Machine Learning for Early Detection of Cognitive Decline in Parkinson’s Disease Using Multimodal Biomarker and Clinical Data
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Outcome Definition
2.3. Input Variables
2.4. Data Imputation and Transformation
2.5. Feature Selection
2.6. Statistical Analysis and ML Methods
3. Results
3.1. Baseline Characteristics and Descriptive Statistics by Outcome
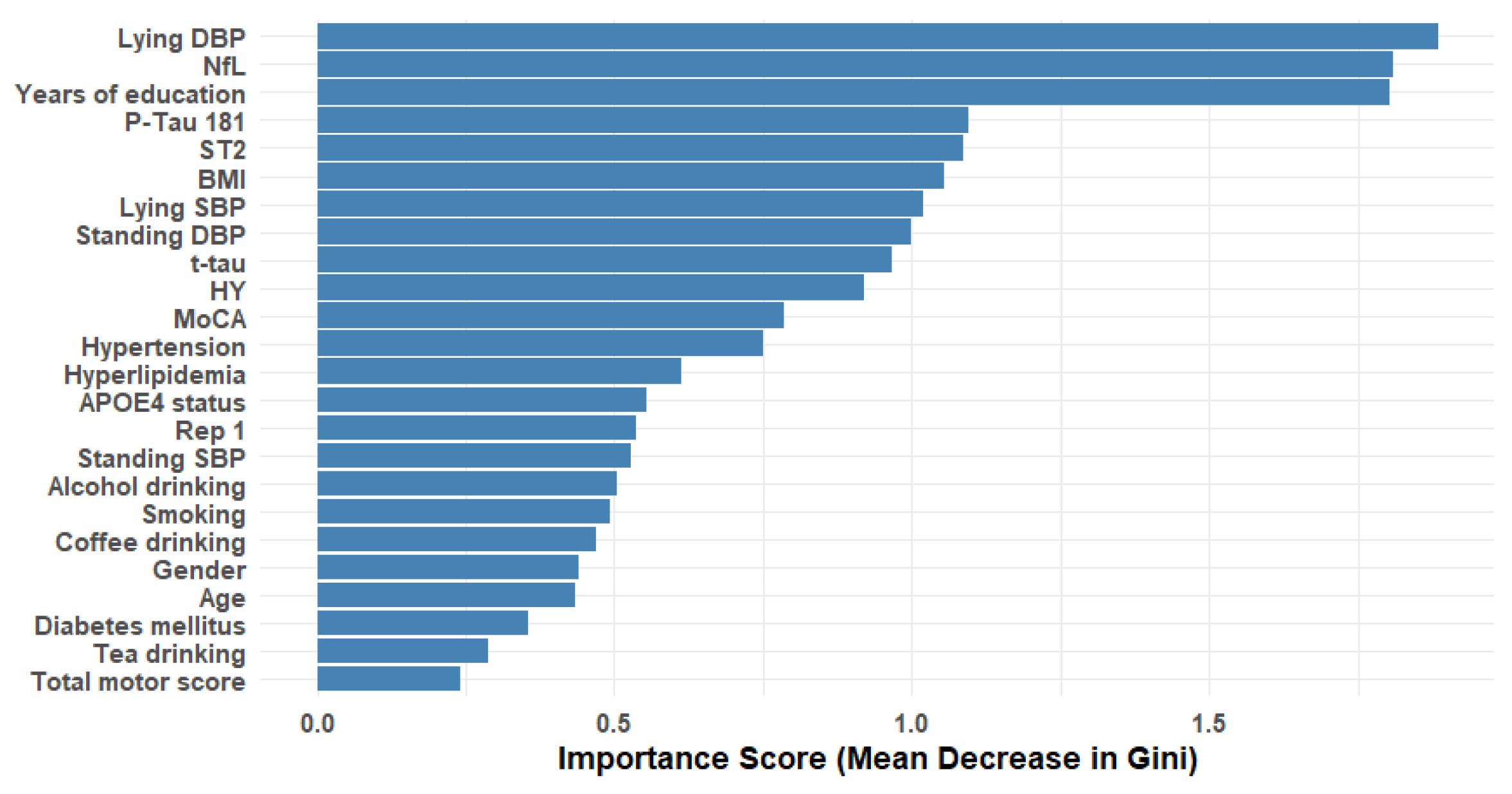
3.2. Feature Selection Analysis
3.3. Model Performance and ROC Analysis
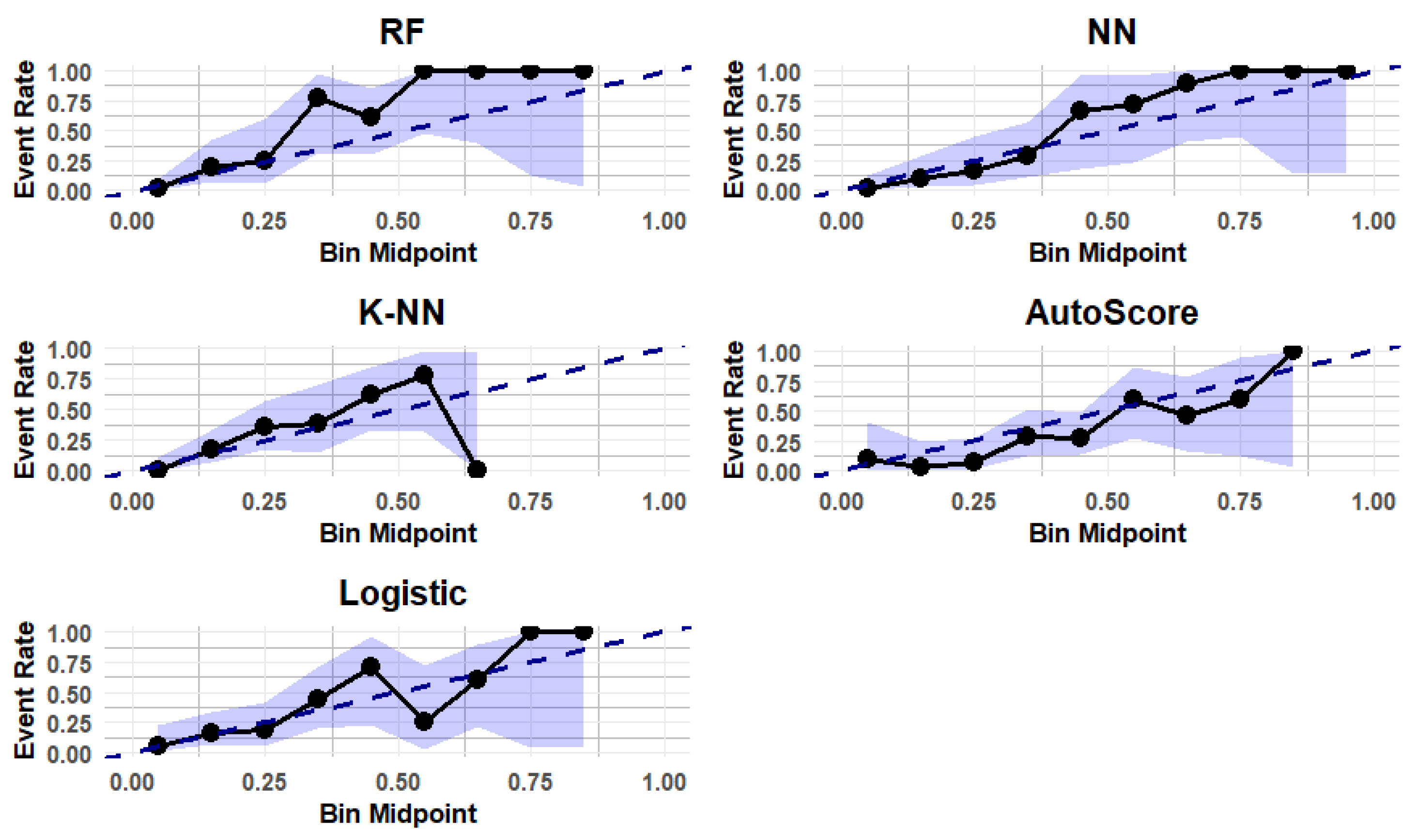
3.4. Calibration of the Predictive Models
3.5. Score of Risk Factors Based on AutoScore Algorithm
3.6. Summary of Key Findings
4. Discussion
Limitations and Future Avenues
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PD | Parkinson’s disease |
| CD | Cognitive decline |
| MoCA | Montreal Cognitive Assessment |
| AUC | Area under the curve |
| MCI | Mild cognitive impairment |
| PDD | Parkinson’s disease dementia |
| ML | Machine learning |
| RF | Random forest |
| CSF | Cerebrospinal fluid |
| PPMI | Parkinson’s Progression Markers Initiative |
| ApoA1 | Apolipoprotein A1 |
| TG | Triglycerides |
| HY | Hoehn and Yahr |
| MDS-UPDRS | Movement Disorder Society-Unified Parkinson’s disease Rating Scale |
| BMI | Body mass index |
| SBP | Systolic blood pressure |
| DBP | Diastolic blood pressure |
| ST2 | Suppression of tumorigenicity 2 |
| NfL | Neurofilament light chain |
| t-tau | Total tau |
| p-tau 181 | Phosphorylated tau at threonine 181 |
| APOE | Apolipoprotein E |
| REP1 | Alpha-synuclein gene promoter |
| OR | Odds ratio |
| SD | Standard deviation |
| CI | Confidence interval |
| KNN | K-nearest neighbors |
| NN | Neural network |
| ROC | Receiver operating characteristic |
| AD | Alzheimer’s disease |
| FTD | Frontotemporal dementia |
References
- Aarsland, D.; Creese, B.; Politis, M.; Chaudhuri, K.R.; Ffytche, D.H.; Weintraub, D.; Ballard, C. Cognitive decline in Parkinson disease. Nat. Rev. Neurol. 2017, 13, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Ciucci, M.R.; Grant, L.M.; Rajamanickam, E.S.P.; Hilby, B.L.; Blue, K.V.; Jones, C.A.; Kelm-Nelson, C.A. Early Identification and Treatment of Communication and Swallowing Deficits in Parkinson Disease; Semin. Speech Lang; Thieme Medical Publishers: New York, NY, USA, 2013; pp. 185–202. [Google Scholar]
- Zhang, J.; Zhou, W.; Yu, H.; Wang, T.; Wang, X.; Liu, L.; Wen, Y. Prediction of Parkinson’s Disease Using Machine Learning Methods. Biomolecules 2023, 13, 1761. [Google Scholar] [CrossRef] [PubMed]
- Simuni, T.; Sethi, K. Nonmotor manifestations of Parkinson’s disease. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 2008, 64, S65–S80. [Google Scholar] [CrossRef]
- Williams-Gray, C.H.; Mason, S.L.; Evans, J.R.; Foltynie, T.; Brayne, C.; Robbins, T.W.; Barker, R.A. The CamPaIGN study of Parkinson’s disease: 10-year outlook in an incident population-based cohort. J. Neurol. Neurosurg. Psychiatry 2013, 84, 1258–1264. [Google Scholar] [CrossRef] [PubMed]
- Riedel, O.; Klotsche, J.; Spottke, A.; Deuschl, G.; Förstl, H.; Henn, F.; Heuser, I.; Oertel, W.; Reichmann, H.; Riederer, P. Frequency of dementia, depression, and other neuropsychiatric symptoms in 1,449 outpatients with Parkinson’s disease. J. Neurol. 2010, 257, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Saffari, S.E.; Xiao, B.; Ng, S.Y.E.; Chia, N.; Choi, X.; Heng, D.L.; Ng, E.; Xu, Z.; Tay, K.-Y. Disease Progression of Data-Driven Subtypes of Parkinson’s Disease: 5-Year Longitudinal Study from the Early Parkinson’s Disease Longitudinal Singapore (PALS) Cohort. JPD 2024, 14, 1051–1059. [Google Scholar] [CrossRef]
- Deng, X.; Saffari, S.E.; Xiao, B.; Ng, S.Y.E.; Chia, N.; Choi, X.; Heng, D.L.; Xu, Z.; Tay, K.-Y.; Au, W.-L. Disease progression in Parkinson’s disease patients with mild cognitive impairment: 5-year longitudinal study from the early Parkinson’s disease longitudinal Singapore (PALS) cohort. Aging 2024, 16, 11491. [Google Scholar] [CrossRef]
- Battaglia, S.; Avenanti, A.; Vécsei, L.; Tanaka, M. Neurodegeneration in cognitive impairment and mood disorders for experimental, clinical and translational neuropsychiatry. Biomedicines 2024, 12, 574. [Google Scholar] [CrossRef]
- Fang, C.; Lv, L.; Mao, S.; Dong, H.; Liu, B. Cognition deficits in Parkinson’s disease: Mechanisms and treatment. Parkinsons Dis. 2020, 2020, 2076942. [Google Scholar] [CrossRef]
- Kandiah, N.; Mak, E.; Ng, A.; Huang, S.; Au, W.L.; Sitoh, Y.Y.; Tan, L.C.S. Cerebral white matter hyperintensity in Parkinson’s disease: A major risk factor for mild cognitive impairment. Park. Relat. Disord. 2013, 19, 680–683. [Google Scholar] [CrossRef]
- Pigott, K.; Rick, J.; Xie, S.X.; Hurtig, H.; Chen-Plotkin, A.; Duda, J.E.; Morley, J.F.; Chahine, L.M.; Dahodwala, N.; Akhtar, R.S. Longitudinal study of normal cognition in Parkinson disease. Neurology 2015, 85, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Hely, M.A.; Reid, W.G.; Adena, M.A.; Halliday, G.M.; Morris, J.G. The Sydney multicenter study of Parkinson’s disease: The inevitability of dementia at 20 years. Mov. Disord. 2008, 23, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Lawson, R.A.; Yarnall, A.J.; Duncan, G.W.; Breen, D.P.; Khoo, T.K.; Williams-Gray, C.H.; Barker, R.A.; Burn, D.J. Stability of mild cognitive impairment in newly diagnosed Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2017, 88, 648–652. [Google Scholar] [CrossRef]
- Poletti, M.; Emre, M.; Bonuccelli, U. Mild cognitive impairment and cognitive reserve in Parkinson’s disease. Park. Relat. Disord. 2011, 17, 579–586. [Google Scholar] [CrossRef]
- Chua, C.Y.; Koh, M.R.E.; Chia, N.S.-Y.; Ng, S.Y.-E.; Saffari, S.E.; Wen, M.-C.; Chen, R.Y.-Y.; Choi, X.; Heng, D.L.; Neo, S.X. Subjective cognitive complaints in early Parkinson’s disease patients with normal cognition are associated with affective symptoms. Park. Relat. Disord. 2021, 82, 24–28. [Google Scholar] [CrossRef]
- Pressley, J.C.; Louis, E.D.; Tang, M.X.; Cote, L.; Cohen, P.D.; Glied, S.; Mayeux, R. The impact of comorbid disease and injuries on resource use and expenditures in parkinsonism. Neurology 2003, 60, 87–93. [Google Scholar] [CrossRef]
- Greenland, J.C.; Williams-Gray, C.H.; Barker, R.A. The clinical heterogeneity of Parkinson’s disease and its therapeutic implications. Eur. J. Neurosci. 2019, 49, 328–338. [Google Scholar] [CrossRef]
- Shen, J.; Amari, N.; Zack, R.; Skrinak, R.T.; Unger, T.L.; Posavi, M.; Tropea, T.F.; Xie, S.X.; Van Deerlin, V.M.; Dewey, R.B., Jr.; et al. Plasma MIA, CRP, and albumin predict cognitive decline in Parkinson’s disease. Ann. Neurol. 2022, 92, 255–269. [Google Scholar] [CrossRef]
- Martin, F.L.; Williamson, S.J.M.; Paleologou, K.E.; Allsop, D.; El-Agnaf, O.M.A. α-Synuclein and the pathogenesis of Parkinson’s disease. Protein Pept. Lett. 2004, 11, 229–237. [Google Scholar] [CrossRef]
- Alves, G.; Brønnick, K.; Aarsland, D.; Blennow, K.; Zetterberg, H.; Ballard, C.; Kurz, M.W.; Andreasson, U.; Tysnes, O.-B.; Larsen, J.P. CSF amyloid-β and tau proteins, and cognitive performance, in early and untreated Parkinson’s Disease: The Norwegian ParkWest study. J. Neurol. Neurosurg. Psychiatry 2010, 81, 1080–1086. [Google Scholar] [CrossRef]
- Deng, X.; Saffari, S.E.; Liu, N.; Xiao, B.; Allen, J.C.; Ng, S.Y.E.; Chia, N.; Tan, Y.J.; Choi, X.; Heng, D.L. Biomarker characterization of clinical subtypes of Parkinson Disease. NPJ Park. Dis. 2022, 8, 109. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Saffari, S.E.; Ng, S.Y.E.; Chia, N.; Tan, J.Y.; Choi, X.; Heng, D.L.; Xu, Z.; Tay, K.-Y.; Au, W.-L. Blood lipid biomarkers in early Parkinson’s disease and Parkinson’s disease with mild cognitive impairment. J. Park. Dis. 2022, 12, 1937–1943. [Google Scholar] [CrossRef] [PubMed]
- Hoogland, J.; De Bie, R.M.A.; Williams-Gray, C.H.; Muslimović, D.; Schmand, B.; Post, B. Catechol-O-methyltransferase val158met and cognitive function in Parkinson’s disease. Mov. Disord. 2010, 25, 2550–2554. [Google Scholar] [CrossRef]
- Kim, R.; Kim, H.J.; Shin, J.H.; Lee, C.Y.; Jeon, S.H.; Jeon, B. Serum inflammatory markers and progression of nonmotor symptoms in early Parkinson’s disease. Mov. Disord. 2022, 37, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Michael, J.; Fox Foundation. FDA Issues Letter of Support Encouraging Use of Synuclein-Based Biomarker (Asyn-SAA) in Clinical Trials 2024. Available online: https://web.archive.org/web/20240930072011/https://www.michaeljfox.org/publication/fda-issues-letter-support-encouraging-use-synuclein-based-biomarker-asyn-saa-clinical (accessed on 30 September 2024).
- Hu, X.; Yang, Y.; Gong, D. Changes of cerebrospinal fluid Aβ 42, t-tau, and p-tau in Parkinson’s disease patients with cognitive impairment relative to those with normal cognition: A meta-analysis. Neurol. Sci. 2017, 38, 1953–1961. [Google Scholar] [CrossRef]
- Mollenhauer, B.; Bibl, M.; Wiltfang, J.; Steinacker, P.; Ciesielczyk, B.; Neubert, K.; Trenkwalder, C.; Otto, M. Total tau Protein, Phosphorylated tau (181p) Protein, β-Amyloid1–42, and β-Amyloid1–40 in Cerebrospinal Fluid of Patients with Dementia with Lewy Bodies; De Gruyter: Berlin, Germany, 2006. [Google Scholar]
- Siderowf, A.; Xie, S.X.; Hurtig, H.; Weintraub, D.; Duda, J.; Chen-Plotkin, A.; Shaw, L.M.; Van Deerlin, V.; Trojanowski, J.Q.; Clark, C. CSF amyloid β 1–42 predicts cognitive decline in Parkinson disease. Neurology 2010, 75, 1055–1061. [Google Scholar] [CrossRef]
- Lerche, S.; Wurster, I.; Röben, B.; Zimmermann, M.; Machetanz, G.; Wiethoff, S.; Dehnert, M.; Rietschel, L.; Riebenbauer, B.; Deuschle, C. CSF NFL in a longitudinally assessed PD cohort: Age effects and cognitive trajectories. Mov. Disord. 2020, 35, 1138–1144. [Google Scholar] [CrossRef]
- Parnetti, L.; Gaetani, L.; Eusebi, P.; Paciotti, S.; Hansson, O.; El-Agnaf, O.; Mollenhauer, B.; Blennow, K.; Calabresi, P. CSF and blood biomarkers for Parkinson’s disease. Lancet Neurol. 2019, 18, 573–586. [Google Scholar] [CrossRef]
- Youssef, P.; Hughes, L.; Kim, W.S.; Halliday, G.M.; Lewis, S.J.; Cooper, A.; Dzamko, N. Evaluation of plasma levels of NFL, GFAP, UCHL1 and tau as Parkinson’s disease biomarkers using multiplexed single molecule counting. Sci. Rep. 2023, 13, 5217. [Google Scholar] [CrossRef]
- Kim, R.; Park, S.; Yoo, D.; Jun, J.-S.; Jeon, B. Association of physical activity and APOE genotype with longitudinal cognitive change in early Parkinson disease. Neurology 2021, 96, e2429–e2437. [Google Scholar] [CrossRef]
- Chen, N.-C.; Chen, H.-L.; Li, S.-H.; Chang, Y.-H.; Chen, M.-H.; Tsai, N.-W.; Yu, C.-C.; Yang, S.-Y.; Lu, C.-H.; Lin, W.-C. Plasma levels of α-synuclein, Aβ-40 and T-tau as biomarkers to predict cognitive impairment in Parkinson’s disease. Front. Aging Neurosci. 2020, 12, 112. [Google Scholar] [CrossRef] [PubMed]
- Pellecchia, M.T.; Savastano, R.; Moccia, M.; Picillo, M.; Siano, P.; Erro, R.; Vallelunga, A.; Amboni, M.; Vitale, C.; Santangelo, G. Lower serum uric acid is associated with mild cognitive impairment in early Parkinson’s disease: A 4-year follow-up study. J. Neural Transm. 2016, 123, 1399–1402. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, H.; Tsuji, A.; Hashimoto, Y.; Takata, M.; Koga, S.; Nishida, K.; Futamura, N.; Kawamoto, M.; Kohara, N.; Dickson, D.W. Discrepancy between distribution of alpha-synuclein oligomers and Lewy-related pathology in Parkinson’s disease. Acta Neuropathol. Commun 2022, 10, 133. [Google Scholar] [CrossRef] [PubMed]
- Aamodt, W.W.; Waligorska, T.; Shen, J.; Tropea, T.F.; Siderowf, A.; Weintraub, D.; Grossman, M.; Irwin, D.; Wolk, D.A.; Xie, S.X. Neurofilament light chain as a biomarker for cognitive decline in Parkinson disease. Mov. Disord. 2021, 36, 2945–2950. [Google Scholar] [CrossRef]
- Chen-Plotkin, A.S.; Hu, W.T.; Siderowf, A.; Weintraub, D.; Goldmann Gross, R.; Hurtig, H.I.; Xie, S.X.; Arnold, S.E.; Grossman, M.; Clark, C.M. Plasma epidermal growth factor levels predict cognitive decline in Parkinson disease. Ann. Neurol. 2011, 69, 655–663. [Google Scholar] [CrossRef]
- Ma, L.-Z.; Zhang, C.; Wang, H.; Ma, Y.-H.; Shen, X.-N.; Wang, J.; Tan, L.; Dong, Q.; Yu, J.-T. Serum neurofilament dynamics predicts cognitive progression in de novo Parkinson’s disease. J. Park. Dis. 2021, 11, 1117–1127. [Google Scholar] [CrossRef]
- Schrag, A.; Siddiqui, U.F.; Anastasiou, Z.; Weintraub, D.; Schott, J.M. Clinical variables and biomarkers in prediction of cognitive impairment in patients with newly diagnosed Parkinson’s disease: A cohort study. Lancet Neurol. 2017, 16, 66–75. [Google Scholar] [CrossRef]
- Almgren, H.; Camacho, M.; Hanganu, A.; Kibreab, M.; Camicioli, R.; Ismail, Z.; Forkert, N.D.; Monchi, O. Machine learning-based prediction of longitudinal cognitive decline in early Parkinson’s disease using multimodal features. Sci. Rep. 2023, 13, 13193. [Google Scholar] [CrossRef]
- Deng, X.; Ning, Y.; Saffari, S.E.; Xiao, B.; Niu, C.; Ng, S.Y.E.; Chia, N.; Choi, X.; Heng, D.L.; Tan, Y.J. Identifying clinical features and blood biomarkers associated with mild cognitive impairment in Parkinson disease using machine learning. Eur. J. Neurol. 2023, 30, 1658–1666. [Google Scholar] [CrossRef]
- Ng, S.Y.-E.; Chia, N.S.-Y.; Abbas, M.M.; Saffari, E.S.; Choi, X.; Heng, D.L.; Xu, Z.; Tay, K.-Y.; Au, W.-L.; Tan, E.-K. Physical activity improves anxiety and apathy in early Parkinson’s disease: A longitudinal follow-up study. Front. Neurol. 2021, 11, 625897. [Google Scholar] [CrossRef]
- Yong, A.C.W.; Tan, Y.J.; Zhao, Y.; Lu, Z.; Ng, E.Y.L.; Ng, S.Y.E.; Chia, N.S.Y.; Choi, X.; Heng, D.; Neo, S. SNCA Rep1 microsatellite length influences non-motor symptoms in early Parkinson’s disease. Aging 2020, 12, 20880. [Google Scholar] [CrossRef] [PubMed]
- Stekhoven, D.J. Using the missForest package. R Package 2011, 1–11. [Google Scholar] [CrossRef]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Niculescu-Mizil, A.; Caruana, R. Predicting good probabilities with supervised learning. In Proceedings of the Twenty-Second International Conference (ICML 2005), Bonn, Germany, 7–11 August 2005; pp. 625–632. [Google Scholar]
- Saffari, S.E.; Ning, Y.; Xie, F.; Chakraborty, B.; Volovici, V.; Vaughan, R.; Ong, M.E.H.; Liu, N. AutoScore-Ordinal: An interpretable machine learning framework for generating scoring models for ordinal outcomes. BMC Med. Res. Methodol. 2022, 22, 286. [Google Scholar] [CrossRef]
- Roheger, M.; Kalbe, E.; Liepelt-Scarfone, I. Progression of cognitive decline in Parkinson’s disease. J. Park. Dis. 2018, 8, 183–193. [Google Scholar] [CrossRef]
- Forbes, E.; Tropea, T.F.; Mantri, S.; Xie, S.X.; Morley, J.F. Modifiable comorbidities associated with cognitive decline in Parkinson’s disease. Mov. Disord. Clin. Pract. 2021, 8, 254–263. [Google Scholar] [CrossRef]
- Zhang, L.; Gu, L.-Y.; Dai, S.-B.; Zheng, R.; Jin, C.-Y.; Fang, Y.; Yang, W.-Y.; Tian, J.; Yin, X.-Z.; Zhao, G.-H. Associations of body mass index-metabolic phenotypes with cognitive decline in Parkinson’s disease. Eur. Neurol. 2022, 85, 24–30. [Google Scholar] [CrossRef]
- Yoo, H.S.; Chung, S.J.; Lee, P.H.; Sohn, Y.H.; Kang, S.Y. The influence of body mass index at diagnosis on cognitive decline in Parkinson’s disease. J. Clin. Neurol. 2019, 15, 517–526. [Google Scholar] [CrossRef]
- Kim, H.J.; Oh, E.S.; Lee, J.H.; Moon, J.S.; Oh, J.E.; Shin, J.W.; Lee, K.J.; Baek, I.C.; Jeong, S.-H.; Song, H.-J. Relationship between changes of body mass index (BMI) and cognitive decline in Parkinson’s disease (PD). Arch. Gerontol. Geriatr. 2012, 55, 70–72. [Google Scholar] [CrossRef]
- Kwon, K.-Y.; Pyo, S.J.; Lee, H.M.; Seo, W.-K.; Koh, S.-B. Cognition and visit-to-visit variability of blood pressure and heart rate in de novo patients with Parkinson’s disease. J. Mov. Disord 2016, 9, 144. [Google Scholar] [CrossRef]
- Xiao, Y.; Yang, T.; Zhang, L.; Wei, Q.; Ou, R.; Hou, Y.; Liu, K.; Lin, J.; Jiang, Q.; Shang, H. Association between the blood pressure variability and cognitive decline in Parkinson’s disease. Brain Behav. 2023, 13, e3319. [Google Scholar] [CrossRef] [PubMed]
- Doiron, M.; Langlois, M.; Dupré, N.; Simard, M. The influence of vascular risk factors on cognitive function in early Parkinson’s disease. Int. J. Geriatr. Psychiatry 2018, 33, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, M.; De Micco, R.; Trojano, L.; De Stefano, M.; Baiano, C.; Passaniti, C.; De Mase, A.; Russo, A.; Tedeschi, G.; Tessitore, A. Cognitive impairment is associated with Hoehn and Yahr stages in early, de novo Parkinson disease patients. Park. Relat. Disord. 2017, 41, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Pagano, G.; Boess, F.G.; Taylor, K.I.; Ricci, B.; Mollenhauer, B.; Poewe, W.; Boulay, A.; Anzures-Cabrera, J.; Vogt, A.; Marchesi, M. A phase II study to evaluate the safety and efficacy of prasinezumab in early Parkinson’s disease (PASADENA): Rationale, design, and baseline data. Front. Neurol. 2021, 12, 705407. [Google Scholar] [CrossRef]
- Jackson, H.; Anzures-Cabrera, J.; Taylor, K.I.; Pagano, G.; Investigators, P.; Prasinezumab Study, G. Hoehn and Yahr stage and striatal Dat-SPECT uptake are predictors of Parkinson’s disease motor progression. Front. Neurosci. 2021, 15, 765765. [Google Scholar] [CrossRef]
- Wang, X.; Yang, X.; He, W.; Song, X.; Zhang, G.; Niu, P.; Chen, T. The association of serum neurofilament light chains with early symptoms related to Parkinson’s disease: A cross-sectional study. J. Affect. Disord. 2023, 343, 144–152. [Google Scholar] [CrossRef]
- Welton, T.; Tan, Y.J.; Saffari, S.E.; Ng, S.Y.; Chia, N.S.; Yong, A.C.; Choi, X.; Heng, D.L.; Shih, Y.-C.; Hartono, S. Plasma neurofilament light concentration is associated with diffusion-tensor MRI-based measures of neurodegeneration in early Parkinson’s disease. J. Park. Dis. 2022, 12, 2135–2146. [Google Scholar] [CrossRef]
- Ng, A.S.L.; Tan, Y.J.; Yong, A.C.W.; Saffari, S.E.; Lu, Z.; Ng, E.Y.; Ng, S.Y.E.; Chia, N.S.Y.; Choi, X.; Heng, D. Utility of plasma Neurofilament light as a diagnostic and prognostic biomarker of the postural instability gait disorder motor subtype in early Parkinson’s disease. Mol. Neurodegener. 2020, 15, 1–8. [Google Scholar] [CrossRef]
- Batzu, L.; Rota, S.; Hye, A.; Heslegrave, A.; Trivedi, D.; Gibson, L.L.; Farrell, C.; Zinzalias, P.; Rizos, A.; Zetterberg, H. Plasma p-tau181, neurofilament light chain and association with cognition in Parkinson’s disease. npj Park. Dis. 2022, 8, 154. [Google Scholar] [CrossRef]
- Tao, M.; Dou, K.; Xie, Y.; Hou, B.; Xie, A. The associations of cerebrospinal fluid biomarkers with cognition, and rapid eye movement sleep behavior disorder in early Parkinson’s disease. Front. Neurosci. 2022, 16, 1049118. [Google Scholar] [CrossRef]
- Terrelonge, M.; Marder, K.S.; Weintraub, D.; Alcalay, R.N. CSF β-amyloid 1–42 predicts progression to cognitive impairment in newly diagnosed Parkinson disease. J. Mol. Neurosci. 2016, 58, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.J.; Saffari, S.E.; Zhao, Y.; Ng, E.Y.; Yong, A.C.; Ng, S.Y.; Chia, N.S.; Choi, X.; Heng, D.; Neo, S. Longitudinal Study of SNCA Rep1 Polymorphism on Executive Function in Early Parkinson’s Disease. J. Park. Dis. 2022, 12, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.J.; Siow, I.; Saffari, S.E.; Ting, S.K.S.; Li, Z.; Kandiah, N.; Tan, L.C.S.; Tan, E.K.; Ng, A.S.L. Plasma soluble ST2 levels are higher in neurodegenerative disorders and associated with poorer cognition. J. Alzheimer’s Dis. 2023, 92, 573–580. [Google Scholar] [CrossRef] [PubMed]
| Total | No Progression | Progression | OR (95% CIs) | p-Value | |
|---|---|---|---|---|---|
| N = 193 | N = 149 | N = 44 | |||
| Demographic Characteristics | |||||
| Male Gender | 112 (58.0%) | 85 (57.0%) | 27 (61.4%) | 1.2 (0.6, 2.4) | 0.737 |
| Smoker | 56 (29.0%) | 43 (28.9%) | 13 (29.5%) | 1.0 (0.5, 2.1) | 1.000 |
| Years of education (≥10 years) | 135 (69.9%) | 112 (75.2%) | 23 (52.3%) | 0.4 (0.2, 0.7) | 0.006 |
| Tea drinking | 180 (93.3%) | 138 (92.6%) | 42 (95.5%) | 1.6 (0.4, 11.5) | 0.736 |
| Coffee drinking | 175 (90.7%) | 135 (90.6%) | 40 (90.9%) | 1.0 (0.3, 3.8) | 1.000 |
| Alcohol drinking | 125 (64.8%) | 101 (67.8%) | 24 (54.5%) | 0.6 (0.3, 1.1) | 0.151 |
| BMI (>25 kg/m2) | 64 (33.2%) | 48 (32.2%) | 16 (36.4%) | 1.2 (0.6, 2.4) | 0.740 |
| Age (>65 years) | 97 (50.3%) | 72 (48.3%) | 25 (56.8%) | 1.4 (0.7, 2.8) | 0.413 |
| Clinical Assessments | |||||
| Lying SBP (≥140 mmHg) | 95 (49.2%) | 67 (45.0%) | 28 (63.6%) | 2.1 (1.1, 4.3) | 0.045 |
| Lying DBP (≥80 mmHg) | 67 (34.7%) | 44 (29.5%) | 23 (52.3%) | 2.6 (1.3, 5.2) | 0.009 |
| Standing SBP (≥140 mmHg) | 85 (44.0%) | 63 (42.3%) | 22 (50.0%) | 1.4 (0.7, 2.7) | 0.463 |
| Standing DBP (≥80 mmHg) | 95 (49.2%) | 67 (45.0%) | 28 (63.6%) | 2.1 (1.1, 4.3) | 0.045 |
| Diabetes mellitus | 31 (16.1%) | 25 (16.8%) | 6 (13.6%) | 0.8 (0.3, 2.0) | 0.791 |
| Hypertension | 88 (45.6%) | 68 (45.6%) | 20 (45.5%) | 1.0 (0.5, 2.0) | 1.000 |
| Hyperlipidemia | 92 (47.7%) | 73 (49.0%) | 19 (43.2%) | 0.8 (0.4, 1.6) | 0.613 |
| MoCA | 26 [23.0, 28.0] | 26 [23.0, 28.0] | 26 [23.0, 28.0] | 1.0 (0.9, 1.1) | 0.771 |
| Total motor score | 20.0 [15.0; 26.0] | 19.0 [15.0; 26.0] | 22.0 [17.0; 29.0] | 1.0 (1.0, 1.1) | 0.062 |
| HY | 2.00 [1.0; 3.0] | 2.00 [1.50; 2.0] | 2.00 [2.00; 2.0] | 2.0 (0.8, 4.8) | 0.112 |
| Blood Biomarkers | |||||
| APOE4 (Non-carriers) | 153 (79.3%) | 120 (80.5%) | 33 (75.0%) | 0.7 (0.3, 1.7) | 0.559 |
| REP1 (Short) | 88 (45.6%) | 66 (44.3%) | 22 (50.0%) | 1.3 (0.6, 2.5) | 0.620 |
| ST2 | 11,600 [8750; 14,800] | 11,500 [8400; 14,900] | 12,600 [9430; 14,800] | 1.0 (1.0, 1.0) | 0.375 |
| NfL | 13.7 [10.1; 18.9] | 13.9 [10.2; 18.7] | 13.3 [9.9; 21.7] | 1.0 (1.0, 1.1) | 0.702 |
| t-tau | 1.17 [0.9; 1.5] | 1.1 [0.9; 1.6] | 1.3 [0.9; 1.5] | 1.3 (0.9, 1.8) | 0.350 |
| p-tau181 | 20.3 [15.7; 24.8] | 20.50 [15.4; 24.3] | 20.1 [15.8; 28.9] | 1.0 (1.0, 1.1) | 0.666 |
| Algorithm | AUC (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|
| Model 1: All Variables | |||
| AutoScore | 0.797 (0.720, 0.8736) | 0.636 (0.500, 0.773) | 0.825 (0.765, 0.879) |
| RF | 0.999 (0.997, 1.000) | 1.000 (0.920, 1.000) | 0.987 (0.952, 0.998) |
| KNN | 0.766 (0.690, 0.842) | 0.750 (0.597, 0.868) | 0.678 (0.596, 0.752) |
| NN | 0.996 (0.989, 1.000) | 0.977 (0.880, 0.999) | 0.987 (0.952, 0.998) |
| Logistic | 0.806 (0.731,0.881) | 0.682 (0.524, 0.814) | 0.819 (0.747, 0.877) |
| Model 2: Top Ten Variables | |||
| AutoScore | 0.771 (0.691,0.851) | 0.818 (0.705, 0.909) | 0.631(0.557, 0.705) |
| RF | 0.930 (0.889,0.971) | 0.818 (0.673, 0.918) | 0.872 (0.808, 0.921) |
| KNN | 0.843 (0.788,0.899) | 0.818 (0.673, 0.918) | 0.711 (0.632, 0.783) |
| NN | 0.918 (0.872,0.965) | 0.841 (0.699, 0.934) | 0.832 (0.762, 0.888) |
| Logistic | 0.770 (0.690, 0.849) | 0.795 (0.647, 0.902) | 0.631 (0.548, 0.708) |
| Variable | Interval | Partial Score |
|---|---|---|
| Lying DBP | Normal | 0 |
| High | 16 | |
| NfL | Normal | 0 |
| High | 18 | |
| Years of education | ≥10 | 0 |
| <10 | 16 | |
| p-tau 181 | Normal | 0 |
| High | 11 | |
| ST2 | Normal | 0 |
| High | 9 | |
| BMI | <25 | 0 |
| ≥25 | 3 | |
| Lying SBP | Normal | 0 |
| High | 1 | |
| Standing DBP | Normal | 0 |
| High | 11 | |
| t-tau | Normal | 0 |
| High | 11 | |
| HY | <2 | 0 |
| ≥2 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammadi, R.; Ng, S.Y.E.; Tan, J.Y.; Ng, A.S.L.; Deng, X.; Choi, X.; Heng, D.L.; Neo, S.; Xu, Z.; Tay, K.-Y.; et al. Machine Learning for Early Detection of Cognitive Decline in Parkinson’s Disease Using Multimodal Biomarker and Clinical Data. Biomedicines 2024, 12, 2758. https://doi.org/10.3390/biomedicines12122758
Mohammadi R, Ng SYE, Tan JY, Ng ASL, Deng X, Choi X, Heng DL, Neo S, Xu Z, Tay K-Y, et al. Machine Learning for Early Detection of Cognitive Decline in Parkinson’s Disease Using Multimodal Biomarker and Clinical Data. Biomedicines. 2024; 12(12):2758. https://doi.org/10.3390/biomedicines12122758
Chicago/Turabian StyleMohammadi, Raziyeh, Samuel Y. E. Ng, Jayne Y. Tan, Adeline S. L. Ng, Xiao Deng, Xinyi Choi, Dede L. Heng, Shermyn Neo, Zheyu Xu, Kay-Yaw Tay, and et al. 2024. "Machine Learning for Early Detection of Cognitive Decline in Parkinson’s Disease Using Multimodal Biomarker and Clinical Data" Biomedicines 12, no. 12: 2758. https://doi.org/10.3390/biomedicines12122758
APA StyleMohammadi, R., Ng, S. Y. E., Tan, J. Y., Ng, A. S. L., Deng, X., Choi, X., Heng, D. L., Neo, S., Xu, Z., Tay, K.-Y., Au, W.-L., Tan, E.-K., Tan, L. C. S., Steyerberg, E. W., Greene, W., & Saffari, S. E. (2024). Machine Learning for Early Detection of Cognitive Decline in Parkinson’s Disease Using Multimodal Biomarker and Clinical Data. Biomedicines, 12(12), 2758. https://doi.org/10.3390/biomedicines12122758









