatr. Y Salud Ment. 2022, 16, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Manca, R.; De Marco, M.; Ince, P.G.; Venneri, A. Heterogeneity in Regional Damage Detected by Neuroimaging and Neuropathological Studies in Older Adults With COVID-19: A Cognitive-Neuroscience Systematic Review to Inform the Long-Term Impact of the Virus on Neurocognitive Trajectories. Front. Aging Neurosci. 2021, 13, 646908. [Google Scholar] [CrossRef] [PubMed]
- Orrù, G.; Conversano, C.; Malloggi, E.; Francesconi, F.; Ciacchini, R.; Gemignani, A. Neurological Complications of COVID-19 and Possible Neuroinvasion Pathways: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 6688. [Google Scholar] [CrossRef]
- Hugon, J.; Queneau, M.; Ortiz, M.S.; Msika, E.F.; Farid, K.; Paquet, C. Cognitive decline and brainstem hypometabolism in long COVID: A case series. Brain Behav. 2022, 12, e2513. [Google Scholar] [CrossRef]
- Shan, D.; Li, S.; Xu, R.; Nie, G.; Xie, Y.; Han, J.; Gao, X.; Zheng, Y.; Xu, Z.; Dai, Z. Post-COVID-19 human memory impairment: A PRISMA-based systematic review of evidence from brain imaging studies. Front. Aging Neurosci. 2022, 14, 1077384. [Google Scholar] [CrossRef] [PubMed]
- Meyer, P.T.; Hellwig, S.; Blazhenets, G.; Hosp, J.A. Molecular Imaging Findings on Acute and Long-Term Effects of COVID-19 on the Brain: A Systematic review. J. Nucl. Med. 2022, 63, 971–980. [Google Scholar] [CrossRef]
- Guedj, E.; Campion, J.Y.; Dudouet, P.; Kaphan, E.; Bregeon, F.; Tissot-Dupont, H.; Guis, S.; Barthelemy, F.; Habert, P.; Ceccaldi, M.; et al. 18F-FDG brain PET hypometabolism in patients with long COVID. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2823–2833. [Google Scholar] [CrossRef] [PubMed]
- Cull, O.; Qadi, L.A.; Stadler, J.; Martin, M.; Helou, A.E.; Wagner, J.; Maillet, D.; Chamard-Witkowski, L. Radiological markers of neurological manifestations of post-acute sequelae of SARS-CoV-2 infection: A mini-review. Front. Neurol. 2023, 14, 1233079. [Google Scholar] [CrossRef] [PubMed]
- Theofilis, P.; Oikonomou, E.; Vasileiadou, M.; Tousoulis, D. A Narrative Review on Prolonged Neuropsychiatric Consequences of COVID-19: A Serious Concern. Heart Mind 2024, 8, 177–183. [Google Scholar] [CrossRef]
- Cecchetti, G.; Agosta, F.; Canu, E.; Basaia, S.; Barbieri, A.; Cardamone, R.; Bernasconi, M.P.; Castelnovo, V.; Cividini, C.; Cursi, M.; et al. Cognitive, EEG, and MRI features of COVID-19 survivors: A 10-month study. J. Neurol. 2022, 269, 3400–3412. [Google Scholar] [CrossRef]
- Douaud, G.; Lee, S.; Alfaro-Almagro, F.; Arthofer, C.; Wang, C.; McCarthy, P.; Lange, F.; Andersson, J.L.R.; Griffanti, L.; Duff, E.; et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature 2022, 604, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Plummer, A.M.; Matos, Y.L.; Lin, H.C.; Ryman, S.G.; Birg, A.; Quinn, D.K.; Parada, A.N.; Vakhtin, A.A. Gut-brain pathogenesis of post-acute COVID-19 neurocognitive symptoms. Front. Neurosci. 2023, 17, 1232480. [Google Scholar] [CrossRef]
- Tizenberg, B.N.; Brenner, L.A.; Lowry, C.A.; Okusaga, O.O.; Benavides, D.R.; Hoisington, A.J.; Benros, M.E.; Stiller, J.W.; Kessler, R.C.; Postolache, T.T. Biological and Psychological Factors Determining Neuropsychiatric Outcomes in COVID-19. Curr. Psychiatry Rep. 2021, 23, 68. [Google Scholar] [CrossRef] [PubMed]
- Spinicci, M.; Graziani, L.; Tilli, M.; Nkurunziza, J.; Vellere, I.; Borchi, B.; Mencarini, J.; Campolmi, I.; Gori, L.; Giovannoni, L.; et al. Infection with SARS-CoV-2 Variants Is Associated with Different Long COVID Phenotypes. Viruses 2022, 14, 2367. [Google Scholar] [CrossRef]
- Bai, F.; Santoro, A.; Hedberg, P.; Tavelli, A.; De Benedittis, S.; De Morais Caporali, J.F.; Marinho, C.C.; Leite, A.S.; Santoro, M.M.; Silberstein, F.C.; et al. The Omicron Variant Is Associated with a Reduced Risk of the Post COVID-19 Condition and Its Main Phenotypes Compared to the Wild-Type Virus: Results from the EuCARE-POSTCOVID-19 Study. Viruses 2024, 16, 1500. [Google Scholar] [CrossRef]
- Canas, L.S.; Molteni, E.; Deng, J.; Sudre, C.H.; Murray, B.; Kerfoot, E.; Antonelli, M.; Rjoob, K.; Pujol, J.C.; Polidori, L.; et al. Profiling post-COVID-19 condition across different variants of SARS-CoV-2: A prospective longitudinal study in unvaccinated wild-type, unvaccinated alpha-variant, and vaccinated delta-variant populations. Lancet Digit. Health 2023, 5, e421–e434. [Google Scholar] [CrossRef]
- Fernández-De-Las-Peñas, C.; Notarte, K.I.; Peligro, P.J.; Velasco, J.V.; Ocampo, M.J.; Henry, B.M.; Arendt-Nielsen, L.; Torres-Macho, J.; Plaza-Manzano, G. Long-COVID Symptoms in Individuals Infected with Different SARS-CoV-2 Variants of Concern: A Systematic Review of the Literature. Viruses 2022, 14, 2629. [Google Scholar] [CrossRef] [PubMed]
- Sahin, A.; Karadag-Oncel, E.; Buyuksen, O.; Ekemen-Keles, Y.; Ustundag, G.; Elvan-Tuz, A.; Tasar, S.; Didinmez-Taskirdi, E.; Baykan, M.; Kara-Aksay, A.; et al. The diversity in the clinical features of children hospitalized with COVID-19 during the nonvariant, Alpha (B.1.1.7), Delta (B.1.617.2), and Omicron (B.1.1.529) variant periods of SARS CoV-2: Caution for neurological symptoms in Omicron variant. J. Med. Virol. 2023, 95, e28628. [Google Scholar] [CrossRef]
- Gao, P.; Liu, J.; Liu, M. Effect of COVID-19 Vaccines on Reducing the Risk of Long COVID in the Real World: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 12422. [Google Scholar] [CrossRef]
- Maier, H.E.; Kowalski-Dobson, T.; Eckard, A.; Gherasim, C.; Manthei, D.; Meyers, A.; Davis, D.; Bakker, K.; Lindsey, K.; Chu, Z.; et al. Reduction in long-COVID symptoms and symptom severity in vaccinated compared to unvaccinated adults. Open Forum Infect. Dis. 2024, 11, ofae039. [Google Scholar] [CrossRef]
- Ayoubkhani, D.; Bermingham, C.; Pouwels, K.B.; Glickman, M.; Nafilyan, V.; Zaccardi, F.; Khunti, K.; Alwan, N.A.; Walker, A.S. Trajectory of long covid symptoms after covid-19 vaccination: Community based cohort study. BMJ 2022, 377, e069676. [Google Scholar] [CrossRef]
- Engelmann, P.; Reinke, M.; Stein, C.; Salzmann, S.; Löwe, B.; Toussaint, A.; Shedden-Mora, M. Psychological factors associated with Long COVID: A systematic review and meta-analysis. EClinicalMedicine 2024, 74, 102756. [Google Scholar] [CrossRef]
- Engelmann, P.; Büchel, C.; Frommhold, J.; Klose, H.F.E.; Lohse, A.W.; Maehder, K.; Nestoriuc, Y.; Scherer, M.; Suling, A.; Toussaint, A.; et al. Psychological risk factors for Long COVID and their modification: Study protocol of a three-arm, randomised controlled trial (SOMA.COV). BJPsych Open 2023, 9, e207. [Google Scholar] [CrossRef] [PubMed]
- Milde, C.; Glombiewski, J.A.; Wilhelm, M.; Schemer, L. Psychological Factors Predict Higher Odds and Impairment of Post-COVID Symptoms: A Prospective Study. Psychosom. Med. 2023, 85, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Bravo, R.G.; Infanti, A.; Billieux, J.; Ritzen, M.; Vögele, C.; Benoy, C. The psychological syndrome associated with Long-COVID: A study protocol. Front. Epidemiol. 2023, 3, 1193369. [Google Scholar] [CrossRef]
- Engelmann, P.; Löwe, B.; Brehm, T.T.; Weigel, A.; Ullrich, F.; Addo, M.M.; Wiesch, J.S.Z.; Lohse, A.W.; Toussaint, A. Risk factors for worsening of somatic symptom burden in a prospective cohort during the COVID-19 pandemic. Front. Psychol. 2022, 13, 1022203. [Google Scholar] [CrossRef]
- Hüsing, P.; Smakowski, A.; Löwe, B.; Kleinstäuber, M.; Toussaint, A.; Shedden-Mora, M.C. The framework for systematic reviews on psychological risk factors for persistent somatic symptoms and related syndromes and disorders (PSY-PSS). Front. Psychiatry 2023, 14, 1142484. [Google Scholar] [CrossRef] [PubMed]
- Kube, T.; Rozenkrantz, L.; Rief, W.; Barsky, A. Understanding persistent physical symptoms: Conceptual integration of psychological expectation models and predictive processing accounts. Clin. Psychol. Rev. 2020, 76, 101829. [Google Scholar] [CrossRef] [PubMed]
- Molero, P.; Reina, G.; Blom, J.D.; Martínez-González, M.Á.; Reinken, A.; De Kloet, E.R.; Molendijk, M.L. COVID-19 risk, course and outcome in people with mental disorders: A systematic review and meta-analyses. Epidemiol. Psychiatr. Sci. 2023, 32, e61. [Google Scholar] [CrossRef] [PubMed]
- Oppenauer, C.; Burghardt, J.; Kaiser, E.; Riffer, F.; Sprung, M. Psychological Distress During the COVID-19 Pandemic in Patients With Mental or Physical Diseases. Front. Psychol. 2021, 12, 703488. [Google Scholar] [CrossRef]
- Mulchandani, R.; Taylor-Philips, S.; Jones, H.E.; Ades, A.; Borrow, R.; Linley, E.; Kirwan, P.D.; Stewart, R.; Moore, P.; Boyes, J.; et al. Self assessment overestimates historical COVID-19 disease relative to sensitive serological assays: Cross-sectional study in UK key workers. MedRxiv 2020, 22, 2020–2108. [Google Scholar] [CrossRef]
- Matta, J.; Wiernik, E.; Robineau, O.; Carrat, F.; Touvier, M.; Severi, G.; De Lamballerie, X.; Blanché, H.; Deleuze, J.-F.; Gouraud, C.; et al. Association of Self-reported COVID-19 Infection and SARS-CoV-2 Serology Test Results With Persistent Physical Symptoms Among French Adults During the COVID-19 Pandemic. JAMA Intern. Med. 2021, 182, 19. [Google Scholar] [CrossRef]
- Ayling, K.; Jia, R.; Coupland, C.; Chalder, T.; Massey, A.; Broadbent, E.; Vedhara, K. Psychological Predictors of Self-reported COVID-19 Outcomes: Results From a Prospective Cohort Study. Ann. Behav. Med. 2022, 56, 484–497. [Google Scholar] [CrossRef]
- Kuppuswamy, A. The Neurobiology of Pathological Fatigue: New Models, New Questions. Neurosci. 2021, 28, 238–253. [Google Scholar] [CrossRef]
- Thomas, B.; Pattinson, R.; Bundy, C.; Davies, J.L. Somatosensory processing in long COVID fatigue and its relations with physiological and psychological factors. Exp. Physiol. 2024, 109, 1637–1649. [Google Scholar] [CrossRef]
- Fietsam, A.C.; Bryant, A.D.; Rudroff, T. Fatigue and perceived fatigability, not objective fatigability, are prevalent in people with post-COVID-19. Exp. Brain Res. 2022, 241, 211–219. [Google Scholar] [CrossRef]
- Scharfenberg, D.; Schild, A.-K.; Warnke, C.; Maier, F. A network perspective on neuropsychiatric and cognitive symptoms of the post-COVID syndrome. Eur. J. Psychol. 2022, 18, 350–356. [Google Scholar] [CrossRef]
- Benke, C.; Autenrieth, L.K.; Asselmann, E.; Pané-Farré, C.A. Lockdown, quarantine measures, and social distancing: Associations with depression, anxiety and distress at the beginning of the COVID-19 pandemic among adults from Germany. Psychiatry Res. 2020, 293, 113462. [Google Scholar] [CrossRef]
- Loades, M.E.; Chatburn, E.; Higson-Sweeney, N.; Reynolds, S.; Shafran, R.; Brigden, A.; Linney, C.; McManus, M.N.; Borwick, C.; Crawley, E. Rapid Systematic Review: The Impact of Social Isolation and Loneliness on the Mental Health of Children and Adolescents in the Context of COVID-19. J. Am. Acad. Child Adolesc. Psychiatry 2020, 59, 1218–1239.e3. [Google Scholar] [CrossRef]
- Banerjee, D.; Vaishnav, M.; Rao, T.S.; Raju, M.; Dalal, P.K.; Javed, A.; Saha, G.; Mishra, K.K.; Kumar, V.; Jagiwala, M.P. Impact of the COVID-19 pandemic on psychosocial health and well-being in South-Asian (World Psychiatric Association zone 16) countries: A systematic and advocacy review from the Indian Psychiatric Society. Indian J. Psychiatry 2020, 62, 343. [Google Scholar] [CrossRef]
- Dubey, S.; Biswas, P.; Ghosh, R.; Chatterjee, S.; Dubey, M.J.; Chatterjee, S.; Lahiri, D.; Lavie, C.J. Psychosocial impact of COVID-19. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Lipsitz, O.; Nasri, F.; Lui, L.M.W.; Gill, H.; Phan, L.; Chen-Li, D.; Iacobucci, M.; Ho, R.; Majeed, A.; et al. Impact of COVID-19 pandemic on mental health in the general population: A systematic review. J. Affect. Disord. 2020, 277, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Riepenhausen, A.; Veer, I.M.; Wackerhagen, C.; Reppmann, Z.C.; Köber, G.; Ayuso-Mateos, J.L.; Bögemann, S.A.; Corrao, G.; Felez-Nobrega, M.; Abad, J.M.H.; et al. Coping with COVID: Risk and resilience factors for mental health in a German representative panel study. Psychol. Med. 2022, 53, 3897–3907. [Google Scholar] [CrossRef]
- Grunden, N.; Calabria, M.; García-Sánchez, C.; Pons, C.; Arroyo, J.A.; Gómez-Ansón, B.; Del Carmen Estévez-García, M.; Belvís, R.; Morollón, N.; Cordero-Carcedo, M.; et al. Evolving trends in neuropsychological profiles of post COVID-19 condition: A 1-year follow-up in individuals with cognitive complaints. PLoS ONE 2024, 19, e0302415. [Google Scholar] [CrossRef] [PubMed]
- Braga, L.W.; Oliveira, S.B.; Moreira, A.S.; Pereira, M.E.M.d.S.M.; Serio, A.S.S.; Carneiro, V.d.S.; Freitas, L.d.F.P.; Souza, L.M.D.N. Long COVID neuropsychological follow-up: Is cognitive rehabilitation relevant? Neurorehabilitation 2023, 53, 517–534. [Google Scholar] [CrossRef]
- Arneth, B.M. Gut–brain axis biochemical signalling from the gastrointestinal tract to the central nervous system: Gut dysbiosis and altered brain function. Postgrad. Med. J. 2018, 94, 446–452. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, L.; Feng, Y.; Liu, Y.-F.; Si, T.L.; Su, Z.; Cheung, T.; Ungvari, G.S.; Ng, C.H.; Xiang, Y.-T. Brain-gut axis and psychiatric disorders: A perspective from bibliometric and visual analysis. Front. Immunol. 2022, 13, 1047007. [Google Scholar] [CrossRef]
- Bonaz, B.; Bazin, T.; Pellissier, S. The Vagus Nerve at the Interface of the Microbiota-Gut-Brain Axis. Front. Neurosci. 2018, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Breit, S.; Kupferberg, A.; Rogler, G.; Hasler, G. Vagus Nerve as Modulator of the Brain–Gut Axis in Psychiatric and Inflammatory Disorders. Front. Psychiatry 2018, 9, 44. [Google Scholar] [CrossRef]
- Scarpelli, S.; De Santis, A.; Alfonsi, V.; Gorgoni, M.; Morin, C.M.; Espie, C.; Merikanto, I.; Chung, F.; Penzel, T.; Bjorvatn, B.; et al. The role of sleep and dreams in long-COVID. J. Sleep Res. 2022, 32, e13789. [Google Scholar] [CrossRef]
- Scarpelli, S.; Gorgoni, M.; Alfonsi, V.; Annarumma, L.; Di Natale, V.; Pezza, E.; De Gennaro, L. The impact of the end of COVID confinement on pandemic dreams, as assessed by a weekly sleep diary: A longitudinal investigation in Italy. J. Sleep Res. 2021, 31, e13429. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’em, Y.; Redfield, S.; 1064 Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef] [PubMed]
- Huth, D.; Bräscher, A.-K.; Tholl, S.; Fiess, J.; Birke, G.; Herrmann, C.; Jöbges, M.; Mier, D.; Witthöft, M. Cognitive-behavioral therapy for patients with post-COVID-19 condition (CBT-PCC): A feasibility trial. Psychol. Med. 2023, 54, 1122–1132. [Google Scholar] [CrossRef] [PubMed]
- Kuut, T.A.; Müller, F.; Csorba, I.; Braamse, A.; Aldenkamp, A.; Appelman, B.; Assmann-Schuilwerve, E.; Geerlings, S.E.; Gibney, K.B.; Kanaan, R.; et al. Efficacy of Cognitive-Behavioral Therapy Targeting Severe Fatigue Following Coronavirus Disease 2019: Results of a Randomized Controlled Trial. Clin. Infect. Dis. 2023, 77, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Borba, V.; Martínez-García, L.; Peris-Baquero, Ó.; Osma, J.; Del Corral-Beamonte, E. Guiding future research on psychological interventions in people with COVID-19 and post COVID syndrome and comorbid emotional disorders based on a systematic review. Front. Public Health 2024, 11, 1305463. [Google Scholar] [CrossRef] [PubMed]
- Nikrah, N.; Bahari, F.; Shiri, A. Effectiveness of the acceptance and commitment therapy on resilience and quality of life in patients with post-acute COVID-19 syndrome. Appl. Nurs. Res. 2023, 73, 151723. [Google Scholar] [CrossRef] [PubMed]
- Samad, F.D.A.; Pereira, X.V.; Chong, S.K.; Latif, M.H.B.A. Interpersonal psychotherapy for traumatic grief following a loss due to COVID-19: A case report. Front. Psychiatry 2023, 14, 1218715. [Google Scholar] [CrossRef]
- Mahendru, K.; Pandit, A.; Singh, V.; Choudhary, N.; Mohan, A.; Bhatnagar, S. Effect of Meditation and Breathing Exercises on the Well-being of Patients with SARS-CoV-2 Infection under Institutional Isolation: A Randomized Control Trial. Indian J. Palliat. Care 2021, 27, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Situmorang, D.D.B. “When the first session may be the last!”: A case report of the implementation of “rapid tele-psychotherapy” with single-session music therapy in the COVID-19 outbreak. Palliat. Support. Care 2021, 20, 290–295. [Google Scholar] [CrossRef]
| Keywords |
|---|
| Post-Acute COVID-19 Syndrome, PACS, PASC, Long COVID, Post-COVID, Pathology, Neuroinflammation, Brain Imaging, Brain Fog, Cognitive Dysfunction, Memory Impairment, Executive Function, Neuropsychiatric Symptoms, Neuropsychological Deficits, Quarantine Impact, Psychological Stress, Social Isolation, Loneliness, Mental Health, Fatigue, Headache, Insomnia, Depression, Immune Response, Autonomic Dysfunction, Viral Persistence |
| Neurological Symptom | Percentage of Affected Patients |
|---|---|
| Fatigue | Up to 58% |
| Sleep disturbances | Up to 30% |
| Headaches | Up to 44% |
| Dizziness and Vertigo | Up to 5% |
| POTS | Up to 14% |
| ME/CFS | Up to 51% |
| Gastrointestinal disturbances | Up to 22% |
| Neurocognitive Symptom | Percentage of Affected Patients |
|---|---|
| Cognitive Impairment | Up to 65% |
| Persistent Fatigue | Up to 46.6% |
| Brain Fog | Up to 31.9% |
| Attention Deficits | Up to 55% |
| Memory Impairment | Up to 40% |
| Executive Function Deficits | Up to 59% |
| Language Difficulties | Up to 93% |
| Neuropsychiatric Symptom | Percentage of Affected Patients |
|---|---|
| Anxiety | Up to 29.6% |
| Depression | Up to 35% |
| PTSD | Up to 16% |
| Psychotic Symptoms | Up to 4% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malioukis, A.; Snead, R.S.; Marczika, J.; Ambalavanan, R. Pathophysiological, Neuropsychological, and Psychosocial Influences on Neurological and Neuropsychiatric Symptoms of Post-Acute COVID-19 Syndrome: Impacts on Recovery and Symptom Persistence. Biomedicines 2024, 12, 2831. https://doi.org/10.3390/biomedicines12122831
Malioukis A, Snead RS, Marczika J, Ambalavanan R. Pathophysiological, Neuropsychological, and Psychosocial Influences on Neurological and Neuropsychiatric Symptoms of Post-Acute COVID-19 Syndrome: Impacts on Recovery and Symptom Persistence. Biomedicines. 2024; 12(12):2831. https://doi.org/10.3390/biomedicines12122831
Chicago/Turabian StyleMalioukis, Alex, R Sterling Snead, Julia Marczika, and Radha Ambalavanan. 2024. "Pathophysiological, Neuropsychological, and Psychosocial Influences on Neurological and Neuropsychiatric Symptoms of Post-Acute COVID-19 Syndrome: Impacts on Recovery and Symptom Persistence" Biomedicines 12, no. 12: 2831. https://doi.org/10.3390/biomedicines12122831
APA StyleMalioukis, A., Snead, R. S., Marczika, J., & Ambalavanan, R. (2024). Pathophysiological, Neuropsychological, and Psychosocial Influences on Neurological and Neuropsychiatric Symptoms of Post-Acute COVID-19 Syndrome: Impacts on Recovery and Symptom Persistence. Biomedicines, 12(12), 2831. https://doi.org/10.3390/biomedicines12122831







