IL-4R and CXCR2 Contribute to Downregulating Neutrophil-Mediated Response in the Early Stage of Fungal Extract-Induced Allergic Airway Inflammation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Ethics Statement
2.2. Induction of Allergic Airway Inflammation
2.3. Blood Collection
2.4. Blood Cell Analysis by Flow Cytometry
2.5. Quantitation of Total IgE
2.6. Bronchoalveolar Lavage (BAL) Collection
2.7. Cytokine Evaluation
2.8. Bone Marrow Cell Extraction and Analysis by Flow Cytometry
2.9. Neutrophil Isolation and Analysis by Flow Cytometry
2.10. Neutrophil ROS Production Detection
2.11. Detection of NET Formation or Necrosis
2.12. Neutrophil Chemotactic Activity Estimation
2.13. Whole-Mount Conducting Airway Specimen Preparation and Staining
2.14. Confocal Laser Scanning Microscopy
2.15. Statistical Analysis
3. Results
3.1. CD-1 Outbred Mice Are Prone to Eosinophil-Mediated Immune Response
3.2. Eosinophils Replaced Neutrophils in the Peripheral Blood of Mice with Allergic Airway Inflammation
3.3. Neutrophils Did Not Migrate to BAL and Lung Tissues During Progression of A. fumigatus-Induced Allergic Airway Inflammation
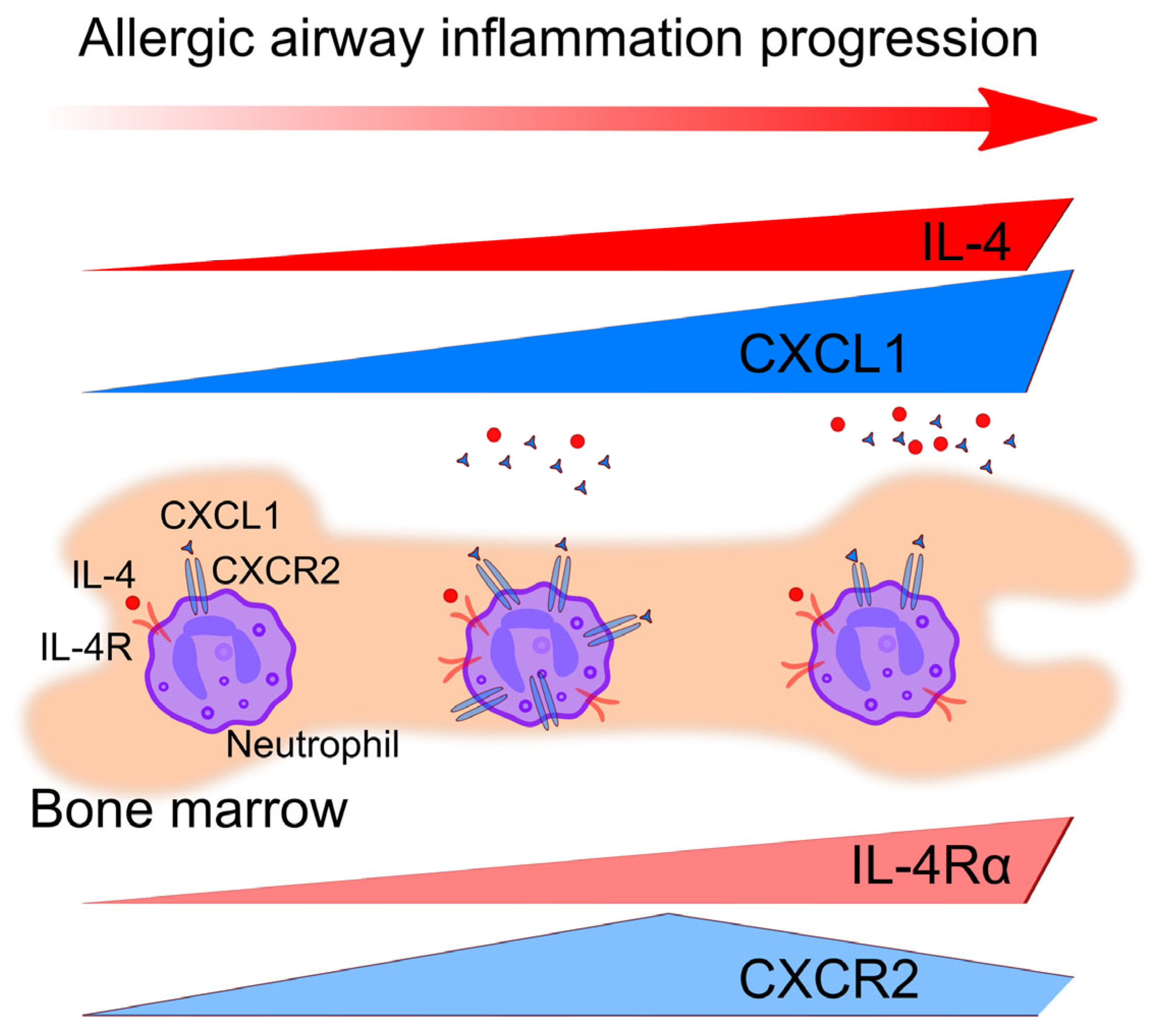
3.4. Circulating Neutrophil Numbers Are Altered in the Bone Marrow of Mice with Allergic Airway Inflammation
3.5. Bone Marrow Neutrophils from Mice with Allergic Airway Inflammation Possess Elevated Motility but Decreased Ability for ROS Production
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agache, I.; Akdis, C.; Jutel, M.; Virchow, J.C. Untangling Asthma Phenotypes and Endotypes. Allergy 2012, 67, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Kuruvilla, M.E.; Lee, F.E.-H.; Lee, G.B. Understanding Asthma Phenotypes, Endotypes, and Mechanisms of Disease. Clin. Rev. Allergy Immunol. 2019, 56, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Fahy, J.V. Type 2 Inflammation in Asthma-Present in Most, Absent in Many. Nat. Rev. Immunol. 2015, 15, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Hammad, H.; Lambrecht, B.N. The Basic Immunology of Asthma. Cell 2021, 184, 1469–1485. [Google Scholar] [CrossRef] [PubMed]
- Moss, R.B. Treatment options in severe fungal asthma and allergic bronchopulmonary aspergillosis. Eur. Respir. J. 2014, 43, 1487–1500. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, M.A.; Bogorodskiy, A.O.; Troyanova, N.I.; Servuli, E.A.; Bolkhovitina, E.L.; Büldt, G.; Fahlke, C.; Gordeliy, V.I.; Gensch, T.; Borshchevskiy, V.I.; et al. Aspergillus fumigatus Infection-Induced Neutrophil Recruitment and Location in the Conducting Airway of Immunocompetent, Neutropenic, and Immunosuppressed Mice. J. Immunol. Res. 2018, 2018, 5379085. [Google Scholar] [CrossRef] [PubMed]
- Templeton, S.; Buskirk, A.; Green, B.; Beezhold, D.; Schmechel, D. Murine models of airway fungal exposure and allergic sensitization. Med. Mycol. 2010, 48, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Fei, M.; Bhatia, S.; Oriss, T.B.; Yarlagadda, M.; Khare, A.; Akira, S.; Saijod, S.; Iwakura, Y.; Fallert Junecko, B.A.; Reinhart, T.A.; et al. TNF-α from Inflammatory Dendritic Cells (DCs) Regulates Lung IL-17A/IL-5 Levels and Neutrophilia versus Eosinophilia during Persistent Fungal Infection. Proc. Natl. Acad. Sci. USA 2011, 108, 5360–5365. [Google Scholar] [CrossRef] [PubMed]
- Mirkov, I.; Stojanovic, I.; Glamoclija, J.; Stosic-Grujicic, S.; Zolotarevski, L.; Kataranovski, D.; Kataranovski, M. Differential mechanisms of resistance to sublethal systemic Aspergillus fumigatus infection in immunocompetent BALB/c and C57BL/6 mice. Immunobiology 2011, 216, 234–242. [Google Scholar] [CrossRef]
- Van Nevel, S.; van Ovost, J.; Holtappels, G.; De Ruyck, N.; Zhang, N.; Braun, H.; Maes, T.; Bachert, C.; Krysko, O. Neutrophils Affect IL-33 Processing in Response to the Respiratory Allergen Alternaria alternata. Front. Immunol. 2021, 12, 677848. [Google Scholar] [CrossRef]
- Egholm, C.; Özcan, A.; Breu, D.; Boyman, O. Type 2 Immune Predisposition Results in Accelerated Neutrophil Aging Causing Susceptibility to Bacterial Infection. Sci. Immunol. 2022, 7, eabi9733. [Google Scholar] [CrossRef] [PubMed]
- Elsakkar, M.G.; Sharaki, O.A.; Abdallah, D.M.; Mostafa, D.K.; Shekondali, F.T. Adalimumab ameliorates OVA-induced airway inflammation in mice: Role of CD4(+) CD25(+) FOXP3(+) regulatory T-cells. Eur. J. Pharmacol. 2016, 786, 100–108. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Ichinose, T.; Yoshida, S.; Takano, H.; Nishikawa, M.; Sun, G.; Shibamoto, T. Induction of immune tolerance and reduction of aggravated lung eosinophilia by co-exposure to Asian sand dust and ovalbumin for 14 weeks in mice. Allergy Asthma Clin. Immunol. 2013, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Assaf, S.M.; Hanania, N.A. Eosinophilic vs. Neutrophilic Asthma. Curr. Pulmonol. Rep. 2020, 9, 28–35. [Google Scholar] [CrossRef]
- Yamasaki, A.; Okazaki, R.; Harada, T. Neutrophils and Asthma. Diagnostics 2022, 12, 1175. [Google Scholar] [CrossRef]
- Shilovskiy, I.P.; Nikolskii, A.A.; Kurbacheva, O.M.; Khaitov, M.R. Modern View of Neutrophilic Asthma Molecular Mechanisms and Therapy. Biochemistry 2020, 85, 854–868. [Google Scholar] [CrossRef]
- Weng, Q.; Zhu, C.; Zheng, K.; Wu, Y.; Dong, L.; Wu, Y.; Li, M.; Shen, J.; Ying, S.; Shen, H.; et al. Early Recruited Neutrophils Promote Asthmatic Inflammation Exacerbation by Release of Neutrophil Elastase. Cell. Immunol. 2020, 352, 104101. [Google Scholar] [CrossRef]
- Patel, D.F.; Peiró, T.; Bruno, N.; Vuononvirta, J.; Akthar, S.; Puttur, F.; Pyle, C.J.; Suveizdyte, K.; Walker, S.A.; Singanayagam, A.; et al. Neutrophils Restrain Allergic Airway Inflammation by Limiting ILC2 Function and Monocyte-Dendritic Cell Antigen Presentation. Sci. Immunol. 2019, 4, eaax7006. [Google Scholar] [CrossRef]
- Whitehead, G.S.; Thomas, S.Y.; Nakano, K.; Royer, D.J.; Burke, C.G.; Nakano, H.; Cook, D.N. A Neutrophil/TGF-β Axis Limits the Pathogenicity of Allergen-Specific CD4+ T Cells. JCI Insight 2022, 7, e150251. [Google Scholar] [CrossRef]
- Gregory, L.G.; Causton, B.; Murdoch, J.R.; Mathie, S.A.; O’Donnell, V.; Thomas, C.P.; Priest, F.M.; Quint, D.J.; Lloyd, C.M. Inhaled House Dust Mite Induces Pulmonary T Helper 2 Cytokine Production. Clin. Exp. Allergy 2009, 39, 1597–1610. [Google Scholar] [CrossRef]
- Lommatzsch, M.; Julius, P.; Kuepper, M.; Garn, H.; Bratke, K.; Irmscher, S.; Luttmann, W.; Renz, H.; Braun, A.; Virchow, J.C. The Course of Allergen-Induced Leukocyte Infiltration in Human and Experimental Asthma. J. Allergy Clin. Immunol. 2006, 118, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Lambrecht, B.N.; Hammad, H.; Fahy, J.V. The Cytokines of Asthma. Immunity 2019, 50, 975–991. [Google Scholar] [CrossRef] [PubMed]
- Steinke, J.W.; Borish, L. Th2 Cytokines and Asthma. Interleukin-4: Its Role in the Pathogenesis of Asthma, and Targeting It for Asthma Treatment with Interleukin-4 Receptor Antagonists. Respir. Res. 2001, 2, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Hurdayal, R.; Brombacher, F. Interleukin-4 Receptor Alpha: From Innate to Adaptive Immunity in Murine Models of Cutaneous Leishmaniasis. Front. Immunol. 2017, 8, 1354. [Google Scholar] [CrossRef] [PubMed]
- Gour, N.; Wills-Karp, M. IL-4 and IL-13 Signaling in Allergic Airway Disease. Cytokine 2015, 75, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Bitton, A.; Avlas, S.; Reichman, H.; Itan, M.; Karo-Atar, D.; Azouz, N.P.; Rozenberg, P.; Diesendruck, Y.; Nahary, L.; Rothenberg, M.E.; et al. A Key Role for IL-13 Signaling via the Type 2 IL-4 Receptor in Experimental Atopic Dermatitis. Sci. Immunol. 2020, 5, eaaw2938. [Google Scholar] [CrossRef] [PubMed]
- Woytschak, J.; Keller, N.; Krieg, C.; Impellizzieri, D.; Thompson, R.W.; Wynn, T.A.; Zinkernagel, A.S.; Boyman, O. Type 2 Interleukin-4 Receptor Signaling in Neutrophils Antagonizes Their Expansion and Migration during Infection and Inflammation. Immunity 2016, 45, 172–184. [Google Scholar] [CrossRef]
- Impellizzieri, D.; Ridder, F.; Raeber, M.E.; Egholm, C.; Woytschak, J.; Kolios, A.G.A.; Legler, D.F.; Boyman, O. IL-4 Receptor Engagement in Human Neutrophils Impairs Their Migration and Extracellular Trap Formation. J. Allergy Clin. Immunol. 2019, 144, 267–279.e4. [Google Scholar] [CrossRef]
- Russkamp, D.; Aguilar-Pimentel, A.; Alessandrini, F.; Gailus-Durner, V.; Fuchs, H.; Ohnmacht, C.; Chaker, A.; de Angelis, M.H.; Ollert, M.; Schmidt-Weber, C.B.; et al. IL-4 Receptor α Blockade Prevents Sensitization and Alters Acute and Long-Lasting Effects of Allergen-Specific Immunotherapy of Murine Allergic Asthma. Allergy 2019, 74, 1549–1560. [Google Scholar] [CrossRef]
- Barrientos, L.; Marin-Esteban, V.; de Chaisemartin, L.; Le-Moal, V.L.; Sandré, C.; Bianchini, E.; Nicolas, V.; Pallardy, M.; Chollet-Martin, S. An Improved Strategy to Recover Large Fragments of Functional Human Neutrophil Extracellular Traps. Front. Immunol. 2013, 4, 52996. [Google Scholar] [CrossRef]
- Idzko, M.; Hammad, H.; Van Nimwegen, M.; Kool, M.; Willart, M.A.M.; Muskens, F.; Hoogsteden, H.C.; Luttmann, W.; Ferrari, D.; Di Virgilio, F.; et al. Extracellular ATP Triggers and Maintains Asthmatic Airway Inflammation by Activating Dendritic Cells. Nat. Med. 2007, 13, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Gu, Y.; Shin, A.; Zhang, S.; Ginhoux, F. Analysis of Myeloid Cells in Mouse Tissues with Flow Cytometry. STAR Protoc. 2020, 1, 100029. [Google Scholar] [CrossRef] [PubMed]
- Bogorodskiy, A.O.; Bolkhovitina, E.L.; Gensch, T.; Troyanova, N.I.; Mishin, A.V.; Okhrimenko, I.S.; Braun, A.; Spies, E.; Gordeliy, V.I.; Sapozhnikov, A.M.; et al. Murine Intraepithelial Dendritic Cells Interact With Phagocytic Cells During Aspergillus fumigatus-Induced Inflammation. Front. Immunol. 2020, 11, 298. [Google Scholar] [CrossRef] [PubMed]
- Khoyratty, T.E.; Ai, Z.; Ballesteros, I.; Eames, H.L.; Mathie, S.; Martín-Salamanca, S.; Wang, L.; Hemmings, A.; Willemsen, N.; von Werz, V.; et al. Distinct Transcription Factor Networks Control Neutrophil-Driven Inflammation. Nat. Immunol. 2021, 22, 1093–1106. [Google Scholar] [CrossRef] [PubMed]
- Hasenberg, A.; Hasenberg, M.; Männ, L.; Neumann, F.; Borkenstein, L.; Stecher, M.; Kraus, A.; Engel, D.R.; Klingberg, A.; Seddigh, P.; et al. Catchup: A mouse model for imaging-based tracking and modulation of neutrophil granulocytes. Nat. Methods 2015, 12, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Evrard, M.; Kwok, I.W.H.; Chong, S.Z.; Teng, K.W.W.; Becht, E.; Chen, J.; Sieow, J.L.; Penny, H.L.; Ching, G.C.; Devi, S.; et al. Developmental Analysis of Bone Marrow Neutrophils Reveals Populations Specialized in Expansion, Trafficking, and Effector Functions. Immunity 2018, 48, 364–379. [Google Scholar] [CrossRef]
- Iwasaki, A.; Foxman, E.F.; Molony, R.D. Early Local Immune Defences in the Respiratory Tract. Nat. Rev. Immunol. 2017, 17, 7–20. [Google Scholar] [CrossRef]
- Martin, C.; Burdon, P.C.; Bridger, G.; Gutierrez-Ramos, J.C.; Williams, T.J.; Rankin, S.M. Chemokines Acting via CXCR2 and CXCR4 Control the Release of Neutrophils from the Bone Marrow and Their Return Following Senescence. Immunity 2003, 19, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Pelleymounter, M.A.; Cullen, M.J.; Healy, D.; Hecht, R.; Winters, D.; McCaleb, M. Efficacy of Exogenous Recombinant Murine Leptin in Lean and Obese 10- to 12-Mo-Old Female CD-1 Mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1998, 275, R950–R959. [Google Scholar] [CrossRef]
- Lloyd, C.M.; Saglani, S. Eosinophils in the Spotlight: Finding the Link between Obesity and Asthma. Nat. Med. 2013, 19, 976–977. [Google Scholar] [CrossRef]
- Brusselle, G.; Maes, T.; Bracke, K. Eosinophils in the Spotlight: Eosinophilic airway inflammation in nonallergic asthma. Nat. Med. 2013, 19, 977–979. [Google Scholar] [CrossRef] [PubMed]
- Eash, K.J.; Greenbaum, A.M.; Gopalan, P.K.; Link, D.C. CXCR2 and CXCR4 Antagonistically Regulate Neutrophil Trafficking from Murine Bone Marrow. J. Clin. Investig. 2010, 120, 2423–2431. [Google Scholar] [CrossRef] [PubMed]
- Vats, R.; Kaminski, T.W.; Brzoska, T.; Leech, J.A.; Tutuncuoglu, E.; Katoch, O.; Jonassaint, J.; Tejero, J.; Novelli, E.M.; Pradhan-Sundd, T.; et al. Liver-to-lung microembolic NETs promote gasdermin D-dependent inflammatory lung injury in sickle cell disease. Blood 2022, 9, 1020–1037. [Google Scholar] [CrossRef] [PubMed]
- Petzold, T.; Zhang, Z.; Ballesteros, I.; Saleh, I.; Polzin, A.; Thienel, M.; Liu, L.; Ain, Q.U.; Ehreiser, V.; Weber, C.; et al. Neutrophil “plucking” on megakaryocytes drives platelet production and boosts cardiovascular disease. Immunity 2022, 12, 2285–2299.e7. [Google Scholar] [CrossRef] [PubMed]
- Castanheira, F.V.E.S.; Nguyen, R.; Willson, M.; Davoli-Ferreira, M.; David, B.A.; Kelly, M.M.; Lee, W.-Y.; Kratofil, R.M.; Zhang, W.X.; Bui-Marinos, M.P.; et al. Intravital imaging of three different microvascular beds in SARS-CoV-2-infected mice. Blood Adv. 2023, 7, 4170–4181. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, T.W.; Brzoska, T.; Li, X.; Vats, R.; Katoch, O.; Dubey, R.K.; Bagale, K.; Watkins, S.C.; McVerry, B.J.; Pradhan-Sundd, T.; et al. Lung microvascular occlusion by platelet-rich neutrophil-platelet aggregates promotes cigarette smoke-induced severe flu. JCI Insight 2024, 9, 167299. [Google Scholar] [CrossRef]
- Korbecki, J.; Maruszewska, A.; Bosiacki, M.; Chlubek, D.; Baranowska-Bosiacka, I. The Potential Importance of CXCL1 in the Physiological State and in Noncancer Diseases of the Cardiovascular System, Respiratory System and Skin. Int. J. Mol. Sci. 2022, 24, 205. [Google Scholar] [CrossRef]
- Mattos, M.S.; Ferrero, M.R.; Kraemer, L.; Lopes, G.A.O.; Reis, D.C.; Cassali, G.D.; Oliveira, F.M.S.; Brandolini, L.; Allegretti, M.; Garcia, C.C.; et al. CXCR1 and CXCR2 Inhibition by Ladarixin Improves Neutrophil-Dependent Airway Inflammation in Mice. Front. Immunol. 2020, 11, 566953. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shevchenko, M.A.; Servuli, E.A.; Murova, D.E.; Vavilova, J.D.; Bolkhovitina, E.L.; Chursanova, E.N.; Sapozhnikov, A.M. IL-4R and CXCR2 Contribute to Downregulating Neutrophil-Mediated Response in the Early Stage of Fungal Extract-Induced Allergic Airway Inflammation. Biomedicines 2024, 12, 2743. https://doi.org/10.3390/biomedicines12122743
Shevchenko MA, Servuli EA, Murova DE, Vavilova JD, Bolkhovitina EL, Chursanova EN, Sapozhnikov AM. IL-4R and CXCR2 Contribute to Downregulating Neutrophil-Mediated Response in the Early Stage of Fungal Extract-Induced Allergic Airway Inflammation. Biomedicines. 2024; 12(12):2743. https://doi.org/10.3390/biomedicines12122743
Chicago/Turabian StyleShevchenko, Marina A., Ekaterina A. Servuli, Dina E. Murova, Julia D. Vavilova, Elena L. Bolkhovitina, Ekaterina N. Chursanova, and Alexander M. Sapozhnikov. 2024. "IL-4R and CXCR2 Contribute to Downregulating Neutrophil-Mediated Response in the Early Stage of Fungal Extract-Induced Allergic Airway Inflammation" Biomedicines 12, no. 12: 2743. https://doi.org/10.3390/biomedicines12122743
APA StyleShevchenko, M. A., Servuli, E. A., Murova, D. E., Vavilova, J. D., Bolkhovitina, E. L., Chursanova, E. N., & Sapozhnikov, A. M. (2024). IL-4R and CXCR2 Contribute to Downregulating Neutrophil-Mediated Response in the Early Stage of Fungal Extract-Induced Allergic Airway Inflammation. Biomedicines, 12(12), 2743. https://doi.org/10.3390/biomedicines12122743





