The Crosstalk Between Cartilage and Bone in Skeletal Growth
Abstract
1. Introduction
2. Physiology of Bone Growth
2.1. The Growth Plate and Endochondral Ossification
2.2. Structure of the Bone
2.3. Cellular Components and Their Roles in Bone Growth
3. Signaling Pathways and Cell–Cell Interactions in Bone Growth
3.1. Local Signaling Pathways Controlling Bone Growth
3.1.1. Ihh-PTHrh Feedback Loop in Growth Plate Development
3.1.2. Wnt Signaling
3.1.3. TGF-β/BMP Signaling
3.1.4. FGF Signaling
3.1.5. IGF1 Signaling
3.2. Cell–Cell Interactions
3.2.1. Chondrocytes–Endothelial Cells
3.2.2. Chondrocytes–Osteoblasts and Cell Transdifferentiation
3.2.3. Chondrocytes–Osteoclasts
3.2.4. Chondrocytes–Osteocytes
3.2.5. Osteoblasts–Osteoclasts
3.2.6. Osteoblasts–Osteocytes
4. Disorders of Bone Growth: Clinical Implications and Future Perspectives
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nichols, J. UpToDate. 2022. p. 1–50 Normal Growth Patterns in Infants and Prepubertal Children. Available online: https://www.uptodate.cn/contents/normal-growth-patterns-in-infants-and-prepubertal-children (accessed on 25 July 2024).
- Polidori, N.; Castorani, V.; Mohn, A.; Chiarelli, F. Deciphering short stature in children. Ann. Pediatr. Endocrinol. Metab. 2020, 25, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Graber, E.G. Physical Growth of Infants and Children. In MSD Manual, Professional Version; Merck & Co, Inc.: Rahway, NJ, USA, 2023. [Google Scholar]
- Bogarín, R.; Richmond, E.; Rogol, A.D. A new approach to the diagnosis of short stature. Minerva Pediatr. 2020, 72, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Chiarelli, F.; Primavera, M.; Mastromauro, C. Evaluation and management of a child with short stature. Minerva Pediatr. 2021, 72, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Blumer, M.J.F. Bone tissue and histological and molecular events during development of the long bones. Ann. Anat. 2021, 235, 151704. [Google Scholar] [CrossRef]
- Ağırdil, Y. The growth plate: A physiologic overview. EFORT Open Rev. 2020, 5, 498–507. [Google Scholar] [CrossRef]
- Csukasi, F.; Bosakova, M.; Barta, T.; Martin, J.H.; Arcedo, J.; Barad, M.; Rico-Llanos, G.A.; Zieba, J.; Becerra, J.; Krejci, P.; et al. Skeletal diseases caused by mutations in PTH1R show aberrant differentiation of skeletal progenitors due to dysregulation of DEPTOR. Front. Cell Dev. Biol. 2023, 10, 963389. [Google Scholar] [CrossRef]
- Helfrich, M.H. Osteoclast diseases. Microsc. Res. Tech. 2003, 61, 514–532. [Google Scholar] [CrossRef]
- Kronenberg, H.M. Developmental regulation of the growth plate. Nature 2003, 423, 332–336. [Google Scholar] [CrossRef]
- Coates, P. Bone turnover markers. Aust. Fam. Physician 2013, 42, 285–287. [Google Scholar]
- Capulli, M.; Paone, R.; Rucci, N. Osteoblast and osteocyte: Games without frontiers. Arch. Biochem. Biophys. 2014, 561, 3–12. [Google Scholar] [CrossRef]
- Ansari, N.; Sims, N.A. The Cells of Bone and Their Interactions. In Bone Regulators and Osteoporosis Therapy; Springer: Cham, Switzerland, 2019; pp. 1–25. [Google Scholar] [CrossRef]
- Šromová, V.; Sobola, D.; Kaspar, P. A Brief Review of Bone Cell Function and Importance. Cells 2023, 12, 2576. [Google Scholar] [CrossRef] [PubMed]
- Florencio-Silva, R.; Sasso, G.R.S.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. Biomed. Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef] [PubMed]
- Dallas, S.L.; Bonewald, L.F. Dynamics of the transition from osteoblast to osteocyte. Ann. N. Y. Acad. Sci. 2010, 1192, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Greene, C.A.; Green, C.R.; Sherwin, T. Transdifferentiation of chondrocytes into neuron-like cells induced by neuronal lineage specifying growth factors. Cell Biol. Int. 2015, 39, 185–191. [Google Scholar] [CrossRef]
- Kobayashi, T. Indian hedgehog stimulates periarticular chondrocyte differentiation to regulate growth plate length independently of PTHrP. J. Clin. Investig. 2005, 115, 1734–1742. [Google Scholar] [CrossRef]
- Fakhry, M.; Roszkowska, M.; Briolay, A.; Bougault, C.; Guignandon, A.; Diaz-Hernandez, J.I.; Diaz-Hernandez, M.; Pikula, S.; Buchet, R.; Hamade, E.; et al. TNAP stimulates vascular smooth muscle cell trans-differentiation into chondrocytes through calcium deposition and BMP-2 activation: Possible implication in atherosclerotic plaque stability. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2017, 1863, 643–653. [Google Scholar] [CrossRef]
- Chung, U.; Schipani, E.; McMahon, A.P.; Kronenberg, H.M. Indian hedgehog couples chondrogenesis to osteogenesis in endochondral bone development. J. Clin. Investig. 2001, 107, 295–304. [Google Scholar] [CrossRef]
- Chung, U.; Lanske, B.; Lee, K.; Li, E.; Kronenberg, H. The parathyroid hormone/parathyroid hormone-related peptide receptor coordinates endochondral bone development by directly controlling chondrocyte differentiation. Proc. Natl. Acad. Sci. USA 1998, 95, 13030–13035. [Google Scholar] [CrossRef]
- Guasto, A.; Cormier-Daire, V. Signaling Pathways in Bone Development and Their Related Skeletal Dysplasia. Int. J. Mol. Sci. 2021, 22, 4321. [Google Scholar] [CrossRef]
- Wu, S.; Yu, Q.; Lai, A.; Tian, J. Pulsed electromagnetic field induces Ca2+-dependent osteoblastogenesis in C3H10T1/2 mesenchymal cells through the Wnt-Ca2+/Wnt-β-catenin signaling pathway. Biochem. Biophys. Res. Commun. 2018, 503, 715–721. [Google Scholar] [CrossRef]
- Li, X.; Han, Y.; Li, G.; Zhang, Y.; Wang, J.; Feng, C. Role of Wnt signaling pathway in joint development and cartilage degeneration. Front. Cell Dev. Biol. 2023, 11, 1181619. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wan, X.; Le, Q. Cross-regulation between SOX9 and the canonical Wnt signalling pathway in stem cells. Front. Mol. Biosci. 2023, 10, 1250530. [Google Scholar] [CrossRef] [PubMed]
- Mak, K.K.; Chen, M.H.; Day, T.F.; Chuang, P.T.; Yang, Y. Wnt/β-catenin signaling interacts differentially with Ihh signaling in controlling endochondral bone and synovial joint formation. Development 2006, 133, 3695–3707. [Google Scholar] [CrossRef]
- Matsuura, V.K.S.K.; Yoshida, C.A.; Komori, H.; Sakane, C.; Yamana, K.; Jiang, Q.; Komori, T. Expression of a Constitutively Active Form of Hck in Chondrocytes Activates Wnt and Hedgehog Signaling Pathways, and Induces Chondrocyte Proliferation in Mice. Int. J. Mol. Sci. 2020, 21, 2682. [Google Scholar] [CrossRef]
- Chen, G.; Deng, C.; Li, Y.P. TGF-β and BMP Signaling in Osteoblast Differentiation and Bone Formation. Int. J. Biol. Sci. 2012, 8, 272–288. [Google Scholar] [CrossRef]
- Nilsson, O.; Parker, E.A.; Hegde, A.; Chau, M.; Barnes, K.M.; Baron, J. Gradients in bone morphogenetic protein-related gene expression across the growth plate. J. Endocrinol. 2007, 193, 75–84. [Google Scholar] [CrossRef]
- Gründer, T.; Gaissmaier, C.; Fritz, J.; Stoop, R.; Hortschansky, P.; Mollenhauer, J.; Aicher, W.K. Bone morphogenetic protein (BMP)-2 enhances the expression of type II collagen and ACAN in chondrocytes embedded in alginate beads. Osteoarthr. Cartil. 2004, 12, 559–567. [Google Scholar] [CrossRef]
- Minina, E.; Wenzel, H.M.; Kreschel, C.; Karp, S.; Gaffield, W.; McMahon, A.P.; Vortkamp, A. BMP and Ihh/PTHrP signaling interact to coordinate chondrocyte proliferation and differentiation. Development 2001, 128, 4523–4534. [Google Scholar] [CrossRef]
- Lowery, J.W.; Rosen, V. The BMP Pathway and Its Inhibitors in the Skeleton. Physiol. Rev. 2018, 98, 2431–2452. [Google Scholar] [CrossRef]
- Chen, M.; Zhu, M.; Awad, H.; Li, T.F.; Sheu, T.J.; Boyce, B.F.; Chen, D.; O’Keefe, R.J. Inhibition of β-catenin signaling causes defects in postnatal cartilage development. J. Cell Sci. 2008, 121, 1455–1465. [Google Scholar] [CrossRef]
- Fu, H.D.; Wang, H.R.; Li, D.H. BMP-7 accelerates the differentiation of rabbit mesenchymal stem cells into cartilage through the Wnt/β-catenin pathway. Exp. Ther. Med. 2017, 14, 5424–5428. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lavine, K.J.; Hung, I.H.; Ornitz, D.M. FGF18 is required for early chondrocyte proliferation, hypertrophy and vascular invasion of the growth plate. Dev. Biol. 2007, 302, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Maes, C. Signaling pathways effecting crosstalk between cartilage and adjacent tissues. Semin. Cell Dev. Biol. 2017, 62, 16–33. [Google Scholar] [CrossRef] [PubMed]
- Montero, A.; Okada, Y.; Tomita, M.; Ito, M.; Tsurukami, H.; Nakamura, T.; Doetschman, T.; Coffin, J.D.; Hurley, M.M. Disruption of the fibroblast growth factor-2 gene results in decreased bone mass and bone formation. J. Clin. Investig. 2000, 105, 1085–1093. [Google Scholar] [CrossRef]
- Santos, F.; Fuente, R.; Mejia, N.; Mantecon, L.; Gil-Peña, H.; Ordoñez, F.A. Hypophosphatemia and growth. Pediatr. Nephrol. 2013, 28, 595–603. [Google Scholar] [CrossRef]
- Simic, P.; Babitt, J.L. Regulation of FGF23: Beyond Bone. Curr. Osteoporos. Rep. 2021, 19, 563–573. [Google Scholar] [CrossRef]
- Fuente, R.; García-Bengoa, M.; Fernández-Iglesias, Á.; Gil-Peña, H.; Santos, F.; López, J.M. Cellular and Molecular Alterations Underlying Abnormal Bone Growth in X-Linked Hypophosphatemia. Int. J. Mol. Sci. 2022, 23, 934. [Google Scholar] [CrossRef]
- Ornitz, D.M.; Itoh, N. Fibroblast growth factors. Genome Biol. 2001, 2, reviews3005.1. [Google Scholar] [CrossRef]
- Wang, Y.; Menendez, A.; Fong, C.; ElAlieh, H.Z.; Chang, W.; Bikle, D.D. Ephrin B2/EphB4 mediates the actions of IGF-I signaling in regulating endochondral bone formation. J. Bone Miner. Res. 2014, 29, 1900–1913. [Google Scholar] [CrossRef]
- Yakar, S.; Isaksson, O. Regulation of skeletal growth and mineral acquisition by the GH/IGF-1 axis: Lessons from mouse models. Growth Horm. IGF Res. 2016, 28, 26–42. [Google Scholar] [CrossRef]
- Scuruchi, M.; Aliquò, F.; Avenoso, A.; Mandraffino, G.; Vermiglio, G.; Minuti, A.; Campo, S.; Campo, G.M.; D’ascola, A. Endocan Knockdown Down-Regulates the Expression of Angiogenesis-Associated Genes in Il-1ß Activated Chondrocytes. Biomolecules 2023, 13, 851. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Maes, C.; Stockmans, I.; Moermans, K.; Van Looveren, R.; Smets, N.; Carmeliet, P.; Bouillon, R.; Carmeliet, G. Soluble VEGF isoforms are essential for establishingepiphyseal vascularization and regulating chondrocyte development and survival. J. Clin. Investig. 2004, 113, 188–199. [Google Scholar] [CrossRef]
- Zelzer, E.; Mamluk, R.; Ferrara, N.; Johnson, R.S.; Schipani, E.; Olsen, B.R. VEGFA is necessary for chondrocyte survival during bone development. Development 2004, 131, 2161–2171. [Google Scholar] [CrossRef] [PubMed]
- Ornitz, D.M.; Itoh, N. New developments in the biology of fibroblast growth factors. WIREs Mech. Dis. 2022, 14, e1549. [Google Scholar] [CrossRef] [PubMed]
- Leire, E.; Olson, J.; Isaacs, H.; Nizet, V.; Hollands, A. Role of hypoxia inducible factor-1 in keratinocyte inflammatory response and neutrophil recruitment. J. Inflamm. 2013, 10, 28. [Google Scholar] [CrossRef]
- Schipani, E.; Ryan, H.E.; Didrickson, S.; Kobayashi, T.; Knight, M.; Johnson, R.S. Hypoxia in cartilage: HIF-1α is essential for chondrocyte growth arrest and survival. Genes Dev. 2001, 15, 2865–2876. [Google Scholar] [CrossRef]
- Mayer, H.; Bertram, H.; Lindenmaier, W.; Korff, T.; Weber, H.; Weich, H. Vascular endothelial growth factor (VEGF-A) expression in human mesenchymal stem cells: Autocrine and paracrine role on osteoblastic and endothelial differentiation. J. Cell. Biochem. 2005, 95, 827–839. [Google Scholar] [CrossRef]
- Stickens, D.; Behonick, D.J.; Ortega, N.; Heyer, B.; Hartenstein, B.; Yu, Y.; Fosang, A.J.; Schorpp-Kistner, M.; Angel, P.; Werb, Z. Altered endochondral bone development in matrix metalloproteinase 13-deficient mice. Development 2004, 131, 5883–5895. [Google Scholar] [CrossRef]
- Ferrara, N.; Gerber, H.P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef]
- Grosso, A.; Lunger, A.; Burger, M.G.; Briquez, P.S.; Mai, F.; Hubbell, J.A.; Schaefer, D.J.; Banfi, A.; Di Maggio, N. VEGF dose controls the coupling of angiogenesis and osteogenesis in engineered bone. NPJ Regen. Med. 2023, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Street, J.; Bao, M.; de Guzman, L.; Bunting, S.; Peale, F.V., Jr.; Ferrara, N.; Steinmetz, H.; Hoeffel, J.; Cleland, J.L.; Daugherty, A.; et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc. Natl. Acad. Sci. USA 2002, 99, 9656–9661. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Tsang, K.Y.; Tang, H.C.; Chan, D.; Cheah, K.S. HC can become osteoblasts and osteocytes in endochondral bone formation. Proc. Natl. Acad. Sci. USA 2014, 111, 12097–12102. [Google Scholar] [CrossRef] [PubMed]
- Mi, M.; Jin, H.; Wang, B.; Yukata, K.; Sheu, T.J.; Ke, Q.H.; Tong, P.; Im, H.J.; Xiao, G.; Chen, D. Chondrocyte BMP2 signaling plays an essential role in bone fracture healing. Gene 2013, 512, 211–218. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, T.L.; Li, X.; Guo, Y. Functional analyses reveal the essential role of SOX6 and RUNX2 in the communication of chondrocyte and osteoblast. Osteoporos. Int. 2015, 26, 553–561. [Google Scholar] [CrossRef]
- Yue, R.; Shen, B.; Morrison, S.J. Clec11a/osteolectin is an osteogenic growth factor that promotes the maintenance of the adult skeleton. eLife 2016, 5, e18782. [Google Scholar] [CrossRef]
- Ishikawa, M.; Yamada, Y. The Role of Pannexin 3 in Bone Biology. J. Dent. Res. 2017, 96, 372–379. [Google Scholar] [CrossRef]
- Lu, C.; Wan, Y.; Cao, J.; Zhu, X.; Yu, J.; Zhou, R.; Yao, Y.; Zhang, L.; Zhao, H.; Li, H.; et al. Wnt-mediated reciprocal regulation between cartilage and bone development during endochondral ossification. Bone 2013, 53, 566–574. [Google Scholar] [CrossRef]
- Qin, X.; Jiang, Q.; Komori, H.; Sakane, C.; Fukuyama, R.; Matsuo, Y.; Ito, K.; Miyazaki, T.; Komori, T. Runt-related transcription factor-2 (RUNX2) is required for bone matrix protein gene expression in committed osteoblasts in mice. J. Bone Miner. Res. 2021, 36, 2081–2095. [Google Scholar] [CrossRef]
- Liu, Q.; Li, M.; Wang, S.; Xiao, Z.; Xiong, Y.; Wang, G. Recent Advances of Osterix Transcription Factor in Osteoblast Differentiation and Bone Formation. Front. Cell Dev. Biol. 2020, 8, 601224. [Google Scholar] [CrossRef]
- Marcadet, L.; Bouredji, Z.; Argaw, A.; Frenette, J. The Roles of RANK/RANKL/OPG in Cardiac, Skeletal, and Smooth Muscles in Health and Disease. Front. Cell Dev. Biol. 2022, 10, 903657. [Google Scholar] [CrossRef] [PubMed]
- Maurizi, A.; Rucci, N. The Osteoclast in Bone Metastasis: Player and Target. Cancers 2018, 10, 218. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Jing, J.; Ye, L.; Liu, X.; Harris, S.E.; Hinton, R.J.; Feng, J.Q. Chondrogenesis and osteogenesis are one continuous developmental and lineage defined biological process. Sci. Rep. 2017, 7, 10020. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Piatetzky-Shapiro, I.I.; Petrakova, K.V. Osteogenesis in transplants of bone marrow cells. J. Embryol. Exp. Morphol. 1966, 16, 381–390. [Google Scholar] [CrossRef]
- Bonewald, L.F. The amazing osteocyte. J. Bone Miner. Res. 2011, 26, 229–238. [Google Scholar] [CrossRef]
- Xu, J.; Yu, L.; Liu, F.; Wan, L.; Deng, Z. The effect of cytokines on osteoblasts and osteoclasts in bone remodeling in osteoporosis: A review. Front. Immunol. 2023, 14, 1222129. [Google Scholar] [CrossRef]
- Inada, M.; Wang, Y.; Byrne, M.H.; Rahman, M.U.; Miyaura, C.; López-Otín, C.; Krane, S.M. Critical roles for collagenase-3 (Mmp13) in development of growth plate cartilage and in endochondral ossification. Proc. Natl. Acad. Sci. USA 2004, 101, 17192–17197. [Google Scholar] [CrossRef]
- Docheva, D.; Popov, C.; Alberton, P.; Aszodi, A. Integrin signaling in skeletal development and function. Birth Defects Res. C Embryo Today 2014, 102, 13–36. [Google Scholar] [CrossRef]
- Rellmann, Y.; Eidhof, E.; Hansen, U.; Fleischhauer, L.; Vogel, J.; Clausen-Schaumann, H.; Aszodi, A.; Dreier, R. ER Stress in ERp57 Knockout Knee Joint Chondrocytes Induces Osteoarthritic Cartilage Degradation and Osteophyte Formation. Int. J. Mol. Sci. 2021, 23, 182. [Google Scholar] [CrossRef]
- Winkler, D.G.; Sutherland, M.K.; Geoghegan, J.C.; Yu, C.; Hayes, T.; Skonier, J.E.; Shpektor, D.; Jonas, M.; Kovacevich, B.R.; Staehling-Hampton, K.; et al. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J. 2003, 22, 6267–6276. [Google Scholar] [CrossRef]
- Kusu, N.; Laurikkala, J.; Imanishi, M.; Usui, H.; Konishi, M.; Miyake, A.; Thesleff, I.; Itoh, N. Sclerostin is a novel secreted osteoclast-derived bone morphogenetic protein antagonist with unique ligand specificity. J Biol. Chem. 2003, 278, 24113–24117. [Google Scholar] [CrossRef] [PubMed]
- Yakar, S.; Werner, H.; Rosen, C.J. Insulin-like growth factors: Actions on the skeleton. J. Mol. Endocrinol. 2018, 61, T115–T137. [Google Scholar] [CrossRef] [PubMed]
- Bonewald, L.F.; Wacker, M.J. FGF23 production by osteocytes. Pediatr. Nephrol. 2013, 28, 563–568. [Google Scholar] [CrossRef] [PubMed]
- van Bezooijen, R.L.; Roelen, B.A.; Visser, A.; van der Wee-Pals, L.; de Wilt, E.; Karperien, M.; Hamersma, H.; Papapoulos, S.E.; Dijke, P.T.; Löwik, C.W. Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J. Exp. Med. 2004, 199, 805–814. [Google Scholar] [CrossRef]
- Elango, J.; Bao, B.; Wu, W. The hidden secrets of soluble RANKL in bone biology. Cytokine 2021, 144, 155559. [Google Scholar] [CrossRef]
- Zhao, C.; Irie, N.; Takada, Y.; Shimoda, K.; Miyamoto, T.; Nishiwaki, T.; Suda, T.; Matsuo, K. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab. 2006, 4, 111–121. [Google Scholar] [CrossRef]
- Maeda, K.; Kobayashi, Y.; Udagawa, N.; Uehara, S.; Ishihara, A.; Mizoguchi, T.; Kikuchi, Y.; Takada, I.; Kato, S.; Kani, S.; et al. Wnt5a-Ror2 signaling between osteoblast-lineage cells and osteoclast precursors enhances osteoclastogenesis. Nat. Med. 2012, 18, 405–412. [Google Scholar] [CrossRef]
- Negishi-Koga, T.; Shinohara, M.; Komatsu, N.; Bito, H.; Kodama, T.; Friedel, R.H.; Takayanagi, H. Suppression of bone formation by osteoclastic expression of semaphorin 4D. Nat. Med. 2011, 17, 1473–1480. [Google Scholar] [CrossRef]
- Marie, P.J.; Cohen-Solal, M. The Expanding Life and Functions of Osteogenic Cells: From Simple Bone-Making Cells to Multifunctional Cells and Beyond. J. Bone Miner. Res. 2018, 33, 199–210. [Google Scholar] [CrossRef]
- Wang, X.; Tian, Y.; Liang, X.; Yin, C.; Huai, Y.; Zhao, Y.; Huang, Q.; Chu, X.; Wang, W.; Qian, A.-R. Bergamottin promotes osteoblast differentiation and bone formation via activating the Wnt/β-catenin signaling pathway. Food Funct. 2022, 13, 2913–2924. [Google Scholar] [CrossRef]
- Li, W.; Xiong, Y.; Chen, W.; Wu, L. Wnt/β-catenin signaling may induce senescence of chondrocytes in osteoarthritis. Exp. Ther. Med. 2020, 20, 2631–2638. [Google Scholar] [CrossRef]
- Lei, J.; Fu, Y.; Zhuang, Y.; Zhang, K.; Lu, D. miR-382-3p suppressed IL-1β induced inflammatory response of chondrocytes via the TLR4/MyD88/NF-κB signaling pathway by directly targeting CX43. J. Cell Physiol. 2019, 234, 23160–23168. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wang, Y.; Huang, P. Association of Four VEGFA Gene Variants with Rheumatoid Arthritis Risk: A Meta-analysis and Trial Sequential Analysis. Biochem. Genet. 2024. [Google Scholar] [CrossRef] [PubMed]
- Karaplis, A.C.; He, B.; Nguyen, M.T.A.; Young, I.D.; Semeraro, D.; Ozawa, H.; Amizuka, N. Inactivating Mutation in the Human Parathyroid Hormone Receptor Type 1 Gene in Blomstrand Chondrodysplasia. Endocrinology 1998, 139, 5255–5258. [Google Scholar] [CrossRef] [PubMed]
- Schipani, E.; Kruse, K.; Jüppner, H. A constitutively active mutant PTH-PTHrP receptor in Jansen-type metaphyseal chondrodysplasia. Science 1995, 268, 98–100. [Google Scholar] [CrossRef]
- Gao, B.; Guo, J.; She, C.; Shu, A.; Yang, M.; Tan, Z.; Yang, X.; Guo, S.; Feng, G.; He, L. Mutations in IHH, encoding Indian hedgehog, cause brachydactyly type A-1. Nat. Genet. 2001, 28, 386–388. [Google Scholar] [CrossRef]
- Hellemans, J.; Coucke, P.J.; Giedion, A.; De Paepe, A.; Kramer, P.; Beemer, F.; Mortier, G.R. Homozygous mutations in IHH cause acrocapitofemoral dysplasia, an autosomal recessive disorder with cone-shaped epiphyses in hands and hips. Am. J. Hum. Genet. 2003, 72, 1040–1046. [Google Scholar] [CrossRef]
- Pignolo, R.J.; Shore, E.M.; Kaplan, F.S. Fibrodysplasia ossificans progressiva: Diagnosis, management, and therapeutic horizons. Pediatr. Endocrinol. Rev. 2013, 10 (Suppl. S2), 437–448. [Google Scholar]
- Blaney Davidson, E.N.; Vitters, E.L.; van der Kraan, P.M.; van den Berg, W.B. Expression of transforming growth factor—(TGF) and the TGF signalling molecule SMAD-2P in spontaneous and instability-induced osteoarthritis: Role in cartilage degradation, chondrogenesis and osteophyte formation. Ann. Rheum. Dis. 2006, 65, 1414–1421. [Google Scholar] [CrossRef]
- Blaney Davidson, E.N.; Scharstuhl, A.; Vitters, E.L.; van der Kraan, P.M.; van den Berg, W.B. Reduced transforming growth factor-beta signaling in cartilage of old mice: Role in impaired repair capacity. Arthritis Res. Ther. 2005, 7, R1338–R1347. [Google Scholar] [CrossRef]
- Song, I.W.; Nagamani, S.C.; Nguyen, D.; Grafe, I.; Sutton, V.R.; Gannon, F.H.; Munivez, E.; Jiang, M.M.; Tran, A.; Wallace, M.; et al. Targeting TGF-β for treatment of osteogenesis imperfecta. J. Clin. Investig. 2022, 132, e152571. [Google Scholar] [CrossRef] [PubMed]
- Cooper, K.L.; Oh, S.; Sung, Y.; Dasari, R.R.; Kirschner, M.W.; Tabin, C.J. Multiple phases of chondrocyte enlargement underlie differences in skeletal proportions. Nature 2013, 495, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Worster, A.A.; Brower-Toland, B.D.; Fortier, L.A.; Bent, S.J.; Williams, J.; Nixon, A.J. Chondrocytic differentiation of mesenchymal stem cells sequentially exposed to transforming growth factor-beta1 in monolayer and insulin-like growth factor-I in a three-dimensional matrix. J. Orthop. Res. 2001, 19, 738–749. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xuan, S.; Bouxsein, M.L.; Von Stechow, D.; Akeno, N.; Faugere, M.C.; Malluche, H.; Zhao, G.; Rosen, C.J.; Efstratiadis, A.; et al. Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. J. Biol. Chem. 2002, 277, 44005–44012. [Google Scholar] [CrossRef]
- Kornak, U.; Mundlos, S. Genetic disorders of the skeleton: A developmental approach. Am. J. Hum. Genet. 2003, 73, 447–474. [Google Scholar] [CrossRef]
- Hodson, E.M.; Willis, N.S.; Craig, J.C. Growth hormone for children with chronic kidney disease. Cochrane Database Syst. Rev. 2012, 2012, CD003264. [Google Scholar] [CrossRef]
- Witkowska-Sędek, E.; Pyrżak, B. Chronic inflammation and the growth hormone/insulin-like growth factor-1 axis. Cent. Eur. J. Immunol. 2020, 45, 469–475. [Google Scholar] [CrossRef]
- Fazeli, P.K.; Klibanski, A. Determinants of GH resistance in malnutrition. J. Endocrinol. 2014, 220, R57–R65. [Google Scholar] [CrossRef]
- Soendergaard, C.; Young, J.; Kopchick, J. Growth Hormone Resistance—Special Focus on Inflammatory Bowel Disease. Int. J. Mol. Sci. 2017, 18, 1019. [Google Scholar] [CrossRef]
- Drube, J.; Wan, M.; Bonthuis, M.; Wühl, E.; Bacchetta, J.; Santos, F.; Grenda, R.; Edefonti, A.; Harambat, J.; Shroff, R.; et al. Clinical practice recommendations for growth hormone treatment in children with chronic kidney disease. Nat. Rev. Nephrol. 2019, 15, 577–589. [Google Scholar] [CrossRef]
- Fernández-Iglesias, Á.; Fuente, R.; Gil-Peña, H.; Alonso-Durán, L.; García-Bengoa, M.; Santos, F.; López, J.M. Innovative Three-Dimensional Microscopic Analysis of Uremic Growth Plate Discloses Alterations in the Process of Chondrocyte Hypertrophy: Effects of Growth Hormone Treatment. Int. J. Mol. Sci. 2020, 21, 4519. [Google Scholar] [CrossRef] [PubMed]
- Mahesh, S.; Kaskel, F. Growth hormone axis in chronic kidney disease. Pediatr. Nephrol. 2008, 23, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, E.A.; Carter, C.E.; Mak, R.H. The Role of Growth Hormone in Chronic Kidney Disease. Semin. Nephrol. 2021, 41, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Ludin, A.; Sela, J.J.; Schroeder, A.; Samuni, Y.; Nitzan, D.W.; Amir, G. Injection of vascular endothelial growth factor into knee joints induces osteoarthritis in mice. Osteoarthr. Cartil. 2013, 21, 491–497. [Google Scholar] [CrossRef]
- Hamilton, J.L.; Nagao, M.; Levine, B.R.; Chen, D.; Olsen, B.R.; Im, H.J. Targeting VEGF and Its Receptors for the Treatment of Osteoarthritis and Associated Pain. J. Bone Miner. Res. 2016, 31, 911–924. [Google Scholar] [CrossRef]
- Pauli, R.M. Achondroplasia: A comprehensive clinical review. Orphanet. J. Rare Dis. 2019, 14, 1. [Google Scholar] [CrossRef]
- Ornitz, D.M.; Legeai-Mallet, L. Achondroplasia: Development, pathogenesis, and therapy. Dev. Dyn. 2017, 246, 291–309. [Google Scholar] [CrossRef]
- Högler, W.; Ward, L.M. New developments in the management of achondroplasia. Wien. Med. Wochenschr. 2020, 170, 104–111. [Google Scholar] [CrossRef]
- Saltarelli, M.A.; Quarta, A.; Chiarelli, F. Growth plate extracellular matrix defects and short stature in children. Ann. Pediatr. Endocrinol. Metab. 2022, 27, 247–255. [Google Scholar] [CrossRef]
- Bechtold, S.; Simon, D. Growth abnormalities in children and adolescents with juvenile idiopathic arthritis. Rheumatol. Int. 2014, 34, 1483–1488. [Google Scholar] [CrossRef]
- MacRae, V.E.; Wong, S.C.; Farquharson, C.; Ahmed, S.F. Cytokine actions in growth disorders associated with pediatric chronic inflammatory diseases. Int. J. Mol. Med. 2006, 18, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. European Medicines Agency. 2024. Evenity. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/evenity (accessed on 1 October 2024).
- Vasiliadis, E.S.; Evangelopoulos, D.S.; Kaspiris, A.; Benetos, I.S.; Vlachos, C.; Pneumaticos, S.G. The Role of Sclerostin in Bone Diseases. J. Clin. Med. 2022, 11, 806. [Google Scholar] [CrossRef] [PubMed]
- Balemans, W.; Van Hul, W. Identification of the disease-causing gene in sclerosteosis—Discovery of a novel bone anabolic targer? J. Musculoskelet. Neuronal Interact. 2004, 4, 139–142. [Google Scholar]
- Komori, T. Regulation of osteoblast differentiation by transcription factors. J. Cell Biochem. 2006, 99, 1233–1239. [Google Scholar] [CrossRef]
- Tintut, Y.; Abedin, M.; Cho, J.; Choe, A.; Lim, J.; Demer, L.L. Regulation of RANKL-induced osteoclastic differentiation by vascular cells. J. Mol. Cell. Cardiol. 2005, 39, 389–393. [Google Scholar] [CrossRef]
- Miyagawa, K.; Tenshin, H.; Mulcrone, P.L.; Delgado-Calle, J.; Subler, M.A.; Windle, J.J.; Chirgwin, J.M.; Roodman, G.D.; Kurihara, N. Osteoclast-derived IGF1 induces RANKL production in osteocytes and contributes to pagetic lesion formation. JCI Insight 2023, 8, e159838. [Google Scholar] [CrossRef]
- Tanaka, S.; Tanaka, Y. RANKL as a therapeutic target of rheumatoid arthritis. J. Bone Miner. Metab. 2021, 39, 106–112. [Google Scholar] [CrossRef]
- Hadji, P.; Papaioannou, N.; Gielen, E.; Tepie, M.F.; Zhang, E.; Frieling, I.; Geusens, P.; Makras, P.; Resch, H.; Möller, G.; et al. Persistence, adherence, and medication-taking behavior in women with postmenopausal osteoporosis receiving denosumab in routine practice in Germany, Austria, Greece, and Belgium: 12-month results from a European non-interventional study. Osteoporos. Int. 2015, 26, 2479–2489. [Google Scholar] [CrossRef]
- Wöhrle, S.; Henninger, C.; Bonny, O.; Thuery, A.; Beluch, N.; E Hynes, N.; Guagnano, V.; Sellers, W.R.; Hofmann, F.; Kneissel, M.; et al. Pharmacological inhibition of fibroblast growth factor (FGF) receptor signaling ameliorates FGF23-mediated hypophosphatemic rickets. J. Bone Miner. Res. 2013, 28, 899–911. [Google Scholar] [CrossRef]
- Ünsal, Y.; Atar, S. Evaluation of Clinical Characteristics and Growth Hormone Response in a Rare Skeletal Dysplasia: Pycnodysostosis. Cureus 2023, 15, e44823. [Google Scholar] [CrossRef]
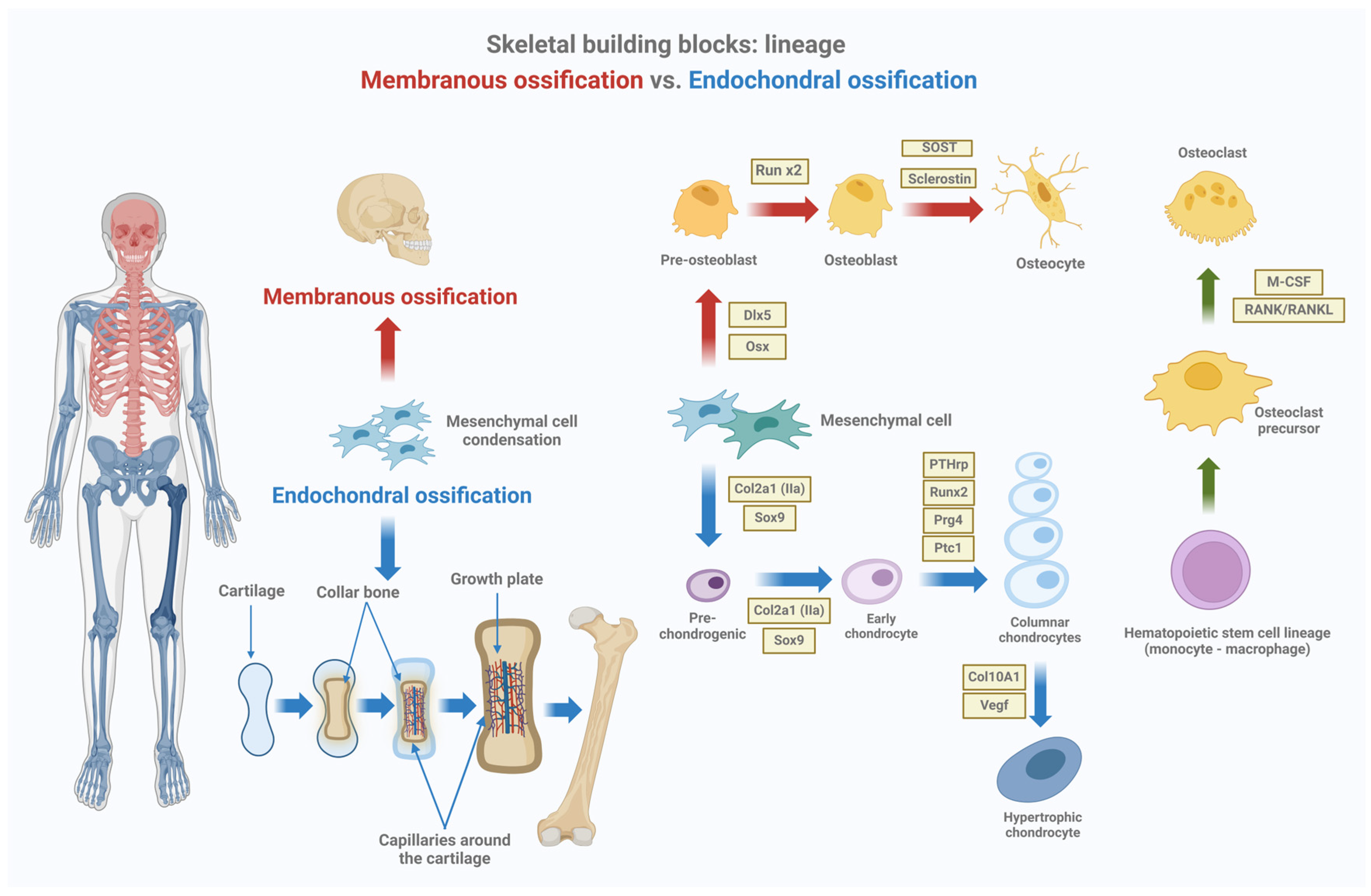
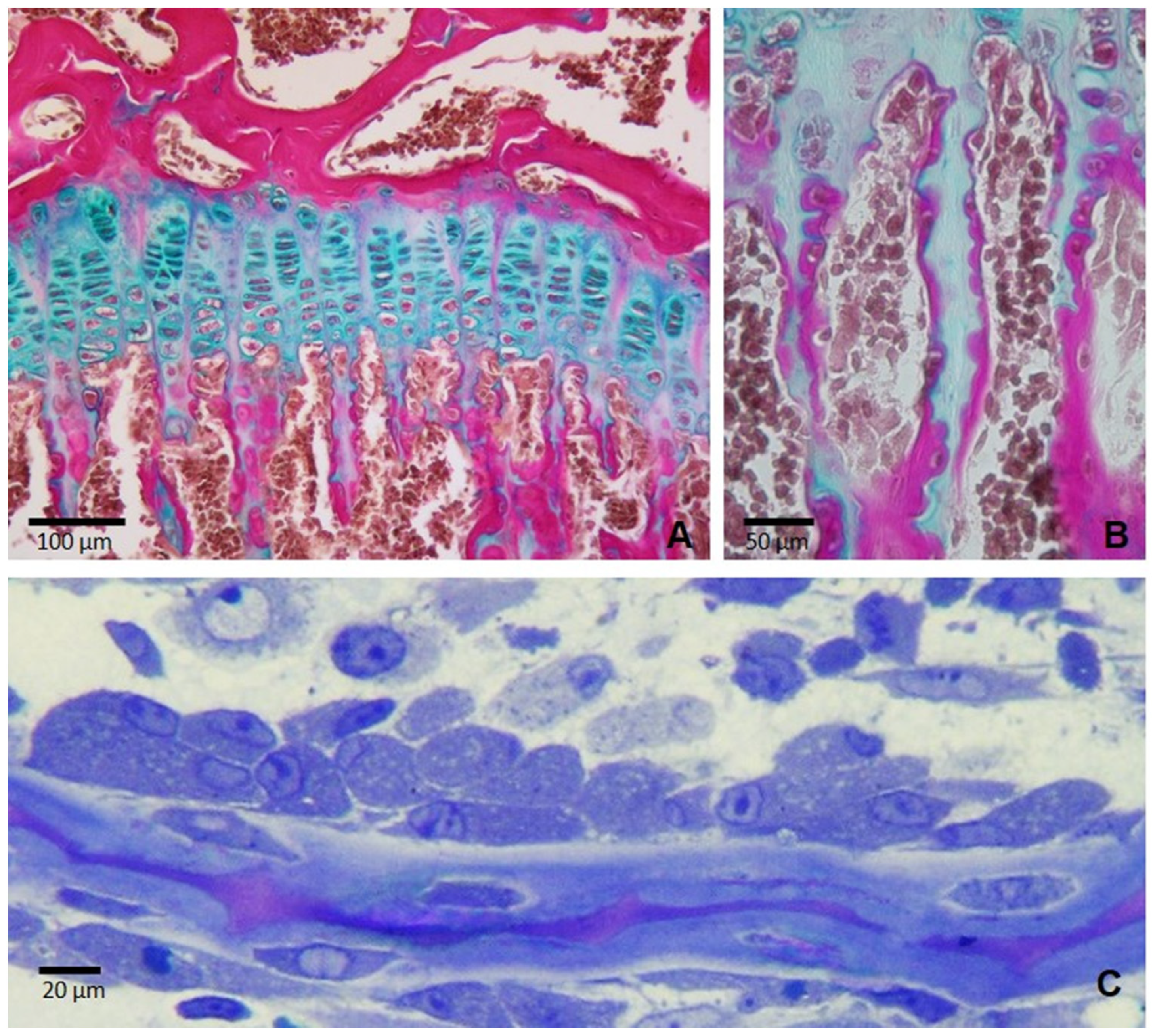




| Cell Type | Role in Bone Growth and Remodeling | Key Markers |
|---|---|---|
| Chondrocytes | Primary cells in growth plate; produce and maintain cartilage matrix, undergo proliferation, hypertrophy, and apoptosis | SOX 9, COL2A1, and ACAN (early development); Col10a1, RUNX2, and MMP13 (hypertrophy and bone formation) |
| Osteoblasts | Bone-forming cells derived from MSCs; synthesize organic bone matrix and facilitate mineralization | RUNX2, Osx, Col1, ALPL, OCN, PHOSPHO-1, PTH1R, MEPE, DMP1, PHEX, SOST, and FGF23 |
| Osteoclasts | Multinucleated cells from hematopoietic stem cells; responsible for bone resorption | RANK, TRAF6, CTSK, and TRAP |
| Osteocytes | Mature osteoblasts embedded in bone matrix; act as mechanosensors and regulate bone formation and resorption | OPN, DMP1, BSP, OPG, SOST, FGF23, and MEPE |
| Vascular cells | Support vascular invasion in hypertrophic zone, deliver nutrients and osteoprogenitor cells, and are necessary for ossification | CD34 and CD31 (endothelial cells); CD133, CXCR4, and VEGFR-2 (endothelial progenitor cells) |
| Involved Pathway | Disease | Genetic Origin | Clinical Manifestations | Authorized Therapy |
|---|---|---|---|---|
| PTH/PTHrP | Blomstrand Chondro-osteodystrophy | PTH1R gene | Prenatal lethal disorder Shortened limbs Premature ossification of bones | No |
| Jansen Metaphyseal Chondrodysplasia | Abnormal bone growth Short stature Hypercalcemia Metaphyseal widening | Calcium management | ||
| Ihh | Brachydactyly Type A-1 | Ihh gene | Shortened/absent middle phalanges | No |
| Acrocapitofemoral Dysplasia | Short stature Brachydactyly Narrow thorax Short limbs | No | ||
| FGF | Achondroplasia | FGFR3 gene | Shortened limbs Large head Spinal stenosis Hypoplasia of the foramen magnum | Anti-VEGF Anti-FGFR3 Orthopedic surgery |
| XLH | PHEX gene | Rickets Bone pain Skeletal deformities Impaired bone mineralization | Phosphate supplementation Active vitamin D analogues Anti-FGF23 * Orthopedic surgery | |
| VEGF | OA | VEGFA gene [SNP] | Joint pain Cartilage loss Decreased joint function | No Anti-VEGF * |
| RA | VEGFA gene [SNPs] [86] | Tenderness Nocturnal pain Limited joint motion | Anti-VEGF | |
| BMP/TGFβ | FOP | ACVR1 gene | Progressive ossification of soft tissues Restricted movement Joint pain | No Anti- BMP * |
| OA | ALDH1A2, COLGALT2, GDF5, MGP, NCOA3 PLEC, RUNX2, RWDD2B, TGFB1, and WNT9A genes [Methylation: SUPT3H, NCOA3, and DOT1L genes] | Joint pain Cartilage loss Decreased joint function | Anti-TGFβ * | |
| OI | COL1A2 gene | Fragile bones Fractures | Anti-TGFβ | |
| GH/IGF-1 | PIGFD | IGF1 gene | Growth failure Delayed bone growth | IGF-1 supplementation |
| CKD-MBD | Secondary to primary or acquired disorders | Bone formation deficits Growth retardation Secondary hyperparathyroidism | Phosphate control GH therapy | |
| Wnt/β-catenin | CCD | RUNX2 gene | Underdeveloped clavicles Dental abnormalities Delayed bone ossification | No Orthopedic surgery |
| Sclerosteosis and Van Buchem Disease | SOST gene | Excessive bone growth Thickened skull Neural complications | No Anti-sclerostin * | |
| OA | ALDH1A2, COLGALT2, GDF5, MGP, NCOA3 PLEC, RUNX2, RWDD2B, TGFB1, and WNT9A genes [Epigenetic association: SUPT3H, NCOA3, and DOT1L genes] | Joint pain Cartilage loss Decreased joint function | NSAIDs Anti-sclerostin * | |
| RANK/RANKL/OPG | Paget’s Disease of Bone | RANKL gene | Pain Enlarged and deformed bones Increased risk of fractures | Bisphosphonates Calcitonin RANKL inhibitors |
| Osteopetrosis | TCIRG1, CLCN7, OSTM1, PLEKHM1, SNX10, TNFSF11, and TNFRSF11A genes | Increased bone density Brittle bones Nerve compression issues | Bone marrow transplant | |
| Pycnodysostosis | CTSK gene | Osteosclerosis Fractures Short stature Increased bone density | GH therapy Orthopedic surgery |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-García, F.; Fernández-Iglesias, Á.; Rodríguez Suárez, J.; Gil Peña, H.; López, J.M.; Pérez, R.F. The Crosstalk Between Cartilage and Bone in Skeletal Growth. Biomedicines 2024, 12, 2662. https://doi.org/10.3390/biomedicines12122662
Hernández-García F, Fernández-Iglesias Á, Rodríguez Suárez J, Gil Peña H, López JM, Pérez RF. The Crosstalk Between Cartilage and Bone in Skeletal Growth. Biomedicines. 2024; 12(12):2662. https://doi.org/10.3390/biomedicines12122662
Chicago/Turabian StyleHernández-García, Frank, Ángela Fernández-Iglesias, Julián Rodríguez Suárez, Helena Gil Peña, José M. López, and Rocío Fuente Pérez. 2024. "The Crosstalk Between Cartilage and Bone in Skeletal Growth" Biomedicines 12, no. 12: 2662. https://doi.org/10.3390/biomedicines12122662
APA StyleHernández-García, F., Fernández-Iglesias, Á., Rodríguez Suárez, J., Gil Peña, H., López, J. M., & Pérez, R. F. (2024). The Crosstalk Between Cartilage and Bone in Skeletal Growth. Biomedicines, 12(12), 2662. https://doi.org/10.3390/biomedicines12122662








