Sex-Related Differences in Heart Failure Development in Patients After First Myocardial Infarction: The Role of Galectin-3
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Laboratory Analysis
2.3. Echocardiography
2.4. Coronary Angiography, Percutaneous Coronary Intervention and Medication
2.5. Reverse Transcripion–Quantitative Real-Time PCR (RT-qPCR)
2.6. Quantification of pGal-3 Levels
2.7. Statistical Analysis
3. Results
3.1. Sex Differences Between Clinical, Doppler Echocardiographic and Angiographic Data
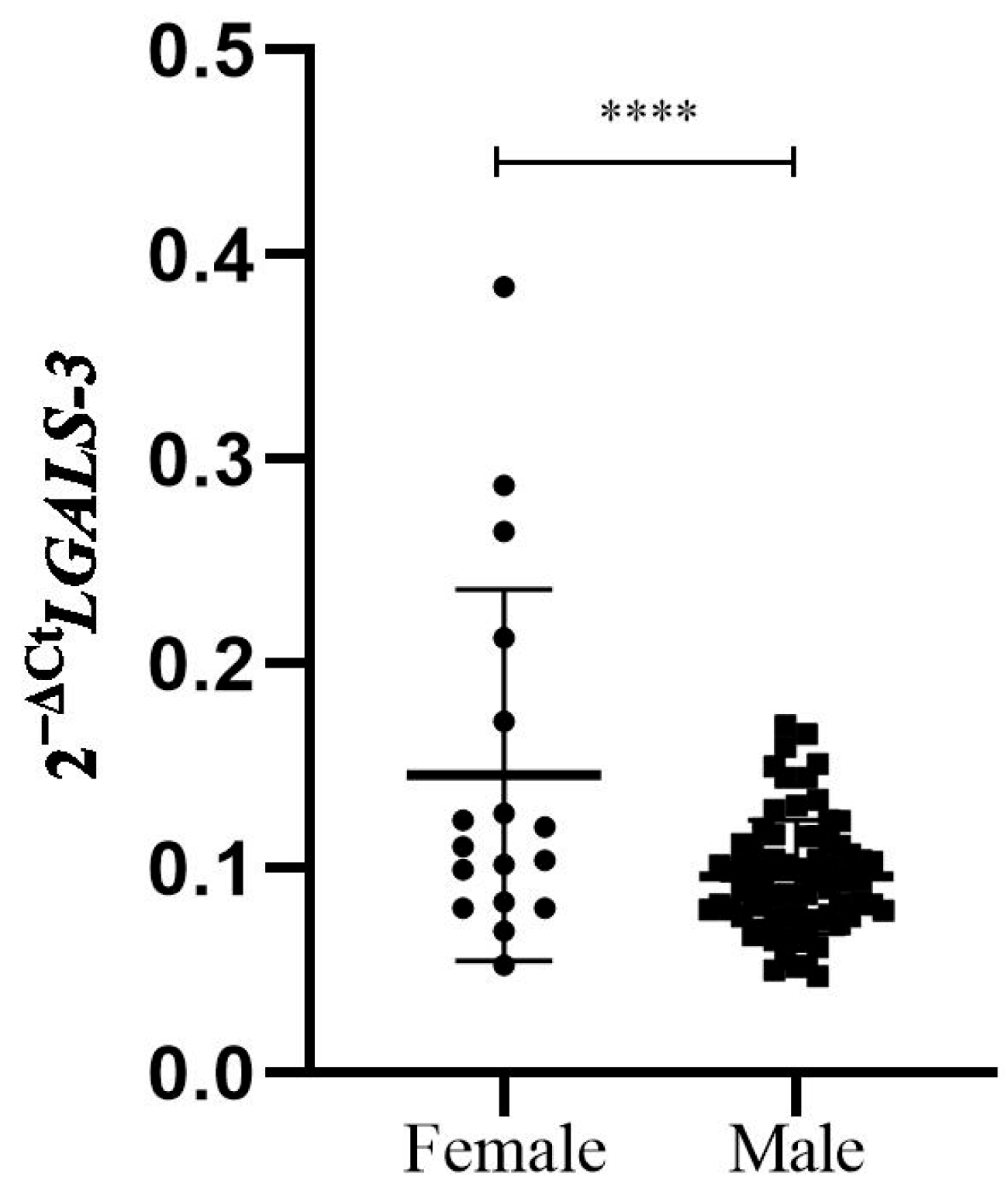
3.2. Sex Differences Between Cardiac Biomarkers in Study Patients
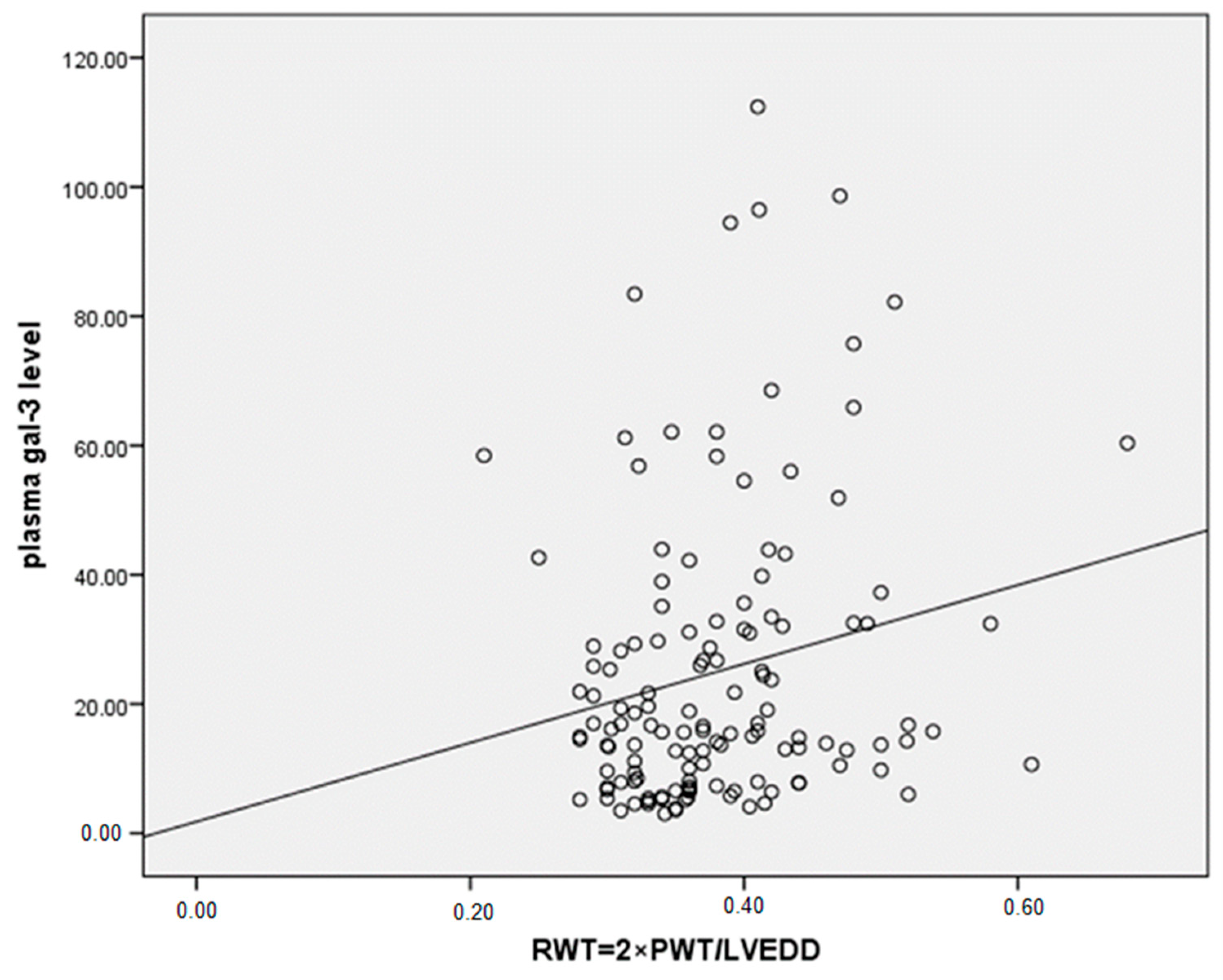
3.3. Correlations Between Gal-3 Levels and Parameters of LV Remodeling and Function, with Sex Differences
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bucholz, E.M.; Butala, N.M.; Rathore, S.S.; Dreyer, R.P.; Lansky, A.J.; Krumholz, H.M. Sex differences in long-term mortality after myocardial infarction: A systematic review. Circulation 2014, 130, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Pancholy, S.B.; Shantha, G.P.; Patel, T.; Cheskin, L.J. Sex differences in short-term and long-term all-cause mortality among patients with ST-segment elevation myocardial infarction treated by primary percutaneous intervention: A meta-analysis. JAMA Intern. Med. 2014, 174, 1822–1830. [Google Scholar] [CrossRef] [PubMed]
- Dewan, P.; Rørth, R.; Jhund, P.S.; Shen, L.; Raparelli, V.; Petrie, M.C.; Abraham, W.T.; Desai, A.S.; Dickstein, K.; Køber, L.; et al. Differential Impact of Heart Failure with Reduced Ejection Fraction on Men and Women. J. Am. Coll. Cardiol. 2019, 73, 29–40. [Google Scholar] [CrossRef]
- Duca, F.; Zotter-Tufaro, C.; Kammerlander, A.A.; Aschauer, S.; Binder, C.; Mascherbauer, J.; Bonderman, D. Gender-related differences in heart failure with preserved ejection fraction. Sci. Rep. 2018, 8, 1080. [Google Scholar] [CrossRef] [PubMed]
- De Boer, R.A.; Verweij, N.; van Veldhuisen, D.J.; Westra, H.J.; Bakker, S.J.; Gansevoort, R.T.; Muller Kobold, A.C.; van Gilst, W.H.; Franke, L.; Mateo Leach, I.; et al. A genome-wide association study of circulating galectin-3. PLoS ONE 2012, 7, e47385. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef]
- Ho, J.E.; Liu, C.; Lyass, A.; Courchesne, P.; Pencina, M.J.; Vasan, R.S.; Larson, M.G.; Levy, D. Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J. Am. Coll. Cardiol. 2012, 60, 1249–1256. [Google Scholar] [CrossRef]
- Melin, E.O.; Dereke, J.; Hillman, M. Female sex, high soluble CD163, and low HDL-cholesterol were associated with high galectin-3 binding protein in type 1 diabetes. Biol. Sex. Differ. 2019, 10, 51. [Google Scholar] [CrossRef]
- Steg, P.G.; James, S.K.; Atar, D.; Badano, L.P.; Blömstrom-Lundqvist, C.; Borger, M.A.; Di Mario, C.; Dickstein, K.; Ducrocq, G.; Fernandez-Aviles, F.; et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC). Eur. Heart J. 2012, 33, 2569–2619. [Google Scholar] [CrossRef]
- Nagueh, S.F. Classification of Left Ventricular Diastolic Dysfunction and Heart Failure Diagnosis and Prognosis. J. Am. Soc. Echocardiogr. 2018, 31, 1209–1211. [Google Scholar] [CrossRef] [PubMed]
- Geyer, H.; Caracciolo, G.; Abe, H.; Wilansky, S.; Carerj, S.; Gentile, F.; Nesser, H.J.; Khandheria, B.; Narula, J.; Sengupta, P.P. Assessment of myocardial mechanics using speckle tracking echocardiography: Fundamentals and clinical applications. J. Am. Soc. Echocardiogr. 2010, 23, 351–369, quiz 453–455. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef] [PubMed]
- Partow-Navid, R.; Prasitlumkum, N.; Mukherjee, A.; Varadarajan, P.; Pai, R.G. Management of ST Elevation Myocardial Infarction (STEMI) in Different Settings. Int. J. Angiol. 2021, 30, 67–75. [Google Scholar] [CrossRef]
- Bundhun, P.K.; Sookharee, Y.; Bholee, A.; Huang, F. Application of the SYNTAX score in interventional cardiology: A systematic review and meta-analysis. Medicine 2017, 96, e7410. [Google Scholar] [CrossRef]
- Djordjevic, A.; Zivkovic, M.; Stankovic, A.; Zivotic, I.; Koncar, I.; Davidovic, L.; Alavantic, D.; Djuric, T. Genetic Variants in the Vicinity of LGALS-3 Gene and LGALS-3 mRNA Expression in Advanced Carotid Atherosclerosis: An Exploratory Study. J. Clin. Lab. Anal. 2016, 30, 1150–1157. [Google Scholar] [CrossRef]
- Djordjevic, A.; Zivkovic, M.; Boskovic, M.; Dekleva, M.; Stankovic, G.; Stankovic, A.; Djuric, T. Variants Tagging LGALS-3 Haplotype Block in Association with First Myocardial Infarction and Plasma Galectin-3 Six Months after the Acute Event. Genes 2022, 14, 109. [Google Scholar] [CrossRef] [PubMed]
- De Boer, R.A.; van Veldhuisen, D.J.; Gansevoort, R.T.; Muller Kobold, A.C.; van Gilst, W.H.; Hillege, H.L.; Bakker, S.J.; van der Harst, P. The fibrosis marker galectin-3 and outcome in the general population. J. Intern. Med. 2012, 272, 55–64. [Google Scholar] [CrossRef]
- Garcia, M.; Mulvagh, S.L.; Merz, C.N.; Buring, J.E.; Manson, J.E. Cardiovascular Disease in Women: Clinical Perspectives. Circ. Res. 2016, 118, 1273–1293. [Google Scholar] [CrossRef]
- Wenger, N.K. Adverse Cardiovascular Outcomes for Women-Biology, Bias, or Both? JAMA Cardiol. 2020, 5, 27–28. [Google Scholar] [CrossRef]
- Mallat, Z.; Fornes, P.; Costagliola, R.; Esposito, B.; Belmin, J.; Lecomte, D.; Tedgui, A. Age and gender effects on cardiomyocyte apoptosis in the normal human heart. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M719–M723. [Google Scholar] [CrossRef] [PubMed]
- Canali, E.; Masci, P.; Bogaert, J.; Bucciarelli Ducci, C.; Francone, M.; McAlindon, E.; Carbone, I.; Lombardi, M.; Desmet, W.; Janssens, S.; et al. Impact of gender differences on myocardial salvage and post-ischaemic left ventricular remodelling after primary coronary angioplasty: New insights from cardiovascular magnetic resonance. Eur. Heart J. Cardiovasc. Imaging 2012, 13, 948–953. [Google Scholar] [CrossRef]
- Biondi-Zoccai, G.G.; Abate, A.; Bussani, R.; Camilot, D.; Giorgio, F.D.; Marino, M.P.; Silvestri, F.; Baldi, F.; Biasucci, L.M.; Baldi, A. Reduced post-infarction myocardial apoptosis in women: A clue to their different clinical course? Heart 2005, 91, 99–101. [Google Scholar] [CrossRef] [PubMed]
- Patten, R.D. Models of Gender Differences in Cardiovascular Disease. Drug Discov. Today Dis. Models 2007, 4, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.F.; Claggett, B.L.; McMurray, J.J.V.; Packer, M.; Lefkowitz, M.P.; Rouleau, J.L.; Liu, J.; Shi, V.C.; Zile, M.R.; Desai, A.S.; et al. Health-Related Quality of Life Outcomes in PARADIGM-HF. Circ. Heart Fail. 2017, 10, e003430. [Google Scholar] [CrossRef]
- Simpson, J.; Jhund, P.S.; Lund, L.H.; Padmanabhan, S.; Claggett, B.L.; Shen, L.; Petrie, M.C.; Abraham, W.T.; Desai, A.S.; Dickstein, K.; et al. Prognostic Models Derived in PARADIGM-HF and Validated in ATMOSPHERE and the Swedish Heart Failure Registry to Predict Mortality and Morbidity in Chronic Heart Failure. JAMA Cardiol. 2020, 5, 432–441. [Google Scholar] [CrossRef]
- Chimed, S.; van der Bijl, P.; Lustosa, R.; Fortuni, F.; Montero-Cabezas, J.M.; Ajmone Marsan, N.; Gersh, B.J.; Delgado, V.; Bax, J.J. Functional classification of left ventricular remodelling: Prognostic relevance in myocardial infarction. ESC Heart Fail. 2022, 9, 912–924. [Google Scholar] [CrossRef]
- Sofia, R.R.; Serra, A.J.; Silva, J.A., Jr.; Antonio, E.L.; Manchini, M.T.; Oliveira, F.A.; Teixeira, V.P.; Tucci, P.J. Gender-based differences in cardiac remodeling and ILK expression after myocardial infarction. Arq. Bras. Cardiol. 2014, 103, 124–130. [Google Scholar] [CrossRef]
- Grandin, E.W.; Jarolim, P.; Murphy, S.A.; Ritterova, L.; Cannon, C.P.; Braunwald, E.; Morrow, D.A. Galectin-3 and the development of heart failure after acute coronary syndrome: Pilot experience from PROVE IT-TIMI 22. Clin. Chem. 2012, 58, 267–273. [Google Scholar] [CrossRef]
- Suthahar, N.; Meijers, W.C.; Silljé, H.H.W.; Ho, J.E.; Liu, F.T.; de Boer, R.A. Galectin-3 Activation and Inhibition in Heart Failure and Cardiovascular Disease: An Update. Theranostics 2018, 8, 593–609. [Google Scholar] [CrossRef]
- Kirk, J.A.; Frangogiannis, N.G. Galectin-3 in the pathogenesis of heart failure: A causative mediator or simply a biomarker? Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H1256–H1258. [Google Scholar] [CrossRef] [PubMed]
- Kosmidou, I.; Redfors, B.; Selker, H.P.; Thiele, H.; Patel, M.R.; Udelson, J.E.; Magnus Ohman, E.; Eitel, I.; Granger, C.B.; Maehara, A.; et al. Infarct size, left ventricular function, and prognosis in women compared to men after primary percutaneous coronary intervention in ST-segment elevation myocardial infarction: Results from an individual patient-level pooled analysis of 10 randomized trials. Eur. Heart J. 2017, 38, 1656–1663. [Google Scholar] [CrossRef] [PubMed]
- Leistner, D.M.; Dietrich, S.; Erbay, A.; Steiner, J.; Abdelwahed, Y.; Siegrist, P.T.; Schindler, M.; Skurk, C.; Haghikia, A.; Sinning, D.; et al. Association of left ventricular end-diastolic pressure with mortality in patients undergoing percutaneous coronary intervention for acute coronary syndromes. Catheter. Cardiovasc. Interv. 2020, 96, E439–E446. [Google Scholar] [CrossRef] [PubMed]
- Ansari, U.; Behnes, M.; Hoffmann, J.; Natale, M.; Fastner, C.; El-Battrawy, I.; Rusnak, J.; Kim, S.H.; Lang, S.; Hoffmann, U.; et al. Galectin-3 Reflects the Echocardiographic Grades of Left Ventricular Diastolic Dysfunction. Ann. Lab. Med. 2018, 38, 306–315. [Google Scholar] [CrossRef]
- Suarez, G.; Meyerrose, G. Heart failure and galectin 3. Ann. Transl. Med. 2014, 2, 86. [Google Scholar] [CrossRef]
- Wu, C.K.; Lee, J.K.; Chiang, F.T.; Yang, C.H.; Huang, S.W.; Hwang, J.J.; Lin, J.L.; Tseng, C.D.; Chen, J.J.; Tsai, C.T. Plasma levels of tumor necrosis factor-α and interleukin-6 are associated with diastolic heart failure through downregulation of sarcoplasmic reticulum Ca2+ ATPase. Crit. Care Med. 2011, 39, 984–992. [Google Scholar] [CrossRef]
- D’Onofrio, G.; Safdar, B.; Lichtman, J.H.; Strait, K.M.; Dreyer, R.P.; Geda, M.; Spertus, J.A.; Krumholz, H.M. Sex differences in reperfusion in young patients with ST-segment-elevation myocardial infarction: Results from the VIRGO study. Circulation 2015, 131, 1324–1332. [Google Scholar] [CrossRef]
- Mendirichaga, R.; Jacobs, A.K. Sex Differences in Ischemic Heart Disease-the Paradox Persists. JAMA Cardiol. 2020, 5, 754–756. [Google Scholar] [CrossRef]



| Variable | Gender | p Value | |
|---|---|---|---|
| Male (n = 137) | Female (n = 44) | ||
| Age (years) | 54.8 ± 8.2 | 57.8 ± 7.6 | 0.034 |
| Hypertension, n (%) | 78 (57.8) | 25 (58.1) | 0.967 |
| Cigarette smoking, n (%) | 82 (62.6) | 25 (62.5) | 0.991 |
| Diabetes mellitus, n (%) | 48 (34.8) | 14 (31.8) | 0.718 |
| Family history, n (%) | 80 (62.5) | 27 (67.5) | 0.566 |
| BMI (kg/m2) | 27.8 ± 3.6 | 26.7 ± 4.4 | 0.125 |
| NSTEMI, n (%) | 28 (20.7) | 7 (15.9) | 0.483 |
| Advanced Killip class, n (%) | 15 (11.6) | 4 (9.5) | 1.000 |
| Systolic blood pressure (mmHg) | 129.9 ± 25.2 | 129.8 ± 23.8 | 0.988 |
| Diastolic blood pressure (mmHg) | 80.7 ± 16.1 | 79.7 ± 12.9 | 0.705 |
| Total cholesterol (mmol/L) | 5.42 ± 0.99 | 6.09 ± 1.37 | 0.004 |
| LDL cholesterol (mmol/L) | 3.54 ± 0.93 | 4.03 ± 1.27 | 0.027 |
| Triglycerides (mmol/L) | 1.93 ± 1.15 | 1.79 ± 1.02 | 0.546 |
| Glucose (mmol/L) | 8.63 ± 4.23 | 8.56 ± 4.54 | 0.646 |
| CRP (mg/L) | 10.1 (3.9–26.3) | 8.1 (3.6–30.2) | 0.991 |
| Troponine (ng/mL) | 149.00 | 114.80 | 0.728 |
| Hematocrit (mL/L) | 42.9 ± 3.7 | 39.6 ± 3.9 | <0.001 |
| Variable | Gender | p Value | |
|---|---|---|---|
| Male | Female | ||
| Infarct/related artery | |||
| No sign lesion, n (%) | 2 (1.5) | 0 | 0.958 |
| LAD and LMCA, n (%) | 46 (34.8) | 17 (38.6) | |
| Lcx, n (%) | 29 (22.0) | 9 (20.5) | |
| RCA, n (%) | 55 (41.7) | 18 (40.9) | |
| Number of CAD | |||
| Normal, n (%) | 1 (0.7) | 0 | 0.593 |
| One vessel, n (%) | 66 (49.3) | 25 (56.8) | |
| Two vessel, n (%) | 47 (35.1) | 12 (27.3) | |
| Three vessel, n (%) | 20 (14.9) | 7 (15.9) | |
| Only infarct related, n (%) | 65 (49.6) | 23 (53.5) | 0.660 |
| TIMI after PPCI (0–1), n (%) | 12 (10.6) | 4 (9.8) | 1.000 |
| PPCI of non infarction, n (%) | 5 (3.8) | 1 (2.4) | 1.000 |
| Syntax score culpit | 8 (5–14) | 8 (5–10.5) | 0.638 |
| Syntax score total | 13 (7–22) | 13 (8–20) | 0.973 |
| Aspirin, n (%) | 135 (99.3) | 43 (100) | 1.000 |
| Clopidogrel, n (%) | 134 (98.5) | 43 (100) | 1.000 |
| LMWH, n (%) | 131 (96.3) | 42 (97.7) | 1.000 |
| ACE-I or ARBs, n (%) | 131 (96.3) | 38 (88.4) | 0.062 |
| β-blockers, n (%) | 108 (80.0) | 38 (88.4) | 0.260 |
| Diuretic, n (%) | 25 (18.4) | 6 (14.0) | 0.504 |
| Statins, n (%) | 134 (98.5) | 43 (100) | 1.000 |
| Variable | Gender | p Value | |
|---|---|---|---|
| Male | Female | ||
| LVEDVi (mL/m2) | |||
| First week | 58.2 ± 13.1 | 46.3 ± 11.1 | <0.001 |
| 6 months | 60.2 ± 15.3 | 49.2 ± 16.2 | <0.001 |
| Δ | 1.48 ± 12.4 | 2.62 ± 12.4 | 0.610 |
| LVESVi (mL/m2) | |||
| First week | 33.7 ± 9.5 | 27.0 ± 9.2 | <0.001 |
| 6 months | 35.3 ± 13.7 | 28.1 ± 13.6 | 0.004 |
| Δ | 0.83 ± 8.82 | 0.94 ± 9.26 | 0.944 |
| LVEF (%) | |||
| First week | 44.2 ± 6.6 | 44.8 ± 8.5 | 0.591 |
| 6 months | 45.6 ± 8.0 * | 46.8 ± 10.3 * | 0.492 |
| Δ | 1.26 ± 6.96 | 2.33 ± 7.18 | 0.395 |
| SV (mL) | |||
| First week | 75.9 ± 17.1 | 66.5 ± 17.4 | 0.004 |
| 6 months | 80.2 ± 17.6 * | 71.6 ± 14.3 | 0.006 |
| Δ | 4.62 ± 19.21 | 3.19 ± 15.66 | 0.692 |
| VmaxA (cm/s) | |||
| First week | 66.48 ± 14.96 | 77.18 ± 15.96 * | <0.001 |
| 6 months | 67.14 ± 15.99 | 74.34 ± 19.37 * | 0.015 |
| Δ | 0.66 ± 0.059 | −2.84 ± 0.071 | 0.316 |
| E/e (ratio) | |||
| First week | 9.87 ± 3.06 | 10.78 ± 3.10 | 0.104 |
| 6 months | 10.16 ± 3.04 | 11.54 ± 3.53 | 0.015 * |
| Δ | 0.489 ± 2.520 | 0.344 ± 3.416 | 0.777 |
| SPVD (mmHg) | |||
| First week | 33.4 ± 6.7 | 35.7 ± 8.4 | 0.099 |
| 6 months | 34.2 ± 7.2 | 37.6 ± 10.3 | 0.024* |
| Δ | 1.03 ± 8.08 | 2.09 ± 11.78 | 0.567 |
| GLS (%) | |||
| First week | −8.41 ± 2.97 | −9.34 ± 3.88 | 0.164 |
| 6 months | −9.59 ± 3.08 * | −9.76 ± 3.13 | 0.769 |
| Δ | −0.91 ± 2.97 | −0.38 ± 4.06 | 0.377 |
| GRS (%) | |||
| First week | 17.75 ± 7.50 | 20.62 ± 8.88 | 0.052 |
| 6 months | 20.75 ± 8.26 * | 21.67 ± 8.81 | 0.551 |
| Δ | 3.00 ± 7.93 | 0.87 ± 8.80 | 0.181 |
| DD, n (%) | |||
| First week | 7 (5.11) | 3 (6.81) | 0.48 |
| 6 months | 8 (11.68) | 8 (18.18) | 0.014 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dekleva, M.; Djuric, T.; Djordjevic, A.; Soldatovic, I.; Stankovic, A.; Suzic Lazic, J.; Zivkovic, M. Sex-Related Differences in Heart Failure Development in Patients After First Myocardial Infarction: The Role of Galectin-3. Biomedicines 2024, 12, 2661. https://doi.org/10.3390/biomedicines12122661
Dekleva M, Djuric T, Djordjevic A, Soldatovic I, Stankovic A, Suzic Lazic J, Zivkovic M. Sex-Related Differences in Heart Failure Development in Patients After First Myocardial Infarction: The Role of Galectin-3. Biomedicines. 2024; 12(12):2661. https://doi.org/10.3390/biomedicines12122661
Chicago/Turabian StyleDekleva, Milica, Tamara Djuric, Ana Djordjevic, Ivan Soldatovic, Aleksandra Stankovic, Jelena Suzic Lazic, and Maja Zivkovic. 2024. "Sex-Related Differences in Heart Failure Development in Patients After First Myocardial Infarction: The Role of Galectin-3" Biomedicines 12, no. 12: 2661. https://doi.org/10.3390/biomedicines12122661
APA StyleDekleva, M., Djuric, T., Djordjevic, A., Soldatovic, I., Stankovic, A., Suzic Lazic, J., & Zivkovic, M. (2024). Sex-Related Differences in Heart Failure Development in Patients After First Myocardial Infarction: The Role of Galectin-3. Biomedicines, 12(12), 2661. https://doi.org/10.3390/biomedicines12122661







