In Situ Light-Source Delivery During 5-Aminulevulinic Acid-Guided High-Grade Glioma Resection: Spatial, Functional and Oncological Informed Surgery
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Main Applications
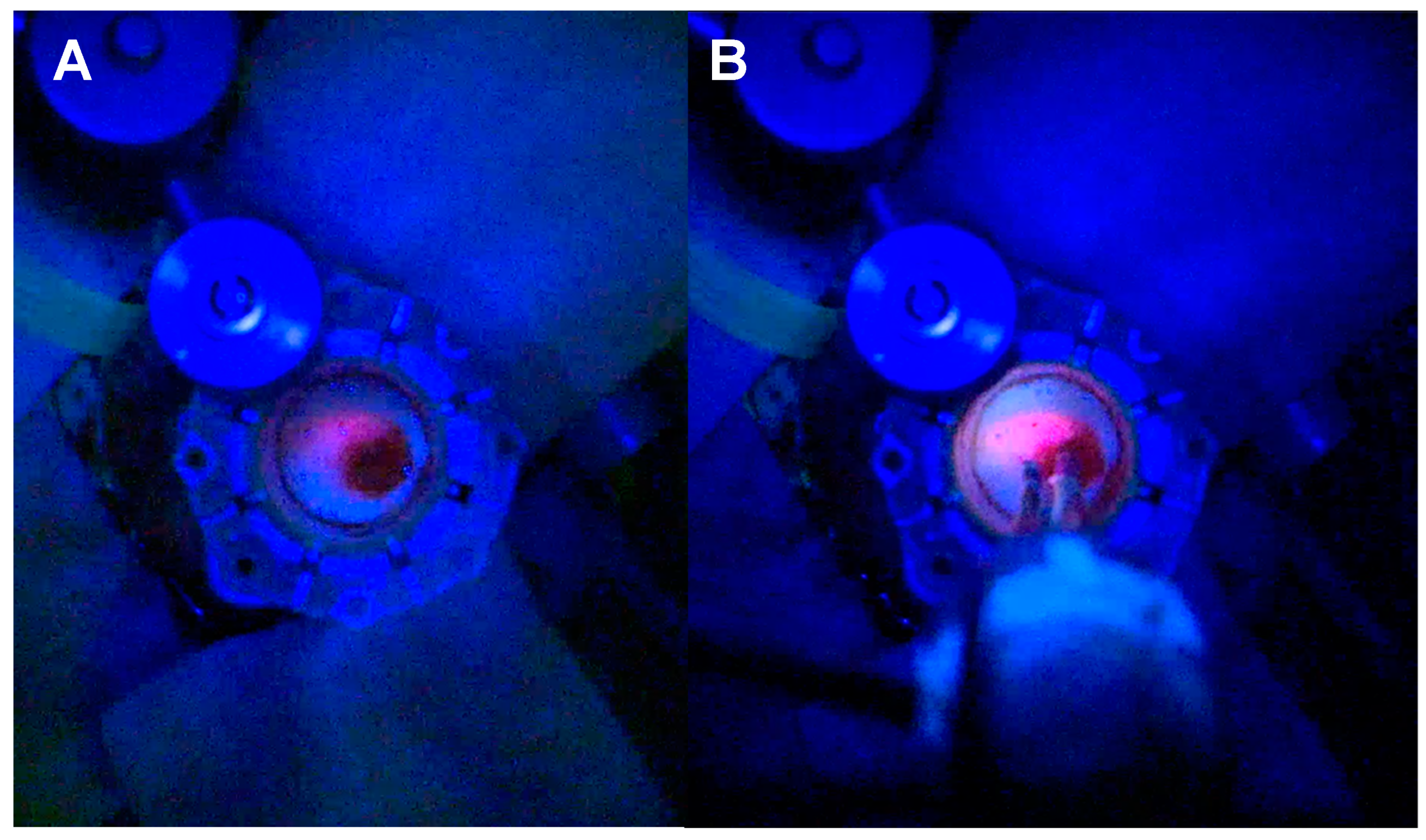
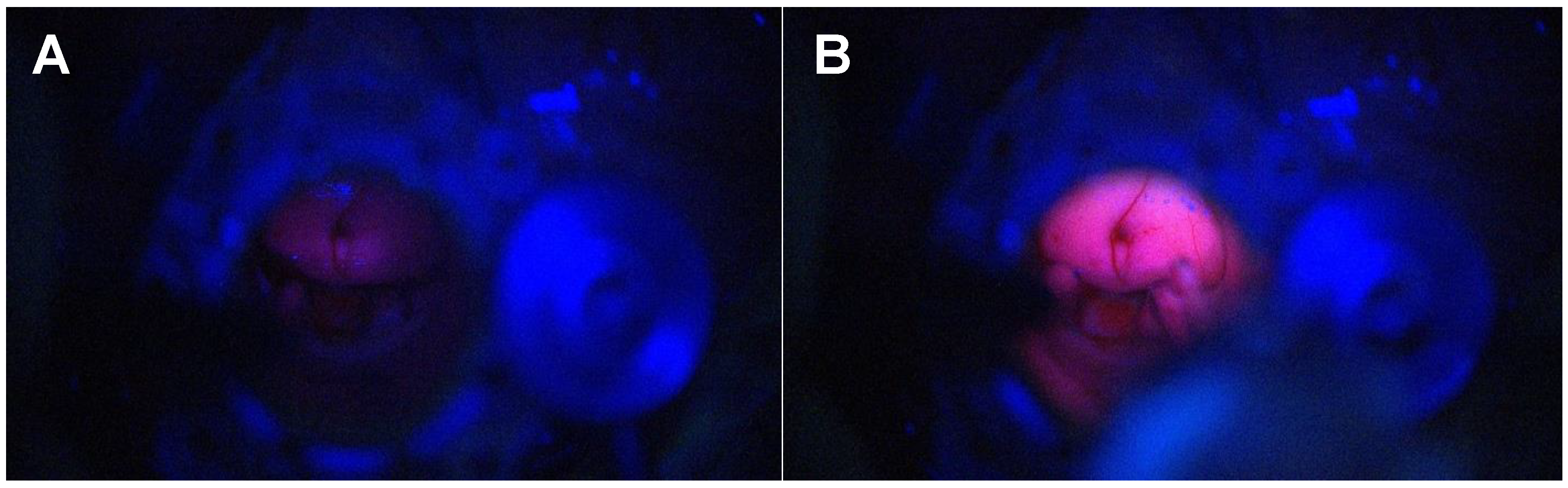
3.1.1. Tubular Retractor-Minimally Invasive Parafascicular Surgery (tr-MIPS)
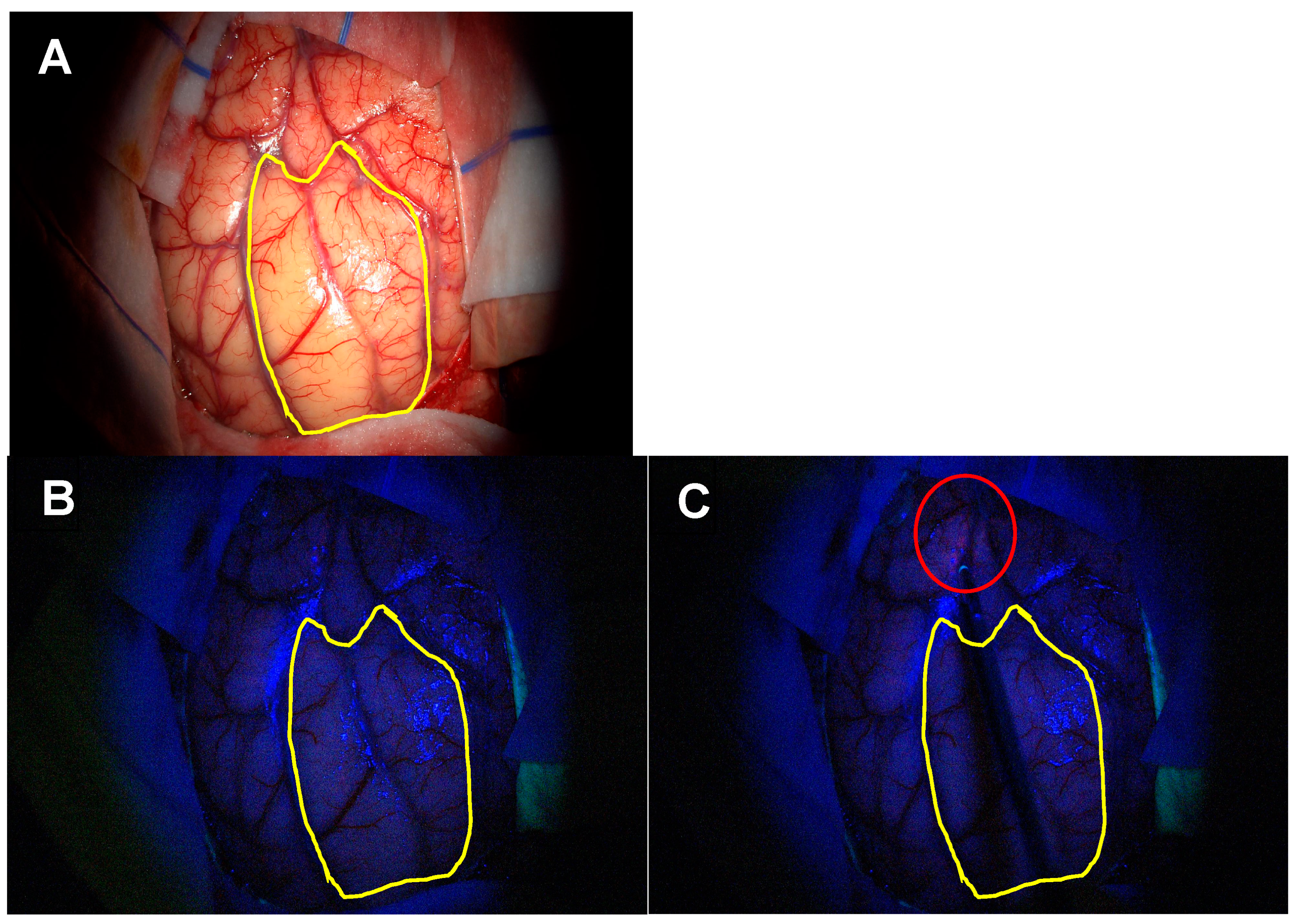
3.1.2. Extent of Resection (EoR) in Patients Eligible for Gross Total Resection
3.1.3. Satellite Foci Identification and Resection
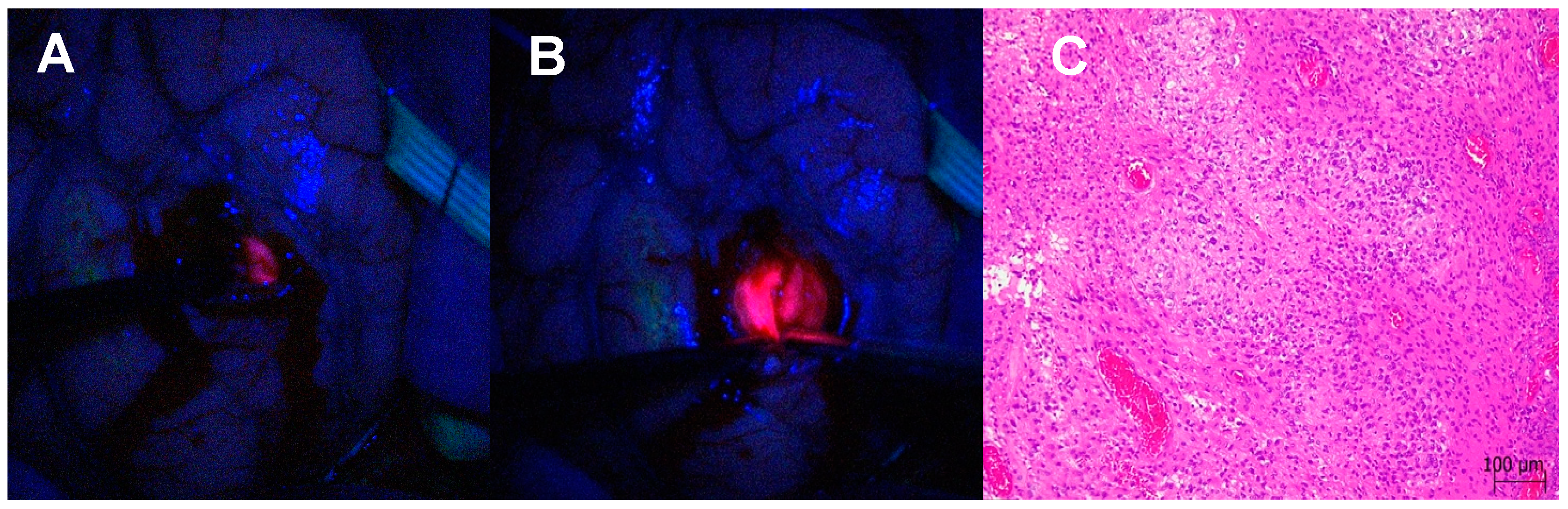
3.1.4. Differentiation Between Necrosis and Recurrence in Redo Surgery
3.1.5. Identification of Normal Ependyma Versus Pathological Ependymal and SubepenDymal Tissue
3.2. Surgical Workflow During 5-ALA-Guided Resection
- Microscope—White Light; Spectra—Blue Light;
- Microscope—Blue Light; Spectra—White Light;
- Microscope—Blue Light; Spectra—Blue Light.
3.3. Spatially, Functionally and Oncologically Informed Tissue Collection
4. Discussion
Strength and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.-J.; ALA-Glioma Study Group. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.; Reulen, H.J.; Meinel, T.; Pichlmeier, U.; Schumacher, W.; Tonn, J.-C.; Rohde, V.; Oppel, F.; Turowski, B.; Woiciechowsky, C.; et al. Extent of resection and survival in glioblastoma multiforme: Identification of and adjustment for bias. Neurosurgery 2008, 62, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Hadjipanayis, C.G.; Widhalm, G.; Stummer, W. What is the surgical benefit of utilizing 5-aminolevulinic acid for fluorescence-guided surgery of malignant gliomas? Neurosurgery 2015, 77, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Coburger, J.; Hagel, V.; Wirtz, C.R.; König, R. Surgery for glioblastoma: Impact of the combined use of 5-aminolevulinic acid and intraoperative MRI on extent of resection and survival. PLoS ONE 2015, 10, e0131872. [Google Scholar] [CrossRef] [PubMed]
- Schupper, A.J.; Baron, R.B.; Cheung, W.; Rodriguez, J.; Kalkanis, S.N.; Chohan, M.O.; Andersen, B.J.; Chamoun, R.; Nahed, B.V.; Zacharia, B.E.; et al. 5-Aminolevulinic acid for enhanced surgical visualization of high-grade gliomas: A prospective, multicenter study. J. Neurosurg. 2022, 136, 1525–1534. [Google Scholar] [CrossRef]
- Gandhi, S.; Meybodi, A.T.; Belykh, E.; Cavallo, C.; Zhao, X.; Syed, M.P.; Moreira, L.B.; Lawton, M.T.; Nakaji, P.; Preul, M.C. Survival outcomes among patients with high-grade glioma treated with 5-aminolevulinic acid–guided surgery: A systematic review and meta-analysis. Front. Oncol. 2019, 9, 620. [Google Scholar] [CrossRef]
- Michael, A.P.; Watson, V.L.; Ryan, D.; Delfino, K.R.; Bekker, S.V.; Cozzens, J.W. Effects of 5-ALA dose on resection of glioblastoma. J. Neurooncol. 2019, 141, 523–531. [Google Scholar] [CrossRef]
- Picart, T.; Pallud, J.; Berthiller, J.; Dumot, C.; Berhouma, M.; Ducray, F.; Armoiry, X.; Margier, J.; Guerre, P.; Varlet, P.; et al. Use of 5-ALA fluorescence-guided surgery versus white-light conventional microsurgery for the resection of newly diagnosed glioblastomas (RESECT study): A French multicenter randomized phase III study. J. Neurosurg. 2024, 140, 987–1000. [Google Scholar] [CrossRef]
- Haider, S.A.; Lim, S.; Kalkanis, S.N.; Lee, I.Y. The impact of 5-aminolevulinic acid on extent of resection in newly diagnosed high grade gliomas: A systematic review and single institutional experience. J. Neurooncol. 2019, 141, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Senders, J.T.; Muskens, I.S.; Schnoor, R.; Karhade, A.V.; Cote, D.J.; Smith, T.R.; Broekman, M.L. Agents for fluorescence-guided glioma surgery: A systematic review of preclinical and clinical results. Acta Neurochir. (Wien) 2017, 159, 151–167. [Google Scholar] [CrossRef] [PubMed]
- McCracken, D.J.; Schupper, A.J.; Lakomkin, N.; Malcolm, J.; Bray, D.P.; Hadjipanayis, C.G. Turning on the light for brain tumor surgery: A 5-aminolevulinic acid story. Neuro Oncol 2022, 24, S52–S61. [Google Scholar] [CrossRef] [PubMed]
- Eatz, T.A.; Eichberg, D.G.; Lu, V.M.; Di, L.; Komotar, R.J.; Ivan, M.E. Intraoperative 5-ALA fluorescence-guided resection of high-grade glioma leads to greater extent of resection with better outcomes: A systematic review. J. Neurooncol. 2022, 156, 233–256. [Google Scholar] [CrossRef]
- Nikova, A.S.; Vlotinou, P.; Karelis, L.; Karanikas, M.; Birbilis, T.A. Gross total resection with fluorescence could lead to improved overall survival rates: A systematic review and meta-analysis. Br. J. Neurosurg. 2022, 36, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Hadjipanayis, C.G.; Stummer, W. 5-ALA and FDA approval for glioma surgery. J. Neurooncol. 2019, 141, 479–486. [Google Scholar] [CrossRef]
- Fontana, A.O.; Piffaretti, D.; Marchi, F.; Burgio, F.; Faia-Torres, A.B.; Paganetti, P.; Pinton, S.; Pieles, U.; Reinert, M. Epithelial growth factor receptor expression influences 5-ALA induced glioblastoma fluorescence. J. Neurooncol. 2017, 133, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Reinert, M.; Piffaretti, D.; Wilzbach, M.; Hauger, C.; Guckler, R.; Marchi, F.; D’Angelo, M.L. Quantitative modulation of PpiX fluorescence and improved glioma visualization. Front. Surg. 2019, 6, 41, Erratum in Front. Surg. 2020, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Mazevet, M.; Oberli, C.; Marinelli, S.; Zaed, I.; Bauer, S.; Kaelin-Lang, A.; Marchi, F.; Gardenghi, R.; Reinert, M.; Cardia, A. Automated online safety margin (GLIOVIS) for glioma surgery model OPEN ACCESS EDITED BY. Front. Oncol. Front. Org Front. Oncol. 2024, 14, 1361022. [Google Scholar] [CrossRef]
- Bettag, C.; Schregel, K.; Langer, P.; Thomas, C.; Behme, D.; Stadelmann, C.; Rohde, V.; Mielke, D. Endoscope-assisted fluorescence-guided resection allowing supratotal removal in glioblastoma surgery. Neurosurg. Focus 2021, 50, E3. [Google Scholar] [CrossRef]
- Bettag, C.; Hussein, A.; Behme, D.; Maragkou, T.; Rohde, V.; Mielke, D. Endoscopic fluorescence-guided resection increases radicality in glioblastoma surgery. Oper. Neurosurg. 2020, 18, 41–46. [Google Scholar] [CrossRef]
- Keric, N.; Krenzlin, H.; Kurz, E.; Wesp, D.M.A.; Kalasauskas, D.; Ringel, F. Evaluation of 3D Robotic-Guided Exoscopic Visualization in Microneurosurgery. Front. Surg. 2022, 8, 791427. [Google Scholar] [CrossRef]
- Henderson, F.; Belykh, E.; Ramos, A.D.; Schwartz, T.H. Qualitative head-to-head comparison of headlamp and microscope for visualizing 5-ALA fluorescence during resection of glioblastoma. Neurosurg. Focus Video 2022, 6, V7. [Google Scholar] [CrossRef] [PubMed]
- Giantini-Larsen, A.M.; Parker, W.E.; Cho, S.S.; Goldberg, J.L.; Carnevale, J.A.; Michael, A.P.; Teng, C.W.; De Ravin, E.; Brennan, C.W.; Lee, J.Y.; et al. The Evolution of 5-Aminolevulinic Acid Fluorescence Visualization: Time for a Headlamp/Loupe Combination. World Neurosurg. 2022, 159, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, M.; Szczepanek, D.; Orzyłowska, A.; Rola, R. Analysis of Factors Affecting 5-ALA Fluorescence Intensity in Visualizing Glial Tumor Cells—Literature Review. Int. J. Mol. Sci. 2022, 23, 926. [Google Scholar] [CrossRef] [PubMed]
- Kamp, M.A.; Knipps, J.; Neumann, L.M.; Mijderwijk, H.-J.; Dibué-Adjei, M.; Steiger, H.-J.; Slotty, P.J.; Rapp, M.; Cornelius, J.-F.; Sabel, M. Is the Intensity of 5-Aminolevulinic Acid–Derived Fluorescence Related to the Light Source? World Neurosurg. 2019, 131, E271–E276. [Google Scholar] [CrossRef]
- Pala, A.; Reske, S.N.; Eberhardt, N.; Scheuerle, A.; König, R.; Schmitz, B.; Beer, A.J.; Wirtz, C.R.; Coburger, J. Diagnostic accuracy of intraoperative perfusion-weighted MRI and 5-aminolevulinic acid in relation to contrast-enhanced intraoperative MRI and 11 C-methionine positron emission tomography in resection of glioblastoma: A prospective study. Neurosurg. Rev. 2019, 42, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Tsugu, A.; Ishizaka, H.; Mizokami, Y.; Osada, T.; Baba, T.; Yoshiyama, M.; Nishiyama, J.; Matsumae, M. Impact of the combination of 5-aminolevulinic acid-induced fluorescence with intraoperative magnetic resonance imaging-guided surgery for glioma. World Neurosurg. 2011, 76, 120–127. [Google Scholar] [CrossRef]
- Shimizu, K.; Tamura, K.; Hara, S.; Inaji, M.; Tanaka, Y.; Kobayashi, D.; Sugawara, T.; Wakimoto, H.; Nariai, T.; Ishii, K.; et al. Correlation of Intraoperative 5-ALA-Induced Fluorescence Intensity and Preoperative 11C-Methionine PET Uptake in Glioma Surgery. Cancers 2022, 14, 1449. [Google Scholar] [CrossRef]
- Kim, J.K.; Jung, T.Y.; Jung, S.; Kim, I.Y.; Jang, W.Y.; Moon, K.S.; Kim, S.K.; Kim, J.H.; Lee, K.H. Relationship between tumor cell infiltration and 5-aminolevulinic acid fluorescence signals after resection of MR-enhancing lesions and its prognostic significance in glioblastoma. Clin. Transl. Oncol. 2021, 23, 459–467. [Google Scholar] [CrossRef]
- Certo, F.; Altieri, R.; Maione, M.; Schonauer, C.; Sortino, G.; Fiumanò, G.; Tirrò, E.; Massimino, M.; Broggi, G.; Vigneri, P.; et al. FLAIRectomy in Supramarginal Resection of Glioblastoma Correlates with Clinical Outcome and Survival Analysis: A Prospective, Single Institution, Case Series. Oper. Neurosurg. 2021, 20, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, G.M.; Maione, M.; Peschillo, S.; Signorelli, F.; Visocchi, M.; Sortino, G.; Fiumanò, G.; Certo, F. Intraoperative computed tomography, navigated ultrasound, 5-amino-levulinic acid fluorescence and neuromonitoring in brain tumor surgery: Overtreatment or useful tool combination? J. Neurosurg. Sci. 2024, 68, 31–43. [Google Scholar] [CrossRef]
- Utsuki, S.; Oka, H.; Sato, S.; Shimizu, S.; Suzuki, S.; Tanizaki, Y.; Kondo, K.; Miyajima, Y.; Fujii, K. Histological examination of false positive tissue resection using 5-aminolevulinic acid-induced fluorescence guidance. Neurol. Med. Chir. (Tokyo) 2007, 47, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Karschnia, P.; Young, J.S.; Dono, A.; Häni, L.; Sciortino, T.; Bruno, F.; Juenger, S.T.; Teske, N.; Morshed, R.A.; Haddad, A.F.; et al. Prognostic validation of a new classification system for extent of resection in glioblastoma: A report of the RANO resect group. Neuro Oncol. 2023, 25, 940–954. [Google Scholar] [CrossRef] [PubMed]
- Karschnia, P.; Dono, A.; Young, J.S.; Jünger, S.T.; Teske, N.; Häni, L.; Sciortino, T.; Mau, C.Y.; Bruno, F.; Weller, M.; et al. Evaluation Of Surgical Resection for Recurrent Glioblastoma Using The RANO Classification For Extent Of Resection: A Report Of The RANO Resect Group. Brain Spine 2023, 3, 101849. [Google Scholar] [CrossRef]
- Bjorland, L.S.; Mahesparan, R.; Fluge, Ø.; Gilje, B.; Kurz, K.D.; Farbu, E. Impact of extent of resection on outcome from glioblastoma using the RANO resect group classification system: A retrospective, population-based cohort study. Neuro-Oncol. Adv. 2023, 5, vdad126, Erratum in Neuro-Oncol. Adv. 2023, 5, vdad126. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Young, R.J.; Karimi, S.; Sood, S.; Zhang, Z.; Mo, Q.; Gutin, P.; Holodny, A.; Lassman, A. Isolated diffusion restriction precedes the development of enhancing tumor in a subset of patients with glioblastoma. Am. J. Neuroradiol. 2011, 32, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Kamble, A.N.; Agrawal, N.K.; Koundal, S.; Bhargava, S.; Joyner, D.A.; Kalelioglu, T.; Patel, S.H.; Jain, R. Imaging-based stratification of adult gliomas prognosticates survival and correlates with the 2021 WHO classification. Neuroradiology 2023, 65, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Farid, N.; Almeida-Freitas, D.B.; White, N.S.; McDonald, C.R.; Kuperman, J.M.; Almutairi, A.A.; Muller, K.A.; VandenBerg, S.R.; Kesari, S.; Dale, A.M. Combining diffusion and perfusion differentiates tumor from bevacizumab-related imaging abnormality (bria). J. Neurooncol. 2014, 120, 539–546. [Google Scholar] [CrossRef]
- Raabe, A.; Beck, J.; Schucht, P.; Seidel, K. Continuous dynamic mapping of the corticospinal tract during surgery of motor eloquent brain tumors: Evaluation of a new method: Clinical article. J. Neurosurg. 2014, 120, 1015–1024. [Google Scholar] [CrossRef]
- Soumpasis, C.; Díaz-Baamonde, A.; Ghimire, P.; Mirza, A.B.; Borri, M.; Jarosz, J.; Gullan, R.; Ashkan, K.; Bhangoo, R.; Vergani, F.; et al. Intraoperative Neuromonitoring of the Visual Pathway in Asleep Neuro-Oncology Surgery. Cancers 2023, 15, 3943. [Google Scholar] [CrossRef] [PubMed]
- Al-Adli, N.N.; Young, J.S.; Sibih, Y.E.; Berger, M.S. Technical Aspects of Motor and Language Mapping in Glioma Patients. Cancers 2023, 15, 2173. [Google Scholar] [CrossRef]
- Mirza, A.B.; Christodoulides, I.; Lavrador, J.P.; Giamouriadis, A.; Vastani, A.; Boardman, T.; Ahmed, R.; Norman, I.; Murphy, C.; Devi, S.; et al. 5-Aminolevulinic acid-guided resection improves the overall survival of patients with glioblastoma—A comparative cohort study of 343 patients. Neurooncol. Adv. 2021, 3, vdab047. [Google Scholar] [CrossRef]
- Gallagher, M.J.; Lavrador, J.P.; Coelho, P.; Mirallave-Pescador, A.; Bleil, C.; Gullan, R.; Ashkan, K.; Vergani, F.; Bhangoo, R. Continuous Microdebrider-Based Dynamic Subcortical Motor Mapping: A Technical Advance in Tubular Retractor–Assisted Surgery. Oper. Neurosurg. 2022, 23, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, M.M.; Sekerak, P.; Mark, J.; Bailes, J. Use of a novel navigable tubular retractor system in 1826 minimally invasive parafascicular surgery (MIPS) cases involving deep-seated brain tumors, hemorrhages and malformations. Interdiscip. Neurosurg. 2021, 23, 100919. [Google Scholar] [CrossRef]
- Zammar, S.G.; Cappelli, J.; Zacharia, B.E. Utility of Tubular Retractors Augmented with Intraoperative Ultrasound in the Resection of Deep-seated Brain Lesions: Technical Note. Cureus 2019, 11, e4272. [Google Scholar] [CrossRef] [PubMed]
- Eliyas, J.K.; Glynn, R.; Kulwin, C.G.; Rovin, R.; Young, R.; Alzate, J.; Pradilla, G.; Shah, M.V.; Kassam, A.; Ciric, I.; et al. Minimally Invasive Transsulcal Resection of Intraventricular and Periventricular Lesions Through a Tubular Retractor System: Multicentric Experience and Results. World Neurosurg. 2016, 90, 556–564. [Google Scholar] [CrossRef]
- Haddad, A.F.; Young, J.S.; Berger, M.S.; Tarapore, P.E. Preoperative Applications of Navigated Transcranial Magnetic Stimulation. Front. Neurol. 2021, 11, 628903. [Google Scholar] [CrossRef]
- Luna, L.P.; Sherbaf, F.G.; Sair, H.I.; Mukherjee, D.; Oliveira, I.B.; Köhler, C.A. Can preoperative mapping with functional MRI reduce morbidity in brain tumor resection? A systematic review and meta-analysis of 68 observational studies. Radiology 2021, 300, 338–349. [Google Scholar] [CrossRef]
- Haddad, A.F.; Aghi, M.K.; Butowski, N. Novel intraoperative strategies for enhancing tumor control: Future directions. Neuro Oncol. 2022, 24, S25–S32. [Google Scholar] [CrossRef]
- Belykh, E.; Miller, E.J.; Patel, A.A.; Bozkurt, B.; Yağmurlu, K.; Robinson, T.R.; Nakaji, P.; Spetzler, R.F.; Lawton, M.T.; Nelson, L.Y.; et al. Optical Characterization of Neurosurgical Operating Microscopes: Quantitative Fluorescence and Assessment of PpIX Photobleaching. Sci. Rep. 2018, 8, 12543. [Google Scholar] [CrossRef]
- Piloni, M.; Bailo, M.; Gagliardi, F.; Mortini, P. Resection of Intracranial Tumors with a Robotic-Assisted Digital Microscope: A Preliminary Experience with Robotic Scope. World Neurosurg. 2021, 152, e205–e211. [Google Scholar] [CrossRef]
- Farhat, M.; Fuller, G.N.; Wintermark, M.; Chung, C.; Kumar, V.A.; Chen, M. Multifocal and multicentric glioblastoma: Imaging signature, molecular characterization, patterns of spread, and treatment. Neuroradiol. J. 2023. [Google Scholar] [CrossRef] [PubMed]
- Baro, V.; Cerretti, G.; Todoverto, M.; Della Puppa, A.; Chioffi, F.; Volpin, F.; Causin, F.; Busato, F.; Fiduccia, P.; Landi, A.; et al. Newly Diagnosed Multifocal GBM: A Monocentric Experience and Literature Review. Curr. Oncol. 2022, 29, 3472–3488. [Google Scholar] [CrossRef]
- Broekx, S.; Weyns, F.; De Vleeschouwer, S. 5-Aminolevulinic acid for recurrent malignant gliomas: A systematic review. Clin Neurol. Neurosurg. 2020, 195, 105913. [Google Scholar] [CrossRef] [PubMed]
- Tejada-Solís, S.; Aldave-Orzaiz, G.; Pay-Valverde, E.; Marigil-Sánchez, M.; Idoate-Gastearena, M.A.; Díez-Valle, R. Prognostic value of ventricular wall fluorescence during 5-aminolevulinic-guided surgery for glioblastoma. Acta Neurochir. (Wien) 2012, 154, 1997–2002. [Google Scholar] [CrossRef] [PubMed]
- Müther, M.; Stummer, W. Ependymal fluorescence in fluorescence-guided resection of malignant glioma: A systematic review. Acta Neurochir. (Wien) 2020, 162, 365–372. [Google Scholar] [CrossRef]
- Moiyadi, A.V.; Shetty, P.; Sridhar, E. Periventricular glioblastomas and ependymal involvement interrogated using intraoperative fluorescence–a pathological correlative study. Br. J. Neurosurg. 2017, 31, 107–112. [Google Scholar] [CrossRef]
- Otani, Y.; Kurozumi, K.; Ishida, J.; Hiramatsu, M.; Kameda, M.; Ichikawa, T.; Date, I. Combination of the tubular retractor and brain spatulas provides an adequate operative field in surgery for deep seated lesions: Case series and technical note. Surg. Neurol. Int. 2018, 9, 220. [Google Scholar] [CrossRef] [PubMed]
- Recinos, P.F.; Raza, S.M.; Jallo, G.I.; Recinos, V.R. Use of a minimally invasive tubular retraction system for deep-seated tumors in pediatric patients: Technical note. J. Neurosurg. Pediatr. 2011, 7, 516–521. [Google Scholar] [CrossRef]
- Bander, E.D.; Jones, S.H.; Kovanlikaya, I.; Schwartz, T.H. Utility of tubular retractors to minimize surgical brain injury in the removal of deep intraparenchymal lesions: A quantitative analysis of FLAIR hyperintensity and apparent diffusion coeffiient maps. J. Neurosurg. 2016, 124, 1053–1060. [Google Scholar] [CrossRef]
- Shapiro, S.Z.; Sabacinski, K.A.; Mansour, S.A.; Echeverry, N.B.; Shah, S.S.; Stein, A.A.; Snelling, B.M. Use of Vycor Tubular Retractors in the Management of Deep Brain Lesions: A Review of Current Studies. World Neurosurg. 2020, 133, 283–290. [Google Scholar] [CrossRef]
- Ratre, S.; Yadav, Y.R.; Parihar, V.S.; Kher, Y. Microendoscopic Removal of Deep-Seated Brain Tumors Using Tubular Retraction System. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2016, 77, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.S.; Prevedello, D.M.; Elder, J.B. Comparison of endoscope- versus microscope-assisted resection of deep-seated intracranial lesions using a minimally invasive port retractor system. J. Neurosurg. 2016, 124, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Eichberg, D.G.; Buttrick, S.; Brusko, G.D.; Ivan, M.; Starke, R.M.; Komotar, R.J.; Eichberg, D.G.; Buttrick, S.; Brusko, G.D.; Ivan, M.; et al. Use of Tubular Retractor for Resection of Deep-Seated Cerebral Tumors and Colloid Cysts: Single Surgeon Experience and Review of the Literature. World Neurosurg. 2018, 112, e50–e60. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, J.; Kitagawa, T.; Miyaoka, R.; Suzuki, K.; Takamatsu, S.; Saito, T.; Nakano, Y. 5-Aminolevulinic acid: Pitfalls of Fluorescence-guided resection for Malignant Gliomas and application for Malignant Glioma therapy. J UOEH 2020, 42, 27–34. [Google Scholar] [CrossRef]
- Kamp, M.A.; Krause Molle, Z.; Munoz-Bendix, C.; Rapp, M.; Sabel, M.; Steiger, H.J.; Cornelius, J.F. Various shades of red—A systematic analysis of qualitative estimation of ALA-derived fluorescence in neurosurgery. Neurosurg. Rev. 2018, 41, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, M. Towards the Development of Personalized Medicine: A Novel Tissue Preservation System for the Automation and Standardization of Brain Tumor Harvesting in a Surgical Setting. FASEB J. 2017, 31, lb516. [Google Scholar] [CrossRef]










| Sex | Age | Tumour Location | Procedure | Diagnosis | Extent of Resection | |
|---|---|---|---|---|---|---|
| 1 | M | 60 | Temporal | Right fronto-temporal craniotomy | Glioblastoma, IDH-wildtype, CNS WHO grade 4 | GTR, no residual fluorescence |
| 2 | M | 66 | Temporo-parietal | Awake left temporo-parietal craniotomy | Glioblastoma, IDH-wildtype, CNS WHO grade 4 | STR due to eloquent area, residual fluorescence |
| 3 | F | 56 | Paracentral lobule | Left parasagittal fronto-parietal craniotomy | Metastatic non-small cell carcinoma | GTR, no residual fluorescence |
| 4 | M | 39 | Temporal | Redo right temporal craniotomy | Residual/recurrent Glioblastoma, IDH-wildtype, CNS WHO grade 4 | GTR, no residual fluorescence |
| 5 | F | 58 | Temporo-parietal | Awake left fronto-temporo-parietal craniotomy for tr-MIPS | Glioblastoma, IDH-wildtype, CNS WHO grade 4 | STR due to eloquent area, residual fluorescence |
| 6 | M | 72 | Parieto-occipital | Redo right parieto-occipital craniotomy | Marked reactive/therapy-related changes with a tiny cluster of residual metastatic adenocarcinoma | GTR, no residual fluorescence |
| 7 | M | 60 | Frontal | Left frontal craniotomy | Meningioma (in the context of BAP1 tumour predisposition syndrome) | GTR, no residual fluorescence |
| 8 | M | 61 | Temporal | Left fronto-temporal craniotomy | Glioblastoma, IDH-wildtype, CNS WHO grade 4 | STR due to invasion of vascular structure, residual fluorescence |
| 9 | F | 45 | Temporal | Right temporal craniotomy for tr-MIPS | Subependymoma WHO grade 1 | GTR, no residual fluorescence |
| 10 | F | 73 | Temporal | Left fronto-temporal craniotomy | Glioblastoma, IDH-wildtype, CNS WHO grade 4 | GTR, no residual fluorescence |
| 11 | F | 70 | Temporal | Right fronto-temporal craniotomy | Glioblastoma, IDH-wildtype, CNS WHO grade 4 | STR due to invasion of vascular structure, residual fluorescence |
| 12 | F | 52 | Temporo-parietal | Redo right temporo-parietal craniotomy | Residual/recurrent glioblastoma, IDH-wildtype, CNS WHO grade 4 | GTR, no residual fluorescence |
| 13 | F | 63 | Parietal | Right parietal craniotomy for tr-MIPS | Glioblastoma, IDH-wildtype, CNS WHO grade 4 | STR due to invasion of vascular structure, residual fluorescence |
| 14 | M | 55 | Temporal | Left temporo-parietal craniotomy | Glioblastoma, IDH-wildtype, CNS WHO grade 4 | GTR, no residual fluorescence |
| 15 | M | 35 | Temporal | Right fronto-temporal craniotomy | Glioblastoma, IDH-wildtype, CNS WHO grade 4 | GTR, no residual fluorescence |
| 16 | F | 23 | Frontal | Awake redo left frontal craniotomy | Recurrent astrocytoma, IDH-mutant, CNS WHO grade 3, transformed from lower-grade astrocytoma | STR due to eloquent area, residual fluorescence |
| 17 | M | 51 | Temporal | Left temporal craniotomy for tr-MIPS | Glioblastoma, IDH-wildtype, CNS WHO grade 4 | STR due to eloquent area, residual fluorescence |
| 18 | F | 51 | Frontal | Awake right frontal craniotomy | Glioblastoma, IDH-wildtype, CNS WHO grade 4 | GTR, no residual fluorescence |
| 19 | F | 34 | Frontal | Awake right fronto-temporo-parietal craniotomy | Oligodendroglioma, IDH-mutant and 1p/19q co-deleted, CNS WHO grade 2 | STR due to eloquent area, no fluorescence |
| 20 | M | 79 | Frontal | Right frontal craniotomy | Glioblastoma, IDH-wildtype, CNS WHO grade 4 | STR due to eloquent area, residual fluorescence |
| 21 | M | 66 | Parietal | Awake right fronto-temporo-parietal craniotomy | Glioblastoma, IDH-wildtype, CNS WHO grade 4 | STR due to eloquent area, residual fluorescence |
| 22 | F | 52 | Fronto-temporo-parietal | Awake right fronto-temporo-parietal craniotomy | Glioblastoma, IDH-wildtype, CNS WHO grade 4 | STR due to eloquent area, residual fluorescence |
| 23 | F | 29 | Frontal | Awake right frontal craniotomy | Diffuse low-grade glioma, IDH-mutant, favouring oligodendroglioma CNS WHO grade 2 | GTR, no fluorescence |
| 24 | M | 79 | Parietal | Right parietal craniotomy | Metastatic mucinous adenocarcinoma, in keeping with primary lung origin | GTR, no residual fluorescence |
| 25 | M | 35 | Frontal | Awake right frontal craniotomy | Oligodendroglioma, IDH-mutant and 1p/19q-codeleted, CNS WHO grade 2 | STR due to eloquent area, no fluorescence |
| 26 | M | 31 | Thalamic-intraventricular | Left parietal craniotomy for tr-MIPS | Low-grade glioneuronal tumour, favouring ganglioglioma, CNS WHO grade 1 | STR due to eloquent area, no fluorescence |
| 27 | M | 35 | Temporal | Right fronto-temporal craniotomy | Glioblastoma, IDH-wildtype, CNS WHO grade 4 | GTR, no residual fluorescence |
| 28 | M | 59 | Temporal | Right fronto-temporal craniotomy | Glioblastoma, IDH-wildtype, CNS WHO grade 4 | GTR, no residual fluorescence |
| 29 | F | 44 | Temporal | Awake right fronto-temporal craniotomy | Glioblastoma, IDH-wildtype, CNS WHO grade 4 | STR due to eloquent area, residual fluorescence |
| 30 | M | 54 | Temporal | Right fronto-temporal craniotomy for tr-MIPS | Glioblastoma, IDH-wildtype, CNS WHO grade 4 | GTR, no residual fluorescence |
| 31 | M | 56 | Frontal | Left frontal craniotomy | Glioblastoma, IDH-wildtype, CNS WHO grade 4 | GTR, no residual fluorescence |
| 32 | M | 30 | Frontal | Left frontal craniotomy for tr-MIPS | High-grade astrocytoma with piloid features | GTR, no residual fluorescence |
| 33 | F | 62 | Frontal | Awake left frontal craniotomy | Glioblastoma, IDH-wildtype, CNS WHO grade 4 | GTR, no residual fluorescence |
| 34 | M | 72 | Posterior insula | Awake left parietal craniotomy for tr-MIPS | Diffuse large B-cell lymphoma (DLBCL), strongly suggestive of a non-germinal centre B-cell-like (non-GCB) subtype | STR due to eloquent area and intraop results, no fluorescence |
| 35 | F | 34 | Left cerebellar | Suboccipital craniotomy | Medulloblastoma | GTR, no residual fluorescence |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lavrador, J.P.; Marchi, F.; Elhag, A.; Kalyal, N.; Mthunzi, E.; Awan, M.; Wroe-Wright, O.; Díaz-Baamonde, A.; Mirallave-Pescador, A.; Reisz, Z.; et al. In Situ Light-Source Delivery During 5-Aminulevulinic Acid-Guided High-Grade Glioma Resection: Spatial, Functional and Oncological Informed Surgery. Biomedicines 2024, 12, 2748. https://doi.org/10.3390/biomedicines12122748
Lavrador JP, Marchi F, Elhag A, Kalyal N, Mthunzi E, Awan M, Wroe-Wright O, Díaz-Baamonde A, Mirallave-Pescador A, Reisz Z, et al. In Situ Light-Source Delivery During 5-Aminulevulinic Acid-Guided High-Grade Glioma Resection: Spatial, Functional and Oncological Informed Surgery. Biomedicines. 2024; 12(12):2748. https://doi.org/10.3390/biomedicines12122748
Chicago/Turabian StyleLavrador, José Pedro, Francesco Marchi, Ali Elhag, Nida Kalyal, Engelbert Mthunzi, Mariam Awan, Oliver Wroe-Wright, Alba Díaz-Baamonde, Ana Mirallave-Pescador, Zita Reisz, and et al. 2024. "In Situ Light-Source Delivery During 5-Aminulevulinic Acid-Guided High-Grade Glioma Resection: Spatial, Functional and Oncological Informed Surgery" Biomedicines 12, no. 12: 2748. https://doi.org/10.3390/biomedicines12122748
APA StyleLavrador, J. P., Marchi, F., Elhag, A., Kalyal, N., Mthunzi, E., Awan, M., Wroe-Wright, O., Díaz-Baamonde, A., Mirallave-Pescador, A., Reisz, Z., Gullan, R., Vergani, F., Ashkan, K., & Bhangoo, R. (2024). In Situ Light-Source Delivery During 5-Aminulevulinic Acid-Guided High-Grade Glioma Resection: Spatial, Functional and Oncological Informed Surgery. Biomedicines, 12(12), 2748. https://doi.org/10.3390/biomedicines12122748