Innovative Approaches in Knee Osteoarthritis Treatment: A Comprehensive Review of Bone Marrow-Derived Products
Abstract
1. Introduction
2. Methods
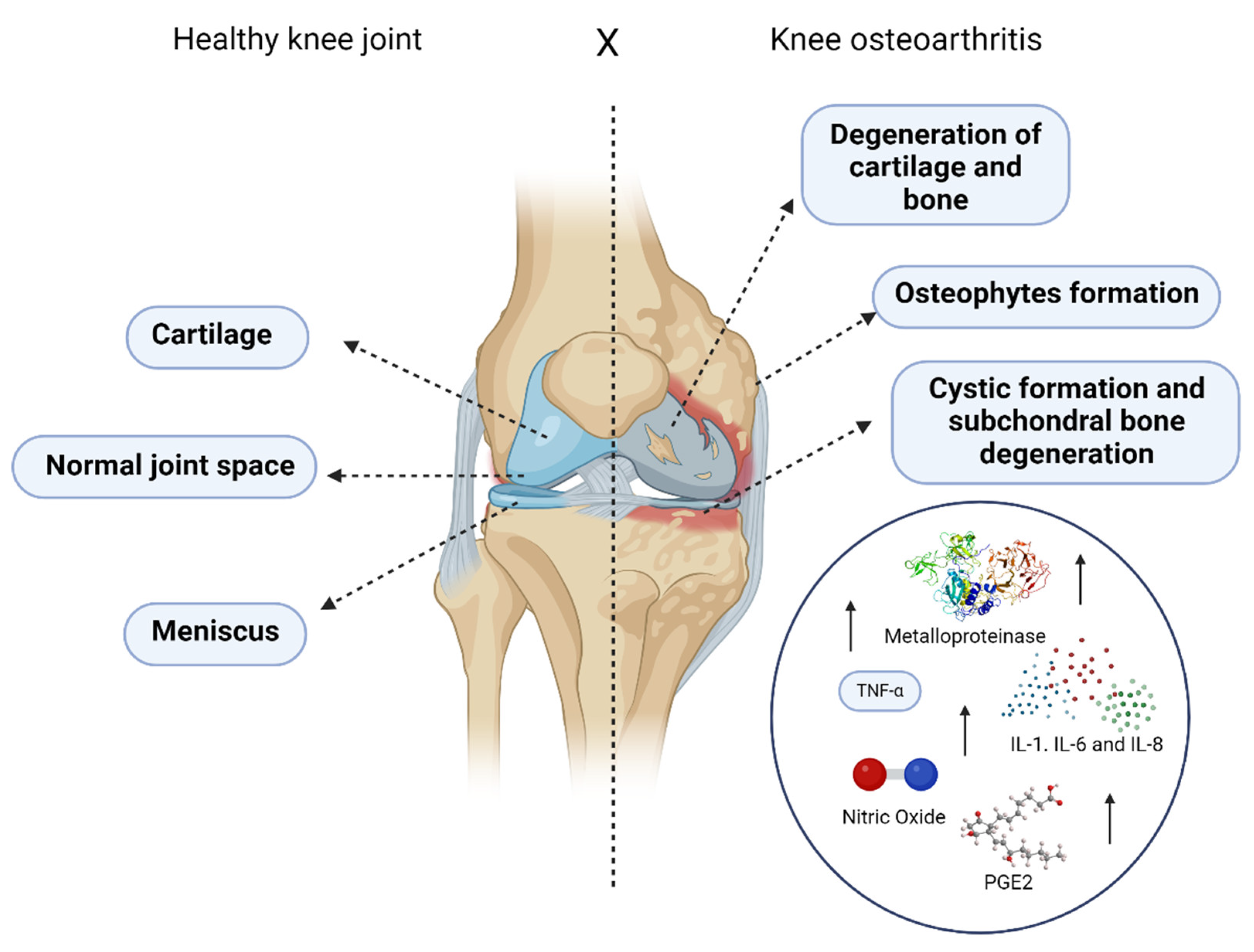
2.1. Osteoarthritis: An Overview
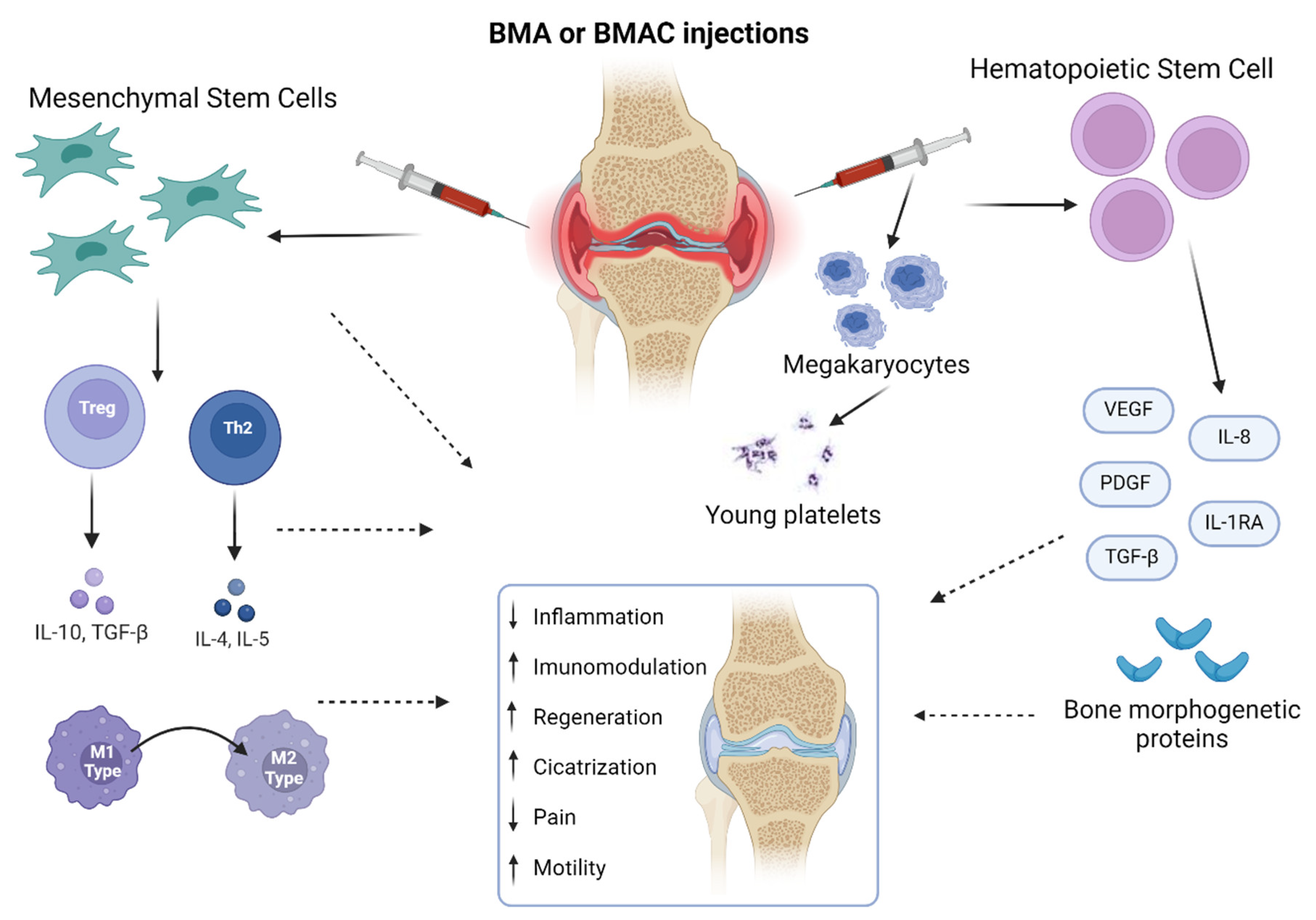
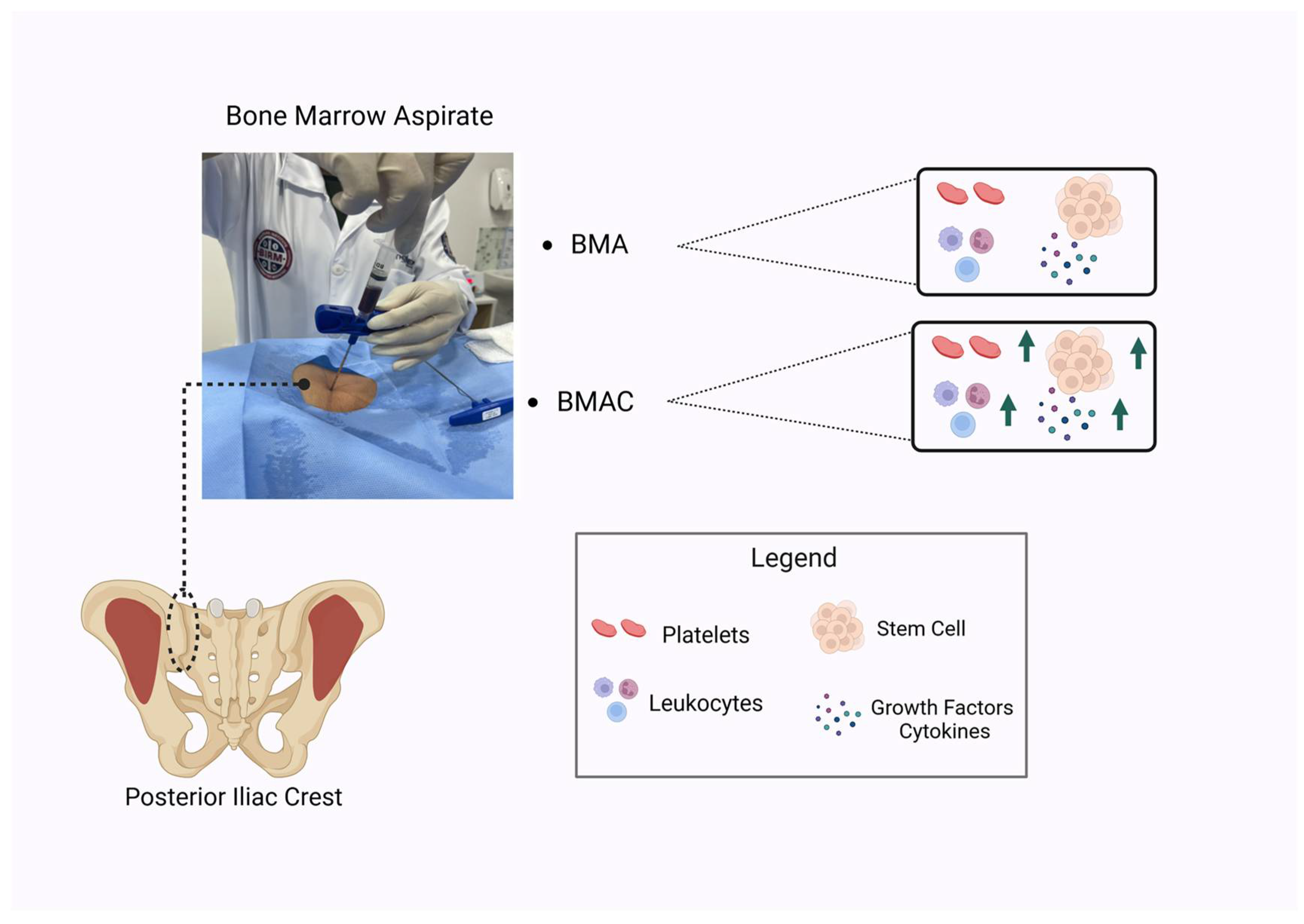
2.2. Bone Marrow Aspirate
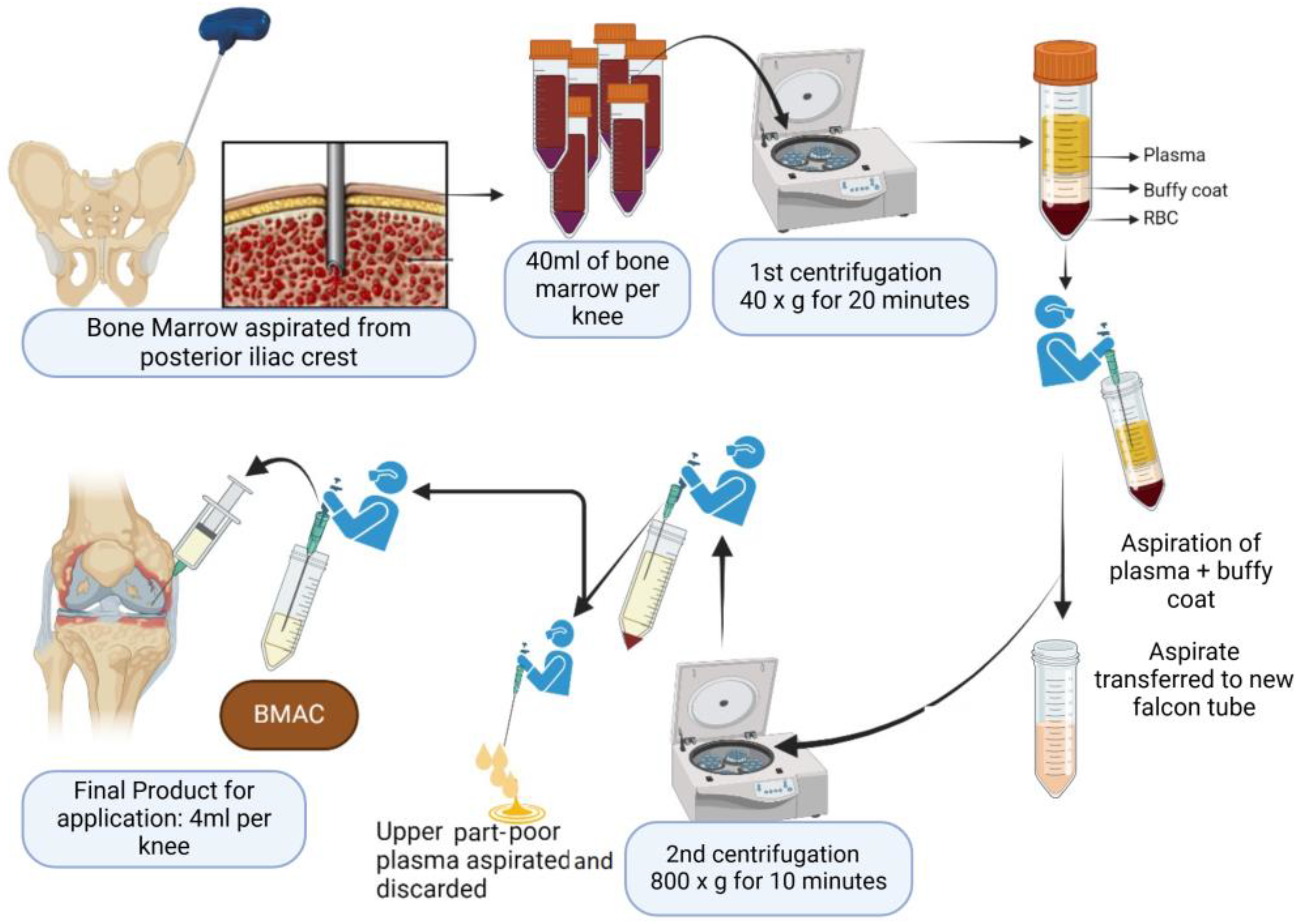
2.3. Bone Marrow Aspirate Concentrate
2.4. Implications and Challenges
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Di Nicola, V. Degenerative Osteoarthritis a Reversible Chronic Disease. Regen. Ther. 2020, 15, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Ege, F. Pathogenesis, Pathology and Genetics of Osteoarthritis. In Rheumatoid Arthritis; IntechOpen: London, UK, 2021; ISBN 978-1-83969-672-5. [Google Scholar]
- Zhang, K.; Fu, W. HIF-1α: Linking Subchondral Bone and Cartilage as a Therapeutic Target in Osteoarthritis. Biomater. Transl. 2024, 5, 89–91. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, Z.; Alexander, P.G.; Ocasio-Nieves, B.D.; Yocum, L.; Lin, H.; Tuan, R.S. Pathogenesis of Osteoarthritis: Risk Factors, Regulatory Pathways in Chondrocytes, and Experimental Models. Biology 2020, 9, 194. [Google Scholar] [CrossRef] [PubMed]
- Fontanella, C.G.; Belluzzi, E.; Pozzuoli, A.; Scioni, M.; Olivotto, E.; Reale, D.; Ruggieri, P.; De Caro, R.; Ramonda, R.; Carniel, E.L.; et al. Exploring Anatomo-Morphometric Characteristics of Infrapatellar, Suprapatellar Fat Pad, and Knee Ligaments in Osteoarthritis Compared to Post-Traumatic Lesions. Biomedicines 2022, 10, 1369. [Google Scholar] [CrossRef]
- Palazzo, C.; Nguyen, C.; Lefevre-Colau, M.-M.; Rannou, F.; Poiraudeau, S. Risk Factors and Burden of Osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 134–138. [Google Scholar] [CrossRef]
- El-Tallawy, S.N.; Nalamasu, R.; Salem, G.I.; LeQuang, J.A.K.; Pergolizzi, J.V.; Christo, P.J. Management of Musculoskeletal Pain: An Update with Emphasis on Chronic Musculoskeletal Pain. Pain Ther. 2021, 10, 181–209. [Google Scholar] [CrossRef]
- Sharma, A.; Kudesia, P.; Shi, Q.; Gandhi, R. Anxiety and Depression in Patients with Osteoarthritis: Impact and Management Challenges. Open Access Rheumatol. 2016, 8, 103–113. [Google Scholar] [CrossRef]
- Wang, S.-T.; Ni, G.-X. Depression in Osteoarthritis: Current Understanding. Neuropsychiatr. Dis. Treat. 2022, 18, 375. [Google Scholar] [CrossRef]
- Wilcox, S.; Brenes, G.A.; Levine, D.; Sevick, M.A.; Shumaker, S.A.; Craven, T. Factors Related to Sleep Disturbance in Older Adults Experiencing Knee Pain or Knee Pain with Radiographic Evidence of Knee Osteoarthritis. J. Am. Geriatr. Soc. 2000, 48, 1241–1251. [Google Scholar] [CrossRef]
- Sutton, J.S.; Muran, A.; Zaslav, K.; Grande, D. Orthobiologics: An Updated Definition. Open J. Regen. Med. 2023, 12, 36–48. [Google Scholar] [CrossRef]
- Noback, P.C.; Donnelley, C.A.; Yeatts, N.C.; Parisien, R.L.; Fleischli, J.E.; Ahmad, C.S.; Moorman, C.T.; Trofa, D.P.; Saltzman, B.M. Utilization of Orthobiologics by Sports Medicine Physicians: A Survey-Based Study. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2021, 5, e20.00185. [Google Scholar] [CrossRef]
- Dhillon, M.S.; Behera, P.; Patel, S.; Shetty, V. Orthobiologics and Platelet Rich Plasma. Indian J. Orthop. 2014, 48, 1–9. [Google Scholar] [CrossRef]
- Moreno-Garcia, A.; Rodriguez-Merchan, E.C. Orthobiologics: Current Role in Orthopedic Surgery and Traumatology. Arch. Bone Jt. Surg. 2022, 10, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Malgieri, A.; Kantzari, E.; Patrizi, M.P.; Gambardella, S. Bone Marrow and Umbilical Cord Blood Human Mesenchymal Stem Cells: State of the Art. Int. J. Clin. Exp. Med. 2010, 3, 248–269. [Google Scholar] [PubMed]
- Sugaya, H.; Yoshioka, T.; Kato, T.; Taniguchi, Y.; Kumagai, H.; Hyodo, K.; Ohneda, O.; Yamazaki, M.; Mishima, H. Comparative Analysis of Cellular and Growth Factor Composition in Bone Marrow Aspirate Concentrate and Platelet-Rich Plasma. Bone Marrow Res. 2018, 2018, 1549826. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraman, M.; Bingi, S.K.; Muthu, S.; Jeyaraman, N.; Packkyarathinam, R.P.; Ranjan, R.; Sharma, S.; Jha, S.K.; Khanna, M.; Rajendran, S.N.S.; et al. Impact of the Process Variables on the Yield of Mesenchymal Stromal Cells from Bone Marrow Aspirate Concentrate. Bioengineering 2022, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Muthu, S.; Jeyaraman, M.; Narula, A.; Ravi, V.R.; Gandi, A.; Khanna, M.; Maffulli, N.; Gupta, A. Factors Influencing the Yield of Progenitor Cells in Bone Marrow Aspiration Concentrate-A Retrospective Analysis of 58 Patients. Biomedicines 2023, 11, 738. [Google Scholar] [CrossRef]
- Lucas, D. Structural Organization of the Bone Marrow and Its Role in Hematopoiesis. Curr. Opin. Hematol. 2021, 28, 36–42. [Google Scholar] [CrossRef]
- Mapara, M.; Thomas, B.S.; Bhat, K.M. Rabbit as an Animal Model for Experimental Research. Dent. Res. J. 2012, 9, 111–118. [Google Scholar] [CrossRef]
- Han, I.; Kwon, B.-S.; Park, H.-K.; Kim, K.S. Differentiation Potential of Mesenchymal Stem Cells Is Related to Their Intrinsic Mechanical Properties. Int. Neurourol. J. 2017, 21, S24–S31. [Google Scholar] [CrossRef]
- Tan, L.; Liu, X.; Dou, H.; Hou, Y. Characteristics and Regulation of Mesenchymal Stem Cell Plasticity by the Microenvironment—Specific Factors Involved in the Regulation of MSC Plasticity. Genes Dis. 2022, 9, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Mesenchymal Stem Cells: Time to Change the Name! Stem Cells Transl. Med. 2017, 6, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Hosseiniyan Khatibi, S.M.; Kheyrolahzadeh, K.; Barzegari, A.; Rahbar Saadat, Y.; Zununi Vahed, S. Medicinal Signaling Cells: A Potential Antimicrobial Drug Store. J. Cell. Physiol. 2020, 235, 7731–7746. [Google Scholar] [CrossRef]
- Caplan, A.I. Medicinal Signalling Cells: They Work, so Use Them. Nature 2019, 566, 39. [Google Scholar] [CrossRef] [PubMed]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 Polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef]
- Chen, S.; Saeed, A.F.U.H.; Liu, Q.; Jiang, Q.; Xu, H.; Xiao, G.G.; Rao, L.; Duo, Y. Macrophages in Immunoregulation and Therapeutics. Sig. Transduct. Target. Ther. 2023, 8, 1–35. [Google Scholar] [CrossRef]
- Abdelrazik, H.; Giordano, E.; Barbanti Brodano, G.; Griffoni, C.; De Falco, E.; Pelagalli, A. Substantial Overview on Mesenchymal Stem Cell Biological and Physical Properties as an Opportunity in Translational Medicine. Int. J. Mol. Sci. 2019, 20, 5386. [Google Scholar] [CrossRef]
- Steens, J.; Klein, D. HOX Genes in Stem Cells: Maintaining Cellular Identity and Regulation of Differentiation. Front. Cell Dev. Biol. 2022, 10, 1002909. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Hong, S.-H. Hematopoietic Stem Cells and Their Roles in Tissue Regeneration. Int. J. Stem Cells 2019, 13, 1–12. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, C.; Li, J.; Han, J.; Liu, X.; Yang, H. The Physical Microenvironment of Hematopoietic Stem Cells and Its Emerging Roles in Engineering Applications. Stem Cell Res. Ther. 2019, 10, 327. [Google Scholar] [CrossRef]
- Salamanna, F.; Contartese, D.; Nicoli Aldini, N.; Barbanti Brodano, G.; Griffoni, C.; Gasbarrini, A.; Fini, M. Bone Marrow Aspirate Clot: A Technical Complication or a Smart Approach for Musculoskeletal Tissue Regeneration? J. Cell. Physiol. 2017, 233, 2723–2732. [Google Scholar] [CrossRef] [PubMed]
- Fennema, E.M.; Renard, A.J.S.; Leusink, A.; van Blitterswijk, C.A.; de Boer, J. The Effect of Bone Marrow Aspiration Strategy on the Yield and Quality of Human Mesenchymal Stem Cells. Acta Orthop. 2009, 80, 618–621. [Google Scholar] [CrossRef]
- Lana, J.F.; Navani, A.; Jeyaraman, M.; Santos, N.; Pires, L.; Santos, G.S.; Rodrigues, I.J.; Santos, D.; Mosaner, T.; Azzini, G.; et al. Sacral Bioneuromodulation: The Role of Bone Marrow Aspirate in Spinal Cord Injuries. Bioengineering 2024, 11, 461. [Google Scholar] [CrossRef] [PubMed]
- Santos Duarte Lana, J.F.; Furtado da Fonseca, L.; Mosaner, T.; Tieppo, C.E.; Marques Azzini, G.O.; Ribeiro, L.L.; Setti, T.; Purita, J. Bone Marrow Aspirate Clot: A Feasible Orthobiologic. J. Clin. Orthop. Trauma 2020, 11, S789–S794. [Google Scholar] [CrossRef]
- Tomaiuolo, M.; Brass, L.F.; Stalker, T.J. Regulation of Platelet Activation and Coagulation and Its Role in Vascular Injury and Arterial Thrombosis. Interv. Cardiol. Clin. 2017, 6, 1–12. [Google Scholar] [CrossRef]
- Ferrari, G.; Cusella-De Angelis, G.; Coletta, M.; Paolucci, E.; Stornaiuolo, A.; Cossu, G.; Mavilio, F. Muscle Regeneration by Bone Marrow-Derived Myogenic Progenitors. Science 1998, 279, 1528–1530. [Google Scholar] [CrossRef]
- Lana, J.F.; da Fonseca, L.F.; Azzini, G.; Santos, G.; Braga, M.; Cardoso Junior, A.M.; Murrell, W.D.; Gobbi, A.; Purita, J.; Percope de Andrade, M.A. Bone Marrow Aspirate Matrix: A Convenient Ally in Regenerative Medicine. Int. J. Mol. Sci. 2021, 22, 2762. [Google Scholar] [CrossRef]
- Opneja, A.; Kapoor, S.; Stavrou, E.X. Contribution of Platelets, the Coagulation and Fibrinolytic Systems to Cutaneous Wound Healing. Thromb. Res. 2019, 179, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Lim, Z.X.H.; Rai, B.; Tan, T.C.; Ramruttun, A.K.; Hui, J.H.; Nurcombe, V.; Teoh, S.H.; Cool, S.M. Autologous Bone Marrow Clot as an Alternative to Autograft for Bone Defect Healing. Bone Jt. Res. 2019, 8, 107–117. [Google Scholar] [CrossRef]
- Knapik, D.M.; Evuarherhe, A.; Frank, R.M.; Steinwachs, M.; Rodeo, S.; Mumme, M.; Cole, B.J. Nonoperative and Operative Soft-Tissue and Cartilage Regeneration and Orthopaedic Biologics of the Knee: An Orthoregeneration Network (ON) Foundation Review. Arthrosc. J. Arthrosc. Relat. Surg. 2021, 37, 2704–2721. [Google Scholar] [CrossRef]
- Wells, K.; Klein, M.; Hurwitz, N.; Santiago, K.; Cheng, J.; Abutalib, Z.; Beatty, N.; Lutz, G. Cellular and Clinical Analyses of Autologous Bone Marrow Aspirate Injectate for Knee Osteoarthritis: A Pilot Study. PM R 2021, 13, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, T.; Hoit, G.; Lee, A.; Watkins, E.; Henry, P.; Leroux, T.; Veillette, C.; Theodoropoulos, J.; Ogilvie-Harris, D.; Chahal, J. Injection of Bone Marrow Aspirate for Glenohumeral Joint Osteoarthritis: A Pilot Randomized Control Trial. Arthrosc. Sports Med. Rehabil. 2021, 3, e1431–e1440. [Google Scholar] [CrossRef] [PubMed]
- Tsitsilianos, N.; Shirazi, Z.; Lu, J.; Singh, J.R. Bone Marrow Aspirate Injection for Osteoarthritis of the Hip; A Pilot Study. Interv. Pain Med. 2022, 1, 100163. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraman, M.; Karthik, K.S.; Choudary, D.; Jeyaraman, N.; Nallakumarasamy, A.; Ramasubramian, S. Autologous Bone Marrow Aspiration Concentrate (BMAC) Therapy for Primary Knee Osteoarthritis—An Observational and Dose Escalation Study. Indian J. Orthop. 2024, 58, 1016–1026. [Google Scholar] [CrossRef]
- Jeyaraman, M.; Jeyaraman, N.; Ramasubramanian, S.; Ranjan, R.; Jha, S.K.; Gupta, A. Bone Marrow Aspirate Concentrate for Treatment of Primary Knee Osteoarthritis: A Prospective, Single-Center, Non-Randomized Study with 2-Year Follow-Up. Indian J. Orthop. 2024, 58, 894–904. [Google Scholar] [CrossRef]
- Chahla, J.; Dean, C.S.; Moatshe, G.; Pascual-Garrido, C.; Serra Cruz, R.; LaPrade, R.F. Concentrated Bone Marrow Aspirate for the Treatment of Chondral Injuries and Osteoarthritis of the Knee. Orthop. J. Sports Med. 2016, 4, 2325967115625481. [Google Scholar] [CrossRef]
- El-Kadiry, A.E.-H.; Lumbao, C.; Salame, N.; Rafei, M.; Shammaa, R. Bone Marrow Aspirate Concentrate versus Platelet-Rich Plasma for Treating Knee Osteoarthritis: A One-Year Non-Randomized Retrospective Comparative Study. BMC Musculoskelet. Disord. 2022, 23, 23. [Google Scholar] [CrossRef]
- Hussein, M.; van Eck, C.F.; Kregar Velikonja, N. Bone Marrow Aspirate Concentrate Is More Effective Than Hyaluronic Acid and Autologous Conditioned Serum in the Treatment of Knee Osteoarthritis: A Retrospective Study of 505 Consecutive Patients. Appl. Sci. 2021, 11, 2932. [Google Scholar] [CrossRef]
- Saw, K.-Y.; Hussin, P.; Loke, S.-C.; Azam, M.; Chen, H.-C.; Tay, Y.-G.; Low, S.; Wallin, K.-L.; Ragavanaidu, K. Articular Cartilage Regeneration with Autologous Marrow Aspirate and Hyaluronic Acid: An Experimental Study in a Goat Model. Arthroscopy 2009, 25, 1391–1400. [Google Scholar] [CrossRef]
- McIlwraith, C.W.; Fortier, L.A.; Frisbie, D.D.; Nixon, A.J. Equine Models of Articular Cartilage Repair. Cartilage 2011, 2, 317–326. [Google Scholar] [CrossRef]
- Dulic, O.; Rasovic, P.; Lalic, I.; Kecojevic, V.; Gavrilovic, G.; Abazovic, D.; Maric, D.; Miskulin, M.; Bumbasirevic, M. Bone Marrow Aspirate Concentrate versus Platelet Rich Plasma or Hyaluronic Acid for the Treatment of Knee Osteoarthritis. Medicina 2021, 57, 1193. [Google Scholar] [CrossRef] [PubMed]
- Vitali, M.; Ometti, M.; Pironti, P.; Salvato, D.; Sandrucci, A.; Leone, O.; Salini, V. Clinical and Functional Evaluation of Bone Marrow Aspirate Concentrate vs Autologous Conditioned Serum in the Treatment of Knee Osteoarthritis. Acta Biomed. 2022, 93, e2022222. [Google Scholar] [CrossRef]
- Cotter, E.J.; Wang, K.C.; Yanke, A.B.; Chubinskaya, S. Bone Marrow Aspirate Concentrate for Cartilage Defects of the Knee: From Bench to Bedside Evidence. Cartilage 2018, 9, 161–170. [Google Scholar] [CrossRef]
- Pabinger, C.; Lothaller, H.; Kobinia, G.S. Intra-Articular Injection of Bone Marrow Aspirate Concentrate (Mesenchymal Stem Cells) in KL Grade III and IV Knee Osteoarthritis: 4 Year Results of 37 Knees. Sci. Rep. 2024, 14, 2665. [Google Scholar] [CrossRef]
- Marongiu, G.; Contini, A.; Cozzi Lepri, A.; Donadu, M.; Verona, M.; Capone, A. The Treatment of Acute Diaphyseal Long-Bones Fractures with Orthobiologics and Pharmacological Interventions for Bone Healing Enhancement: A Systematic Review of Clinical Evidence. Bioengineering 2020, 7, 22. [Google Scholar] [CrossRef]
- Chahla, J.; Mannava, S.; Cinque, M.E.; Geeslin, A.G.; Codina, D.; LaPrade, R.F. Bone Marrow Aspirate Concentrate Harvesting and Processing Technique. Arthrosc. Tech. 2017, 6, e441–e445. [Google Scholar] [CrossRef]
- Brozovich, A.; Sinicrope, B.J.; Bauza, G.; Niclot, F.B.; Lintner, D.; Taraballi, F.; McCulloch, P.C. High Variability of Mesenchymal Stem Cells Obtained via Bone Marrow Aspirate Concentrate Compared with Traditional Bone Marrow Aspiration Technique. Orthop. J. Sports Med. 2021, 9, 23259671211058459. [Google Scholar] [CrossRef]
- Kim, G.B.; Shon, O.-J. Current Perspectives in Stem Cell Therapies for Osteoarthritis of the Knee. Yeungnam Univ. J. Med. 2020, 37, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Ip, H.L.; Nath, D.K.; Sawleh, S.H.; Kabir, M.H.; Jahan, N. Regenerative Medicine for Knee Osteoarthritis—The Efficacy and Safety of Intra-Articular Platelet-Rich Plasma and Mesenchymal Stem Cells Injections: A Literature Review. Cureus 2020, 12, e10575. [Google Scholar] [CrossRef]
- Nelson, P.A.; George, T.; Bowen, E.; Sheean, A.J.; Bedi, A. An Update on Orthobiologics: Cautious Optimism. Am. J. Sports Med. 2024, 52, 242–257. [Google Scholar] [CrossRef]
- Huddleston, H.P.; Maheshwer, B.; Wong, S.E.; Chahla, J.; Cole, B.J.; Yanke, A.B. An Update on the Use of Orthobiologics: Use of Biologics for Osteoarthritis. Oper. Tech. Sports Med. 2020, 28, 150759. [Google Scholar] [CrossRef]
- Shapiro, S.A.; Master, Z.; Arthurs, J.R.; Mautner, K. Tiered Approach to Considering Orthobiologics for Patients with Musculoskeletal Conditions. Br. J. Sports Med. 2023, 57, 179–180. [Google Scholar] [CrossRef] [PubMed]
- Murray, I.R.; Chahla, J.; Wordie, S.J.; Shapiro, S.A.; Piuzzi, N.S.; Frank, R.M.; Halbrecht, J.; Okada, K.; Nakamura, N.; Mandelbaum, B.; et al. Regulatory and Ethical Aspects of Orthobiologic Therapies. Orthop. J. Sports Med. 2022, 10, 23259671221101626. [Google Scholar] [CrossRef] [PubMed]
- Momaya, A.M.; McGee, A.S.; Dombrowsky, A.R.; Wild, A.J.; Faroqui, N.M.; Waldrop, R.P.; He, J.K.; Brabston, E.W.; Ponce, B.A. The Cost Variability of Orthobiologics. Sports Health 2019, 12, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Lee, J.; Chawla, A.; Rajagopalan, V.; Kohler, M.; Moelling, B.; McFarlane, K.H.; Sheth, K.R.; Ratliff, J.K.; Hu, S.S.; et al. A Stepwise Replicable Approach to Negotiating Value-Driven Supply Chain Contracts for Orthobiologics. JAAOS-J. Am. Acad. Orthop. Surg. 2023, 31, 470. [Google Scholar] [CrossRef]
- Purita, J.; Lana, J.F.S.D.; Kolber, M.; Rodrigues, B.L.; Mosaner, T.; Santos, G.S.; Caliari-Oliveira, C.; Huber, S.C. Bone Marrow-Derived Products: A Classification Proposal—Bone Marrow Aspirate, Bone Marrow Aspirate Concentrate or Hybrid? World J. Stem Cells 2020, 12, 241–250. [Google Scholar] [CrossRef]
- Guo, W.-Y.; Wang, W.-H.; Xu, P.-Y.; Kankala, R.K.; Chen, A.-Z. Decellularised Extracellular Matrix-Based Injectable Hydrogels for Tissue Engineering Applications. Biomater. Transl. 2024, 5, 114–128. [Google Scholar] [CrossRef]
| Feature | Bone Marrow Aspirate (BMA) | Bone Marrow Aspirate Concentrate (BMAC) |
|---|---|---|
| Composition | Contains mesenchymal stem cells (MSCs), hematopoietic stem cells (HSCs), endothelial progenitors, other progenitor cells, growth factors (BMPs, PDGF, TGF-β, VEGF, IL-8, IL-1Ra), and megakaryocytes. | Concentrated form of BMA, containing higher concentration of MSCs, HSCs, platelets, chemokines, and cytokines. |
| Preparation | Collected directly from the bone marrow, typically from the posterior iliac crest, without further manipulation. | BMA subjected to laboratory processing and centrifugation to concentrate the cellular and molecular components. |
| MSC Concentration | Low concentration of MSCs (approximately from 0.01% to 0.02%). | Higher concentration of MSCs compared to BMA. |
| Cellular Viability | Greater cellular viability compared to BMAC as it is not subjected to centrifugation or manipulation. | Cellular viability may be affected by centrifugation parameters and manipulation techniques. |
| Regulatory Compliance | Does not require laboratory manipulation, reducing regulatory compliance issues. | Requires laboratory manipulation, necessitating regulatory compliance measures. |
| Coagulation | BMA forms a clot after collection, which can play a role in healing due to platelet activation and growth factor release. | Not explicitly mentioned in the provided text. |
| Clinical Applications | Used in orthopedics and regenerative medicine for tissue regeneration of bones, cartilage, and soft tissues. | Emerging treatment for osteoarthritis (OA) capable of promoting angiogenesis, osteoinduction, osteoconduction, and osteogenesis. Reported improvements in symptoms and quality of life scores for OA patients. |
| Advantages | Easy to collect and apply without laboratory manipulation, reducing costs and regulatory issues. The formed clot can aid in healing. | Higher concentration of MSCs, HSCs, and growth factors, promoting tissue regeneration, angiogenesis, and immunomodulation. Can be used concomitantly with other procedures. |
| Disadvantages | Lower concentration of MSCs compared to BMAC. | Requires laboratory manipulation, which may affect cellular viability and increase regulatory compliance issues. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lana, J.F.; Purita, J.; Jeyaraman, M.; de Souza, B.F.; Rodrigues, B.L.; Huber, S.C.; Caliari, C.; Santos, G.S.; da Fonseca, L.F.; Dallo, I.; et al. Innovative Approaches in Knee Osteoarthritis Treatment: A Comprehensive Review of Bone Marrow-Derived Products. Biomedicines 2024, 12, 2812. https://doi.org/10.3390/biomedicines12122812
Lana JF, Purita J, Jeyaraman M, de Souza BF, Rodrigues BL, Huber SC, Caliari C, Santos GS, da Fonseca LF, Dallo I, et al. Innovative Approaches in Knee Osteoarthritis Treatment: A Comprehensive Review of Bone Marrow-Derived Products. Biomedicines. 2024; 12(12):2812. https://doi.org/10.3390/biomedicines12122812
Chicago/Turabian StyleLana, José Fábio, Joseph Purita, Madhan Jeyaraman, Bianca Freitas de Souza, Bruno Lima Rodrigues, Stephany Cares Huber, Carolina Caliari, Gabriel Silva Santos, Lucas Furtado da Fonseca, Ignacio Dallo, and et al. 2024. "Innovative Approaches in Knee Osteoarthritis Treatment: A Comprehensive Review of Bone Marrow-Derived Products" Biomedicines 12, no. 12: 2812. https://doi.org/10.3390/biomedicines12122812
APA StyleLana, J. F., Purita, J., Jeyaraman, M., de Souza, B. F., Rodrigues, B. L., Huber, S. C., Caliari, C., Santos, G. S., da Fonseca, L. F., Dallo, I., Navani, A., De Andrade, M. A. P., & Everts, P. A. (2024). Innovative Approaches in Knee Osteoarthritis Treatment: A Comprehensive Review of Bone Marrow-Derived Products. Biomedicines, 12(12), 2812. https://doi.org/10.3390/biomedicines12122812










