In Vitro Models of Cardiovascular Disease: Embryoid Bodies, Organoids and Everything in Between
Abstract
1. Introduction
2. Stem Cells for Cardiac Disease Modeling
Stem Cells: Definition, Classification and Disease Modeling
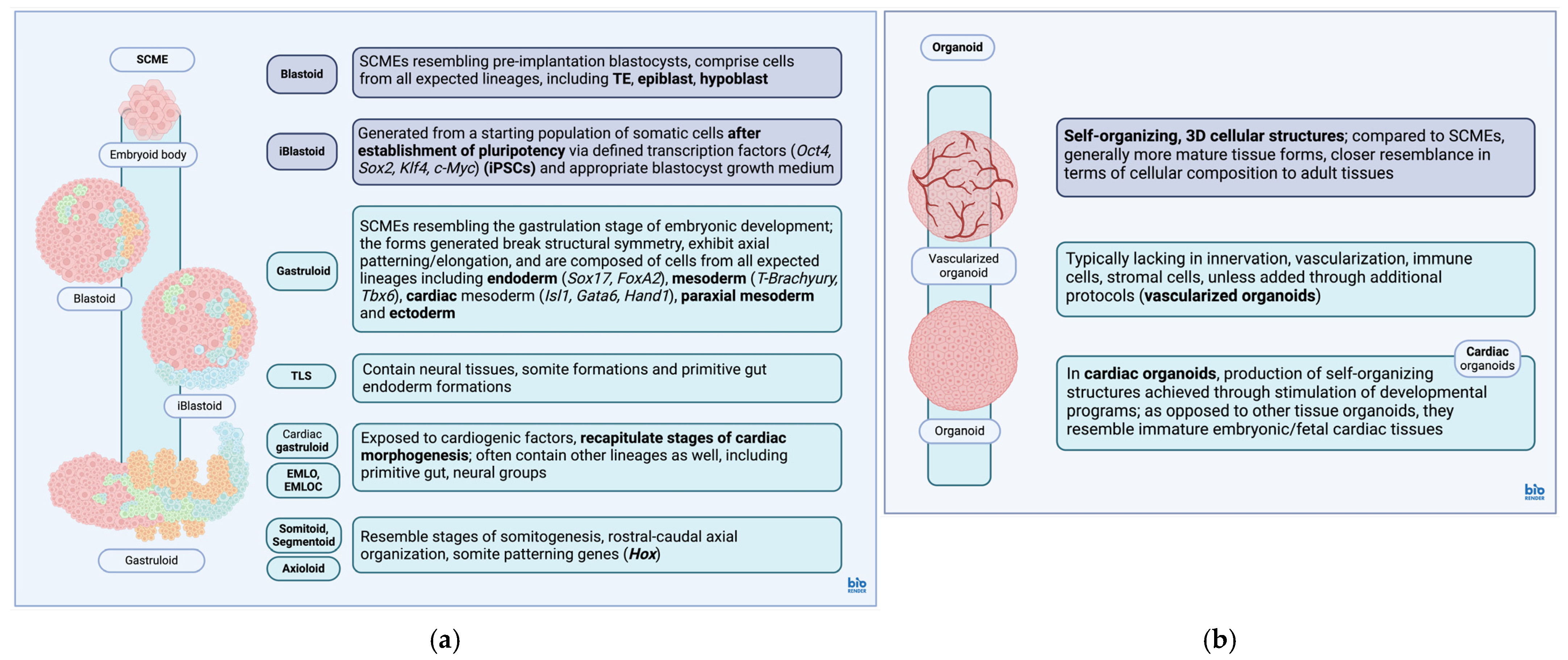
3. Embryoid Bodies, Organoids and Associated Models
3.1. Stem Cell-Based Models of Embryos
3.2. Organoids
| Model | Description | Ref. |
|---|---|---|
| EB | Disorganized 3D ESC aggregations, can organize into early embryonic structures, used as assays of pluripotency/first step during PSC expansion and differentiation protocols, capable of generating both intraembryonic (endoderm, mesoderm and ectoderm) and extraembryonic lineages. | [158,159,160,161] |
| Blastoid | SCMEs resembling pre-implantation blastocysts, derived from totipotent/pluripotent starter cell populations, comprising cells from all expected lineages at this stage of development (TE, epiblast, hypoblast). | [106] |
| iBlastoid: generated from starting populations of somatic cells after reprogramming/establishment of pluripotency via defined transcription factors (Oct4, Sox2, Klf4, c-Myc) within the blastocyst growth medium. | [133] | |
| Gastruloid | SCMEs resembling the gastrulation stage of embryonic development, exhibit characteristics found during this stage (breaking of symmetry, axial patterning, three major body axes, anteroposterior axial elongation, PS formation). | [107,136,143] |
| Comprises cells from all expected lineages at this stage of development, including endoderm (Sox17, FoxA2), mesoderm (Brachyury, Tbx6), cardiac mesoderm (Isl1, Gata6, Hand1), paraxial mesoderm, ectoderm. | [140,162,163] | |
| Cardiac mesoderm, cranial lineage derivatives often underrepresented in general gastruloid models derived via CHIR99021-mediated Wnt signaling stimulation. | [136] | |
| TLS: gastruloids composed of neural tissues, somite formations surrounding a primitive neural tube and primitive gut endoderm formations, resemble the ‘trunk’ area of a developing embryo. | [108,164] | |
| Cardiac gastruloids, EMLOC gastruloids: gastruloids additionally exposed to cardiogenic factors, recapitulate stages of cardiac morphogenesis along with other lineages (multilineage cardiac-neural gastruloids). | [101,102,165] | |
| Somitoids, Axioloids, Segmentoids: gastruloids that recapitulate stages of embryonic somitogenesis with rostral–caudal axial organization, segmentation, expression of genes associated with somitogenesis and anteroposterior somite patterning (Hox). | [143,166,167,168] | |
| Organoids | Self-organizing, 3D cellular structures, more mature tissue forms (cellular composition, tissue architecture) compared to EBs/SCMEs, cardiac organoids resemble more immature embryonic/fetal tissue forms (compared to other tissue organoids), generally lack innervation, vascularization, immune cells, stromal cells and bacterial flora (can be added separately). | [54,56,110,154,169,170,171,172,173] |
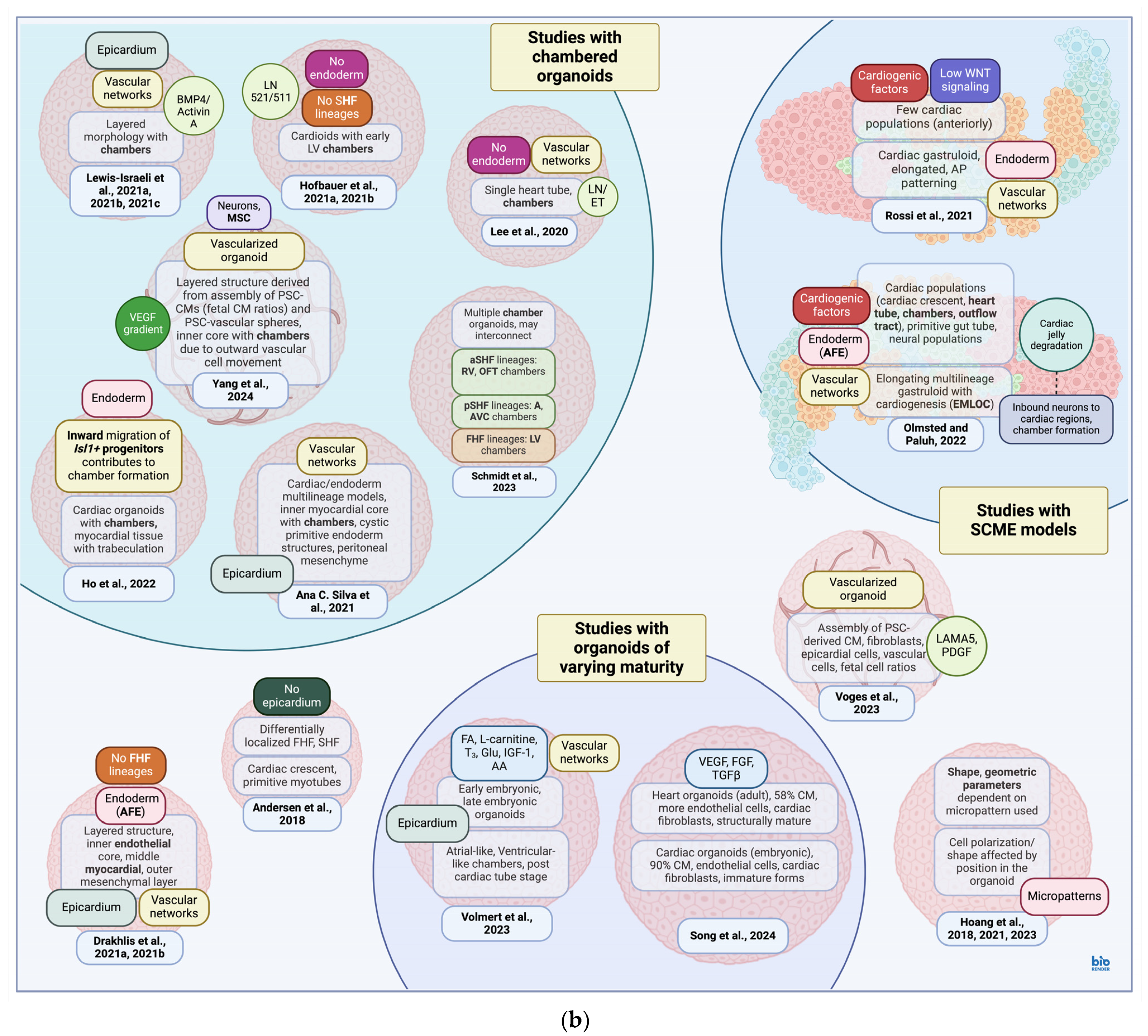
4. Cardiac Models
4.1. Cardiac Models and Signaling Pathways
4.2. Cardiac Models: Production and Composition

5. Cardiac Models of Disease
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- British Heart Foundation. Heart & Circulatory Disease Statistics, 2023 Compendium; British Heart Foundation: London, UK, 2023. [Google Scholar]
- Butler, D.; Reyes, D.R. Heart-on-a-Chip Systems: Disease Modeling and Drug Screening Applications. Lab. A Chip 2024, 24, 1494–1528. [Google Scholar] [CrossRef] [PubMed]
- Farah, E.N.; Hu, R.K.; Kern, C.; Zhang, Q.; Lu, T.-Y.; Ma, Q.; Tran, S.; Zhang, B.; Carlin, D.; Monell, A.; et al. Spatially Organized Cellular Communities Form the Developing Human Heart. Nature 2024, 627, 854–864. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.R.; Ilinykh, A.; Ivey, M.J.; Kuwabara, J.T.; D’Antoni, M.L.; Debuque, R.; Chandran, A.; Wang, L.; Arora, K.; Rosenthal, N.; et al. Revisiting Cardiac Cellular Composition. Circ. Res. 2016, 118, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Swanson, K.; Yildirim, Z.; Liu, W.; Liao, R.; Wu, J.C. Clinical Trials In-a-Dish for Cardiovascular Medicine. Eur. Heart J. 2024, 45, 4275–4290. [Google Scholar] [CrossRef] [PubMed]
- van Loo, B.; ten Den, S.A.; Araújo-Gomes, N.; de Jong, V.; Snabel, R.R.; Schot, M.; Rivera-Arbeláez, J.M.; Veenstra, G.J.C.; Passier, R.; Kamperman, T.; et al. Mass Production of Lumenogenic Human Embryoid Bodies and Functional Cardiospheres Using In-Air-Generated Microcapsules. Nat. Commun. 2023, 14, 6685. [Google Scholar] [CrossRef]
- van Doorn, E.C.H.; Amesz, J.H.; Sadeghi, A.H.; de Groot, N.M.S.; Manintveld, O.C.; Taverne, Y.J.H.J. Preclinical Models of Cardiac Disease: A Comprehensive Overview for Clinical Scientists. Cardiovasc. Eng. Technol. 2024, 15, 232–249. [Google Scholar] [CrossRef]
- Drakhlis, L.; Biswanath, S.; Farr, C.-M.; Lupanow, V.; Teske, J.; Ritzenhoff, K.; Franke, A.; Manstein, F.; Bolesani, E.; Kempf, H.; et al. Human Heart-Forming Organoids Recapitulate Early Heart and Foregut Development. Nat. Biotechnol. 2021, 39, 737–746. [Google Scholar] [CrossRef]
- Lewis-Israeli, Y.R.; Wasserman, A.H.; Gabalski, M.A.; Volmert, B.D.; Ming, Y.; Ball, K.A.; Yang, W.; Zou, J.; Ni, G.; Pajares, N.; et al. Self-Assembling Human Heart Organoids for the Modeling of Cardiac Development and Congenital Heart Disease. Nat. Commun. 2021, 12, 5142. [Google Scholar] [CrossRef]
- Mohammad, M.A.; Stone, G.W.; Koul, S.; Olivecrona, G.K.; Bergman, S.; Persson, J.; Engstrøm, T.; Fröbert, O.; Jernberg, T.; Omerovic, E.; et al. On the Natural History of Coronary Artery Disease: A Longitudinal Nationwide Serial Angiography Study. J. Am. Heart Assoc. 2022, 11, e026396. [Google Scholar] [CrossRef]
- Mola-Caminal, M.; Carrera, C.; Soriano-Tárraga, C.; Giralt-Steinhauer, E.; Díaz-Navarro, R.M.; Tur, S.; Jiménez, C.; Medina-Dols, A.; Cullell, N.; Torres-Aguila, N.P.; et al. PATJ Low Frequency Variants Are Associated With Worse Ischemic Stroke Functional Outcome. Circ. Res. 2019, 124, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Zou, M.; Jia, D.-M.; Shi, S.; Yang, X.; Liu, Q.; Dong, J.; Sheth, K.N.; Wang, X.; Shi, F.-D. tPA Mobilizes Immune Cells That Exacerbate Hemorrhagic Transformation in Stroke. Circ. Res. 2021, 128, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Ganta, V.C.; Choi, M.; Kutateladze, A.; Annex, B.H. VEGF165b Modulates Endothelial VEGFR1–STAT3 Signaling Pathway and Angiogenesis in Human and Experimental Peripheral Arterial Disease. Circ. Res. 2017, 120, 282–295. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.S.; Kameswaran, V.; Bruneau, B.G. Modeling Congenital Heart Disease: Lessons from Mice, hPSC-Based Models, and Organoids. Genes Dev. 2022, 36, 652–663. [Google Scholar] [CrossRef] [PubMed]
- Airhart, N.; Brownstein, B.H.; Cobb, J.P.; Schierding, W.; Arif, B.; Ennis, T.L.; Thompson, R.W.; Curci, J.A. Smooth Muscle Cells from Abdominal Aortic Aneurysms Are Unique and Can Independently and Synergistically Degrade Insoluble Elastin. J. Vasc. Surg. 2014, 60, 1033–1042.e5. [Google Scholar] [CrossRef] [PubMed]
- Alexander, K.C.; Anderson, C.W.; Agala, C.B.; Tasoudis, P.; Collins, E.N.; Ding, Y.; Blackwell, J.W.; Willcox, D.E.; Farivar, B.S.; Kibbe, M.R.; et al. Paradoxical Changes: EMMPRIN Tissue and Plasma Levels in Marfan Syndrome-Related Thoracic Aortic Aneurysms. J. Clin. Med. 2024, 13, 1548. [Google Scholar] [CrossRef]
- Karangelis, D.; Daskalopoulos, M.; Giamouzis, G.; Koufakis, T.; Fragoulis, S.; Papadakis, E.; Kalafati, G.; Tsilimingas, N. Acute Aortic Dissection Is Independent of Weather Conditions but Statistically Correlates with Day of the Week. J. Emergencies Trauma Shock. 2014, 7, 244–246. [Google Scholar] [CrossRef]
- Luo, S.; Kong, C.; Zhao, S.; Tang, X.; Wang, Y.; Zhou, X.; Li, R.; Liu, X.; Tang, X.; Sun, S.; et al. Endothelial HDAC1-ZEB2-NuRD Complex Drives Aortic Aneurysm and Dissection Through Regulation of Protein S-Sulfhydration. Circulation 2023, 147, 1382–1403. [Google Scholar] [CrossRef]
- Drawnel, F.M.; Boccardo, S.; Prummer, M.; Delobel, F.; Graff, A.; Weber, M.; Gérard, R.; Badi, L.; Kam-Thong, T.; Bu, L.; et al. Disease Modeling and Phenotypic Drug Screening for Diabetic Cardiomyopathy Using Human Induced Pluripotent Stem Cells. Cell Rep. 2014, 9, 810–820. [Google Scholar] [CrossRef]
- Choi, H.S.; Won, T.; Hou, X.; Chen, G.; Bracamonte-Baran, W.; Talor, M.V.; Jurčová, I.; Szárszoi, O.; Čurnova, L.; Stříž, I.; et al. Innate Lymphoid Cells Play a Pathogenic Role in Pericarditis. Cell Rep. 2020, 30, 2989–3003.e6. [Google Scholar] [CrossRef]
- Haataja, T.J.K.; Capoulade, R.; Lecointe, S.; Hellman, M.; Merot, J.; Permi, P.; Pentikäinen, U. Critical Structural Defects Explain Filamin A Mutations Causing Mitral Valve Dysplasia. Biophys. J. 2019, 117, 1467–1475. [Google Scholar] [CrossRef]
- Balistreri, C.R.; Crapanzano, F.; Schirone, L.; Allegra, A.; Pisano, C.; Ruvolo, G.; Forte, M.; Greco, E.; Cavarretta, E.; Marullo, A.G.M.; et al. Deregulation of Notch1 Pathway and Circulating Endothelial Progenitor Cell (EPC) Number in Patients with Bicuspid Aortic Valve with and without Ascending Aorta Aneurysm. Sci. Rep. 2018, 8, 13834. [Google Scholar] [CrossRef] [PubMed]
- Diekman, C.O.; Wei, N. Circadian Rhythms of Early Afterdepolarizations and Ventricular Arrhythmias in a Cardiomyocyte Model. Biophys. J. 2021, 120, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Hasaballa, A.I.; Wang, V.Y.; Sands, G.B.; Wilson, A.J.; Young, A.A.; LeGrice, I.J.; Nash, M.P. Microstructurally Motivated Constitutive Modeling of Heart Failure Mechanics. Biophys. J. 2019, 117, 2273–2286. [Google Scholar] [CrossRef] [PubMed]
- Lewalle, A.; Land, S.; Merken, J.J.; Raafs, A.; Sepúlveda, P.; Heymans, S.; Kleinjans, J.; Niederer, S.A. Balance of Active, Passive, and Anatomical Cardiac Properties in Doxorubicin-Induced Heart Failure. Biophys. J. 2019, 117, 2337–2348. [Google Scholar] [CrossRef] [PubMed]
- Azab, B.; Aburizeg, D.; Ji, W.; Jeffries, L.; Isbeih, N.J.; Al-Akily, A.S.; Mohammad, H.; Osba, Y.A.; Shahin, M.A.; Dardas, Z.; et al. TBX5 Variant with the Novel Phenotype of Mixed-type Total Anomalous Pulmonary Venous Return in Holt-Oram Syndrome and Variable Intrafamilial Heart Defects. Mol. Med. Rep. 2022, 25, 210. [Google Scholar] [CrossRef] [PubMed]
- Møller Nielsen, A.K.; Dehn, A.M.; Hjortdal, V.; Larsen, L.A. TBX5 Variants and Cardiac Phenotype: A Systematic Review of the Literature and a Novel Variant. Eur. J. Med. Genet. 2024, 68, 104920. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, F.-X.; Liu, X.-Y.; Hou, J.-Y.; Ni, S.-H.; Wang, J.; Zhao, C.-M.; Zhang, W.; Kong, Y.; Huang, R.-T.; et al. TBX1 Loss-of-Function Mutation Contributes to Congenital Conotruncal Defects. Exp. Ther. Med. 2018, 15, 447–453. [Google Scholar] [CrossRef]
- Pashmforoush, M.; Lu, J.T.; Chen, H.; Amand, T.S.; Kondo, R.; Pradervand, S.; Evans, S.M.; Clark, B.; Feramisco, J.R.; Giles, W.; et al. Nkx2-5 Pathways and Congenital Heart Disease: Loss of Ventricular Myocyte Lineage Specification Leads to Progressive Cardiomyopathy and Complete Heart Block. Cell 2004, 117, 373–386. [Google Scholar] [CrossRef]
- Abadir, S.; Vobecky, S.J.; Rohlicek, C.; Fournier, A.; Roméo, P.; Khairy, P. Left Atrial Inexcitability in Pediatric Patients with Congenital Lupus Induced Complete Atrioventricular Block. Can. J. Cardiol. 2013, 29, S93. [Google Scholar] [CrossRef]
- Mahmoud, M.; Allinson, K.R.; Zhai, Z.; Oakenfull, R.; Ghandi, P.; Adams, R.H.; Fruttiger, M.; Arthur, H.M. Pathogenesis of Arteriovenous Malformations in the Absence of Endoglin. Circ. Res. 2010, 106, 1425–1433. [Google Scholar] [CrossRef]
- Cacheiro, P.; Spielmann, N.; Mashhadi, H.H.; Fuchs, H.; Gailus-Durner, V.; Smedley, D.; de Angelis, M.H. Knockout Mice Are an Important Tool for Human Monogenic Heart Disease Studies. Dis. Models Mech. 2023, 16, dmm049770. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Wakimoto, H.; Paulo, J.A.; Zhang, Q.; Reichart, D.; Toepfer, C.; Sharma, A.; Tai, A.C.; Lun, M.; Gorham, J.; et al. Pathogenesis of Cardiomyopathy Caused by Variants in ALPK3, an Essential Pseudokinase in the Cardiomyocyte Nucleus and Sarcomere. Circulation 2022, 146, 1674–1693. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhao, J.V.; Qi, Y.; Deng, X.; Ji, Z.; Liu, J. A Translational Framework of Genoproteomic Studies for Cardiovascular Drug Discovery. npj Cardiovasc. Health 2024, 1, 12. [Google Scholar] [CrossRef]
- Dimitriou, M.; Moulos, P.; Kalafati, I.P.; Saranti, G.; Rallidis, L.S.; Dedoussis, G.V. Evaluation of Polygenic Risk Scores for Prediction of Coronary Artery Disease in a Greek Case-Control Study. J. Pers. Med. 2024, 14, 565. [Google Scholar] [CrossRef] [PubMed]
- Kot-Leibovich, H.; Fainsod, A. Ethanol Induces Embryonic Malformations by Competing for Retinaldehyde Dehydrogenase Activity during Vertebrate Gastrulation. Dis. Models Mech. 2009, 2, 295. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-Y.; Qin, L.; Li, G.; Wang, Z.; Dahlman, J.E.; Malagon-Lopez, J.; Gujja, S.; Cilfone, N.A.; Kauffman, K.J.; Sun, L.; et al. Endothelial TGF-β Signalling Drives Vascular Inflammation and Atherosclerosis. Nat. Metab. 2019, 1, 912–926. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Choi, D.B.; Im, J.S.; Song, Y.N.; Kim, J.H.; Lee, H.; An, J.; Kim, A.; Choi, H.; Kim, J.-C.; et al. Modeling Acute Myocardial Infarction and Cardiac Fibrosis Using Human Induced Pluripotent Stem Cell-Derived Multi-Cellular Heart Organoids. Cell Death Dis. 2024, 15, 308. [Google Scholar] [CrossRef]
- Khalil, A.; Tanos, R.; El-Hachem, N.; Kurban, M.; Bouvagnet, P.; Bitar, F.; Nemer, G. A HAND to TBX5 Explains the Link Between Thalidomide and Cardiac Diseases. Sci. Rep. 2017, 7, 1416. [Google Scholar] [CrossRef]
- Olvera Lopez, E.; Ballard, B.D.; Jan, A. Cardiovascular Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Ye, L.; Qiu, L.; Zhang, H.; Chen, H.; Jiang, C.; Hong, H.; Liu, J. Cardiomyocytes in Young Infants with Congenital Heart Disease: A Three-Month Window of Proliferation. Sci. Rep. 2016, 6, 23188. [Google Scholar] [CrossRef]
- Wang, L.; Serpooshan, V.; Zhang, J. Engineering Human Cardiac Muscle Patch Constructs for Prevention of Post-Infarction LV Remodeling. Front. Cardiovasc. Med. 2021, 8, 621781. [Google Scholar] [CrossRef]
- Boudou, T.; Legant, W.R.; Mu, A.; Borochin, M.A.; Thavandiran, N.; Radisic, M.; Zandstra, P.W.; Epstein, J.A.; Margulies, K.B.; Chen, C.S. A Microfabricated Platform to Measure and Manipulate the Mechanics of Engineered Cardiac Microtissues. Tissue Eng. Part A 2012, 18, 910–919. [Google Scholar] [CrossRef]
- Vila Cuenca, M.; Cochrane, A.; van den Hil, F.E.; de Vries, A.A.F.; Lesnik Oberstein, S.A.J.; Mummery, C.L.; Orlova, V.V. Engineered 3D Vessel-on-Chip Using hiPSC-Derived Endothelial- and Vascular Smooth Muscle Cells. Stem Cell Rep. 2021, 16, 2159–2168. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, A.; Wang, Y.; Zhao, Y.; Landau, S.; Perera, K.; Lee, J.; Radisic, M. Engineering Organ-on-a-Chip Systems for Vascular Diseases. Arter. Thromb. Vasc. Biol. 2023, 43, 2241–2255. [Google Scholar] [CrossRef] [PubMed]
- Le Guennec, J.-Y.; Champéroux, P.; Gavotto, A.; Cambonie, G.; Goual, L.; Bounasri, E.; Lacampagne, A.; Farès, R.; Thireau, J. Combined in Silico Model of Cardiac Electrophysiological Activity and Modulation by the Autonomic Nervous System. Arch. Cardiovasc. Dis. 2024, 117, S207. [Google Scholar] [CrossRef]
- Derda, R.; Tang, S.K.Y.; Laromaine, A.; Mosadegh, B.; Hong, E.; Mwangi, M.; Mammoto, A.; Ingber, D.E.; Whitesides, G.M. Multizone Paper Platform for 3D Cell Cultures. PLoS ONE 2011, 6, e18940. [Google Scholar] [CrossRef]
- Badie, N.; Bursac, N. Novel Micropatterned Cardiac Cell Cultures with Realistic Ventricular Microstructure. Biophys. J. 2009, 96, 3873. [Google Scholar] [CrossRef]
- Notbohm, J.; Napiwocki, B.N.; deLange, W.J.; Stempien, A.; Saraswathibhatla, A.; Craven, R.J.; Salick, M.R.; Ralphe, J.C.; Crone, W.C. Two-Dimensional Culture Systems to Enable Mechanics-Based Assays for Stem Cell-Derived Cardiomyocytes. Exp. Mech. 2019, 59, 1235–1248. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Kupfer, M.E.; Jung, J.P.; Yang, L.; Zhang, P.; Da Sie, Y.; Tran, Q.; Ajeti, V.; Freeman, B.T.; Fast, V.G.; et al. Myocardial Tissue Engineering With Cells Derived From Human-Induced Pluripotent Stem Cells and a Native-Like, High-Resolution, 3-Dimensionally Printed Scaffold. Circ. Res. 2017, 120, 1318–1325. [Google Scholar] [CrossRef]
- Duval, K.; Grover, H.; Han, L.-H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling Physiological Events in 2D vs. 3D Cell Culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef]
- Boxman, J.; Sagy, N.; Achanta, S.; Vadigepalli, R.; Nachman, I. Integrated Live Imaging and Molecular Profiling of Embryoid Bodies Reveals a Synchronized Progression of Early Differentiation. Sci. Rep. 2016, 6, 31623. [Google Scholar] [CrossRef]
- Voges, H.K.; Foster, S.R.; Reynolds, L.; Parker, B.L.; Devilée, L.; Quaife-Ryan, G.A.; Fortuna, P.R.J.; Mathieson, E.; Fitzsimmons, R.; Lor, M.; et al. Vascular Cells Improve Functionality of Human Cardiac Organoids. Cell Rep. 2023, 42, 112322. [Google Scholar] [CrossRef]
- Recaldin, T.; Steinacher, L.; Gjeta, B.; Harter, M.F.; Adam, L.; Kromer, K.; Mendes, M.P.; Bellavista, M.; Nikolaev, M.; Lazzaroni, G.; et al. Human Organoids with an Autologous Tissue-Resident Immune Compartment. Nature 2024, 633, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; An, J.-H.; Yang, H.-J.; Lee, D.G.; Kim, J.; Koh, H.; Park, Y.-H.; Song, B.-S.; Sim, B.-W.; Lee, H.J.; et al. Human Blood Vessel Organoids Penetrate Human Cerebral Organoids and Form a Vessel-Like System. Cells 2021, 10, 2036. [Google Scholar] [CrossRef] [PubMed]
- Beşikcioğlu, H.E.; Yurteri, Ü.; Ye, L.; Zhang, F.; Moretti, A.; Gürcinar, I.H.; Dogruöz, A.; Karakas, D.; Friess, H.; Ceyhan, G.O.; et al. Protocol for Whole-Mount Immunofluorescence Staining of ECM Gel-Embedded Innervated Pancreatic Organoids. STAR Protoc. 2024, 5, 103132. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Wang, H.; Sato, H.; Honda, S.; Ikeda, S.; Minami, N. MYC–MAX Heterodimerization Is Essential for the Induction of Major Zygotic Genome Activation and Subsequent Preimplantation Development. Sci. Rep. 2023, 13, 16011. [Google Scholar] [CrossRef] [PubMed]
- Lai, F.; Li, L.; Hu, X.; Liu, B.; Zhu, Z.; Liu, L.; Fan, Q.; Tian, H.; Xu, K.; Lu, X.; et al. NR5A2 Connects Zygotic Genome Activation to the First Lineage Segregation in Totipotent Embryos. Cell Res. 2023, 33, 952–966. [Google Scholar] [CrossRef] [PubMed]
- Aoki, F. Zygotic Gene Activation in Mice: Profile and Regulation. J. Reprod. Dev. 2022, 68, 79. [Google Scholar] [CrossRef]
- Zhou, C.Y.; Heald, R. Principles of Genome Activation in the Early Embryo. Curr. Opin. Genet. Dev. 2023, 81, 102062. [Google Scholar] [CrossRef]
- Asami, M.; Lam, B.Y.H.; Ma, M.K.; Rainbow, K.; Braun, S.; VerMilyea, M.D.; Yeo, G.S.H.; Perry, A.C.F. Human Embryonic Genome Activation Initiates at the One-Cell Stage. Cell Stem Cell 2022, 29, 209–216.e4. [Google Scholar] [CrossRef]
- Du, Z.; Lin, M.; Li, Q.; Guo, D.; Xue, Y.; Liu, W.; Shi, H.; Chen, T.; Dan, J. The Totipotent 2C-like State Safeguards Genomic Stability of Mouse Embryonic Stem Cells. J. Cell. Physiol. 2024, 239, e31337. [Google Scholar] [CrossRef]
- Malik, V.; Wang, J. Pursuing Totipotency: Authentic Totipotent Stem Cells in Culture. Trends Genet. 2022, 38, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhao, J.; Ren, Y.; Wang, X.; Lyu, Y.; Xie, B.; Sun, Y.; Yuan, X.; Liu, H.; Yang, W.; et al. Derivation of Totipotent-like Stem Cells with Blastocyst-like Structure Forming Potential. Cell Res. 2022, 32, 513–529. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Yu, H.; Yu, X.; Liang, S.; Hu, Y.; Luo, Y.; Izsvák, Z.; Sun, C.; Wang, J. Chemical-Induced Chromatin Remodeling Reprograms Mouse ESCs to Totipotent-like Stem Cells. Cell Stem Cell 2022, 29, 400–418.e13. [Google Scholar] [CrossRef] [PubMed]
- Okubo, T.; Rivron, N.; Kabata, M.; Masaki, H.; Kishimoto, K.; Semi, K.; Nakajima-Koyama, M.; Kunitomi, H.; Kaswandy, B.; Sato, H.; et al. Hypoblast from Human Pluripotent Stem Cells Regulates Epiblast Development. Nature 2024, 626, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Linneberg-Agerholm, M.; Wong, Y.F.; Romero Herrera, J.A.; Monteiro, R.S.; Anderson, K.G.V.; Brickman, J.M. Naïve Human Pluripotent Stem Cells Respond to Wnt, Nodal and LIF Signalling to Produce Expandable Naïve Extra-Embryonic Endoderm. Development 2019, 146, dev180620. [Google Scholar] [CrossRef]
- Artus, J.; Piliszek, A.; Hadjantonakis, A.-K. The Primitive Endoderm Lineage of the Mouse Blastocyst: Sequential Transcription Factor Activation and Regulation of Differentiation by Sox17. Dev. Biol. 2010, 350, 393. [Google Scholar] [CrossRef]
- Brown, K.; Legros, S.; Artus, J.; Doss, M.X.; Khanin, R.; Hadjantonakis, A.-K.; Foley, A. A Comparative Analysis of Extra-Embryonic Endoderm Cell Lines. PLoS ONE 2010, 5, e12016. [Google Scholar] [CrossRef]
- Panda, A.; Pham, T.X.A.; Khodeer, S.; Pasque, V. Induction of Human Extraembryonic Mesoderm Cells from Naive Pluripotent Stem Cells. Methods Mol. Biol. 2024, 2767, 105–113. [Google Scholar] [CrossRef]
- Thomson, J.A.; Itskovitz-Eldor, J.J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic Stem Cell Lines Derived from Human Blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef]
- Aasen, T.; Raya, A.; Barrero, M.J.; Garreta, E.; Consiglio, A.; Gonzalez, F.; Vassena, R.; Bilić, J.; Pekarik, V.; Tiscornia, G.; et al. Efficient and Rapid Generation of Induced Pluripotent Stem Cells from Human Keratinocytes. Nat. Biotechnol. 2008, 26, 1276–1284. [Google Scholar] [CrossRef]
- Hester, M.E.; Song, S.; Miranda, C.J.; Eagle, A.; Schwartz, P.H.; Kaspar, B.K. Two Factor Reprogramming of Human Neural Stem Cells into Pluripotency. PLoS ONE 2009, 4, e7044. [Google Scholar] [CrossRef]
- Eminli, S.; Utikal, J.; Arnold, K.; Jaenisch, R.; Hochedlinger, K. Reprogramming of Neural Progenitor Cells into Induced Pluripotent Stem Cells in the Absence of Exogenous Sox2 Expression. Stem Cells 2008, 26, 2467–2474. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Panetta, N.J.; Gupta, D.M.; Wilson, K.D.; Lee, A.; Jia, F.; Hu, S.; Cherry, A.M.; Robbins, R.C.; Longaker, M.T.; et al. Feeder-Free Derivation of Induced Pluripotent Stem Cells from Adult Human Adipose Stem Cells. Proc. Natl. Acad. Sci. USA 2009, 106, 15720–15725. [Google Scholar] [CrossRef] [PubMed]
- Cerneckis, J.; Cai, H.; Shi, Y. Induced Pluripotent Stem Cells (iPSCs): Molecular Mechanisms of Induction and Applications. Sig Transduct. Target. Ther. 2024, 9, 112. [Google Scholar] [CrossRef] [PubMed]
- Chakritbudsabong, W.; Chaiwattanarungruengpaisan, S.; Sariya, L.; Pamonsupornvichit, S.; Ferreira, J.N.; Sukho, P.; Gronsang, D.; Tharasanit, T.; Dinnyes, A.; Rungarunlert, S. Exogenous LIN28 Is Required for the Maintenance of Self-Renewal and Pluripotency in Presumptive Porcine-Induced Pluripotent Stem Cells. Front. Cell Dev. Biol. 2021, 9, 709286. [Google Scholar] [CrossRef] [PubMed]
- Chin, M.H.; Mason, M.J.; Xie, W.; Volinia, S.; Singer, M.; Peterson, C.; Ambartsumyan, G.; Aimiuwu, O.; Richter, L.; Zhang, J.; et al. Induced Pluripotent Stem Cells and Embryonic Stem Cells Are Distinguished by Gene Expression Signatures. Cell Stem Cell 2009, 5, 111–123. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. A Decade of Transcription Factor-Mediated Reprogramming to Pluripotency. Nat. Rev. Mol. Cell Biol. 2016, 17, 183–193. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Yang, S.; Hu, H.; Kung, H.; Zou, R.; Dai, Y.; Hu, Y.; Wang, T.; Lv, T.; Yu, J.; Li, F. Organoids: The Current Status and Biomedical Applications. MedComm 2023, 4, e274. [Google Scholar] [CrossRef] [PubMed]
- Matthews, K.R.W.; Wagner, D.S.; Warmflash, A. Stem Cell-Based Models of Embryos: The Need for Improved Naming Conventions. Stem Cell Rep. 2021, 16, 1014–1020. [Google Scholar] [CrossRef]
- Li, R.; Zhong, C.; Yu, Y.; Liu, H.; Sakurai, M.; Yu, L.; Min, Z.; Shi, L.; Wei, Y.; Takahashi, Y.; et al. Generation of Blastocyst-like Structures from Mouse Embryonic and Adult Cell Cultures. Cell 2019, 179, 687–702.e18. [Google Scholar] [CrossRef] [PubMed]
- Sozen, B.; Cox, A.L.; De Jonghe, J.; Bao, M.; Hollfelder, F.; Glover, D.M.; Zernicka-Goetz, M. Self-Organization of Mouse Stem Cells into an Extended Potential Blastoid. Dev. Cell 2019, 51, 698–712.e8. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Yang, Y.; Tan, P.; Zhang, Y.; Han, M.; Yu, J.; Zhang, X.; Jia, Z.; Wang, D.; Yao, K.; et al. Induction of Mouse Totipotent Stem Cells by a Defined Chemical Cocktail. Nature 2023, 617, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Kues, W.A.; Kumar, D. Cocktails of Defined Chemical Compounds: Sufficient to Induce Totipotency in Embryonic Stem Cells. Sig Transduct. Target. Ther. 2022, 7, 330. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Martinez, E.; Suazo-Sanchez, I.; Celis-Romero, M.; Carnero, A. 3D and Organoid Culture in Research: Physiology, Hereditary Genetic Diseases and Cancer. Cell Biosci. 2022, 12, 39. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Deyett, A.; Ilmer, T.; Haendeler, S.; Caballero, A.T.; Novatchkova, M.; Netzer, M.A.; Ginistrelli, L.C.; Juncosa, E.M.; Bhattacharya, T.; et al. Multi-Chamber Cardioids Unravel Human Heart Development and Cardiac Defects. Cell 2023, 186, 5587–5605.e27. [Google Scholar] [CrossRef]
- Lee, J.; Sutani, A.; Kaneko, R.; Takeuchi, J.; Sasano, T.; Kohda, T.; Ihara, K.; Takahashi, K.; Yamazoe, M.; Morio, T.; et al. In Vitro Generation of Functional Murine Heart Organoids via FGF4 and Extracellular Matrix. Nat. Commun. 2020, 11, 4283. [Google Scholar] [CrossRef]
- Andersen, P.; Tampakakis, E.; Jimenez, D.V.; Kannan, S.; Miyamoto, M.; Shin, H.K.; Saberi, A.; Murphy, S.; Sulistio, E.; Chelko, S.P.; et al. Precardiac Organoids Form Two Heart Fields via Bmp/Wnt Signaling. Nat. Commun. 2018, 9, 3140. [Google Scholar] [CrossRef]
- Hoang, P.; Sun, S.; Tarris, B.A.; Ma, Z. Controlling Morphology and Functions of Cardiac Organoids by Two-Dimensional Geometrical Templates. Cells Tissues Organs 2023, 212, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Hoang, P.; Kowalczewski, A.; Sun, S.; Winston, T.S.; Archilla, A.M.; Lemus, S.M.; Ercan-Sencicek, A.G.; Gupta, A.R.; Liu, W.; Kontaridis, M.I.; et al. Engineering Spatial-Organized Cardiac Organoids for Developmental Toxicity Testing. Stem Cell Rep. 2021, 16, 1228–1244. [Google Scholar] [CrossRef]
- Hoang, P.; Wang, J.; Conklin, B.R.; Healy, K.E.; Ma, Z. Generation of Spatial-Patterned Early-Developing Cardiac Organoids Using Human Pluripotent Stem Cells. Nat. Protoc. 2018, 13, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Drakhlis, L.; Devadas, S.B.; Zweigerdt, R. Generation of Heart-Forming Organoids from Human Pluripotent Stem Cells. Nat. Protoc. 2021, 16, 5652–5672. [Google Scholar] [CrossRef] [PubMed]
- Hofbauer, P.; Jahnel, S.M.; Papai, N.; Giesshammer, M.; Deyett, A.; Schmidt, C.; Penc, M.; Tavernini, K.; Grdseloff, N.; Meledeth, C.; et al. Cardioids Reveal Self-Organizing Principles of Human Cardiogenesis. Cell 2021, 184, 3299–3317.e22. [Google Scholar] [CrossRef] [PubMed]
- Hofbauer, P.; Jahnel, S.M.; Mendjan, S. In Vitro Models of the Human Heart. Development 2021, 148, dev199672. [Google Scholar] [CrossRef] [PubMed]
- Lewis-Israeli, Y.R.; Wasserman, A.H.; Aguirre, A. Heart Organoids and Engineered Heart Tissues: Novel Tools for Modeling Human Cardiac Biology and Disease. Biomolecules 2021, 11, 1277. [Google Scholar] [CrossRef]
- Ho, B.X.; Pang, J.K.S.; Chen, Y.; Loh, Y.-H.; An, O.; Yang, H.H.; Seshachalam, V.P.; Koh, J.L.Y.; Chan, W.-K.; Ng, S.Y.; et al. Robust Generation of Human-Chambered Cardiac Organoids from Pluripotent Stem Cells for Improved Modelling of Cardiovascular Diseases. Stem Cell Res. Ther. 2022, 13, 529. [Google Scholar] [CrossRef]
- Volmert, B.; Kiselev, A.; Juhong, A.; Wang, F.; Riggs, A.; Kostina, A.; O’Hern, C.; Muniyandi, P.; Wasserman, A.; Huang, A.; et al. A Patterned Human Primitive Heart Organoid Model Generated by Pluripotent Stem Cell Self-Organization. Nat. Commun. 2023, 14, 8245. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.C.; Matthys, O.B.; Joy, D.A.; Kauss, M.A.; Natarajan, V.; Lai, M.H.; Turaga, D.; Blair, A.P.; Alexanian, M.; Bruneau, B.G.; et al. Co-Emergence of Cardiac and Gut Tissues Promotes Cardiomyocyte Maturation within Human iPSC-Derived Organoids. Cell Stem Cell 2021, 28, 2137–2152.e6. [Google Scholar] [CrossRef]
- Rossi, G.; Broguiere, N.; Miyamoto, M.; Boni, A.; Guiet, R.; Girgin, M.; Kelly, R.G.; Kwon, C.; Lutolf, M.P. Capturing Cardiogenesis in Gastruloids. Cell Stem Cell 2021, 28, 230–240.e6. [Google Scholar] [CrossRef] [PubMed]
- Olmsted, Z.T.; Paluh, J.L. A Combined Human Gastruloid Model of Cardiogenesis and Neurogenesis. Iscience 2022, 25, 104486. [Google Scholar] [CrossRef]
- Yang, J.; Lei, W.; Xiao, Y.; Tan, S.; Yang, J.; Lin, Y.; Yang, Z.; Zhao, D.; Zhang, C.; Shen, Z.; et al. Generation of Human Vascularized and Chambered Cardiac Organoids for Cardiac Disease Modelling and Drug Evaluation. Cell Prolif. 2024, 57, e13631. [Google Scholar] [CrossRef] [PubMed]
- Cardano, M.; Marsoner, F.; Marcatili, M.; Karnavas, T.; Zasso, J.; Lanterna, L.A.; Conti, L. Establishment of Induced Pluripotent Stem Cell (iPSC) Line from 55-Year Old Male Patient with Hemorrhagic Moyamoya Disease. Stem Cell Res. 2016, 17, 623–626. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cardano, M.; Marsoner, F.; Zasso, J.; Marcatili, M.; Karnavas, T.; Lanterna, L.A.; Conti, L. Establishment of Induced Pluripotent Stem Cell (iPSC) Line from an 8-Year Old Female Patient with Ischemic Moyamoya Disease. Stem Cell Res. 2016, 17, 619–622. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Min, Z.; Alsolami, S.; Ma, Z.; Zhang, E.; Chen, W.; Zhong, K.; Pei, W.; Kang, X.; Zhang, P.; et al. Generation of Human Blastocyst-like Structures from Pluripotent Stem Cells. Cell Discov. 2021, 7, 81. [Google Scholar] [CrossRef] [PubMed]
- Beccari, L.; Moris, N.; Girgin, M.; Turner, D.A.; Baillie-Johnson, P.; Cossy, A.-C.; Lutolf, M.P.; Duboule, D.; Arias, A.M. Multi-Axial Self-Organization Properties of Mouse Embryonic Stem Cells into Gastruloids. Nature 2018, 562, 272–276. [Google Scholar] [CrossRef]
- Veenvliet, J.V.; Bolondi, A.; Kretzmer, H.; Haut, L.; Scholze-Wittler, M.; Schifferl, D.; Koch, F.; Guignard, L.; Kumar, A.S.; Pustet, M.; et al. Mouse Embryonic Stem Cells Self-Organize into Trunk-like Structures with Neural Tube and Somites. Science 2020, 370, eaba4937. [Google Scholar] [CrossRef]
- Brickman, J.M.; Serup, P. Properties of Embryoid Bodies. WIREs Dev. Biol. 2017, 6, e259. [Google Scholar] [CrossRef]
- Chen, K.; Li, Y.; Wu, X.; Tang, X.; Zhang, B.; Fan, T.; He, L.; Pei, X.; Li, Y. Establishment of Human Hematopoietic Organoids for Evaluation of Hematopoietic Injury and Regeneration Effect. Stem Cell Res. Ther. 2024, 15, 133. [Google Scholar] [CrossRef]
- Sato, T.; Vries, R.G.; Snippert, H.J.; van de Wetering, M.; Barker, N.; Stange, D.E.; van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 Stem Cells Build Crypt-Villus Structures in Vitro without a Mesenchymal Niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef]
- Mandl, M.; Viertler, H.P.; Hatzmann, F.M.; Brucker, C.; Großmann, S.; Waldegger, P.; Rauchenwald, T.; Mattesich, M.; Zwierzina, M.; Pierer, G.; et al. An Organoid Model Derived from Human Adipose Stem/Progenitor Cells to Study Adipose Tissue Physiology. Adipocyte 2022, 11, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Sumbal, J.; Chiche, A.; Charifou, E.; Koledova, Z.; Li, H. Primary Mammary Organoid Model of Lactation and Involution. Front. Cell Dev. Biol. 2020, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- Caruso, M.; Huang, S.; Mourao, L.; Scheele, C.L.G.J. A Mammary Organoid Model to Study Branching Morphogenesis. Front. Physiol. 2022, 13, 826107. [Google Scholar] [CrossRef] [PubMed]
- Kessler, M.; Hoffmann, K.; Brinkmann, V.; Thieck, O.; Jackisch, S.; Toelle, B.; Berger, H.; Mollenkopf, H.-J.; Mangler, M.; Sehouli, J.; et al. The Notch and Wnt Pathways Regulate Stemness and Differentiation in Human Fallopian Tube Organoids. Nat. Commun. 2015, 6, 8989. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Cha, X.; Xu, R.; Wang, T.; Liang, C.; Chou, J.; Zhang, X.; Li, F.; Wang, S.; Cai, B.; et al. Cisplatin Attenuates Taste Cell Homeostasis and Induces Inflammatory Activation in the Circumvallate Papilla. Theranostics 2023, 13, 2896. [Google Scholar] [CrossRef]
- Ozan, V.B.; Wang, H.; Akshay, A.; Anand, D.; Hibaoui, Y.; Feki, A.; Gote-Schniering, J.; Gheinani, A.H.; Heller, M.; Uldry, A.-C.; et al. Influence of Microenvironmental Orchestration on Multicellular Lung Alveolar Organoid Development from Human Induced Pluripotent Stem Cells. Stem Cell Rev. Rep. 2024, 1–22, ahead of print. [Google Scholar] [CrossRef]
- Zanten, J.v.; Jorritsma-Smit, A.; Westra, H.; Baanstra, M.; de Bruin-Jellema, A.; Allersma, D.; Gareb, B.; Coppes, R.P. Optimization of the Production Process of Clinical-Grade Human Salivary Gland Organoid-Derived Cell Therapy for the Treatment of Radiation-Induced Xerostomia in Head and Neck Cancer. Pharmaceutics 2024, 16, 435. [Google Scholar] [CrossRef] [PubMed]
- Shimonosono, M.; Morimoto, M.; Hirose, W.; Tomita, Y.; Matsuura, N.; Flashner, S.; Ebadi, M.S.; Okayasu, E.H.; Lee, C.Y.; Britton, W.R.; et al. Modeling Epithelial Homeostasis and Perturbation in Three-Dimensional Human Esophageal Organoids. Biomolecules 2024, 14, 1126. [Google Scholar] [CrossRef]
- Cristiani, S.; Bertolini, A.; Carnicelli, V.; Contu, L.; Vitelli, V.; Saba, A.; Saponaro, F.; Chiellini, G.; Sabbatini, A.R.M.; Giambelluca, M.A.; et al. Development and Primary Characterization of a Human Thyroid Organoid in Vitro Model for Thyroid Metabolism Investigation. Mol. Cell. Endocrinol. 2024, 594, 112377. [Google Scholar] [CrossRef]
- Davis, D.R. Cardiac Stem Cells in the Post-Anversa Era. Eur. Heart J. 2019, 40, 1039–1041. [Google Scholar] [CrossRef] [PubMed]
- Pierce, G.B.; Dixon, F.J., Jr.; Verney, E. Testicular Teratomas. II. Teratocarcinoma as an Ascitic Tumor. Cancer 1959, 12, 584–589. [Google Scholar] [CrossRef]
- D’Amour, K.A.; Agulnick, A.D.; Eliazer, S.; Kelly, O.G.; Kroon, E.; Baetge, E.E. Efficient Differentiation of Human Embryonic Stem Cells to Definitive Endoderm. Nat. Biotechnol. 2005, 23, 1534–1541. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Idiris, A.; Miwa, T.; Kumagai, H. Microfabric Vessels for Embryoid Body Formation and Rapid Differentiation of Pluripotent Stem Cells. Sci. Rep. 2016, 6, 31063. [Google Scholar] [CrossRef] [PubMed]
- Wolnik, J.; Adamska, P.; Oleksy, A.; Sanetra, A.M.; Palus-Chramiec, K.; Lewandowski, M.H.; Dulak, J.; Biniecka, M. A Novel 3D Cardiac Microtissue Model for Investigation of Cardiovascular Complications in Rheumatoid Arthritis. Stem Cell Res. Ther. 2024, 15, 382. [Google Scholar] [CrossRef] [PubMed]
- Gordeeva, O.; Gordeev, A.; Erokhov, P. Archetypal Architecture Construction, Patterning, and Scaling Invariance in a 3D Embryoid Body Differentiation Model. Front. Cell Dev. Biol. 2022, 10, 852071. [Google Scholar] [CrossRef]
- Anlas, K.; Baillie-Benson, P.; Arató, K.; Turner, D.A.; Trivedi, V. Gastruloids: Embryonic Organoids from Mouse Embryonic Stem Cells to Study Patterning and Development in Early Mammalian Embryos. Methods Mol. Biol. 2021, 2258, 131–147. [Google Scholar] [CrossRef]
- Yu, L.; Wei, Y.; Duan, J.; Schmitz, D.A.; Sakurai, M.; Wang, L.; Wang, K.; Zhao, S.; Hon, G.C.; Wu, J. Blastocyst-like Structures Generated from Human Pluripotent Stem Cells. Nature 2021, 591, 620–626. [Google Scholar] [CrossRef]
- Pennarossa, G.; Arcuri, S.; Gandolfi, F.; Brevini, T.A.L. Generation of Artificial Blastoids Combining miR-200-Mediated Reprogramming and Mechanical Cues. Cells 2024, 13, 628. [Google Scholar] [CrossRef]
- Rivron, N.C.; Frias-Aldeguer, J.; Vrij, E.J.; Boisset, J.-C.; Korving, J.; Vivié, J.; Truckenmüller, R.K.; van Oudenaarden, A.; van Blitterswijk, C.A.; Geijsen, N. Blastocyst-like Structures Generated Solely from Stem Cells. Nature 2018, 557, 106–111. [Google Scholar] [CrossRef]
- Vrij, E.J.; Reimer, Y.S.S.o.; Aldeguer, J.F.; Guerreiro, I.M.; Kind, J.; Koo, B.-K.; van Blitterswijk, C.A.; Rivron, N.C. Chemically-Defined Induction of a Primitive Endoderm and Epiblast-like Niche Supports Post-Implantation Progression from Blastoids. BioRxiv 2019, 120, 173. [Google Scholar]
- Vrij, E.J.; Scholte op Reimer, Y.S.; Fuentes, L.R.; Guerreiro, I.M.; Holzmann, V.; Aldeguer, J.F.; Sestini, G.; Koo, B.-K.; Kind, J.; van Blitterswijk, C.A.; et al. A Pendulum of Induction between the Epiblast and Extra-Embryonic Endoderm Supports Post-Implantation Progression. Development 2022, 149, dev192310. [Google Scholar] [CrossRef]
- Liu, X.; Tan, J.P.; Schröder, J.; Aberkane, A.; Ouyang, J.F.; Mohenska, M.; Lim, S.M.; Sun, Y.B.Y.; Chen, J.; Sun, G.; et al. Modelling Human Blastocysts by Reprogramming Fibroblasts into iBlastoids. Nature 2021, 591, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Abel, A.; Sozen, B. Shifting Early Embryology Paradigms: Applications of Stem Cell-Based Embryo Models in Bioengineering. Curr. Opin. Genet. Dev. 2023, 81, 102069. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Polo, J.M. Human Blastoid as an in Vitro Model of Human Blastocysts. Curr. Opin. Genet. Dev. 2024, 84, 102135. [Google Scholar] [CrossRef] [PubMed]
- Wehmeyer, A.E.; Schüle, K.M.; Conrad, A.; Schröder, C.M.; Probst, S.; Arnold, S.J. Chimeric 3D Gastruloids—A Versatile Tool for Studies of Mammalian Peri-Gastrulation Development. Development 2022, 149, dev200812. [Google Scholar] [CrossRef]
- Moris, N.; Anlas, K.; van den Brink, S.C.; Alemany, A.; Schröder, J.; Ghimire, S.; Balayo, T.; van Oudenaarden, A.; Martinez Arias, A. An in Vitro Model of Early Anteroposterior Organization during Human Development. Nature 2020, 582, 410–415. [Google Scholar] [CrossRef]
- Martyn, I.; Siggia, E.D.; Brivanlou, A.H. Mapping Cell Migrations and Fates in a Gastruloid Model to the Human Primitive Streak. Development 2019, 146, dev179564. [Google Scholar] [CrossRef]
- Farag, N.; Sacharen, C.; Avni, L.; Nachman, I. Coordination between Endoderm Progression and Mouse Gastruloid Elongation Controls Endodermal Morphotype Choice. Dev. Cell 2024, 59, 2364–2374.e4. [Google Scholar] [CrossRef]
- Underhill, E.J.; Toettcher, J.E. Control of Gastruloid Patterning and Morphogenesis by the Erk and Akt Signaling Pathways. Development 2023, 150, dev201663. [Google Scholar] [CrossRef]
- Budjan, C.; Liu, S.; Ranga, A.; Gayen, S.; Pourquié, O.; Hormoz, S. Paraxial Mesoderm Organoids Model Development of Human Somites. Elife 2022, 11, e68925. [Google Scholar] [CrossRef]
- Turner, D.A.; Nichols, J. Modifying Gastruloids to Dissect Mechanisms of Tissue-Specific Induction. Curr. Opin. Genet. Dev. 2023, 83, 102130. [Google Scholar] [CrossRef]
- Sullivan, A.E.; Santos, S.D. The Ever-Growing World of Gastruloids: Autogenous Models of Mammalian Embryogenesis. Curr. Opin. Genet. Dev. 2023, 82, 102102. [Google Scholar] [CrossRef] [PubMed]
- Maroto, M.; Bone, R.A.; Dale, J.K. Somitogenesis. Development 2012, 139, 2453–2456. [Google Scholar] [CrossRef] [PubMed]
- van den Brink, S.C.; Baillie-Johnson, P.; Balayo, T.; Hadjantonakis, A.-K.; Nowotschin, S.; Turner, D.A.; Martinez Arias, A. Symmetry Breaking, Germ Layer Specification and Axial Organisation in Aggregates of Mouse Embryonic Stem Cells. Development 2014, 141, 4231–4242. [Google Scholar] [CrossRef] [PubMed]
- Ergir, E.; Oliver-De La Cruz, J.; Fernandes, S.; Cassani, M.; Niro, F.; Pereira-Sousa, D.; Vrbský, J.; Vinarský, V.; Perestrelo, A.R.; Debellis, D.; et al. Generation and Maturation of Human iPSC-Derived 3D Organotypic Cardiac Microtissues in Long-Term Culture. Sci. Rep. 2022, 12, 17409. [Google Scholar] [CrossRef] [PubMed]
- Simunovic, M.; Brivanlou, A.H. Embryoids, Organoids and Gastruloids: New Approaches to Understanding Embryogenesis. Development 2017, 144, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Saini, H.; Navaei, A.; Van Putten, A.; Nikkhah, M. 3D Cardiac Microtissues Encapsulated with the Co-Culture of Cardiomyocytes and Cardiac Fibroblasts. Adv. Healthc. Mater. 2015, 4, 1961–1971. [Google Scholar] [CrossRef] [PubMed]
- Abecasis, B.; Canhão, P.G.M.; Almeida, H.V.; Calmeiro, T.; Fortunato, E.; Gomes-Alves, P.; Serra, M.; Alves, P.M. Toward a Microencapsulated 3D hiPSC-Derived in Vitro Cardiac Microtissue for Recapitulation of Human Heart Microenvironment Features. Front. Bioeng. Biotechnol. 2020, 8, 580744. [Google Scholar] [CrossRef]
- Giacomelli, E.; Meraviglia, V.; Campostrini, G.; Cochrane, A.; Cao, X.; Helden, R.W.J.v.; Garcia, A.K.; Mircea, M.; Kostidis, S.; Davis, R.P.; et al. Human-iPSC-Derived Cardiac Stromal Cells Enhance Maturation in 3D Cardiac Microtissues and Reveal Non-Cardiomyocyte Contributions to Heart Disease. Cell Stem Cell 2020, 26, 862–879.e11. [Google Scholar] [CrossRef]
- Min, S.; Kim, S.; Sim, W.-S.; Choi, Y.S.; Joo, H.; Park, J.-H.; Lee, S.-J.; Kim, H.; Lee, M.J.; Jeong, I.; et al. Versatile Human Cardiac Tissues Engineered with Perfusable Heart Extracellular Microenvironment for Biomedical Applications. Nat. Commun. 2024, 15, 2564. [Google Scholar] [CrossRef]
- Drakhlis, L.; Zweigerdt, R. Heart in a Dish—Choosing the Right in Vitro Model. Dis. Models Mech. 2023, 16, dmm049961. [Google Scholar] [CrossRef]
- Whye, D.; Norabuena, E.M.; Srinivasan, G.R.; Wood, D.; Polanco, T.J.; Makhortova, N.R.; Sahin, M.; Buttermore, E.D. A Hybrid 2D-to-3D in Vitro Differentiation Platform Improves Outcomes of Cerebral Cortical Organoid Generation in hiPSCs. Curr. Protoc. 2024, 4, e70022. [Google Scholar] [CrossRef] [PubMed]
- Nwokoye, P.N.; Abilez, O.J. Bioengineering Methods for Vascularizing Organoids. Cell Rep. Methods 2024, 4, 100779. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.B.; Hwangbo, S.; Jang, S.; Jo, Y.K. Bioengineered Co-Culture of Organoids to Recapitulate Host-Microbe Interactions. Mater. Today Bio. 2022, 16, 100345. [Google Scholar] [CrossRef] [PubMed]
- Holloway, E.M.; Capeling, M.M.; Spence, J.R. Biologically Inspired Approaches to Enhance Human Organoid Complexity. Development 2019, 146, dev166173. [Google Scholar] [CrossRef]
- Li, X.-H.; Hu, N.; Chang, Z.-H.; Shi, J.-X.; Fan, X.; Chen, M.-M.; Bao, S.-Q.; Chen, C.; Zuo, J.-C.; Zhang, X.-W.; et al. Brain Organoid Maturation and Implantation Integration Based on Electrical Signals Input. J. Adv. Res. 2024, in press. [Google Scholar] [CrossRef]
- Pettinato, G.; Wen, X.; Zhang, N. Formation of Well-Defined Embryoid Bodies from Dissociated Human Induced Pluripotent Stem Cells Using Microfabricated Cell-Repellent Microwell Arrays. Sci. Rep. 2014, 4, 7402. [Google Scholar] [CrossRef]
- Zhao, X.; Radford, B.N.; Ungrin, M.; Dean, W.; Hemberger, M. The Trophoblast Compartment Helps Maintain Embryonic Pluripotency and Delays Differentiation towards Cardiomyocytes. Int. J. Mol. Sci. 2023, 24, 12423. [Google Scholar] [CrossRef]
- Cho, L.T.Y.; Wamaitha, S.E.; Tsai, I.J.; Artus, J.; Sherwood, R.I.; Pedersen, R.A.; Hadjantonakis, A.-K.; Niakan, K.K. Conversion from Mouse Embryonic to Extra-Embryonic Endoderm Stem Cells Reveals Distinct Differentiation Capacities of Pluripotent Stem Cell States. Development 2012, 139, 2866–2877. [Google Scholar] [CrossRef]
- Conley, B.J.; Denham, M.; Gulluyan, L.; Olsson, F.; Cole, T.J.; Mollard, R. Mouse Embryonic Stem Cell Derivation, and Mouse and Human Embryonic Stem Cell Culture and Differentiation as Embryoid Bodies. Curr. Protoc. Cell Biol. 2005, 28, 23.2.1–23.2.22. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; Giger, S.; Hübscher, T.; Lutolf, M.P. Gastruloids as in Vitro Models of Embryonic Blood Development with Spatial and Temporal Resolution. Sci. Rep. 2022, 12, 13380. [Google Scholar] [CrossRef]
- Tsakiridis, A.; Huang, Y.; Blin, G.; Skylaki, S.; Wymeersch, F.; Osorno, R.; Economou, C.; Karagianni, E.; Zhao, S.; Lowell, S.; et al. Distinct Wnt-Driven Primitive Streak-like Populations Reflect in Vivo Lineage Precursors. Development 2014, 141, 1209–1221. [Google Scholar] [CrossRef] [PubMed]
- Bolondi, A.; Haut, L.; Gassaloglu, S.I.; Burton, P.; Kretzmer, H.; Buschow, R.; Meissner, A.; Herrmann, B.G.; Veenvliet, J.V. Generation of Mouse Pluripotent Stem Cell-Derived Trunk-like Structures: An in Vitro Model of Post-Implantation Embryogenesis. Bio-Protocol 2021, 11, e4042. [Google Scholar] [CrossRef] [PubMed]
- Olmsted, Z.T.; Paredes-Espinosa, M.B.; Paluh, J.L. Generation of Human Elongating Multi-Lineage Organized Cardiac Gastruloids. STAR Protoc. 2022, 3, 101898. [Google Scholar] [CrossRef]
- Sanaki-Matsumiya, M.; Matsuda, M.; Gritti, N.; Nakaki, F.; Sharpe, J.; Trivedi, V.; Ebisuya, M. Periodic Formation of Epithelial Somites from Human Pluripotent Stem Cells. Nat. Commun. 2022, 13, 2325. [Google Scholar] [CrossRef]
- Yamanaka, Y.; Hamidi, S.; Yoshioka-Kobayashi, K.; Munira, S.; Sunadome, K.; Zhang, Y.; Kurokawa, Y.; Ericsson, R.; Mieda, A.; Thompson, J.L.; et al. Reconstituting Human Somitogenesis in Vitro. Nature 2023, 614, 509–520. [Google Scholar] [CrossRef]
- Miao, Y.; Djeffal, Y.; Simone, A.D.; Zhu, K.; Lee, J.G.; Lu, Z.; Silberfeld, A.; Rao, J.; Tarazona, O.A.; Mongera, A.; et al. Reconstruction and Deconstruction of Human Somitogenesis in Vitro. Nature 2022, 614, 500. [Google Scholar] [CrossRef]
- Chen, J.; Horiuchi, S.; Kuramochi, S.; Kawasaki, T.; Kawasumi, H.; Akiyama, S.; Arai, T.; Morinaga, K.; Kimura, T.; Kiyono, T.; et al. Human Intestinal Organoid-Derived PDGFRα + Mesenchymal Stroma Enables Proliferation and Maintenance of LGR4 + Epithelial Stem Cells. Stem Cell Res. Ther. 2024, 15, 16. [Google Scholar] [CrossRef]
- Kim, D.; Lim, H.; Youn, J.; Park, T.-E.; Kim, D.S. Scalable Production of Uniform and Mature Organoids in a 3D Geometrically-Engineered Permeable Membrane. Nat. Commun. 2024, 15, 9420. [Google Scholar] [CrossRef]
- Puschhof, J.; Pleguezuelos-Manzano, C.; Martinez-Silgado, A.; Akkerman, N.; Saftien, A.; Boot, C.; de Waal, A.; Beumer, J.; Dutta, D.; Heo, I.; et al. Intestinal Organoid Cocultures with Microbes. Nat. Protoc. 2021, 16, 4633–4649. [Google Scholar] [CrossRef]
- Gnecco, J.S.; Brown, A.; Buttrey, K.; Ives, C.; Goods, B.A.; Baugh, L.; Hernandez-Gordillo, V.; Loring, M.; Isaacson, K.B.; Griffith, L.G. Organoid Co-Culture Model of the Human Endometrium in a Fully Synthetic Extracellular Matrix Enables the Study of Epithelial-Stromal Crosstalk. Med 2023, 4, 554–579.e9. [Google Scholar] [CrossRef] [PubMed]
- Below, C.R.; Kelly, J.; Brown, A.; Humphries, J.D.; Hutton, C.; Xu, J.; Lee, B.Y.; Cintas, C.; Zhang, X.; Hernandez-Gordillo, V.; et al. A Microenvironment-Inspired Synthetic Three-Dimensional Model for Pancreatic Ductal Adenocarcinoma Organoids. Nat. Mater. 2022, 21, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Chen, X.; Dowbaj, A.M.; Sljukic, A.; Bratlie, K.; Lin, L.; Fong, E.L.S.; Balachander, G.M.; Chen, Z.; Soragni, A.; et al. Organoids. Nat. Rev. Methods Primers 2022, 2, 94. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, M.H.; Krup, A.L.; Muncie, J.M.; Bruneau, B.G. Graded Mesoderm Assembly Governs Cell Fate and Morphogenesis of the Early Mammalian Heart. Cell 2023, 186, 479–496.e23. [Google Scholar] [CrossRef] [PubMed]
- Tyser, R.C.V.; Ibarra-Soria, X.; McDole, K.; Arcot Jayaram, S.; Godwin, J.; van den Brand, T.A.H.; Miranda, A.M.A.; Scialdone, A.; Keller, P.J.; Marioni, J.C.; et al. Characterization of a Common Progenitor Pool of the Epicardium and Myocardium. Science 2021, 371, eabb2986. [Google Scholar] [CrossRef]
- Ghatpande, S.; Ghatpande, A.; Zile, M.; Evans, T. Anterior Endoderm Is Sufficient to Rescue Foregut Apoptosis and Heart Tube Morphogenesis in an Embryo Lacking Retinoic Acid. Dev. Biol. 2000, 219, 59–70. [Google Scholar] [CrossRef]
- Liu, W.; Brown, K.; Legros, S.; Foley, A.C. Nodal Mutant eXtraembryonic ENdoderm (XEN) Stem Cells Upregulate Markers for the Anterior Visceral Endoderm and Impact the Timing of Cardiac Differentiation in Mouse Embryoid Bodies. Biol. Open 2012, 1, 208–219. [Google Scholar] [CrossRef]
- Madabhushi, M.; Lacy, E. Anterior Visceral Endoderm Directs Ventral Morphogenesis and Placement of Head and Heart via BMP2 Expression. Dev. Cell 2011, 21, 907–919. [Google Scholar] [CrossRef]
- Thomas, D.; Choi, S.; Alamana, C.; Parker, K.K.; Wu, J.C. Cellular and Engineered Organoids for Cardiovascular Models. Circ. Res. 2022, 130, 1780–1802. [Google Scholar] [CrossRef]
- Huang, X.; Zhong, L.; Hendriks, J.; Post, J.N.; Karperien, M. The Effects of the WNT-Signaling Modulators BIO and PKF118-310 on the Chondrogenic Differentiation of Human Mesenchymal Stem Cells. Int. J. Mol. Sci. 2018, 19, 561. [Google Scholar] [CrossRef]
- Laco, F.; Woo, T.L.; Zhong, Q.; Szmyd, R.; Ting, S.; Khan, F.J.; Chai, C.L.L.; Reuveny, S.; Chen, A.; Oh, S. Unraveling the Inconsistencies of Cardiac Differentiation Efficiency Induced by the GSK3β Inhibitor CHIR99021 in Human Pluripotent Stem Cells. Stem Cell Rep. 2018, 10, 1851–1866. [Google Scholar] [CrossRef]
- Ai, D.; Fu, X.; Wang, J.; Lu, M.-F.; Chen, L.; Baldini, A.; Klein, W.H.; Martin, J.F. Canonical Wnt Signaling Functions in Second Heart Field to Promote Right Ventricular Growth. Proc. Natl. Acad. Sci. USA 2007, 104, 9319. [Google Scholar] [CrossRef] [PubMed]
- Amel, A.; Rabeling, A.; Rossouw, S.; Goolam, M. Wnt and BMP Signalling Direct Anterior–Posterior Differentiation in Aggregates of Mouse Embryonic Stem Cells. Biol. Open 2023, 12, bio059981. [Google Scholar] [CrossRef]
- Tan, J.Y.; Sriram, G.; Rufaihah, A.J.; Neoh, K.G.; Cao, T. Efficient Derivation of Lateral Plate and Paraxial Mesoderm Subtypes from Human Embryonic Stem Cells Through GSKi-Mediated Differentiation. Stem Cells Dev. 2013, 22, 1893. [Google Scholar] [CrossRef]
- Bone, H.K.; Nelson, A.S.; Goldring, C.E.; Tosh, D.; Welham, M.J. A Novel Chemically Directed Route for the Generation of Definitive Endoderm from Human Embryonic Stem Cells Based on Inhibition of GSK-3. J. Cell Sci. 2011, 124, 1992–2000. [Google Scholar] [CrossRef] [PubMed]
- Amel, A.; Rossouw, S.; Goolam, M. Gastruloids: A Novel System for Disease Modelling and Drug Testing. Stem Cell Rev. Rep. 2023, 19, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Muneer, R.; Qazi, R.-E.-M.; Fatima, A.; Ahmad, W.; Salim, A.; Dini, L.; Khan, I. Wnt Signaling Pathway Inhibitor Promotes Mesenchymal Stem Cells Differentiation into Cardiac Progenitor Cells in Vitro and Improves Cardiomyopathy in Vivo. World J. Stem Cells 2023, 15, 821–841. [Google Scholar] [CrossRef] [PubMed]
- Tsoi, C.; Deng, R.; Kwok, M.; Yan, B.; Lee, C.; Li, H.S.; Ma, C.H.Y.; Luo, R.; Leung, K.T.; Chan, G.C.-F.; et al. Temporal Control of the WNT Signaling Pathway During Cardiac Differentiation Impacts Upon the Maturation State of Human Pluripotent Stem Cell Derived Cardiomyocytes. Front. Mol. Biosci. 2022, 9, 714008. [Google Scholar] [CrossRef]
- Martins-Neves, S.R.; Paiva-Oliveira, D.I.; Fontes-Ribeiro, C.; Bovée, J.V.M.G.; Cleton-Jansen, A.-M.; Gomes, C.M.F. IWR-1, a Tankyrase Inhibitor, Attenuates Wnt/β-Catenin Signaling in Cancer Stem-like Cells and Inhibits in Vivo the Growth of a Subcutaneous Human Osteosarcoma Xenograft. Cancer Lett. 2018, 414, 1–15. [Google Scholar] [CrossRef]
- Willems, L.; Daniëls, A.; Fanton, Y.; Linsen, L.; Evens, L.; Bito, V.; Declercq, J.; Rummens, J.-L.; Hensen, K.; Hendrikx, M. Differentiation of Human Cardiac Atrial Appendage Stem Cells into Adult Cardiomyocytes: A Role for the Wnt Pathway? Int. J. Mol. Sci. 2020, 21, 3931. [Google Scholar] [CrossRef]
- Liu, Y.; Qi, X.; Donnelly, L.; Elghobashi-Meinhardt, N.; Long, T.; Zhou, R.W.; Sun, Y.; Wang, B.; Li, X. Mechanisms and Inhibition of Porcupine-Mediated Wnt Acylation. Nature 2022, 607, 816–822. [Google Scholar] [CrossRef]
- Zhao, M.; Tang, Y.; Zhou, Y.; Zhang, J. Deciphering Role of Wnt Signalling in Cardiac Mesoderm and Cardiomyocyte Differentiation from Human iPSCs: Four-Dimensional Control of Wnt Pathway for hiPSC-CMs Differentiation. Sci. Rep. 2019, 9, 19389. [Google Scholar] [CrossRef] [PubMed]
- Ueno, S.; Weidinger, G.; Osugi, T.; Kohn, A.D.; Golob, J.L.; Pabon, L.; Reinecke, H.; Moon, R.T.; Murry, C.E. Biphasic Role for Wnt/β-Catenin Signaling in Cardiac Specification in Zebrafish and Embryonic Stem Cells. Proc. Natl. Acad. Sci. USA 2007, 104, 9685–9690. [Google Scholar] [CrossRef] [PubMed]
- Deimling, S.J.; Drysdale, T.A. Retinoic Acid Regulates Anterior–Posterior Patterning within the Lateral Plate Mesoderm of Xenopus. Mech. Dev. 2009, 126, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Lewis-Israeli, Y.R.; Volmert, B.D.; Gabalski, M.A.; Huang, A.R.; Aguirre, A. Generating Self-Assembling Human Heart Organoids Derived from Pluripotent Stem Cells. J. Vis. Exp. 2021, 175, e63097. [Google Scholar] [CrossRef]
- Zawada, D.; Kornherr, J.; Meier, A.B.; Santamaria, G.; Dorn, T.; Nowak-Imialek, M.; Ortmann, D.; Zhang, F.; Lachmann, M.; Dreßen, M.; et al. Retinoic Acid Signaling Modulation Guides in Vitro Specification of Human Heart Field-Specific Progenitor Pools. Nat. Commun. 2023, 14, 1722. [Google Scholar] [CrossRef]
- Tsaytler, P.; Liu, J.; Blaess, G.; Schifferl, D.; Veenvliet, J.V.; Wittler, L.; Timmermann, B.; Herrmann, B.G.; Koch, F. BMP4 Triggers Regulatory Circuits Specifying the Cardiac Mesoderm Lineage. Development 2023, 150, dev201450. [Google Scholar] [CrossRef]
- Ornitz, D.M.; Xu, J.; Colvin, J.S.; McEwen, D.G.; MacArthur, C.A.; Coulier, F.; Gao, G.; Goldfarb, M. Receptor Specificity of the Fibroblast Growth Factor Family. J. Biol. Chem. 1996, 271, 15292–15297. [Google Scholar] [CrossRef]
- Zhang, X.; Ibrahimi, O.A.; Olsen, S.K.; Umemori, H.; Mohammadi, M.; Ornitz, D.M. Receptor Specificity of the Fibroblast Growth Factor Family: The Complete Mammalian Fgf Family. J. Biol. Chem. 2006, 281, 15694. [Google Scholar] [CrossRef]
- Maas, R.G.C.; van den Dolder, F.W.; Yuan, Q.; van der Velden, J.; Wu, S.M.; Sluijter, J.P.G.; Buikema, J.W. Harnessing Developmental Cues for Cardiomyocyte Production. Development 2023, 150, dev201483. [Google Scholar] [CrossRef]
- Kattman, S.J.; Witty, A.D.; Gagliardi, M.; Dubois, N.C.; Niapour, M.; Hotta, A.; Ellis, J.; Keller, G. Stage-Specific Optimization of Activin/Nodal and BMP Signaling Promotes Cardiac Differentiation of Mouse and Human Pluripotent Stem Cell Lines. Cell Stem Cell 2011, 8, 228–240. [Google Scholar] [CrossRef]
- Katagiri, T.; Imada, M.; Yanai, T.; Suda, T.; Takahashi, N.; Kamijo, R. Identification of a BMP-Responsive Element in Id1, the Gene for Inhibition of Myogenesis. Genes Cells 2002, 7, 949–960. [Google Scholar] [CrossRef]
- Yu, M.S.; Spiering, S.; Colas, A.R. Generation of First Heart Field-like Cardiac Progenitors and Ventricular-like Cardiomyocytes from Human Pluripotent Stem Cells. J. Vis. Exp. (JoVE) 2018, 136, e57688. [Google Scholar] [CrossRef]
- Münsterberg, A.; Hoppler, S. WNT and BMP Regulate Roadblocks toward Cardiomyocyte Differentiation: Lessons Learned from Embryos Inform Human Stem Cell Differentiation. Stem Cell Investig. 2016, 3, 33. [Google Scholar] [CrossRef][Green Version]
- Blin, G.; Wisniewski, D.; Picart, C.; Thery, M.; Puceat, M.; Lowell, S. Geometrical Confinement Controls the Asymmetric Patterning of Brachyury in Cultures of Pluripotent Cells. Development 2018, 145, dev166025. [Google Scholar] [CrossRef]
- Kilian, K.A.; Bugarija, B.; Lahn, B.T.; Mrksich, M. Geometric Cues for Directing the Differentiation of Mesenchymal Stem Cells. Proc. Natl. Acad. Sci. USA 2010, 107, 4872–4877. [Google Scholar] [CrossRef]
- Loye, A.M.; Kinser, E.R.; Bensouda, S.; Shayan, M.; Davis, R.; Wang, R.; Chen, Z.; Schwarz, U.D.; Schroers, J.; Kyriakides, T.R. Regulation of Mesenchymal Stem Cell Differentiation by Nanopatterning of Bulk Metallic Glass. Sci. Rep. 2018, 8, 8758. [Google Scholar] [CrossRef]
- Gumbiner, B.M.; Kim, N.-G. The Hippo-YAP Signaling Pathway and Contact Inhibition of Growth. J. Cell Sci. 2014, 127, 709. [Google Scholar] [CrossRef]
- Sugi, Y.; Markwald, R.R. Endodermal Growth Factors Promote Endocardial Precursor Cell Formation from Precardiac Mesoderm. Dev. Biol. 2003, 263, 35–49. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ng, W.H.; Varghese, B.; Jia, H.; Ren, X. Alliance of Heart and Endoderm: Multilineage Organoids to Model Co-Development. Circ. Res. 2023, 132, 511–518. [Google Scholar] [CrossRef]
- Aleksandrova, A.; Czirok, A.; Kosa, E.; Galkin, O.; Cheuvront, T.J.; Rongish, B.J. The Endoderm and Myocardium Join Forces to Drive Early Heart Tube Assembly. Dev. Biol. 2015, 404, 40–54. [Google Scholar] [CrossRef] [PubMed]
- McFann, S.E.; Shvartsman, S.Y.; Toettcher, J.E. Chapter Seven—Putting in the Erk: Growth Factor Signaling and Mesoderm Morphogenesis. In Current Topics in Developmental Biology; Soriano, P.M., Ed.; Cell Signaling Pathways in Development; Academic Press: New York, NY, USA, 2022; Volume 149, pp. 263–310. [Google Scholar]
- Zhang, P.; Li, J.; Tan, Z.; Wang, C.; Liu, T.; Chen, L.; Yong, J.; Jiang, W.; Sun, X.; Du, L.; et al. Short-Term BMP-4 Treatment Initiates Mesoderm Induction in Human Embryonic Stem Cells. Blood 2008, 111, 1933–1941. [Google Scholar] [CrossRef] [PubMed]
- Dubois, N.C.; Craft, A.M.; Sharma, P.; Elliott, D.A.; Stanley, E.G.; Elefanty, A.G.; Gramolini, A.; Keller, G. SIRPA Is a Specific Cell-Surface Marker for Isolating Cardiomyocytes Derived from Human Pluripotent Stem Cells. Nat. Biotechnol. 2011, 29, 1011–1018. [Google Scholar] [CrossRef]
- Soh, B.-S.; Ng, S.-Y.; Wu, H.; Buac, K.; Park, J.-H.C.; Lian, X.; Xu, J.; Foo, K.S.; Felldin, U.; He, X.; et al. Endothelin-1 Supports Clonal Derivation and Expansion of Cardiovascular Progenitors Derived from Human Embryonic Stem Cells. Nat. Commun. 2016, 7, 10774. [Google Scholar] [CrossRef]
- Pauklin, S.; Vallier, L. Activin/Nodal Signalling in Stem Cells. Development 2015, 142, 607–619. [Google Scholar] [CrossRef]
- Brade, T.; Pane, L.S.; Moretti, A.; Chien, K.R.; Laugwitz, K.-L. Embryonic Heart Progenitors and Cardiogenesis. Cold Spring Harb. Perspect. Med. 2013, 3, a013847. [Google Scholar] [CrossRef]
- Christoffels, V.M.; Moorman, A.F.M. Development of the Cardiac Conduction System. Circ. Arrhythmia Electrophysiol. 2009, 2, 195–207. [Google Scholar] [CrossRef]
- Nakano, H.; Nakano, A. The Role of Metabolism in Cardiac Development. Curr. Top. Dev. Biol. 2024, 156, 201–243. [Google Scholar] [CrossRef]
- Zubrzycki, M.; Schramm, R.; Costard-Jäckle, A.; Grohmann, J.; Gummert, J.F.; Zubrzycka, M. Cardiac Development and Factors Influencing the Development of Congenital Heart Defects (CHDs): Part I. Int. J. Mol. Sci. 2024, 25, 7117. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, K.; Huang, W.; Staudt, L.M.; Cyster, J.G.; Li, X. Structure of S1PR2–Heterotrimeric G13 Signaling Complex. Sci. Adv. 2022, 8, eabn0067. [Google Scholar] [CrossRef]
- Ye, D.; Xie, H.; Hu, B.; Lin, F. Endoderm Convergence Controls Subduction of the Myocardial Precursors during Heart-Tube Formation. Development 2015, 142, 2928–2940. [Google Scholar] [CrossRef] [PubMed]
- Osborne, N.; Brand-Arzamendi, K.; Ober, E.A.; Jin, S.-W.; Verkade, H.; Holtzman, N.G.; Yelon, D.; Stainier, D.Y. The Spinster Homologue, Two of Hearts, Is Required for Sphingosine 1-Phosphate Signaling in Zebrafish. Curr. Biol. CB 2008, 18, 1882. [Google Scholar] [CrossRef] [PubMed]
- Kupperman, E.; An, S.; Osborne, N.; Waldron, S.; Stainier, D.Y.R. A Sphingosine-1-Phosphate Receptor Regulates Cell Migration during Vertebrate Heart Development. Nature 2000, 406, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, A.; Nishi, T.; Hisano, Y.; Fukui, H.; Yamaguchi, A.; Mochizuki, N. The Sphingolipid Transporter Spns2 Functions in Migration of Zebrafish Myocardial Precursors. Science 2009, 323, 524–527. [Google Scholar] [CrossRef]
- Holtzman, N.G.; Schoenebeck, J.J.; Tsai, H.-J.; Yelon, D. Endocardium Is Necessary for Cardiomyocyte Movement during Heart Tube Assembly. Development 2007, 134, 2379–2386. [Google Scholar] [CrossRef] [PubMed]
- Milgrom-Hoffman, M.; Harrelson, Z.; Ferrara, N.; Zelzer, E.; Evans, S.M.; Tzahor, E. The Heart Endocardium Is Derived from Vascular Endothelial Progenitors. Development 2011, 138, 4777. [Google Scholar] [CrossRef] [PubMed]
- Misfeldt, A.M.; Boyle, S.C.; Tompkins, K.L.; Bautch, V.L.; Labosky, P.A.; Baldwin, H.S. Endocardial Cells Are a Distinct Endothelial Lineage Derived from Flk1+ Multipotent Cardiovascular Progenitors. Dev. Biol. 2009, 333, 78–89. [Google Scholar] [CrossRef]
- Nakano, A.; Nakano, H.; Smith, K.A.; Palpant, N.J. The Developmental Origins and Lineage Contributions of Endocardial Endothelium. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2016, 1863, 1937–1947. [Google Scholar] [CrossRef]
- Xie, H.; Ye, D.; Sepich, D.; Lin, F. S1pr2/Gα13 Signaling Regulates the Migration of Endocardial Precursors by Controlling Endoderm Convergence. Dev. Biol. 2016, 414, 228–243. [Google Scholar] [CrossRef]
- Qu, X.; Baldwin, H.S. The Endocardium as a Master Regulator of Ventricular Trabeculation. In Molecular Mechanism of Congenital Heart Disease and Pulmonary Hypertension; Nakanishi, T., Baldwin, H.S., Fineman, J.R., Yamagishi, H., Eds.; Springer: Singapore, 2020; pp. 331–337. [Google Scholar]
- Haack, T.; Abdelilah-Seyfried, S. The Force within: Endocardial Development, Mechanotransduction and Signalling during Cardiac Morphogenesis. Development 2016, 143, 373–386. [Google Scholar] [CrossRef]
- Saint-Jean, L.; Barkas, N.; Harmelink, C.; Tompkins, K.L.; Oakey, R.J.; Baldwin, H.S. Myocardial Differentiation Is Dependent upon Endocardial Signaling during Early Cardiogenesis in Vitro. Development 2019, 146, dev172619. [Google Scholar] [CrossRef]
- Branco, M.A.; Dias, T.P.; Cabral, J.M.S.; Pinto-do-Ó, P.; Diogo, M.M. Human Multilineage Pro-Epicardium/Foregut Organoids Support the Development of an Epicardium/Myocardium Organoid. Nat. Commun. 2022, 13, 6981. [Google Scholar] [CrossRef] [PubMed]
- Protze, S.I.; Liu, J.; Nussinovitch, U.; Ohana, L.; Backx, P.H.; Gepstein, L.; Keller, G.M. Sinoatrial Node Cardiomyocytes Derived from Human Pluripotent Cells Function as a Biological Pacemaker. Nat. Biotechnol. 2017, 35, 56–68. [Google Scholar] [CrossRef]
- Le, M.N.T.; Takahi, M.; Ohnuma, K. Auto/Paracrine Factors and Early Wnt Inhibition Promote Cardiomyocyte Differentiation from Human Induced Pluripotent Stem Cells at Initial Low Cell Density. Sci. Rep. 2021, 11, 21426. [Google Scholar] [CrossRef] [PubMed]
- Greulich, F.; Rudat, C.; Kispert, A. Mechanisms of T-Box Gene Function in the Developing Heart. Cardiovasc. Res. 2011, 91, 212–222. [Google Scholar] [CrossRef]
- Harrelson, Z.; Kelly, R.G.; Goldin, S.N.; Gibson-Brown, J.J.; Bollag, R.J.; Silver, L.M.; Papaioannou, V.E. Tbx2 Is Essential for Patterning the Atrioventricular Canal and for Morphogenesis of the Outflow Tract during Heart Development. Development 2004, 131, 5041–5052. [Google Scholar] [CrossRef] [PubMed]
- Baillie-Johnson, P.; van den Brink, S.C.; Balayo, T.; Turner, D.A.; Martinez Arias, A. Generation of Aggregates of Mouse Embryonic Stem Cells That Show Symmetry Breaking, Polarization and Emergent Collective Behaviour in Vitro. J. Vis. Exp. 2015, 105, e53252. [Google Scholar] [CrossRef]
- Liang, X.; Wang, G.; Lin, L.; Lowe, J.; Zhang, Q.; Bu, L.; Chen, Y.; Chen, J.; Sun, Y.; Evans, S.M. HCN4 Dynamically Marks the First Heart Field and Conduction System Precursors. Circ. Res. 2013, 113, 399–407. [Google Scholar] [CrossRef]
- Rana, M.S.; Théveniau-Ruissy, M.; De Bono, C.; Mesbah, K.; Francou, A.; Rammah, M.; Domínguez, J.N.; Roux, M.; Laforest, B.; Anderson, R.H.; et al. Tbx1 Coordinates Addition of Posterior Second Heart Field Progenitor Cells to the Arterial and Venous Poles of the Heart. Circ. Res. 2014, 115, 790–799. [Google Scholar] [CrossRef]
- Männer, J.; Yelbuz, T.M. Functional Morphology of the Cardiac Jelly in the Tubular Heart of Vertebrate Embryos. J. Cardiovasc. Dev. Dis. 2019, 6, 12. [Google Scholar] [CrossRef]
- Darabid, H.; Perez-Gonzalez, A.P.; Robitaille, R. Neuromuscular Synaptogenesis: Coordinating Partners with Multiple Functions. Nat. Rev. Neurosci. 2014, 15, 703–718. [Google Scholar] [CrossRef] [PubMed]
- Steinbeck, J.A.; Jaiswal, M.K.; Calder, E.L.; Kishinevsky, S.; Weishaupt, A.; Toyka, K.V.; Goldstein, P.A.; Studer, L. Functional Connectivity under Optogenetic Control Allows Modeling of Human Neuromuscular Disease. Cell Stem Cell 2016, 18, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, I.; Ramirez, S.P.; Salazar, W.V.; Mendivil, S.; Guevara, A.; Patel, A.; Loyola, C.D.; Dorado, Z.N.; Joddar, B. A Semi-Three-Dimensional Bioprinted Neurocardiac System for Tissue Engineering of a Cardiac Autonomic Nervous System Model. Bioengineering 2023, 10, 834. [Google Scholar] [CrossRef]
- Noh, J.-M.; Choi, S.-C.; Song, M.-H.; Kim, K.S.; Jun, S.; Park, J.H.; Kim, J.H.; Kim, K.; Ko, T.H.; Choi, J.-I.; et al. The Activation of the LIMK/Cofilin Signaling Pathway via Extracellular Matrix–Integrin Interactions Is Critical for the Generation of Mature and Vascularized Cardiac Organoids. Cells 2023, 12, 2029. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, K. Roles of Cofilin in Development and Its Mechanisms of Regulation. Dev. Growth Differ. 2015, 57, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Pu, W. Cardiomyocyte Maturation: New Phase in Development. Circ. Res. 2020, 126, 1086–1106. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-Catenin Signalling: Function, Biological Mechanisms, and Therapeutic Opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Shewale, B.; Dubois, N. Of Form and Function: Early Cardiac Morphogenesis across Classical and Emerging Model Systems. Semin. Cell Dev. Biol. 2021, 118, 107–118. [Google Scholar] [CrossRef]
- Horb, M.E.; Thomsen, G.H. Tbx5 Is Essential for Heart Development. Development 1999, 126, 1739–1751. [Google Scholar] [CrossRef]
- Hasson, P.; Del Buono, J.; Logan, M.P.O. Tbx5 Is Dispensable for Forelimb Outgrowth. Development 2007, 134, 85–92. [Google Scholar] [CrossRef]
- Takeuchi, J.K.; Koshiba-Takeuchi, K.; Suzuki, T.; Kamimura, M.; Ogura, K.; Ogura, T. Tbx5 and Tbx4 Trigger Limb Initiation through Activation of the Wnt/Fgf Signaling Cascade. Development 2003, 130, 2729–2739. [Google Scholar] [CrossRef]
- Hill, J.T.; Demarest, B.; Gorsi, B.; Smith, M.; Yost, H.J. Heart Morphogenesis Gene Regulatory Networks Revealed by Temporal Expression Analysis. Development 2017, 144, 3487–3498. [Google Scholar] [CrossRef]
- Siatra, P.; Vatsellas, G.; Chatzianastasiou, A.; Balafas, E.; Manolakou, T.; Papapetropoulos, A.; Agapaki, A.; Mouchtouri, E.-T.; Ruchaya, P.J.; Korovesi, A.G.; et al. Return of the Tbx5; Lineage-Tracing Reveals Ventricular Cardiomyocyte-like Precursors in the Injured Adult Mammalian Heart. Npj Regen. Med. 2023, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Waldron, L.; Steimle, J.D.; Greco, T.M.; Gomez, N.C.; Dorr, K.M.; Kweon, J.; Temple, B.; Yang, X.H.; Wilczewski, C.M.; Davis, I.J.; et al. The Cardiac TBX5 Interactome Reveals a Chromatin Remodeling Network Essential for Cardiac Septation. Dev. Cell 2016, 36, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Steimle, J.D.; Moskowitz, I.P. TBX5: A Key Regulator of Heart Development. Curr. Top. Dev. Biol. 2017, 122, 195–221. [Google Scholar] [CrossRef] [PubMed]
- Calmont, A.; Ivins, S.; Van Bueren, K.L.; Papangeli, I.; Kyriakopoulou, V.; Andrews, W.D.; Martin, J.F.; Moon, A.M.; Illingworth, E.A.; Basson, M.A.; et al. Tbx1 Controls Cardiac Neural Crest Cell Migration during Arch Artery Development by Regulating Gbx2 Expression in the Pharyngeal Ectoderm. Development 2009, 136, 3173–3183. [Google Scholar] [CrossRef] [PubMed]
- Vitelli, F.; Morishima, M.; Taddei, I.; Lindsay, E.A.; Baldini, A. Tbx1 Mutation Causes Multiple Cardiovascular Defects and Disrupts Neural Crest and Cranial Nerve Migratory Pathways. Hum. Mol. Genet. 2002, 11, 915–922. [Google Scholar] [CrossRef]
- Phillips, H.M.; Stothard, C.A.; Shaikh Qureshi, W.M.; Kousa, A.I.; Briones-Leon, J.A.; Khasawneh, R.R.; O’Loughlin, C.; Sanders, R.; Mazzotta, S.; Dodds, R.; et al. Pax9 Is Required for Cardiovascular Development and Interacts with Tbx1 in the Pharyngeal Endoderm to Control 4th Pharyngeal Arch Artery Morphogenesis. Development 2019, 146, dev177618. [Google Scholar] [CrossRef]
- Nowotschin, S.; Liao, J.; Gage, P.J.; Epstein, J.A.; Campione, M.; Morrow, B.E. Tbx1 Affects Asymmetric Cardiac Morphogenesis by Regulating Pitx2 in the Secondary Heart Field. Development 2006, 133, 1565–1573. [Google Scholar] [CrossRef]
- Ren, J.; Miao, D.; Li, Y.; Gao, R. Spotlight on Isl1: A Key Player in Cardiovascular Development and Diseases. Front. Cell Dev. Biol. 2021, 9, 793605. [Google Scholar] [CrossRef]
- Jing, Y.; Ren, Y.; Witzel, H.R.; Dobreva, G. A BMP4-P38 MAPK Signaling Axis Controls ISL1 Protein Stability and Activity during Cardiogenesis. Stem Cell Rep. 2021, 16, 1894–1905. [Google Scholar] [CrossRef]
- Golzio, C.; Havis, E.; Daubas, P.; Nuel, G.; Babarit, C.; Munnich, A.; Vekemans, M.; Zaffran, S.; Lyonnet, S.; Etchevers, H.C. ISL1 Directly Regulates FGF10 Transcription during Human Cardiac Outflow Formation. PLoS ONE 2012, 7, e30677. [Google Scholar] [CrossRef]
- Cai, C.-L.; Liang, X.; Shi, Y.; Chu, P.-H.; Pfaff, S.L.; Chen, J.; Evans, S. Isl1 Identifies a Cardiac Progenitor Population That Proliferates Prior to Differentiation and Contributes a Majority of Cells to the Heart. Dev. Cell 2003, 5, 877. [Google Scholar] [CrossRef] [PubMed]
- Maven, B.E.J.; Gifford, C.A.; Weilert, M.; Gonzalez-Teran, B.; Hüttenhain, R.; Pelonero, A.; Ivey, K.N.; Samse-Knapp, K.; Kwong, W.; Gordon, D.; et al. The Multi-Lineage Transcription Factor ISL1 Controls Cardiomyocyte Cell Fate through Interaction with NKX2.5. Stem Cell Rep. 2023, 18, 2138–2153. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Liang, X.; Cheedipudi, S.; Cordero, J.; Jiang, X.; Zhang, Q.; Caputo, L.; Günther, S.; Kuenne, C.; Ren, Y.; et al. Pioneering Function of Isl1 in the Epigenetic Control of Cardiomyocyte Cell Fate. Cell Res. 2019, 29, 486–501. [Google Scholar] [CrossRef] [PubMed]
- Colombo, S.; de Sena-Tomás, C.; George, V.; Werdich, A.A.; Kapur, S.; MacRae, C.A.; Targoff, K.L. Nkx Genes Establish Second Heart Field Cardiomyocyte Progenitors at the Arterial Pole and Pattern the Venous Pole through Isl1 Repression. Development 2018, 145, dev161497. [Google Scholar] [CrossRef]
- Cao, C.; Li, L.; Zhang, Q.; Li, H.; Wang, Z.; Wang, A.; Liu, J. Nkx2.5: A Crucial Regulator of Cardiac Development, Regeneration and Diseases. Front. Cardiovasc. Med. 2023, 10, 1270951. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Chang, E.W.; Lin, Z.; Shekhar, A.; Bu, L.; Khodadadi-Jamayran, A.; Tsirigos, A.; Cen, Y.; Phoon, C.K.L.; Moskowitz, I.P.; et al. An Anterior Second Heart Field Enhancer Regulates the Gene Regulatory Network of the Cardiac Outflow Tract. Circulation 2023, 148, 1705–1722. [Google Scholar] [CrossRef] [PubMed]
- McFadden, D.G.; Barbosa, A.C.; Richardson, J.A.; Schneider, M.D.; Srivastava, D.; Olson, E.N. The Hand1 and Hand2 Transcription Factors Regulate Expansion of the Embryonic Cardiac Ventricles in a Gene Dosage-Dependent Manner. Development 2005, 132, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, Y.; Yanez, D.A.; Touma, M.; Nakano, H.; Jaroszewicz, A.; Jordan, M.C.; Pellegrini, M.; Roos, K.P.; Nakano, A. Nkx2-5 Suppresses the Proliferation of Atrial Myocytes and Conduction System. Circ. Res. 2014, 114, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Cambier, L.; Plate, M.; Sucov, H.M.; Pashmforoush, M. Nkx2-5 Regulates Cardiac Growth through Modulation of Wnt Signaling by R-Spondin3. Development 2014, 141, 2959–2971. [Google Scholar] [CrossRef]
- Anderson, D.J.; Kaplan, D.I.; Bell, K.M.; Koutsis, K.; Haynes, J.M.; Mills, R.J.; Phelan, D.G.; Qian, E.L.; Leitoguinho, A.R.; Arasaratnam, D.; et al. NKX2-5 Regulates Human Cardiomyogenesis via a HEY2 Dependent Transcriptional Network. Nat. Commun. 2018, 9, 1373. [Google Scholar] [CrossRef] [PubMed]
- Mannan, A.; Dhiamn, S.; Garg, N.; Singh, T.G. Pharmacological Modulation of Sonic Hedgehog Signaling Pathways in Angiogenesis: A Mechanistic Perspective. Dev. Biol. 2023, 504, 58–74. [Google Scholar] [CrossRef] [PubMed]
- Patocka, J.; Wu, Q.; Nepovimova, E.; Kuca, K. Phenytoin—An Anti-Seizure Drug: Overview of Its Chemistry, Pharmacology and Toxicology. Food Chem. Toxicol. 2020, 142, 111393. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Krishnan, H.; Raghu, P. IMPA1 Dependent Regulation of Phosphatidylinositol 4,5-Bisphosphate and Calcium Signalling by Lithium. Life Sci. Alliance 2024, 7, e202302425. [Google Scholar] [CrossRef]
- Meffre, D.; Grenier, J.; Bernard, S.; Courtin, F.; Dudev, T.; Shackleford, G.; Jafarian-Tehrani, M.; Massaad, C. Wnt and Lithium: A Common Destiny in the Therapy of Nervous System Pathologies? Cell. Mol. Life Sci. 2014, 71, 1123–1148. [Google Scholar] [CrossRef]
- Shaikh Qureshi, W.M.; Latif, M.L.; Parker, T.L.; Pratten, M.K. Lithium Carbonate Teratogenic Effects in Chick Cardiomyocyte Micromass System and Mouse Embryonic Stem Cell Derived Cardiomyocyte—Possible Protective Role of Myo-Inositol. Reprod. Toxicol. 2014, 46, 106–114. [Google Scholar] [CrossRef]
- Xia, M.; Zhao, X.; Huang, Q.; Sun, H.; Sun, C.; Yuan, J.; He, C.; Sun, Y.; Huang, X.; Kong, W.; et al. Activation of Wnt/Β-catenin Signaling by Lithium Chloride Attenuates D-galactose-induced Neurodegeneration in the Auditory Cortex of a Rat Model of Aging. FEBS Open Bio 2017, 7, 759–776. [Google Scholar] [CrossRef] [PubMed]
- Ahler, E.; Sullivan, W.J.; Cass, A.; Braas, D.; York, A.G.; Bensinger, S.J.; Graeber, T.G.; Christofk, H.R. Doxycycline Alters Metabolism and Proliferation of Human Cell Lines. PLoS ONE 2013, 8, e64561. [Google Scholar] [CrossRef]
- Yap, C.; Wanga, S.; Wüst, R.C.I.; van Os, B.W.; Pijls, M.M.E.; Keijzer, S.; van Zanten, E.; Koolbergen, D.R.; Driessen, A.H.G.; Balm, R.; et al. Doxycycline Induces Mitochondrial Dysfunction in Aortic Smooth Muscle Cells. Vasc. Pharmacol. 2024, 154, 107279. [Google Scholar] [CrossRef]
- Kim, J.H.; Scialli, A.R. Thalidomide: The Tragedy of Birth Defects and the Effective Treatment of Disease. Toxicol. Sci. 2011, 122, 1–6. [Google Scholar] [CrossRef]
- Belair, D.G.; Lu, G.; Waller, L.E.; Gustin, J.A.; Collins, N.D.; Kolaja, K.L. Thalidomide Inhibits Human iPSC Mesendoderm Differentiation by Modulating CRBN-Dependent Degradation of SALL4. Sci. Rep. 2020, 10, 2864. [Google Scholar] [CrossRef]
- Hiroi, Y.; Kudoh, S.; Monzen, K.; Ikeda, Y.; Yazaki, Y.; Nagai, R.; Komuro, I. Tbx5 Associates with Nkx2-5 and Synergistically Promotes Cardiomyocyte Differentiation. Nat. Genet. 2001, 28, 276–280. [Google Scholar] [CrossRef]
- van der Zanden, S.Y.; Qiao, X.; Neefjes, J. New Insights into the Activities and Toxicities of the Old Anticancer Drug Doxorubicin. FEBS J. 2021, 288, 6095–6111. [Google Scholar] [CrossRef]
- Linders, A.N.; Dias, I.B.; López Fernández, T.; Tocchetti, C.G.; Bomer, N.; Van der Meer, P. A Review of the Pathophysiological Mechanisms of Doxorubicin-Induced Cardiotoxicity and Aging. Npj Aging 2024, 10, 9. [Google Scholar] [CrossRef]
- Singla, D.K.; Ahmed, A.; Singla, R.; Yan, B. Embryonic Stem Cells Improve Cardiac Function in Doxorubicin-Induced Cardiomyopathy Mediated through Multiple Mechanisms. Cell Transplant. 2012, 21, 1919–1930. [Google Scholar] [CrossRef]
- Cao, X.; Sun, M.; Yang, Q.; Wang, Q.; Hou, L.; Wang, J.; Wu, Y.; Ge, L. Risk of Abnormal Pregnancy Outcomes after Using Ondansetron during Pregnancy: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2022, 13, 951072. [Google Scholar] [CrossRef] [PubMed]
- Lemon, L.S.; Bodnar, L.M.; Garrard, W.; Venkataramanan, R.; Platt, R.W.; Marroquin, O.C.; Caritis, S.N. Ondansetron Use in the First Trimester of Pregnancy and the Risk of Neonatal Ventricular Septal Defect. Int. J. Epidemiol. 2019, 49, 648. [Google Scholar] [CrossRef]
- Danielsson, B.; Wikner, B.N.; Källén, B. Use of Ondansetron during Pregnancy and Congenital Malformations in the Infant. Reprod. Toxicol. 2014, 50, 134–137. [Google Scholar] [CrossRef]
- Verma, S.K.; Nandi, A.; Sinha, A.; Patel, P.; Mohanty, S.; Jha, E.; Jena, S.; Kumari, P.; Ghosh, A.; Jerman, I.; et al. The Posterity of Zebrafish in Paradigm of in Vivo Molecular Toxicological Profiling. Biomed. Pharmacother. 2024, 171, 116160. [Google Scholar] [CrossRef]
- Roostalu, U.; Thisted, L.; Skytte, J.L.; Salinas, C.G.; Pedersen, P.J.; Hecksher-Sørensen, J.; Rolin, B.; Hansen, H.H.; MacKrell, J.G.; Christie, R.M.; et al. Effect of Captopril on Post-Infarction Remodelling Visualized by Light Sheet Microscopy and Echocardiography. Sci. Rep. 2021, 11, 5241. [Google Scholar] [CrossRef]
- Gao, D.; Critser, J.K. Mechanisms of Cryoinjury in Living Cells. ILAR J. 2000, 41, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Voges, H.K.; Mills, R.J.; Elliott, D.A.; Parton, R.G.; Porrello, E.R.; Hudson, J.E. Development of a Human Cardiac Organoid Injury Model Reveals Innate Regenerative Potential. Development 2017, 144, 1118–1127. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Zhao, X.; Zhang, Y.; Xia, Z. Abnormalities of Glucose and Lipid Metabolism in Myocardial Ischemia-Reperfusion Injury. Biomed. Pharmacother. 2023, 163, 114827. [Google Scholar] [CrossRef] [PubMed]
- Pittas, K.; Vrachatis, D.A.; Angelidis, C.; Tsoucala, S.; Giannopoulos, G.; Deftereos, S. The Role of Calcium Handling Mechanisms in Reperfusion Injury. Curr. Pharm. Des. 2018, 24, 4077–4089. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, M.; Wang, J.; Wang, Y.; Yang, X.; Fu, S. Effect of Gestational Diabetes Mellitus on the Growth, Development, and Stem Cells of Offspring. Matern.-Fetal Med. 2023, 5, 31. [Google Scholar] [CrossRef]
- Archer, C.R.; Robinson, E.L.; Drawnel, F.M.; Roderick, H.L. Endothelin-1 Promotes Hypertrophic Remodelling of Cardiac Myocytes by Activating Sustained Signalling and Transcription Downstream of Endothelin Type A Receptors. Cell. Signal. 2017, 36, 240–254. [Google Scholar] [CrossRef]
- Tanaka, A.; Yuasa, S.; Mearini, G.; Egashira, T.; Seki, T.; Kodaira, M.; Kusumoto, D.; Kuroda, Y.; Okata, S.; Suzuki, T.; et al. Endothelin-1 Induces Myofibrillar Disarray and Contractile Vector Variability in Hypertrophic Cardiomyopathy–Induced Pluripotent Stem Cell–Derived Cardiomyocytes. J. Am. Heart Assoc. 2014, 3, e001263. [Google Scholar] [CrossRef]
- Freeman, B.D.; Machado, F.S.; Tanowitz, H.B.; Desruisseaux, M.S. Endothelin-1 and Its Role in the Pathogenesis of Infectious Diseases. Life Sci. 2014, 118, 110–119. [Google Scholar] [CrossRef]
- Gautam, M. Endothelial Cells as Regulators of Cytokine Storms during Influenza Infection. Thorax 2012, 67, 617. [Google Scholar] [CrossRef][Green Version]
- Enevoldsen, F.C.; Sahana, J.; Wehland, M.; Grimm, D.; Infanger, M.; Krüger, M. Endothelin Receptor Antagonists: Status Quo and Future Perspectives for Targeted Therapy. J. Clin. Med. 2020, 9, 824. [Google Scholar] [CrossRef]
- Duarte, V.E.; Singh, M.N. Genetic Syndromes Associated with Congenital Heart Disease. Heart 2023, 110, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
| Gene | Effects | Organoid | Ref. |
| Tbx5 KO | Delayed cardiac morphogenesis (25% present at the CCL stage on day 3 versus ~30% at the CCL stage for WT), delay/absence of chamber formation (no chambers for 2 of the 3 KO lines used, 8.3% at the CF stage in only one line on day 9 versus ~40% at the CF stage for WT), Tbx5 expression decreased, Tbx5/Nkx2.5 localization disrupted, myofibrillar architecture disruption (weaker cTnT immunofluorescence for KO line versus WT). | Starter material: EB (ESCs) Factors: LN/ET, FGF4, BMP4, BIO, LIF | [89] |
| Tbx5 KO | Atrial: aSHF marker upregulation, pSHF marker (HoxB1) downregulation, cardiomyocyte differentiation efficiency reduced with Tnnt2, Nppa downregulation, ventricular chamber marker (Irx4) downregulation, loss of spontaneous contractile activity. | Starter material: hPSCs Factors: Mesoderm (FGF2, BMP4, Activin A, CHIR99021), FHF-derived (LV) (BMP4, FGF2, Insulin, WNTi, RA), aSHF-derived (RV, OFT) (WNTi, TGFβi, RA) pSHF-derived (Atrial, AVC) (WNTi, TGFβi, RA) | [88] |
| AVC: cardioid area reduced to almost 0 (day 9.5), aSHF marker upregulation, pSHF marker (HoxB1) downregulation, cardiomyocyte differentiation fails on day 9.5, ventricular chamber marker (Irx4) downregulation, loss of spontaneous contractile activity. | |||
| LV: cardioid area reduced by ~4,000,000 μm2 (day 9.5), dysregulated gene expression with upregulation of Hand2, Fgf10, Tbx2, Wnt5a, downregulation of Nkx2.5, Gata4, cardiomyocyte differentiation efficiency reduced (Tnnt2, Nppa downregulation), loss of spontaneous contractile activity, ventricular chamber marker (Irx4) downregulation. | |||
| RV: cardiomyocyte differentiation efficiency reduced with Tnnt2, Nppa downregulation, upregulation of FoxC2, Tbx2, Wnt5a, ventricular chamber marker (Irx4) downregulation, loss of spontaneous contractile activity. | |||
| Tbx5 KO | Development of FHF (CXCR4-) lineages (LV) affected (0.003 decrease in normalized expression of actc1, no effect on actc1 expression in CXCR4+ lineages). | Starter material: hiPSCs Factors: BMP4, CHIR99021 | [90] |
| Tbx1 KO | Development of SHF (CXCR4+) (aSHF: RV, OFT; pSHF: atria, AVC) lineages affected (~0.5 and ~0.7 decrease in relative proliferation of CXCR+ cells after 24 and 48 h, respectively, no effect on relative proliferation of CXCR4- lineages). | ||
| Isl1 KO | Delayed cardiac morphogenesis (16.7% present at the CCL stage on day 3 versus ~30% at the CCL stage for WT, no HT stage for 1 of 3 KO lines used), delay/absence of chamber formation (no chambers for 2 of 3 KO lines used, 12% at the CF stage in only one line on day 9 versus ~40% at the CF stage for WT), no clear separation of Tbx5/Nkx2.5 immunofluorescence, myofibrillar architecture disruption (weak cTnT immunofluorescence for KO line versus WT). | Starter material: EB (ESCs) Factors: LN/ET, FGF4, BMP4, BIO, LIF | [89] |
| Isl1 KO | OFT: cardioid area reduced by ~900,000 μm2 (day 9.5), downregulation of Hand2, BMP4, upregulation of Tbx5, misregulation of Nr2f2, Rspo3, Wnt5a, Myl7, cardiomyocyte differentiation efficiency reduced (only some Tnnt2 immunofluorescence present) with downregulation of Mef2c, Myocd (cardiac differentiation genes), global shift towards atrial phenotype (positive change in expression of Nr2f2, Hey1, negative change in expression of Wnt5a), severe impairment in chamber formation by day 5.5. | Starter material: hPSCs Factors: Mesoderm (FGF2, LY, BMP4, Activin A, CHIR99021), FHF-derived (LV) (BMP4, FGF2, Insulin, WNTi, RA), aSHF-derived (RV, OFT) (WNTi, TGFβi, RA) pSHF-derived (Atrial, AVC) (WNTi, TGFβi, RA) | [88] |
| Atrial: cardioid area reduced by ~900,000 μm2 (day 9.5), downregulation of HoxB1 (pSHF), misregulation of Nr2f2, Rspo3, Wnt5a, Myl7, cardiomyocyte differentiation efficiency reduced (some Tnnt2 immunofluorescence present) with downregulation of Mef2c, Myocd (cardiac differentiation genes), reduced spontaneous contractile activity (bpm decrease by 10, day 9.5), severe impairment in chamber formation by day 5.5. | |||
| RV: cardioid area only slightly reduced (day 9.5), misregulation of Nr2f2, Rspo3, Wnt5a, Myl7, cardiomyocyte differentiation efficiency reduced (almost no Tnnt2 immunofluorescence) with downregulation of Mef2c, Myocd (cardiac differentiation genes), effects on chamber formation (day 5.5) less severe. | |||
| LV: cardioid area reduced by ~2,000,000 μm2 (day 9.5), misregulation of Nr2f2, Rspo3, Wnt5a, Myl7, cardiomyocyte differentiation efficiency not as affected (Tnnt2 immunofluorescence present) with Mef2c, Myocd (cardiac differentiation genes) upregulated, effects on chamber formation less severe. | |||
| Nkx2.5 KO | Total organoid area increased by ~1 mm2 (day 10), reduction in myocardial layer compaction by 20% compared to control (70%) (day 10) though with maximum compactness similar to control reached by day 13, disruption of intercellular cardiomyocyte adhesions, cardiomyocyte hypertrophy (increased in cardiomyocyte area by ~0.1 mm2), 41.7-fold and 25.9-fold downregulation of NKX2.5 gene targets (Nppa and Irx4, respectively), smooth muscle proliferation (15-fold increase in Myh11 upregulation, 11-fold increase in Nr4a3 upregulation). | Starter material: hPSC Factors: CHIR99021, IWP2 | [8] |
| FoxF1 KO | LV: cardioid area reduced by ~1,600,000 μm2 (day 9.5), downregulation of Eno1 (involved in cardiac contraction), HoxB1, Tbx5 (pSHF) with complete absence of pSHF specification, Isl1, Tbx1 (aSHF), upregulation of Pitx2, Tbx1, reduced spontaneous contractile activity (bpm decreased by 10, day 6.5). | Starter material: hPSCs Factors: Mesoderm (FGF2, LY, BMP4, Activin A, CHIR99021), FHF-derived (LV) (BMP4, FGF2, Insulin, WNTi, RA), aSHF-derived (RV, OFT) (WNTi, TGFβi, RA) pSHF-derived (Atrial, AVC) (WNTi, TGFβi, RA) | [88] |
| AVC: cardioid area reduced by ~500,000 μm2 (day 9.5), cessation of spontaneous contractile activity (day 6.5), no chamber formation. | |||
| Atrial: cardioid area reduction not significant (day 9.5), downregulation of HoxB1 (pSHF) with complete absence of pSHF specification, Isl1, Tbx1 (aSHF), shift towards ventricular phenotype with extensive chamber formation, reduced spontaneous contractile activity (bpm decrease by ~6, day 6.5), earlier chamber formation (day 3.5). | |||
| RV: cardioid area reduction not significant (day 9.5), downregulation of Nppa, HoxB1, Tbx5 (pSHF) with complete absence of pSHF specification, upregulation of Pitx2, Tbx1. | |||
| OFT: cardioid area reduction not significant (day 9.5), downregulation of Nppa. |
| Compounds | Effects | Ref. |
|---|---|---|
| Doxylamine succinate | No effect in cardiac differentiation efficiency, some increase in cardiac tissue (increase in area ratio by ~0.1 with 1 μM), reduced cardiac tissue compaction, decrease in contraction velocity (by ~5 μm/s with 10 μM) and beating rate (by ~10 bpm with 10 μM), similar effects in zWECs | [92] |
| Amoxicillin | No effects on organoid function and structure, similar effects in zWECs | |
| Rifampicin | Arrest of organoid development with high concentrations (100 μM), milder effects in zWECs | |
| Doxycycline | Inhibits cardiac differentiation and cardiac organoid formation (area ratio, organoid height reduced to 0 with 10 and 100 μM, respectively), milder effects in zWECs | |
| Lithium carbonate | Stimulates the Wnt/β-catenin signaling pathway, affects organoid formation with reduction in area ratio (median reduced by 0.1 with 10 μM) and FWHM (median reduced by ~50 with 10 μM), no effects in contractile function, similar effects in zWECs | |
| Phenytoin | Reduction in overall organoid size with decreases in area ratio (median reduced by 0.1 with 10 μM), height (~100 μm with 10 μM) and FWHM (~100 μm with 10 μM), cessation of contractile activity with high doses (100 μM), no disruption in normal tissue architecture, similar effects in zWECs | |
| Tretinoin (All-trans RA) Isotretinoin | No effect/increase in organoid size but with failure of cardiac differentiation (area ratio 0 with 10μM tretinoin) (area ratio 0 with 1μM isotretinoin), cessation of contractile activity (10μM tretinoin) (1 μM, 10 μM isotretinoin), similar effects in zWECs | |
| Acitretin | Severe effects on lineage specification/tissue patterning/cardiac morphogenesis on atrial/AVC/OFT, effects on atrial size with 5 nM (day 4.5) and AVC size (50 nM) (day 4.5), severe effect on OFT size (cardioid area reduced by ~2,000,000 μm2) (50 nM) (day 9.5), severe effect on LV/RV size (cardioid area reduced by ~3,000,000 μm2) (50 nM) (day 9.5), disruption in chamber formation in atrial/AVC/OFT (Tnni1-GFP imaging) (day 9.5), downregulation of OFT-specific genes (Wnt5a), upregulation of ventricular genes (Irx1, Irx4) in OFTs, earlier cardiomyocyte differentiation in OFTs | [88] |
| All-trans retinol | Severe effects in tissue morphology in OFT with no effects on other organoids, downregulation of OFT-specific genes (Wnt5a), upregulation of ventricular genes (Irx4, Irx1) in OFTs, earlier cardiomyocyte differentiation in OFTs | |
| Thalidomide | Impairs early mesendoderm specification, reduction in cardiac differentiation efficiency (progressive reduction in differentiation efficiency, efficiency reduced to 10.83% with 100 μM compared to 63.52% for control), disorganized organoid morphology with reduced size parameters (greatest median height reduction by ~100 μm with 1 μM, greatest median FWHM reduction by ~100 μm with 100 μM), reduced cardiac differentiation (greatest reduction in median area ratio by ~0.2 with 100 μM), no effect on contraction velocity, heart rate variability (increase by ~5 bpm with 1 μM, followed by progressive decrease with increasing dose), milder effects in zWECs | [92] |
| Severe phenotype in AVC (effects on AVC size with 0.1 μg/mL), intermediate phenotype in LV/RV (progressively decreasing size, greatest effect with 10 μg/mL), subtle phenotype in atrial/OFT (progressively decreasing size, greatest effect with 10 μg/mL), downregulation of Nppa (downstream of TBX5) in atrial/AVC/LV, Nr2f2 upregulation in AVC (atrial marker), Irx1 downregulation in RV (RV marker), Irx4 downregulation in LV (LV marker) | [88] | |
| Doxorubicin (anthracycline) | Cardiotoxicity (progressing to cardiomyopathy, congestive heart failure), effects on contractile activity (complete cessation of beating rate when 10 μM applied for 72 h, 50 μM applied for 48 h), decrease by 2 ΔF/F0 in peak Ca2+ amplitude and increase by 0.5 sec in time to peak (1 μM, 10 μM), decreasing cell viability with increasing dose, increasing cell apoptosis (~40% increase in TUNEL+ cells) with increasing dose | [103] |
| Ondansetron (5-HT3 receptor antagonist) | Electrophysiological abnormalities (progressive decrease in beating rate, decreased frequency/amplitude of AP with increasing concentrations due to Na+ channel blockade, QT prolongation), total area occupied by atrial cells unaffected (Myl7), total area occupied by ventricular cells (Myl2) reduced to 0.55-fold, 0.18-fold with 10 μM, 100 μM, respectively, (compared to control), structurally disorganized ventricular chambers (loss of ventricular wall definition, loose chamber separation seen with 100 μM), Myl2 expression decreased to 0.40-fold with 100 μM (compared to control), no effect on apoptosis | [99] |
| Injury | Effects | Ref. |
|---|---|---|
| MI (cryoinjury model) | Local tissue compaction, necrosis and limited apoptosis (TUNEL+ staining cells), extracellular material accumulation composed of fibronectin and COL1A1-secreting fibroblasts, fibroblasts derived from epicardial lineages in tri-lineage models (cardiomyocytes, epicardial cells, cardiac fibroblasts), no COL1A1/fibroblasts and reduced fibronectin accumulation in single lineage models (cardiomyocytes) | [95] |
| MI (cryoinjury model) | Localized cardiomyocyte loss (~15% reduction in GFP-cardiomyocyte expression) associated with 3-fold increase in secreted LDH and cTnI compared to control, post-injury fibrosis (no increase in fibronectin accumulation, effects reduced compared to adult tissues), no significant post-injury hypertrophy (no change in cardiomyocyte area, no significant upregulation in Nppa, Acta1 expression), quantification of cardiomyocyte proliferation (Ki67, pH3) shows variation between cell lines, higher baseline levels of cardiomyocyte proliferation overall (tissue immaturity) with return to baseline function after 2 weeks | [296] |
| MI, Fibrosis (cryoinjury model) | Localized cardiomyocyte loss associated increase in secreted cTnT (from ~25 pg/mL to 125 pg/mL), effects on electrophysiological activity with 40% decrease (decrease from ~5 to 3 ΔF/F0) in amplitude (ΔF/F0) and time to peak (s) increase to ~0.6 s (Ca2+ transients), asynchronous contractile function amongst cardiomyocyte groups, 40% increase in total fibrotic area, upregulation of fibrosis markers (Vim, α-SMA), downregulation of endothelial (PECAM1) and cardiomyocyte (Myh7, Tnnt2) markers, lower degree of fibrosis (~20% total fibrotic area) in non-vascularized organoids | [103] |
| MI, Fibrosis (cryoinjury model, captopril) | Captopril (ACEi) administration restores endothelial, cardiomyocyte and fibrotic marker expression to about pre-injury levels, reversal of fibrotic area not to pre-injury levels (although improved to ~20% of total organoid area), mitigation of electrophysiological disturbances (Ca2+ transient amplitude), synchronization in contractile function amongst cardiomyocyte groups, no favorable effects of captopril administration in non-vascularized organoid fibrosis (non-significant effect on % total fibrotic area) | |
| MI, IR, Fibrosis (CoCl2 and Glc depletion, high Glc and Ca2+, TGFβ) | Cardiomyocyte apoptosis (tissue response not as extensive as observed in mature organoids, non-significant increase in cTnI, CKM release and only 10-fold increase MB release from affected cells), less pronounced sarcomere disintegration, inflammatory response (NF-κB), post-infarction remodeling and post-injury fibrosis | [38] |
| MI, IR, Fibrosis (CoCl2 and Glc depletion, high Glc and Ca2+, TGFβ) | More accurate recreation of MI injury HO constructs, greater decrease in intracellular cTnT, cTnI, greater increase in cTnI (up to 15-fold in 72 h compared to controls), CKM (2-fold in 72 h compared to controls), MB (up to 20-fold in 72 h compared to control) release from affected cells, more pronounced sarcomere disintegration and inflammatory response (NF-κB), post-MI remodeling (upregulation of ERK, JNK, p38, SERCA, alterations in Ca2+ handling), more pronounced post-MI fibrosis with effects on contractile and electrophysiological activity | [38] |
| GDM (Glc, insulin modulation) | Absence of elongation/tissue patterning (4–8 days) with spherical shape and increase in overall size (organoid area at ~1,500,000 µm2 versus less than ~1,500,000 µm2 for controls) (4–8 days), electrophysiological irregularities (mean beating rate increased by 10 bpm), increased rate of glycolysis, reduced mitochondrial numbers (~0.25 mean mitochondria/μm2 versus mean number of 0.5 mitochondria/μm2 in controls), reduced O2 consumption, increased numbers of lipid droplets, disruption in cardiomyocyte populations (mean ventricular cardiomyocyte ratio decreased by ~10%, mean atrial cardiomyocyte ratio increased by ~25%), abnormally localized epicardium | [9] |
| CH (Endothelin-1) | Disruption in myocardial tissue architecture and actin–myosin interactions (skewed sarcomeric z-lines, myofibrillar disarray), myocardial hypertrophy (increase in thickness ~40%, sustained for 3 weeks with 100 ng/mL, lower doses associated with return to baseline after 1 week), effects on contraction frequency (doubles by week 3) and variability (decreases), electrophysiological disruption (decrease in beat duration, Ca2+ transients, depolarization duration), effects on ventricular function (fractional shortening decreased by ~30% with 100 ng/mL) | [98] |
| CS (IFN-γ, poly(I:C), IL-1β and endothelin-1) | Magnitude of inflammation dependent on organoid vascularization, hVCOs (CS) exhibit an increase in time to relaxation and preservation of contractile force, hVCOs (Endothelin-1) exhibit increased time to relaxation with increased contractile force and increased rate of contraction (diastolic contractile dysfunction), effects mitigated by endothelin-1 antagonists (bosentan, sitaxsentan) | [53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stougiannou, T.M.; Christodoulou, K.C.; Karangelis, D. In Vitro Models of Cardiovascular Disease: Embryoid Bodies, Organoids and Everything in Between. Biomedicines 2024, 12, 2714. https://doi.org/10.3390/biomedicines12122714
Stougiannou TM, Christodoulou KC, Karangelis D. In Vitro Models of Cardiovascular Disease: Embryoid Bodies, Organoids and Everything in Between. Biomedicines. 2024; 12(12):2714. https://doi.org/10.3390/biomedicines12122714
Chicago/Turabian StyleStougiannou, Theodora M., Konstantinos C. Christodoulou, and Dimos Karangelis. 2024. "In Vitro Models of Cardiovascular Disease: Embryoid Bodies, Organoids and Everything in Between" Biomedicines 12, no. 12: 2714. https://doi.org/10.3390/biomedicines12122714
APA StyleStougiannou, T. M., Christodoulou, K. C., & Karangelis, D. (2024). In Vitro Models of Cardiovascular Disease: Embryoid Bodies, Organoids and Everything in Between. Biomedicines, 12(12), 2714. https://doi.org/10.3390/biomedicines12122714









