Investigating the Cytotoxic Effects of Artemisia absinthium Extract on Oral Carcinoma Cell Line
Abstract
1. Introduction
2. Materials and Methods
2.1. Artemisia absinthium Extraction
2.2. Phytochemical Analysis of A. absinthium Extract
2.2.1. High-Pressure Liquid Chromatography (HPLC)
2.2.2. Total Phenolic Compounds Quantification
2.3. Establishment of Primary Cultures
2.4. Biocompatibility Assay
2.5. Osteogenic Activity
2.6. Anticancer Activity
2.6.1. Cytotoxicity Assay-CCK8
2.6.2. Gene Expression Level-qPCR
2.7. Statistical Analysis
3. Results
3.1. Phytochemical Analysis of A. absinthium Extract
High-Pressure Liquid Chromatography (HPLC) and Total Phenolic Quantification
3.2. Biological Evaluation
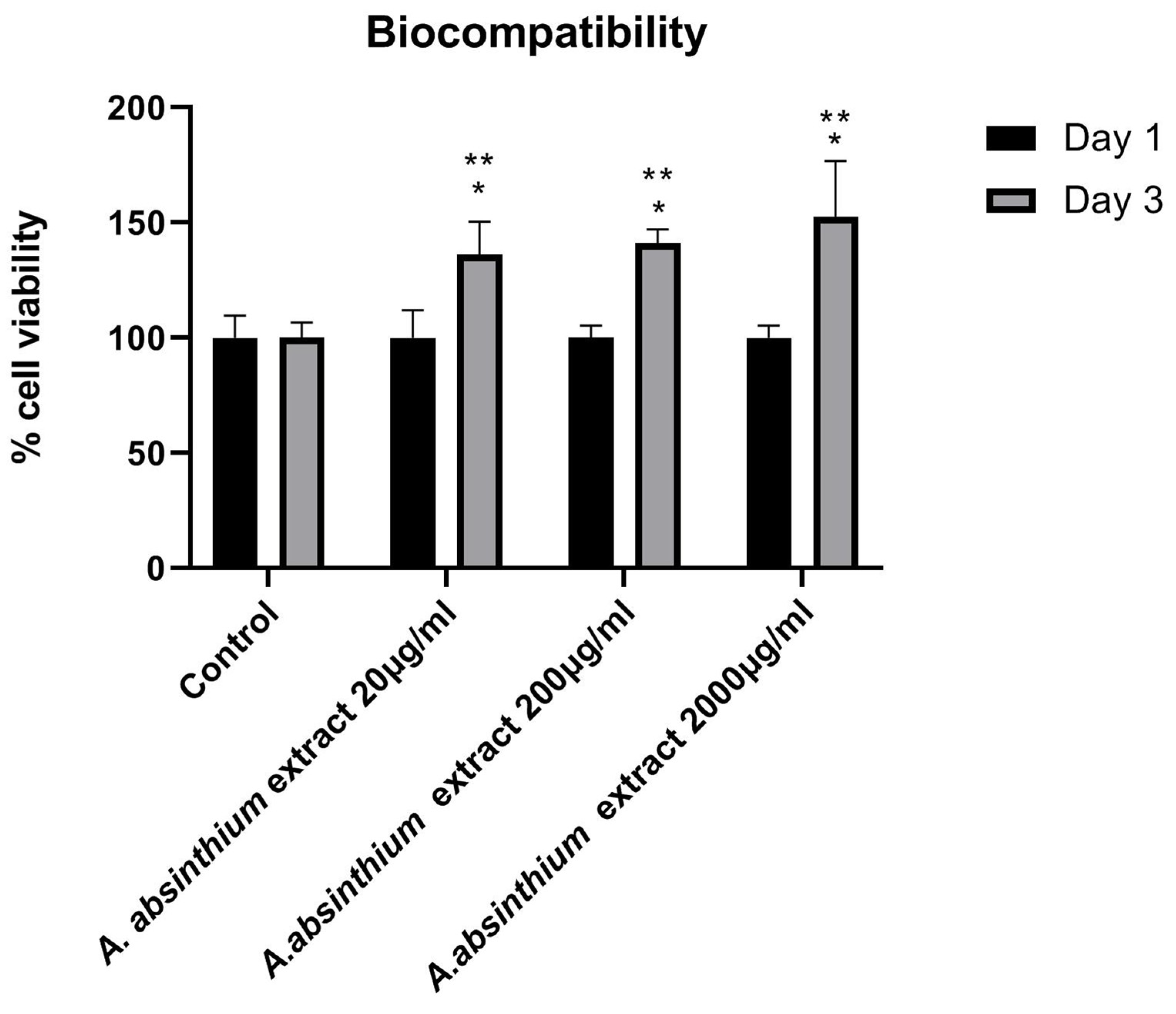
3.2.1. Biocompatibility Assay
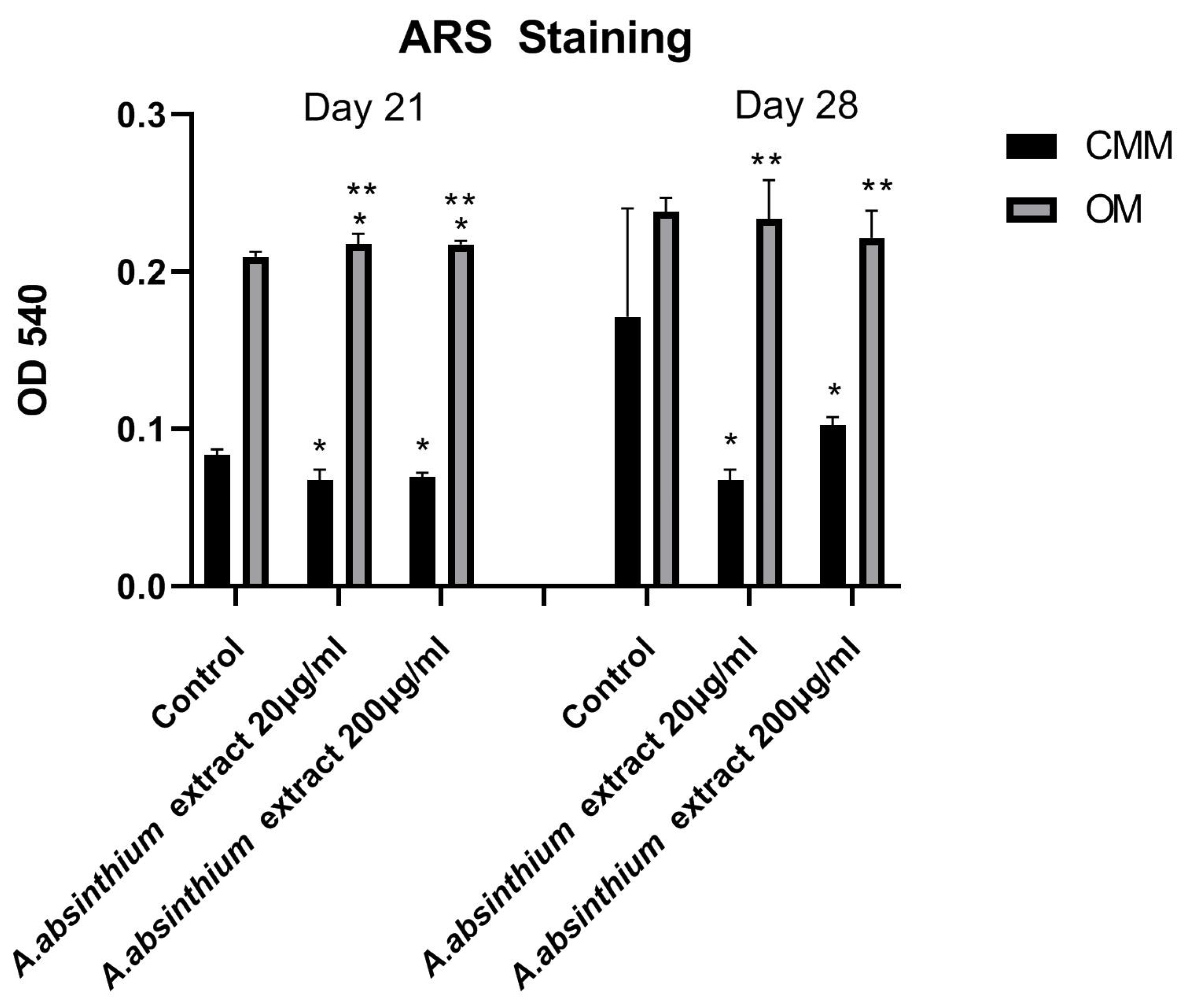
3.2.2. Osteogenic Activity
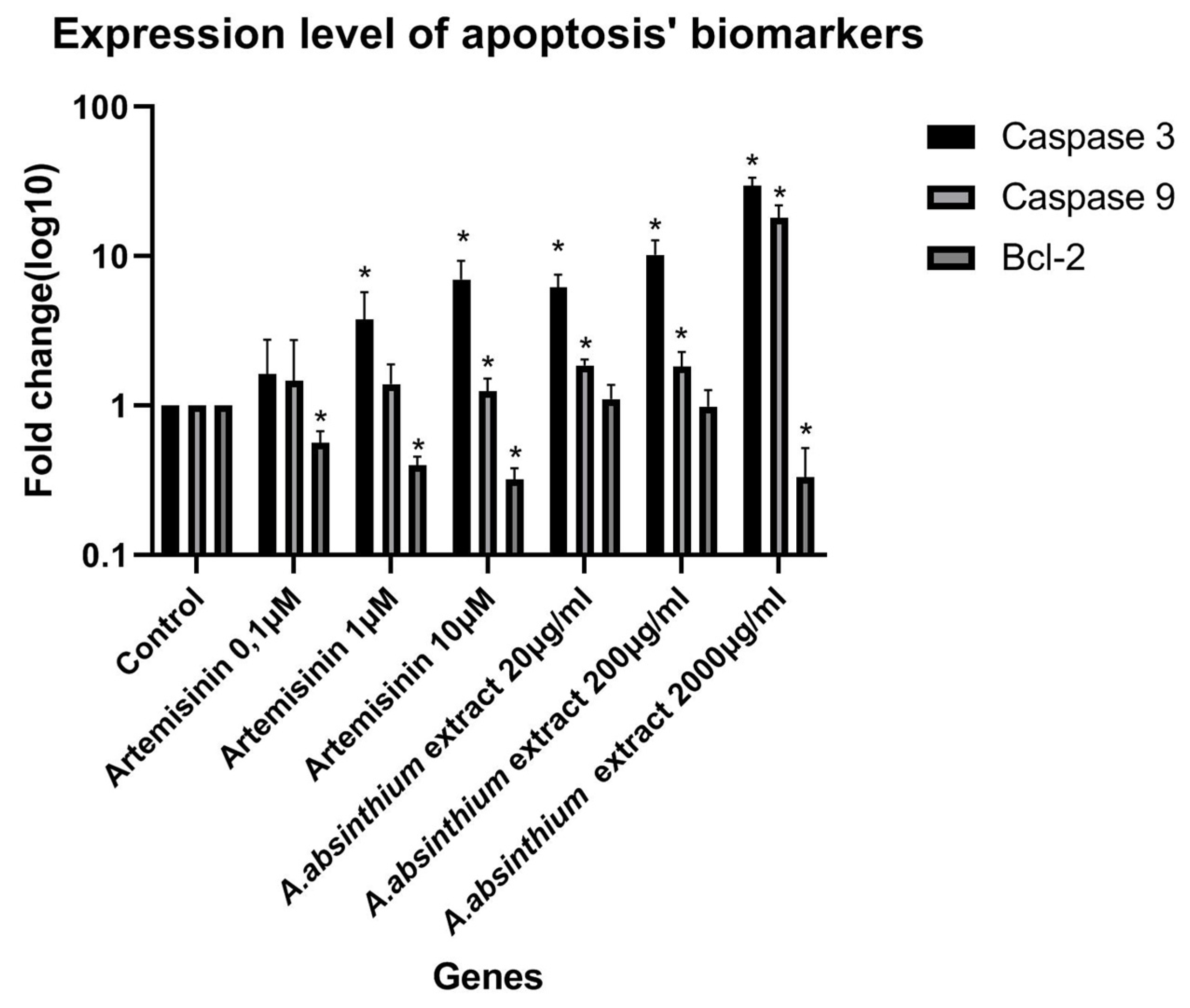
3.2.3. Anticancer Activity
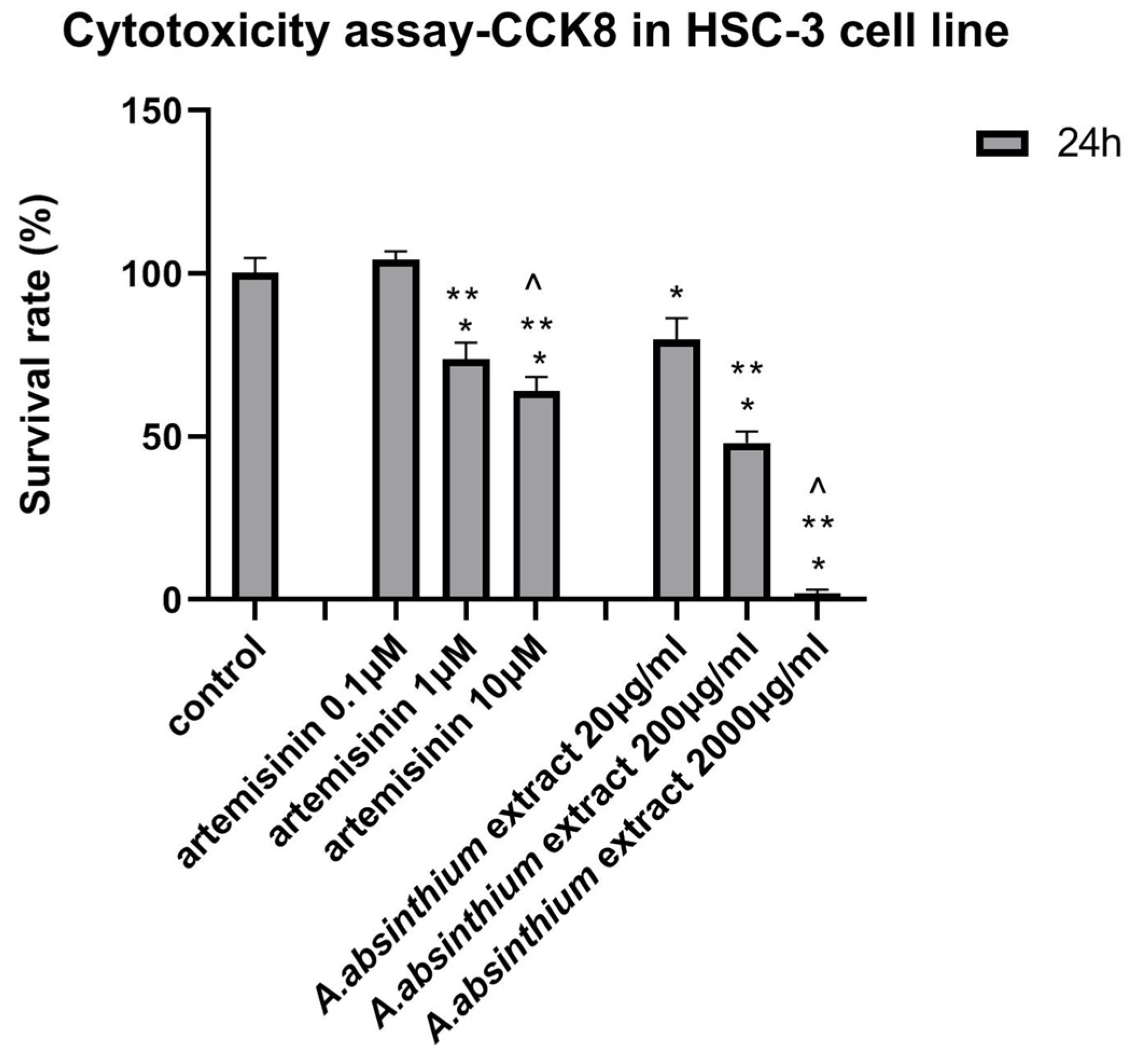
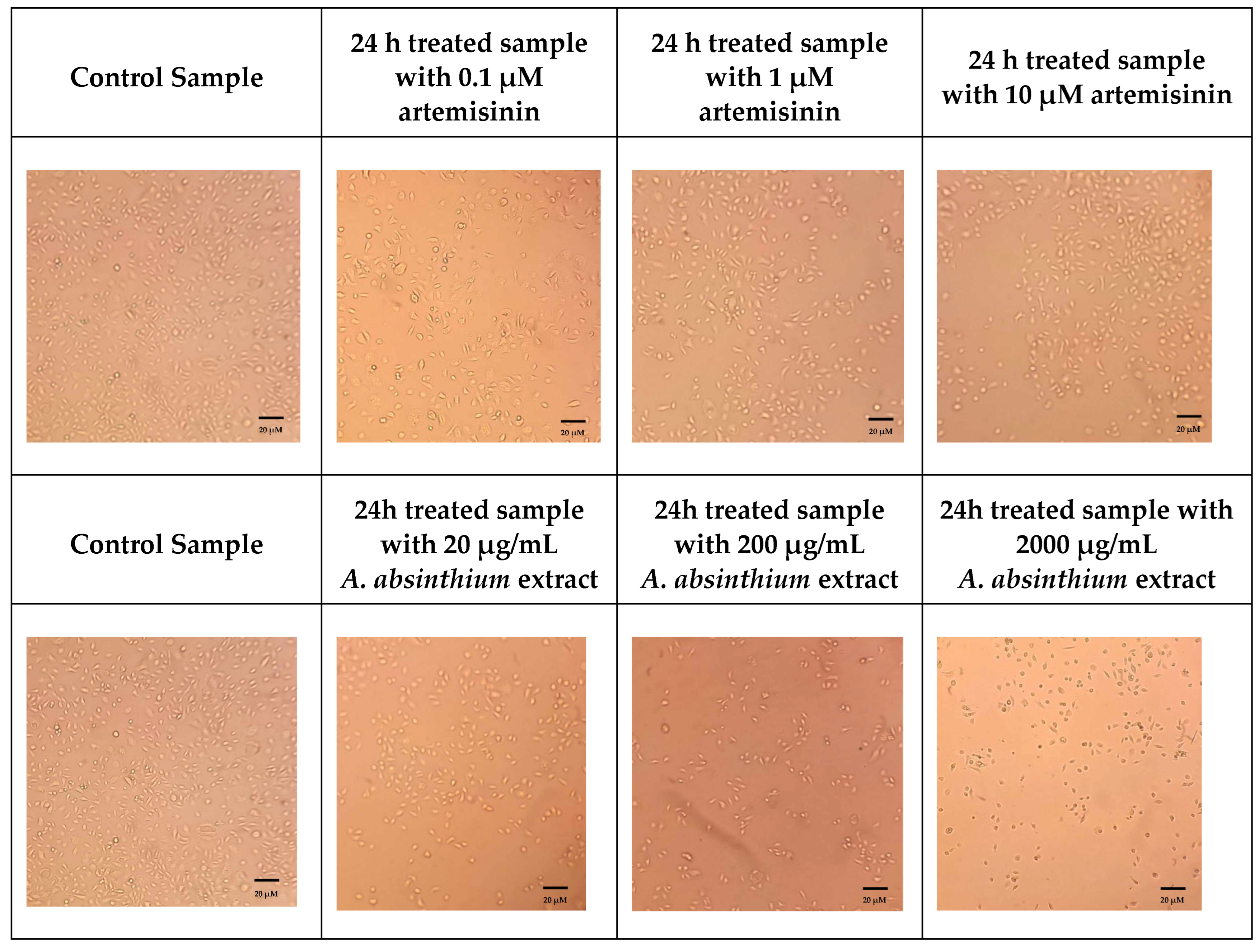
Cytotoxicity Assay-CCK8
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abati, S.; Bramati, C.; Bondi, S.; Lissoni, A.; Trimarchi, M. Oral Cancer and Precancer: A Narrative Review on the Relevance of Early Diagnosis. Int. J. Environ. Res. Public Health 2020, 17, 9160. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S. Living with Oral Cancer: Epidemiology with Particular Reference to Prevalence and Lifestyle Changes that Influence Survival. Oral Oncol. 2010, 46, 407–410. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. Nat. Rev. Dis. Primers 2021, 7, 96. [Google Scholar] [CrossRef] [PubMed]
- Marur, S.; Forastiere, A.A. Head and Neck Squamous Cell Carcinoma: Update on Epidemiology, Diagnosis, and Treatment. Mayo Clin. Proc. 2016, 91, 386–396. [Google Scholar] [CrossRef]
- Elting, L.S.; Keefe, D.M.; Sonis, S.T.; Garden, A.S.; Spijkervet, F.K.L. Burden of Illness Head and Neck Cancer Treatment: Mucositis, Pain, and Dysphagia. Cancer 2008, 113, 2704–2713. [Google Scholar] [CrossRef]
- Peterson, D.E.; Bensadoun, R.-J.; Roila, F. Management of Oral and Gastrointestinal Mucositis: ESMO Clinical Practice Guidelines. Ann. Oncol. 2011, 22, vi78–vi84. [Google Scholar] [CrossRef]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K. Curcumin, a Component of Golden Spice: From Bedside to Bench and Back. Biotechnol. Adv. 2014, 32, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Lin, H.; Seidman, A.; Fornier, M.; D’Andrea, G.; Wesa, K.; Yeung, K.; Cunningham-Rundles, S.; Vickers, A. A Phase I/II Trial of a Standardized Herbal Supplement in Combination with Chemotherapy in Women with Breast Cancer. J. Clin. Oncol. 2007, 25, 4647–4652. [Google Scholar]
- Bora, K.S.; Sharma, A. The genus Artemisia: A comprehensive review. Pharm. Biol. 2011, 49, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Wake, G.; Court, J.; Pickering, A.; Lewis, R.; Wilkins, R.; Perry, E. CNS Acetylcholine Receptor Activity in European Medicinal Plants Traditionally Used to Improve Failing Memory. J. Ethnopharmacol. 2000, 69, 105–114. [Google Scholar] [CrossRef]
- Guarrera, P.M. Traditional phytotherapy in Central Italy (Marche, Abruzzo, and Latium). Fitoterapia 2005, 76, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Sarker, S.D.; Latif, Z.; Gray, A.I. Artemisia absintheum (L.) and Its Bioactive Constituents: An Overview of Their Chemical Composition, Biological Activity, and Applications. Phytochem. Rev. 2012, 11, 263–288. [Google Scholar]
- Augustin, Y.; Staines, H.M.; Krishna, S. Artemisinins as a novel anti-cancer therapy: Targeting a global cancer pandemic through drug repurposing. Pharmacol. Ther. 2020, 216, 107706. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Suo, F.; Haslinger, K.; Quax, W.J. Artemisinin-Type Drugs in Tumor Cell Death: Mechanisms, Combination Treatment with Biologics and Nanoparticle Delivery. Pharmaceutics 2022, 14, 395. [Google Scholar] [CrossRef]
- Choi, E.; Kim, G. Effect of Artemisia species on cellular proliferation and apoptosis in human breast cancer cells via estrogen receptor-related pathway. J. Tradit. Chin. Med. 2013, 33, 658–663. [Google Scholar] [CrossRef]
- Forouhandeh, H.; Tarhriz, V.; Zadehkamand, M.; Asgharian, P. Anti-proliferative activity of Artemisia marschalliana on cancerous cell lines. BMC Complement. Med. Ther. 2023, 23, 119. [Google Scholar] [CrossRef]
- Wei, X.; Xia, L.; Ziyayiding, D.; Chen, Q.; Liu, R.; Xu, X.; Li, J. The Extracts of Artemisia absinthium L. Suppress the growth of hepatocellular carcinoma cells through induction of apoptosis via endoplasmic reticulum stress and mitochondrial-dependent pathway. Molecules 2019, 24, 913. [Google Scholar] [CrossRef]
- Mughees, M.; Wajid, S.; Samim, M. Cytotoxic potential of Artemisia absinthium extract-loaded polymeric nanoparticles against breast cancer cells: Insight into the protein targets. Int. J. Pharm. 2020, 586, 119583. [Google Scholar] [CrossRef]
- He, M.; Yasin, K.; Yu, S.; Li, J.; Xia, L. Total Flavonoids in Artemisia absinthium L. and Evaluation of Its Anticancer Activity. Int. J. Mol. Sci. 2023, 24, 16348. [Google Scholar] [CrossRef]
- Tsamesidis, I.; Reybier, K.; Marchetti, G.; Pau, M.C.; Virdis, P.; Fozza, C.; Nepveu, F.; Low, P.S.; Turrini, F.M.; Pantaleo, A. Syk Kinase Inhibitors Synergize with Artemisinins by Enhancing Oxidative Stress in Plasmodium falciparum-Parasitized Erythrocytes. Antioxidants 2020, 9, 753. [Google Scholar] [CrossRef]
- Ali Abdalla, Y.O.; Subramaniam, B.; Nyamathulla, S.; Shamsuddin, N.; Arshad, N.M.; Mun, K.S.; Awang, K.; Nagoor, N.H. Natural Products for Cancer Therapy: A Review of Their Mechanism of Actions and Toxicity in the Past Decade. J. Trop. Med. 2022, 2022, 5794350. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Z.; Zhou, H.Y.; Zhao, Y. An effective method for fast determination of artemisinin in Artemisia annua L. by high performance liquid chromatography with evaporative light scattering detection. Anal. Chim. Acta 2007, 581, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Al-Farsi, M.; Alasalvar, C.; Morris, A.; Baron, M.; Shahidi, F. Comparison of antioxidant activity, anthocyanins, carotenoids, and phenolics of three native fresh and sun-dried date (Phoenix dactylifera L.) varieties grown in Oman. J. Agric. Food Chem. 2005, 53, 7592–7599. [Google Scholar] [CrossRef]
- Gkiliopoulos, D.; Tsamesidis, I.; Theocharidou, A.; Pouroutzidou, G.K.; Christodoulou, E.; Stalika, E.; Xanthopoulos, K.; Bikiaris, D.; Triantafyllidis, K.; Kontonasaki, E. SBA-15 Mesoporous Silica as Delivery Vehicle for rhBMP-2 Bone Morphogenic Protein for Dental Applications. Nanomaterials 2022, 12, 822. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.S.; Huang, Y.; Fidock, D.A.; Polyak, S.J.; Wagoner, J.; Towler, M.J.; Weathers, P.J. Artemisia annua L. extracts inhibit the in vitro replication of SARS-CoV-2 and two of its variants. J. Ethnopharmacol. 2021, 274, 114016. [Google Scholar] [CrossRef]
- Zhu, W.; Liang, M. Periodontal ligament stem cells: Current status, concerns, and future prospects. Stem Cells Int. 2015, 2015, 972313. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, Q.; Yang, M.; Wang, T.; Yao, J.; Cheng, J.; Yuan, J.; Lin, X.; Zhao, J.; Tickner, J.; et al. Dihydroartemisinin, an Anti-Malaria Drug, Suppresses Estrogen Deficiency-Induced Osteoporosis, Osteoclast Formation, and RANKL-Induced Signaling Pathways. J. Bone Miner. Res. 2016, 31, 964–974. [Google Scholar] [CrossRef]
- Lee, S.K.; Kim, H.; Park, J.; Kim, H.J.; Kim, K.R.; Son, S.H.; Park, K.K.; Chung, W.Y. Artemisia annua extract prevents ovariectomy-induced bone loss by blocking receptor activator of nuclear factor kappa-B ligand-induced differentiation of osteoclasts. Sci. Rep. 2017, 7, 17332. [Google Scholar] [CrossRef]
- Feng, M.X.; Hong, J.X.; Wang, Q.; Fan, Y.Y.; Yuan, C.T.; Lei, X.H.; Zhu, M.; Qin, A.; Chen, H.X.; Hong, D. Dihydroartemisinin prevents breast cancer-induced osteolysis via inhibiting both breast caner cells and osteoclasts. Sci. Rep. 2016, 6, 19074. [Google Scholar] [CrossRef]
- Wei, C.M.; Liu, Q.; Song, F.M.; Lin, X.X.; Su, Y.J.; Xu, J.; Huang, L.; Zong, S.H.; Zhao, J.M. Artesunate inhibits RANKL-induced osteoclastogenesis and bone resorption in vitro and prevents LPS-induced bone loss in vivo. J. Cell. Physiol. 2018, 233, 476–485. [Google Scholar] [CrossRef]
- Li, Y.; Mu, W.; Xu, B.; Ren, J.; Wahafu, T.; Wuermanbieke, S.; Ma, H.; Gao, H.; Liu, Y.; Zhang, K.; et al. Artesunate, an anti-malaria agent, attenuates experimental osteoarthritis by inhibiting bone resorption and CD31HiEMCnHi vessel formation in subchondral bone. Front. Pharmacol. 2019, 10, 685. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J. The osteoprotective effects of artemisinin compounds and the possible mechanisms associated with intracellular iron: A review of in vivo and in vitro studies. Environ. Toxicol. Pharmacol. 2020, 76, 103358. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Kuang, Z.; Gong, Z.; Xue, D.; Zheng, Q. Dihydroartemisinin Promotes the Osteogenesis of Human Mesenchymal Stem Cells via the ERK and Wnt/í µí»½-Catenin Signaling Pathways. BioMed Res. Int. 2019, 2019, 3456719. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.M.; Mao, M.H.; Hu, Y.H.; Zhou, X.C.; Li, S.; Chen, C.F.; Li, C.N.; Yuan, Q.L.; Li, W. Artemisinin protects DPSC from hypoxia and TNF-α mediated osteogenesis impairments through CA9 and Wnt signaling pathway. Life Sci. 2021, 277, 119471. [Google Scholar] [CrossRef]
- Fang, J.; Zhao, X.; Li, S.; Xing, X.; Wang, H.; Lazarovici, P.; Zheng, W. Protective mechanism of artemisinin on rat bone marrow-derived mesenchymal stem cells against apoptosis induced by hydrogen peroxide via activation of c-Raf-Erk1/2-p90rsk-CREB pathway. Stem Cell Res. Ther. 2019, 10, 312. [Google Scholar] [CrossRef]
- Bhat, M.Y.; Gul, M.Z.; Lohamror, L.R.; Qureshi, I.A.; Ghazi, I.A. An in vitro Study of the Antioxidant and Antiproliferative Properties of Artemisia absinthium—A Potent Medicinal Plant. Free Radic. Antioxid. 2017, 8, 18–25. [Google Scholar] [CrossRef]
- Msaada, K.; Salem, N.; Bachriuch, O.; Bousselmi, S.; Tamaar, S.; Alfaify, A. Chemical Composition and Antioxidant and Antimicrobial Activities of Wormwood (Artemisia absinthium L.) Essential Oils and Phenolics. J. Chem. 2015, 2015, 804658. [Google Scholar] [CrossRef]
- Mohammadi, A.; Sani, T.A.; Ameri, A.A.; Imani, M.; Golmakani, E.; Kamali, H. Seasonal variation in the chemical composition, antioxidant activity, and total phenolic content of Artemisia absinthium essential oils. Pharmacogn. Res. 2015, 7, 329. [Google Scholar]
- Bordean, M.E.; Ungur, R.A.; Toc, D.A.; Borda, I.M.; Marțiș, G.S.; Pop, C.R.; Filip, M.; Vlassa, M.; Nasui, B.A.; Pop, A.; et al. Antibacterial and Phytochemical Screening of Artemisia Species. Antioxidants 2023, 12, 596. [Google Scholar] [CrossRef]
- Hajam, Y.A.; Lone, R.; Kumar, R. Role of Plant Phenolics Against Reactive Oxygen Species (ROS) Induced Oxidative Stress and Biochemical Alterations. In Plant Phenolics in Abiotic Stress Management; Springer Nature: Singapore, 2023; pp. 125–147. [Google Scholar] [CrossRef]
- Rajashekar, C.B. Dual Role of Plant Phenolic Compounds as Antioxidants and Prooxidants. Am. J. Plant Sci. 2023, 14, 15–28. [Google Scholar] [CrossRef]
- León-González, A.J.; Auger, C.; Schini-Kerth, V.B. Pro-oxidant activity of polyphenols and its implication on cancer chemoprevention and chemotherapy. Biochem. Pharmacol. 2015, 98, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Nam, W.; Tak, J.; Ryu, J.K.; Jung, M.; Yook, J.I.; Kim, H.J.; Cha, I.H. Effects of artemisinin and its derivatives on growth inhibition and apoptosis of oral cancer cells. Head Neck 2007, 29, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.C.; Singh, N.P.; Sasaki, T. Development of artemisinin compounds for cancer treatment. Investig. New Drugs 2013, 31, 230–246. [Google Scholar] [CrossRef]
- Oda, D.; Habte, T.; Yamachika, E. Artemisinin: An alternative treatment for oral cancer. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2004, 98, 204. [Google Scholar] [CrossRef]
- Yu, X.H.; Wu, J.B.; Fan, H.Y.; Dai, L.; Xian, H.C.; Chen, B.J.; Liao, P.; Huang, M.C.; Pang, X.; Zhang, M.; et al. Artemisinin suppressed tumour growth and induced vascular normalisation in oral squamous cell carcinoma via inhibition of macrophage migration inhibitory factor. Oral Dis. 2024, 30, 363–375. [Google Scholar] [CrossRef]
- Ali, M.; Iqbal, R.; Safdar, M.; Murtaza, S.; Mustafa, M.; Sajjad, M.; Bukhari, S.A.; Huma, T. Antioxidant and antibacterial activities of Artemisia absinthium and Citrus paradisi extracts repress viability of aggressive liver cancer cell line. Mol. Biol. Rep. 2021, 48, 7703–7710. [Google Scholar] [CrossRef]
- Sohail, J.; Zubair, M.; Hussain, K.; Faisal, M.; Ismail, M.; Haider, I.; Mumtaz, R.; Khan, A.A.; Khan, M.A. Pharmacological activities of Artemisia absinthium and control of hepatic cancer by expression regulation of TGFβ1 and MYC genes. PLoS ONE 2023, 18, e0284244. [Google Scholar] [CrossRef]
- Kauser, S.; Mughees, M.; Swami, S.; Wajid, S. Pre-clinical Toxicity Assessment of Artemisia absinthium Extract-Loaded Polymeric Nanoparticles Associated with Their Oral Administration. Front. Pharmacol. 2023, 14, 1196842. [Google Scholar] [CrossRef]

| Voucher No. | 125 |
| Collection date | 14.6.2023 |
| Locality | Crete, Greece |
| GPS coordinates | 35.417416° N, 24.530005° E |
| Dried plant of Artemisia absinthium |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsamesidis, I.; Papadimitriou-Tsantarliotou, A.; Christodoulou, A.; Amanatidou, D.; Avgeros, C.; Stalika, E.; Bousnaki, M.; Michailidou, G.; Beketova, A.; Eleftheriou, P.; et al. Investigating the Cytotoxic Effects of Artemisia absinthium Extract on Oral Carcinoma Cell Line. Biomedicines 2024, 12, 2674. https://doi.org/10.3390/biomedicines12122674
Tsamesidis I, Papadimitriou-Tsantarliotou A, Christodoulou A, Amanatidou D, Avgeros C, Stalika E, Bousnaki M, Michailidou G, Beketova A, Eleftheriou P, et al. Investigating the Cytotoxic Effects of Artemisia absinthium Extract on Oral Carcinoma Cell Line. Biomedicines. 2024; 12(12):2674. https://doi.org/10.3390/biomedicines12122674
Chicago/Turabian StyleTsamesidis, Ioannis, Aliki Papadimitriou-Tsantarliotou, Athanasios Christodoulou, Dionysia Amanatidou, Chrysostomos Avgeros, Evangelia Stalika, Maria Bousnaki, Georgia Michailidou, Anastasia Beketova, Phaedra Eleftheriou, and et al. 2024. "Investigating the Cytotoxic Effects of Artemisia absinthium Extract on Oral Carcinoma Cell Line" Biomedicines 12, no. 12: 2674. https://doi.org/10.3390/biomedicines12122674
APA StyleTsamesidis, I., Papadimitriou-Tsantarliotou, A., Christodoulou, A., Amanatidou, D., Avgeros, C., Stalika, E., Bousnaki, M., Michailidou, G., Beketova, A., Eleftheriou, P., Bikiaris, D. N., Vizirianakis, I. S., & Kontonasaki, E. (2024). Investigating the Cytotoxic Effects of Artemisia absinthium Extract on Oral Carcinoma Cell Line. Biomedicines, 12(12), 2674. https://doi.org/10.3390/biomedicines12122674












