Exploring the Impact of Semaglutide on Cognitive Function and Anxiety-Related Behaviors in a Murine Model of Alzheimer’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
- Group (WT + VEH): C57BL/6J + saline;
- Group (WT + SEM): C57BL/6J + semaglutide;
- Group (AD + VEH): AD + saline;
- Group (AD + SEM): AD + semaglutide.
2.2. Blood Glucose Monitoring
2.3. Weight Monitoring
2.4. Behavioral Studies
2.4.1. Open Field Test (OFT)
2.4.2. Novel Object Recognition Test (NORT)
2.4.3. Social Chamber Test (SCT)
2.4.4. 0-Maze Test (0-MT)
2.5. Statistical Data Processing
3. Results
3.1. Blood Glucose Monitoring
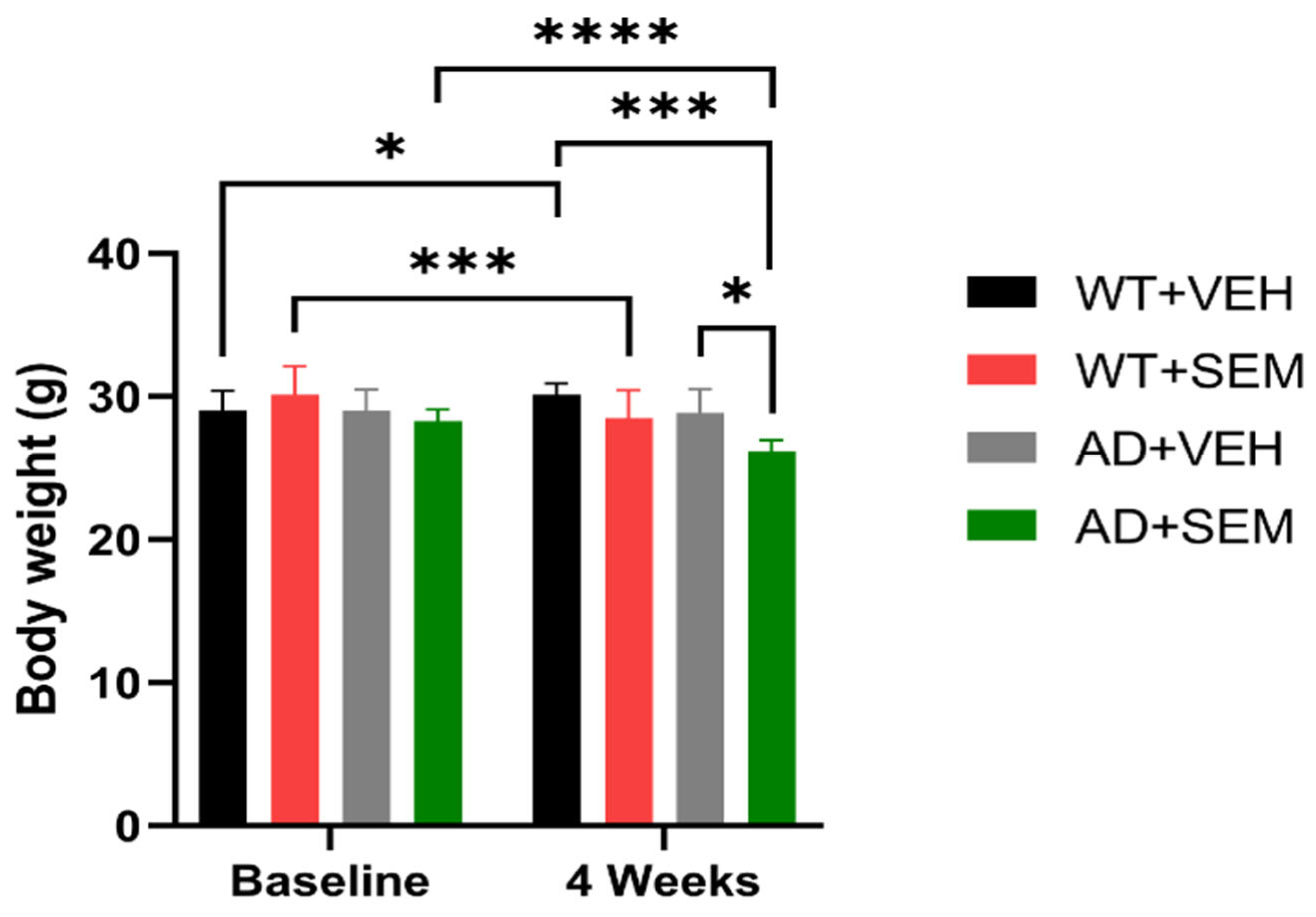
3.2. Weight Monitoring
3.3. Behavioral Studies
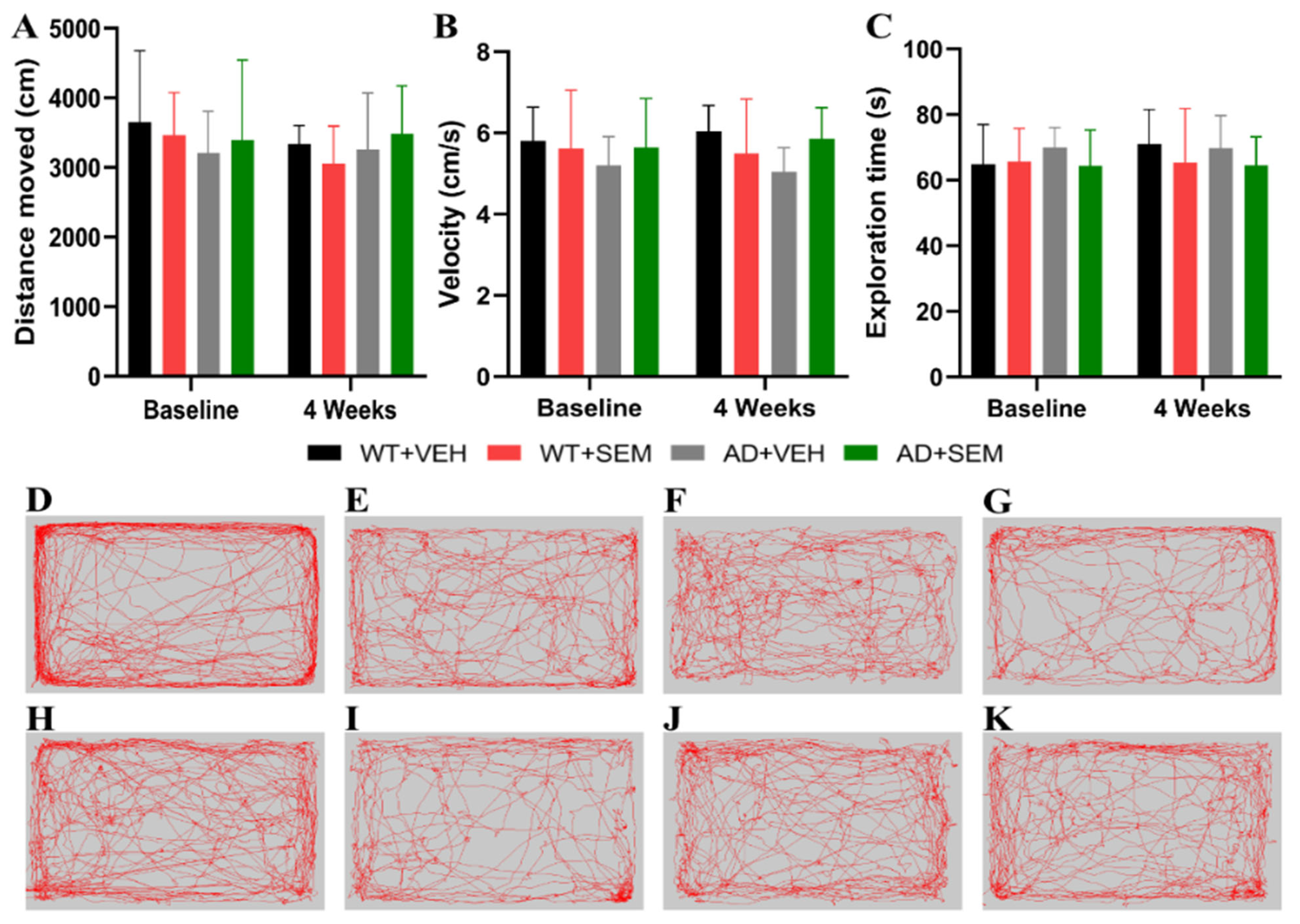
3.3.1. OFT
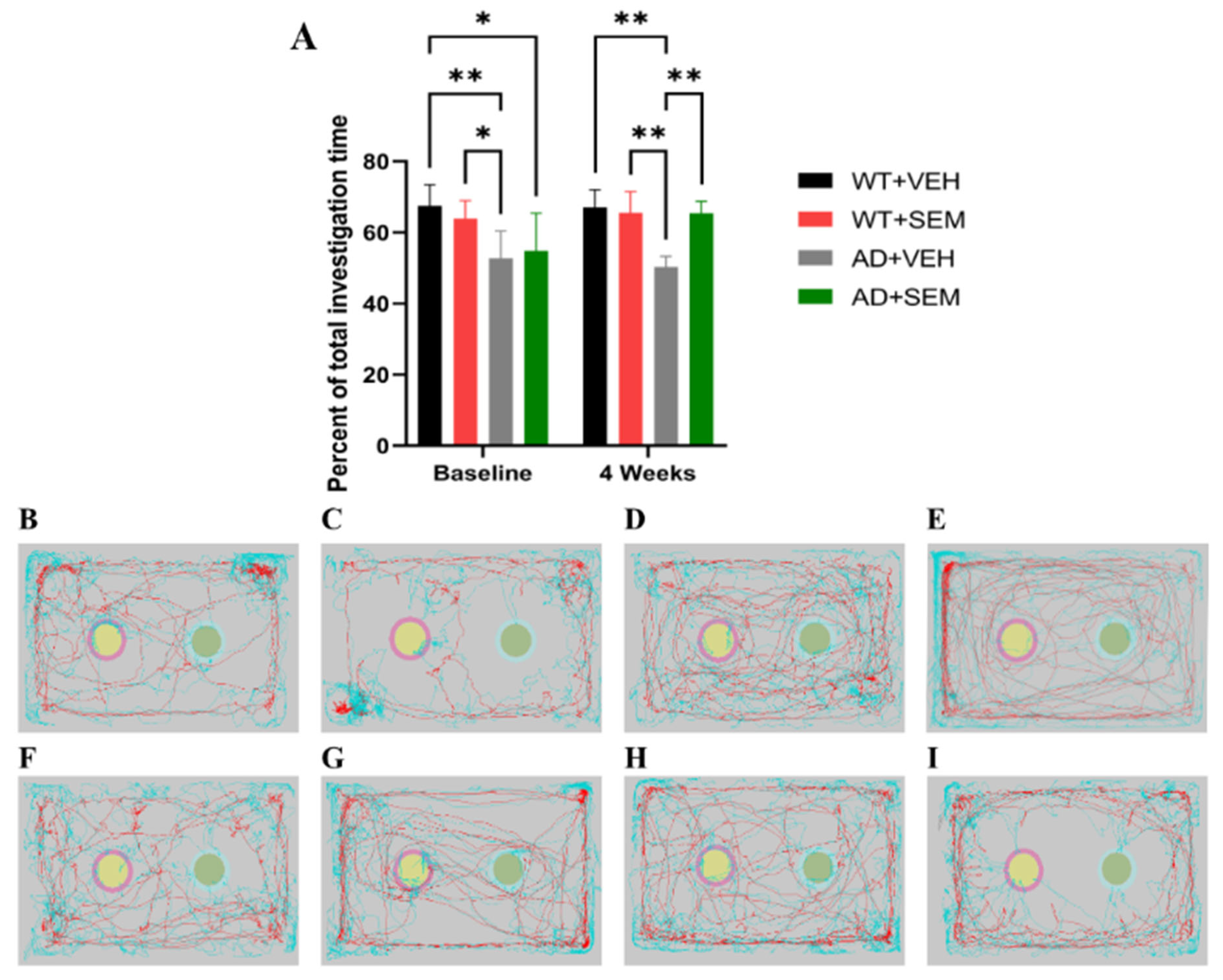
3.3.2. NORT
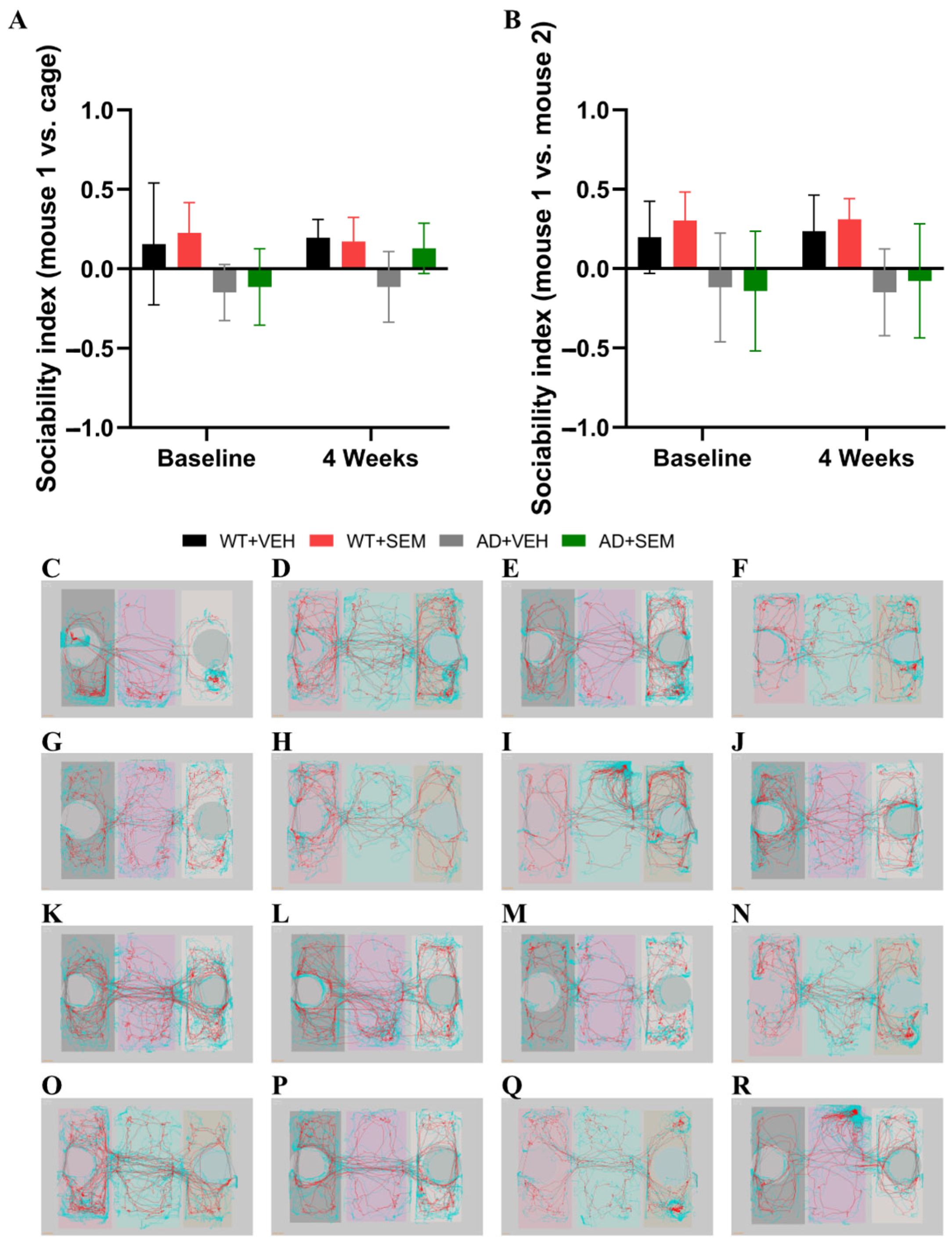
3.3.3. SCT
3.3.4. 0-MT
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perneczky, R.; Dom, G.; Chan, A.; Falkai, P.; Bassetti, C. Anti-amyloid antibody treatments for Alzheimer’s disease. Eur. J. Neurol. 2024, 31, e16049. [Google Scholar] [CrossRef] [PubMed]
- Nasb, M.; Tao, W.; Chen, N. Alzheimer’s Disease Puzzle: Delving into Pathogenesis Hypotheses. Aging Dis. 2024, 15, 43–73. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Lee, C.; Torres, E.R.S.; Carling, G.; Gan, L. Mechanisms of sex differences in Alzheimer’s disease. Neuron 2024, 112, 1208–1221. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Osse, A.M.L.; Cammann, D.; Powell, J.; Chen, J. Anti-Amyloid Monoclonal Antibodies for the Treatment of Alzheimer’s Disease. BioDrugs 2024, 38, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Dissanayaka, D.M.S.; Jayasena, V.; Rainey-Smith, S.R.; Martins, R.N.; Fernando, W.M.A.D.B. The Role of Diet and Gut Microbiota in Alzheimer’s Disease. Nutrients 2024, 16, 412. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Escobar, G.; Manero, R.M.; Fernández-Lebrero, A.; Ois, A.; Navalpotro-Gómez, I.; Puente-Periz, V.; Contador-Muñana, J.; Estragués-Gazquez, I.; Puig-Pijoan, A.; Jiménez-Balado, J. Blood Biomarkers of Alzheimer’s Disease and Cognition: A Literature Review. Biomolecules 2024, 14, 93. [Google Scholar] [CrossRef]
- Alkhalifa, A.E.; Al-Ghraiybah, N.F.; Odum, J.; Shunnarah, J.G.; Austin, N.; Kaddoumi, A. Blood-Brain Barrier Breakdown in Alzheimer’s Disease: Mechanisms and Targeted Strategies. Int. J. Mol. Sci. 2023, 24, 16288. [Google Scholar] [CrossRef]
- Tang, D.; Sun, C.; Yang, J.; Fan, L.; Wang, Y. Advances in the Study of the Pathology and Treatment of Alzheimer’s Disease and Its Association with Periodontitis. Life 2023, 13, 2203. [Google Scholar] [CrossRef]
- Guo, X.; Yan, L.; Zhang, D.; Zhao, Y. Passive immunotherapy for Alzheimer’s disease. Ageing Res. Rev. 2024, 94, 102192. [Google Scholar] [CrossRef]
- Tamayo-Trujillo, R.; Ruiz-Pozo, V.A.; Cadena-Ullauri, S.; Guevara-Ramírez, P.; Paz-Cruz, E.; Zambrano-Villacres, R.; Simancas-Racines, D.; Zambrano, A.K. Molecular mechanisms of semaglutide and liraglutide as a therapeutic option for obesity. Front. Nutr. 2024, 11, 1398059. [Google Scholar] [CrossRef]
- Ding, Y.; Shi, Y.; Guan, R.; Yan, S.; Liu, H.; Wang, Z.; Li, J.; Wang, T.; Cai, W.; Ma, G. Evaluation and comparison of efficacy and safety of tirzepatide and semaglutide in patients with type 2 diabetes mellitus: A Bayesian network meta-analysis. Pharmacol. Res. 2024, 199, 107031. [Google Scholar] [CrossRef] [PubMed]
- Meier, J.J. Efficacy of Semaglutide in a Subcutaneous and an Oral Formulation. Front. Endocrinol. 2021, 12, 645617. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.D.; Yang, Y.Y. Clinical Pharmacokinetics of Semaglutide: A Systematic Review. Drug Des. Dev. Ther. 2024, 18, 2555–2570. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Maleki, M.; Jamialahmadi, T.; Sahebkar, A. Anti-inflammatory benefits of semaglutide: State of the art. J. Clin. Transl. Endocrinol. 2024, 36, 100340. [Google Scholar] [CrossRef] [PubMed]
- Candido, R.; Di Loreto, C.; Desenzani, P.; Pantanetti, P.; Romano, C.; Settembrini, S.; Solerte, S.B.; Fadini, G.P. Suitability and Usefulness of a Flexible Dosing Timing of Oral Semaglutide to Maximize Benefit in Clinical Practice: An Expert Panel. Diabetes Ther. 2024, 15, 1963–1977. [Google Scholar] [CrossRef]
- Koureta, E.; Cholongitas, E. Evolving role of semaglutide in NAFLD: In combination, weekly and oral administration. Front. Pharmacol. 2024, 15, 1343587. [Google Scholar] [CrossRef]
- Alharbi, S.H. Anti-inflammatory role of glucagon-like peptide 1 receptor agonists and its clinical implications. Ther. Adv. Endocrinol. Metab. 2024, 15, 20420188231222367. [Google Scholar] [CrossRef]
- Tipa, R.O.; Balan, D.G.; Georgescu, M.T.; Ignat, L.A.; Vacaroiu, I.A.; Georgescu, D.E.; Raducu, L.; Mihai, D.A.; Chiperi, L.V.; Balcangiu-Stroescu, A.E. A Systematic Review of Semaglutide’s Influence on Cognitive Function in Preclinical Animal Models and Cell-Line Studies. Int. J. Mol. Sci. 2024, 25, 4972. [Google Scholar] [CrossRef]
- Drummond, E.; Wisniewski, T. Alzheimer’s disease: Experimental models and reality. Acta Neuropathol. 2017, 133, 155–175. [Google Scholar] [CrossRef]
- Pádua, M.S.; Guil-Guerrero, J.L.; Prates, J.A.M.; Lopes, P.A. Insights on the Use of Transgenic Mice Models in Alzheimer’s Disease Research. Int. J. Mol. Sci. 2024, 25, 2805. [Google Scholar] [CrossRef]
- Elder, G.A.; Gama Sosa, M.A.; De Gasperi, R. Transgenic mouse models of Alzheimer’s disease. Mt. Sinai J. Med. New York 2010, 77, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.J.; Li, X.R.; Chai, S.F.; Li, W.R.; Li, S.; Hou, M.; Li, J.L.; Ye, Y.C.; Cai, H.Y.; Hölscher, C.; et al. Semaglutide ameliorates cognition and glucose metabolism dysfunction in the 3xTg mouse model of Alzheimer’s disease via the GLP-1R/SIRT1/GLUT4 pathway. Neuropharmacology 2023, 240, 109716. [Google Scholar] [CrossRef] [PubMed]
- Kennard, M.R.; Daniels Gatward, L.F.; Roberts, A.G.; White, E.R.P.; Nandi, M.; King, A.J.F. The use of mice in diabetes research: The impact of experimental protocols. Diabet. Med. A J. Br. Diabet. Assoc. 2021, 38, e14705. [Google Scholar] [CrossRef]
- Boboc IK, S.; Cojocaru, A.; Nedelea, G.; Catalin, B.; Bogdan, M.; Calina, D. Chronic Administration of Ion Channel Blockers Impact Microglia Morphology and Function in a Murine Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 14474. [Google Scholar] [CrossRef]
- Gould, T.D.; Dao, D.T.; Kovacsics, C.E. The open field test. In Mood and Anxiety Related Phenotypes in Mice: Characterization Using Behavioral Tests; Gould, T.D., Ed.; Humana Press: Totowa, NJ, USA; Springer Nature: Berlin/Heidelberg, Germany, 2009; pp. 1–20. [Google Scholar] [CrossRef]
- Choleris, E.; Thomas, A.W.; Kavaliers, M.; Prato, F.S. A detailed ethological analysis of the mouse open field test: Effects of diazepam, chlordiazepoxide and an extremely low frequency pulsed magnetic field. Neurosci. Biobehav. Rev. 2001, 25, 235–260. [Google Scholar] [CrossRef]
- Lueptow, L.M. Novel Object Recognition Test for the Investigation of Learning and Memory in Mice. J. Vis. Exp. JoVE 2017, 126, 55718. [Google Scholar] [CrossRef]
- Antunes, M.; Biala, G. The novel object recognition memory: Neurobiology, test procedure, and its modifications. Cogn. Process. 2012, 13, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Denninger, J.K.; Smith, B.M.; Kirby, E.D. Novel Object Recognition and Object Location Behavioral Testing in Mice on a Budget. J. Vis. Exp. JoVE 2018, 141, 10.3791/58593. [Google Scholar] [CrossRef]
- Yang, M.; Silverman, J.L.; Crawley, J.N. Automated three-chambered social approach task for mice. Curr. Protoc. Neurosci. 2011, 56, 8.26.1–8.26.16. [Google Scholar] [CrossRef]
- Fang, L.P.; Zhao, N.; Caudal, L.C.; Chang, H.F.; Zhao, R.; Lin, C.H.; Hainz, N.; Meier, C.; Bettler, B.; Huang, W.; et al. Impaired bidirectional communication between interneurons and oligodendrocyte precursor cells affects social cognitive behavior. Nat. Commun. 2022, 13, 1394. [Google Scholar] [CrossRef]
- Rein, B.; Ma, K.; Yan, Z. A standardized social preference protocol for measuring social deficits in mouse models of autism. Nat. Protoc. 2020, 15, 3464–3477. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, J.K.; Grewal, S.S.; Fletcher, A.; Bill, D.J.; Dourish, C.T. Behavioural and pharmacological characterisation of the elevated “zero-maze” as an animal model of anxiety. Psychopharmacology 1994, 116, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Heredia, L.; Torrente, M.; Domingo, J.L.; Colomina, M.T. Individual housing and handling procedures modify anxiety levels of Tg2576 mice assessed in the zero maze test. Physiol. Behav. 2012, 107, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Stanley, M.; Macauley, S.L.; Caesar, E.E.; Koscal, L.J.; Moritz, W.; Robinson, G.O.; Roh, J.; Keyser, J.; Jiang, H.; Holtzman, D.M. The Effects of Peripheral and Central High Insulin on Brain Insulin Signaling and Amyloid-β in Young and Old APP/PS1 Mice. J. Neurosci. Off. J. Soc. Neurosci. 2016, 36, 11704–11715. [Google Scholar] [CrossRef] [PubMed]
- Faivre, E.; Hölscher, C. Neuroprotective effects of D-Ala(2)GIP on Alzheimer’s disease biomarkers in an APP/PS1 mouse model. Alzheimer’s Res. Ther. 2013, 5, 20. [Google Scholar] [CrossRef]
- Porquet, D.; Andrés-Benito, P.; Griñán-Ferré, C.; Camins, A.; Ferrer, I.; Canudas, A.M.; Del Valle, J.; Pallàs, M. Amyloid and tau pathology of familial Alzheimer’s disease APP/PS1 mouse model in a senescence phenotype background (SAMP8). Age 2015, 37, 9747. [Google Scholar] [CrossRef]
- Webster, S.J.; Bachstetter, A.D.; Van Eldik, L.J. Comprehensive behavioral characterization of an APP/PS-1 double knock-in mouse model of Alzheimer’s disease. Alzheimer’s Res. Ther. 2013, 5, 28. [Google Scholar] [CrossRef]
- Gabery, S.; Salinas, C.G.; Paulsen, S.J.; Ahnfelt-Rønne, J.; Alanentalo, T.; Baquero, A.F.; Buckley, S.T.; Farkas, E.; Fekete, C.; Frederiksen, K.S.; et al. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight 2020, 5, e133429. [Google Scholar] [CrossRef]
- Pontes-da-Silva, R.M.; de Souza Marinho, T.; de Macedo Cardoso, L.E.; Mandarim-de-Lacerda, C.A.; Aguila, M.B. Obese mice weight loss role on nonalcoholic fatty liver disease and endoplasmic reticulum stress treated by a GLP-1 receptor agonist. Int. J. Obes. 2022, 46, 21–29. [Google Scholar] [CrossRef]
- Yue, L.; Chen, S.; Ren, Q.; Niu, S.; Pan, X.; Chen, X.; Li, Z.; Chen, X. Effects of semaglutide on vascular structure and proteomics in high-fat diet-induced obese mice. Front. Endocrinol. 2022, 13, 995007. [Google Scholar] [CrossRef]
- Poupon-Bejuit, L.; Hughes, M.P.; Liu, W.; Geard, A.; Faour-Slika, N.; Whaler, S.; Massaro, G.; Rahim, A.A. A GLP1 receptor agonist diabetes drug ameliorates neurodegeneration in a mouse model of infantile neurometabolic disease. Sci. Rep. 2022, 12, 13825. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, L.; Li, L.; Hölscher, C. Neuroprotective effects of the novel GLP-1 long acting analogue semaglutide in the MPTP Parkinson’s disease mouse model. Neuropeptides 2018, 71, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, L.; Li, L.; Hölscher, C. Semaglutide is Neuroprotective and Reduces α-Synuclein Levels in the Chronic MPTP Mouse Model of Parkinson’s Disease. J. Park. Dis. 2019, 9, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Meca, A.D.; Boboc, I.K.S.; Mititelu-Tartau, L.; Bogdan, M. Unlocking the Potential: Semaglutide’s Impact on Alzheimer’s and Parkinson’s Disease in Animal Models. Curr. Issues Mol. Biol. 2024, 46, 5929–5949. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boboc, I.K.S.; Dumitrelea, P.-D.; Meca, A.D.; Mititelu-Tartau, L.; Bogdan, M. Exploring the Impact of Semaglutide on Cognitive Function and Anxiety-Related Behaviors in a Murine Model of Alzheimer’s Disease. Biomedicines 2024, 12, 2689. https://doi.org/10.3390/biomedicines12122689
Boboc IKS, Dumitrelea P-D, Meca AD, Mititelu-Tartau L, Bogdan M. Exploring the Impact of Semaglutide on Cognitive Function and Anxiety-Related Behaviors in a Murine Model of Alzheimer’s Disease. Biomedicines. 2024; 12(12):2689. https://doi.org/10.3390/biomedicines12122689
Chicago/Turabian StyleBoboc, Ianis Kevyn Stefan, Petrica-Daniel Dumitrelea, Andreea Daniela Meca, Liliana Mititelu-Tartau, and Maria Bogdan. 2024. "Exploring the Impact of Semaglutide on Cognitive Function and Anxiety-Related Behaviors in a Murine Model of Alzheimer’s Disease" Biomedicines 12, no. 12: 2689. https://doi.org/10.3390/biomedicines12122689
APA StyleBoboc, I. K. S., Dumitrelea, P.-D., Meca, A. D., Mititelu-Tartau, L., & Bogdan, M. (2024). Exploring the Impact of Semaglutide on Cognitive Function and Anxiety-Related Behaviors in a Murine Model of Alzheimer’s Disease. Biomedicines, 12(12), 2689. https://doi.org/10.3390/biomedicines12122689







