Evaluation of BRIP-1 (FANCJ) and FANCI Protein Expression in Ovarian Cancer Tissue
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participation in the Study
2.3. Immunohistochemical (IHC) Expression of FANCI and BRIP-1 (FANCJ) in Ovarian Cancer Tissue Samples
2.4. Statistical Calculations
2.5. Group Characteristics
3. Results
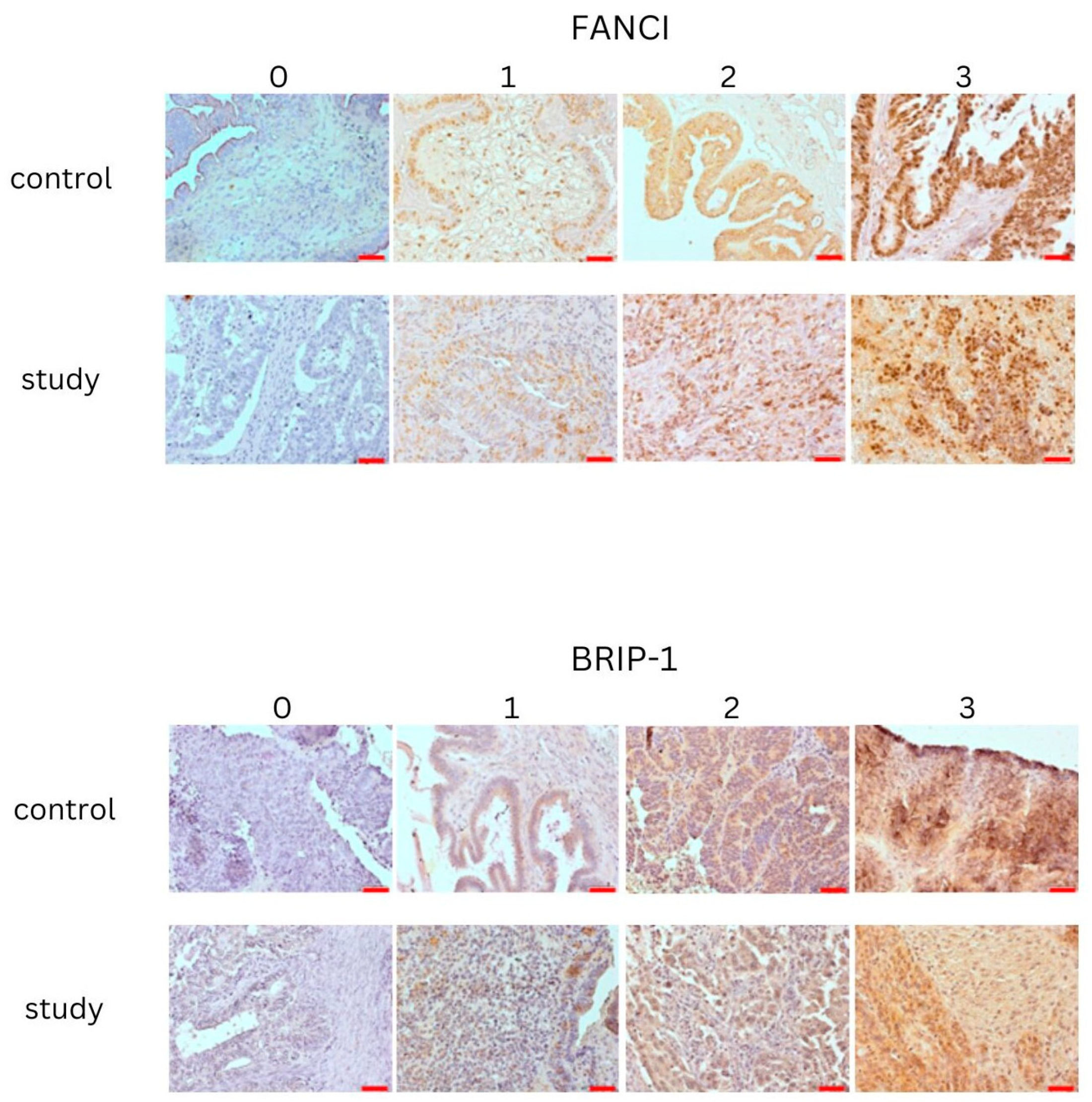
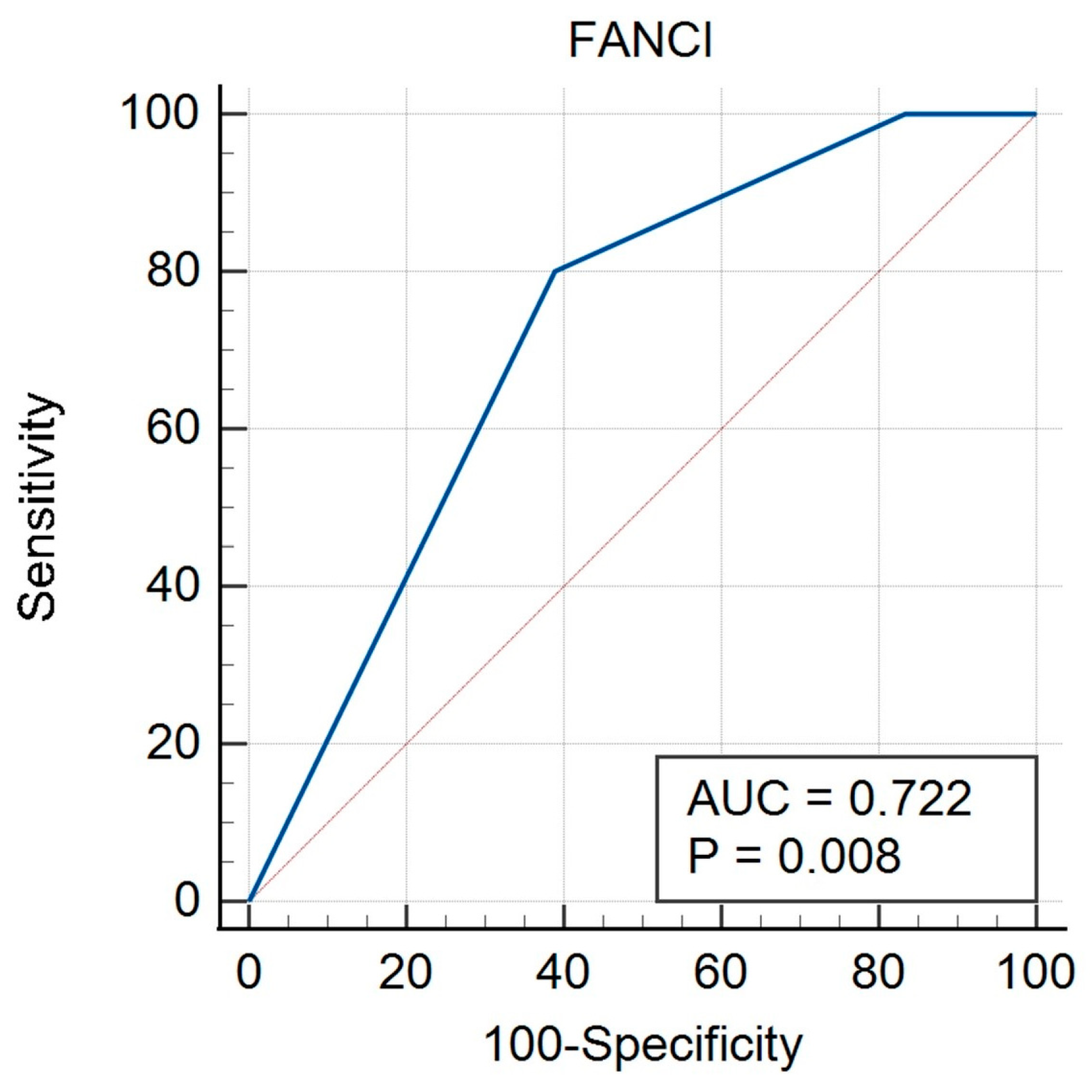
3.1. Tissue Expression of FANCI Protein
3.2. Tissue Expression of BRIP-1 (FANCJ) Protein
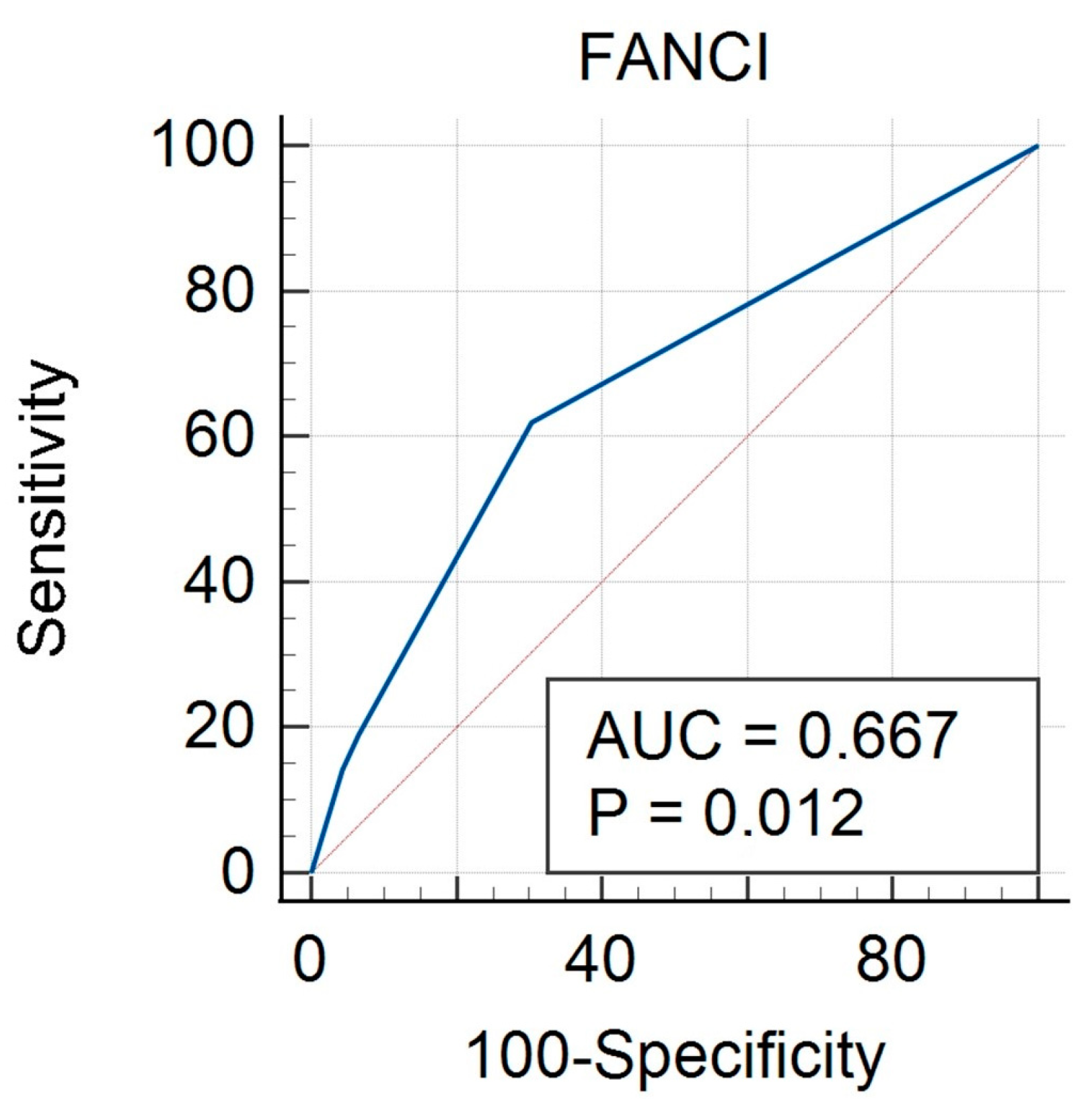
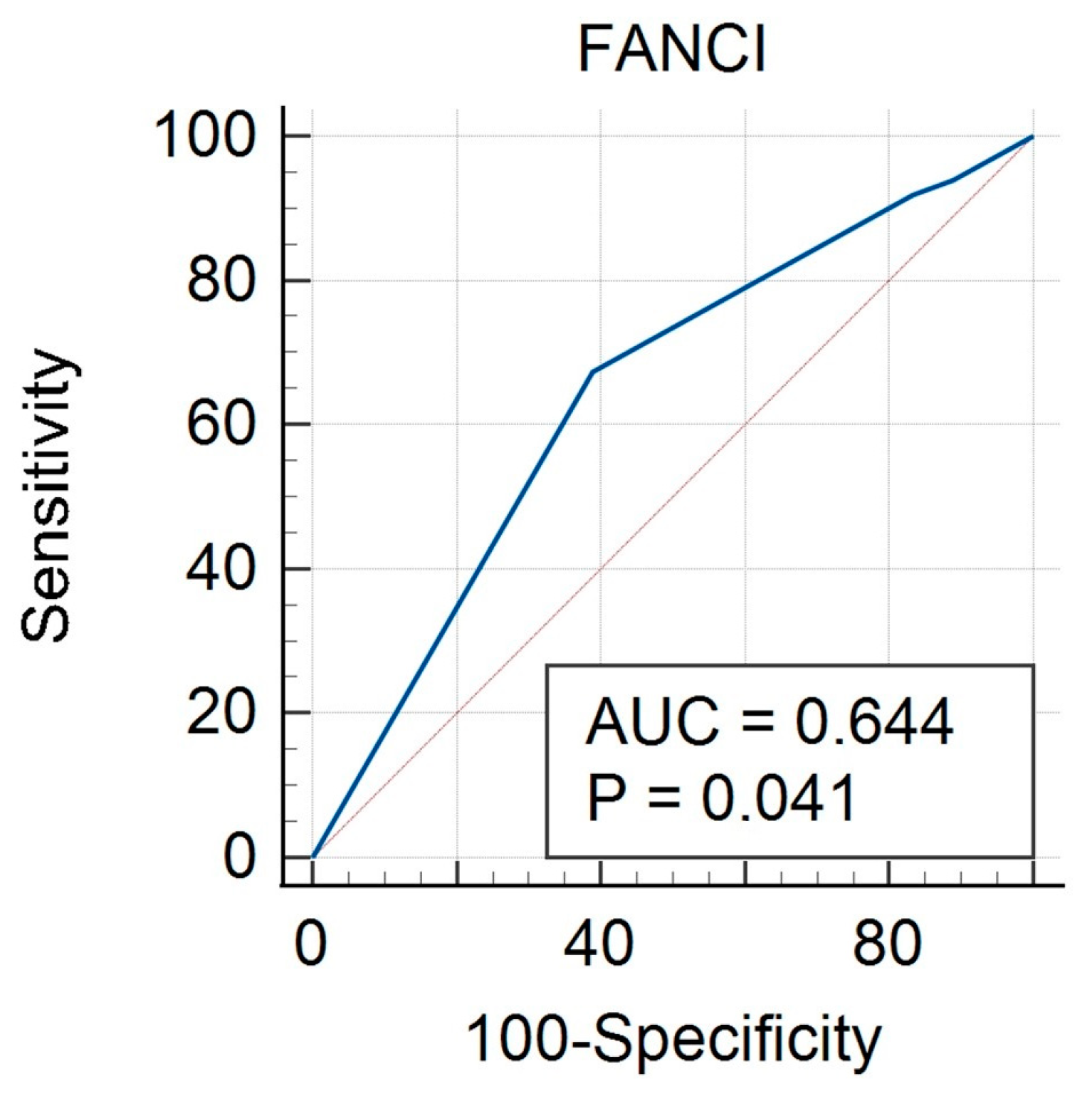
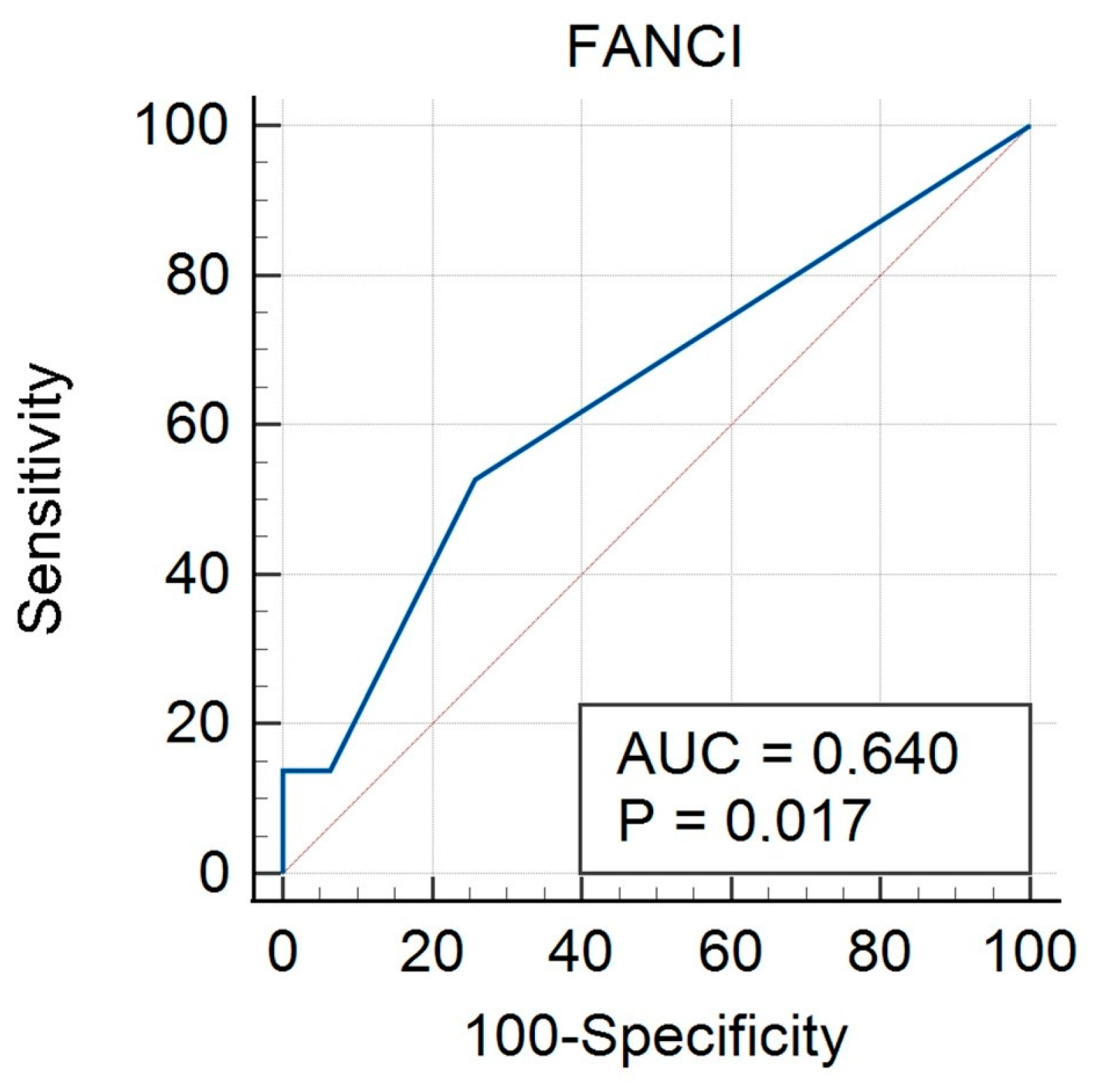
3.3. FANCI Protein Expression in the Differentiation of the Described Clinical Features
3.4. BRIP-1 Protein Expression in the Differentiation of the Described Clinical Features
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Available online: https://www.wcrf.org/cancer-trends/ovarian-cancer-statistics/ (accessed on 23 October 2024).
- Menon, U.; Gentry-Maharaj, A.; Burnell, M.; Singh, N.; Ryan, A.; Karpinskyj, C.; Carlino, G.; Taylor, J.; Massingham, S.K.; Raikou, M.; et al. Ovarian cancer population screening and mortality after long-term follow-up in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): A randomised controlled trial. Lancet 2021, 397, 2182–2193. [Google Scholar] [CrossRef] [PubMed]
- Buys, S.S. Effect of screening on ovarian cancer mortality: The Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening randomized controlled trial. JAMA 2011, 305, 2295–2303. [Google Scholar] [CrossRef] [PubMed]
- Luvero, D.; Milani, A.; Ledermann, J.A. Treatment options in recurrent ovarian cancer: Latest evidence and clinical potential. Ther. Adv. Med. Oncol. 2014, 6, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Lheureux, S.; Braunstein, M.; Oza, A.M. Epithelial ovarian cancer: Evolution of management in the era of precision medicine. CA Cancer J. Clin. 2019, 69, 280–304. [Google Scholar] [CrossRef] [PubMed]
- Hinchcliff, E.; Westin, S.N.; Herzog, T.J. State of the science: Contemporary front-line treatment of advanced ovarian cancer. Gynecol. Oncol. 2022, 166, 18–24. [Google Scholar] [CrossRef]
- Pujade-Lauraine, E.; Ledermann, J.A.; Selle, F.; Gebski, V.; Penson, R.T.; Oza, A.M.; Korach, J.; Huzarski, T.; Poveda, A.; Pignata, S.; et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1274–1284. [Google Scholar] [CrossRef]
- Coleman, R.L.; Oza, A.M.; Lorusso, D.; Aghajanian, C.; Oaknin, A.; Dean, A.; Colombo, N.; Weberpals, J.I.; Clamp, A.; Scambia, G.; et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 390, 1949–1961. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Pautier, P.; Pignata, S.; Pérol, D.; González-Martín, A.; Berger, R.; Fujiwara, K.; Vergote, I.; Colombo, N.; Mäenpää, J.; et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2416–2428. [Google Scholar] [CrossRef]
- Wu, X.; Liu, J.; Wang, J.; Wang, L.; Lin, Z.; Wang, X.; Zhu, J.; Kong, B.; Fei, J.; Tang, Y.; et al. Senaparib as first-line maintenance therapy in advanced ovarian cancer: A randomized phase 3 trial. Nat. Med. 2024, 30, 1612–1621. [Google Scholar] [CrossRef]
- Whitehair, R.; Peres, L.C.; Mills, A.M. Expression of the Immune Checkpoints LAG-3 and PD-L1 in High-grade Serous Ovarian Carcinoma: Relationship to Tumor-associated Lymphocytes and Germline BRCA Status. Int. J. Gynecol. Pathol. 2020, 39, 558–566. [Google Scholar] [CrossRef]
- Blanc-Durand, F.; Genestie, C.; Galende, E.Y.; Gouy, S.; Morice, P.; Pautier, P.; Maulard, A.; Mesnage, S.; Le Formal, A.; Brizais, C.; et al. Distribution of novel immune-checkpoint targets in ovarian cancer tumor microenvironment: A dynamic landscape. Gynecol. Oncol. 2021, 160, 279–284. [Google Scholar] [CrossRef]
- Matsuzaki, J.; Gnjatic, S.; Mhawech-Fauceglia, P.; Beck, A.; Miller, A.; Tsuji, T.; Eppolito, C.; Qian, F.; Lele, S.; Shrikant, P.; et al. Tumor-infiltrating NY-ESO-1–specific CD8 + T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc. Natl. Acad. Sci. USA 2010, 107, 7875–7880. [Google Scholar] [CrossRef] [PubMed]
- Fucikova, J.; Rakova, J.; Hensler, M.; Kasikova, L.; Belicova, L.; Hladikova, K.; Truxova, I.; Skapa, P.; Laco, J.; Pecen, L.; et al. TIM-3 dictates functional orientation of the immune infiltrate in ovarian cancer. Clin. Cancer Res. 2019, 25, 4820–4831. [Google Scholar] [CrossRef] [PubMed]
- London, T.B.C.; Barber, L.J.; Mosedale, G.; Kelly, G.P.; Balasubramanian, S.; Hickson, I.D.; Boulton, S.J.; Hiom, K. FANCJ Is a Structure-specific DNA helicase associated with the maintenance of genomic G/C tracts. J. Biol. Chem. 2008, 283, 36132–36139. [Google Scholar] [CrossRef] [PubMed]
- Andrikopoulou, A.; Zografos, E.; Apostolidou, K.; Kyriazoglou, A.; Papatheodoridi, A.-M.; Kaparelou, M.; Koutsoukos, K.; Liontos, M.; Dimopoulos, M.-A.; Zagouri, F. Germline and somatic variants in ovarian carcinoma: A next-generation sequencing (NGS) analysis. Front. Oncol. 2022, 12, 1030786. [Google Scholar] [CrossRef] [PubMed]
- Nath, S.; Nagaraju, G. FANCJ helicase promotes DNA end resection by facilitating CtIP recruitment to DNA double-strand breaks. PLoS Genet. 2020, 16, e1008701. [Google Scholar] [CrossRef]
- Prakash, R.; Zhang, Y.; Feng, W.; Jasin, M. Homologous recombination and human health: The roles of BRCA1, BRCA2, and associated proteins. Cold Spring Harb. Perspect. Biol. 2015, 7, a016600. [Google Scholar] [CrossRef]
- Mahdi, K.M.; Nassiri, M.R.; Nasiri, K. Hereditary genes and SNPs associated with breast cancer. Asian Pac. J. Cancer Prev. 2013, 14, 3403–3409. [Google Scholar] [CrossRef]
- Guénard, F.; Brcas, I.; Labrie, Y.; Ouellette, G.; Beauparlant, C.J.; Simard, J.; Durocher, F. Mutational analysis of the breast cancer susceptibility gene BRIP1/BACH1/FANCJ in high-risk non-BRCA1/BRCA2 breast cancer families. J. Hum. Genet. 2008, 53, 579–591. [Google Scholar] [CrossRef][Green Version]
- Eelen, G.; Bempt, I.V.; Verlinden, L.; Drijkoningen, M.; Smeets, A.; Neven, P.; Christiaens, M.R.; Marchal, K.; Bouillon, R.; Verstuyf, A. Expression of the BRCA1-interacting protein Brip1/BACH1/FANCJ is driven by E2F and correlates with human breast cancer malignancy. Oncogene 2008, 27, 4233–4241. [Google Scholar] [CrossRef][Green Version]
- Ramus, S.J.; Song, H.; Dicks, E.; Tyrer, J.P.; Rosenthal, A.N.; Intermaggio, M.P.; Fraser, L.; Gentry-Maharaj, A.; Hayward, J.; Philpott, S.; et al. Germline mutations in the BRIP1, BARD1, PALB2, and NBN genes in women with ovarian cancer. J. Natl. Cancer Inst. 2015, 107, djv214. [Google Scholar] [CrossRef] [PubMed]
- Horackova, K.; Janatova, M.; Kleiblova, P.; Kleibl, Z.; Soukupova, J. Early-Onset Ovarian Cancer <30 Years: What Do We Know about Its Genetic Predisposition? Int. J. Mol. Sci. 2023, 24, 17020. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, R.; Ghosh, A.; Mukhopadhyay, S. High-grade serous ovarian carcinoma, the “Achiles’ hill” for clinicians and molecular biologists: A molecular insight. Mol. Biol. Rep. 2023, 50, 9511–9519. [Google Scholar] [CrossRef] [PubMed]
- Moes-Sosnowska, J.; Rzepecka, I.K.; Chodzynska, J.; Dansonka-Mieszkowska, A.; Szafron, L.M.; Balabas, A.; Lotocka, R.; Sobiczewski, P.; Kupryjanczyk, J. Clinical importance of FANCD2, BRIP1, BRCA1, BRCA2 and FANCF expression in ovarian carcinomas. Cancer Biol. Ther. 2019, 20, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Moyer, C.L.; Ivanovich, J.; Gillespie, J.L.; Doberstein, R.; Radke, M.R.; Richardson, M.E.; Kaufmann, S.H.; Swisher, E.M.; Goodfellow, P.J. Rare BRIP1 missense alleles confer risk for ovarian and breast cancer. Cancer Res. 2020, 80, 857–867. [Google Scholar] [CrossRef]
- Ma, X.; Cai, G.; Zou, W.; Huang, Y.; Zhang, J.; Wang, D.; Chen, B. First evidence for the contribution of the genetic variations of BRCA1-interacting protein 1 (BRIP1) to the genetic susceptibility of cervical cancer. Gene 2013, 524, 208–213. [Google Scholar] [CrossRef]
- Ma, X.; Cai, G.; Zou, W.; Huang, Y.; Zhang, J.; Wang, D.; Chen, B. BRIP1 variations analysis reveals their relative importance as genetic susceptibility factor for cervical cancer. Biochem. Biophys. Res. Commun. 2013, 433, 232–236. [Google Scholar] [CrossRef]
- Kote-Jarai, Z.; Jugurnauth, S.; Mulholland, S.; Leongamornlert, D.A.; Guy, M.; Edwards, S.; Tymrakiewitcz, M.; O’brien, L.; Hall, A.; Wilkinson, R.; et al. A recurrent truncating germline mutation in the BRIP1/FANCJ gene and susceptibility to prostate cancer. Br. J. Cancer 2009, 100, 426–430. [Google Scholar] [CrossRef]
- Howlett, N.G.; Taniguchi, T.; Olson, S.; Cox, B.; Waisfisz, Q.; De Die-Smulders, C.; Persky, N.; Grompe, M.; Joenje, H.; Pals, G.; et al. Biallelic inactivation of BRCA2 in fanconi anemia. Science 2002, 297, 606–609. [Google Scholar] [CrossRef]
- Hou, Y.; Li, J.; Yu, A.; Deng, K.; Chen, J.; Wang, Z.; Huang, L.; Ma, S.; Dai, X. FANCI is associated with poor prognosis and immune infiltration in liver hepatocellular carcinoma. Int. J. Med. Sci. 2023, 20, 918–932. [Google Scholar] [CrossRef]
- Cai, Z.; Duan, Y.; Li, W.; Liu, Z.; Gong, Z.; Hong, S.; He, X.; Xuanyuan, X.; Chen, Y.; Bi, X.; et al. FANCI serve as a prognostic biomarker correlated with immune infiltrates in skin cutaneous melanoma. Front. Immunol. 2023, 14, 1295831. [Google Scholar] [CrossRef] [PubMed]
- Kaljunen, H.; Taavitsainen, S.; Kaarijärvi, R.; Takala, E.; Paakinaho, V.; Nykter, M.; Bova, G.S.; Ketola, K. Fanconi anemia pathway regulation by FANCI in prostate cancer. Front. Oncol. 2023, 13, 1260826. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Y.; Yang, Q.; Zhou, X.; Guo, Y.; Ding, F.; Liu, Z.; Luo, A. Silencing of FANCI Promotes DNA Damage and Sensitizes Ovarian Cancer Cells to Carboplatin. Curr. Cancer Drug Targets 2022, 22, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Z.; Sang, M.Y.; Xi, P.W.; Xu, R.X.; Cai, M.Y.; Wang, Z.W.; Zhao, J.Y.; Li, Y.H.; Wei, J.F.; Ding, Q. FANCI Inhibition Induces PARP1 Redistribution to Enhance the Efficacy of PARP Inhibitors in Breast Cancer. Cancer Res. 2024, 84, 3447–3463. [Google Scholar] [CrossRef]
- Ardeljan, D.; Steranka, J.P.; Liu, C.; Li, Z.; Taylor, M.S.; Payer, L.M.; Gorbounov, M.; Sarnecki, J.S.; Deshpande, V.; Hruban, R.H.; et al. Cell fitness screens reveal a conflict between LINE-1 retrotransposition and DNA replication. Nat. Struct. Mol. Biol. 2020, 27, 168–178. [Google Scholar] [CrossRef]
- Mita, P.; Sun, X.; Fenyö, D.; Kahler, D.J.; Li, D.; Agmon, N.; Wudzinska, A.; Keegan, S.; Bader, J.S.; Yun, C.; et al. BRCA1 and S phase DNA repair pathways restrict LINE-1 retrotransposition in human cells. Nat. Struct. Mol. Biol. 2020, 27, 179–191. [Google Scholar] [CrossRef]
- Deans, A.J.; West, S.C. DNA interstrand crosslink repair and cancer. Nat. Rev. Cancer 2011, 11, 467–480. [Google Scholar] [CrossRef]
- Fierheller, C.T.; Guitton-Sert, L.; Alenezi, W.M.; Revil, T.; Oros, K.K.; Gao, Y.; Bedard, K.; Arcand, S.L.; Serruya, C.; Behl, S.; et al. A functionally impaired missense variant identified in French Canadian families implicates FANCI as a candidate ovarian cancer-predisposing gene. Genome Med. 2021, 13, 186. [Google Scholar] [CrossRef]
- Fierheller, C.T.; Alenezi, W.M.; Serruya, C.; Revil, T.; Amuzu, S.; Bedard, K.; Subramanian, D.N.; Fewings, E.; Bruce, J.P.; Prokopec, S.; et al. Molecular Genetic Characteristics of FANCI, a Proposed New Ovarian Cancer Predisposing Gene. Genes 2023, 14, 277. [Google Scholar] [CrossRef]
- Cummings, S.; Roman, S.S.; Saam, J.; Bernhisel, R.; Brown, K.; Lancaster, J.M.; Usha, L. Age of ovarian cancer diagnosis among BRIP1, RAD51C, and RAD51D mutation carriers identified through multi-gene panel testing. J. Ovarian Res. 2021, 14, 61. [Google Scholar] [CrossRef]
- Lheureux, S.; Gourley, C.; Vergote, I.; Oza, A.M. Epithelial ovarian cancer. Lancet 2019, 393, 1240–1253. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.R.; Kamara, D.; Karlan, B.Y.; Pharoah, P.D.; Gayther, S.A. Genetic epidemiology of ovarian cancer and prospects for polygenic risk prediction. Gynecol. Oncol. 2017, 147, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Liu, Y.; Zhang, L.; Dong, L.; Bao, L.; Bai, Q.; Cui, Q.; Xu, J.; Li, M.; Liu, J.; et al. Mutation Landscape of Homologous Recombination Repair Genes in Epithelial Ovarian Cancer in China and Its Relationship with Clinicopathlological Characteristics. Front. Oncol. 2022, 12, 709645. [Google Scholar] [CrossRef] [PubMed]


| Parameters | Total Cohort (n = 68) | Non-HGSOC (n = 28) | HGSOC (n = 40) | p-Value * | OR (95% CI) |
|---|---|---|---|---|---|
| Median (IQR) | |||||
| Age (years old) | 61.0 (20) | 57.0 (19) | 64.5 (15) | 0.03 | |
| BMI (kg/m2) | 25.1 (6.5) | 25.1 (6.9) | 25.4 (6.3) | NS | |
| Number (%) | |||||
| FANCI protein expression intensity | 0.9 (0.2–5.2) | ||||
| 0–1 | 7 (10.3%) | 3 (10.7%) | 4 (11.1%) | 0.5 (0.2–1.5) | |
| 2–3 | 61 (89.7%) | 25 (89.3%) | 36 (88.9%) | ||
| BRIP-1 protein expression intensity | |||||
| 0–1 | 49 (72.1%) | 18 (64.3%) | 31 (77.5%) | ||
| 2–3 | 19 (27.9%) | 10 (35.7%) | 9 (22.5%) | ||
| Age (years old) | |||||
| <65 | 39 (57.3%) | 19 (67.9%) | 20 (50%) | NS | |
| ≥65 | 29 (42.6%) | 9 (32.1%) | 20 (50%) | ||
| BMI | |||||
| <25 | 31 (45.6%) | 13 (46.4%) | 18 (45%) | NS | |
| ≥25 | 37 (54.4%) | 15 (53.6%) | 22 (55%) | ||
| Menopausal status | |||||
| Premenopausal | 22 (32.4%) | 12 (42.9%) | 10 (25%) | NS | |
| Postmenopausal | 46 (67.6%) | 16 (57.1%) | 30 (75%) | ||
| Grade | |||||
| High (G2 + G3) | 50 (73.5%) | 10 (35.7%) | 40 (100%) | 0.0001 | |
| Low (G1) | 18 (26.5%) | 18 (64.3%) | 0 (0%) | ||
| Histological type | |||||
| Serous | 52 (76.5%) | 12 (42.8%) | 40 (100%) | 0.0001 | |
| Non-serous | 16 (23.5%) | 16 (57.2%) | 0 (0%) | ||
| FIGO stage | |||||
| I–II | 21 (31.1%) | 13 (46.4%) | 8 (20%) | 0.02 | |
| III–IV | 47 (69.1%) | 15 (53.6%) | 32 (80%) | ||
| Non-HGSOC (n = 3) | HGSOC (n = 4) | p-Value * | |
|---|---|---|---|
| Group 0–1 of FANCI protein expression intensity | |||
| Grade | |||
| High | 0 (0%) | 4 (100%) | 0.008 |
| Low | 3 (100%) | 0 (0%) | |
| Histological type Serous Non-serous | 3 (100%) 0 (0%) | 4 (100%) 0 (0%) | NS |
| Menopausal status | |||
| Premenopausal | 2 (66.7%) | 0 (0%) | 0.05 |
| Postmenopausal | 1 (33.3%) | 4 (100%) | |
| FIGO stage | |||
| I–II | 3 (100%) | 1 (25%) | 0.04 |
| III–IV | 0 (0%) | 3 (75%) | |
| BMI | |||
| <25 | 1 (33.3%) | 1 (25%) | 0.05 |
| >25 | 2 (66.7%) | 3 (75%) | |
| Age | |||
| <65 years old | 2 (66.7%) | 2 (50%) | NS |
| >65 years old | 1 (33.3%) | 2 (50%) | |
| Non-HGSOC (n = 25) | HGSOC (n = 36) | p-Value * | |
|---|---|---|---|
| Group of 2–3 FANCI protein expression intensity | |||
| Grade | |||
| High | 10 (40%) | 36 (100%) | <0.0001 |
| Low | 15 (60%) | 0 (0%) | |
| Histological type | |||
| Serous | 9 (36%) | 36 (100%) | <0.0001 |
| Non-serous | 16 (64%) | 0 (0%) | |
| Menopausal status | |||
| Premenopausal | 10 (40%) | 10 (27.8%) | NS |
| Postmenopausal | 15 (60%) | 26 (72.2%) | |
| FIGO stage | |||
| I–II | 10 (40%) | 7 (19.4%) | 0.07 |
| III–IV | 15 (60%) | 29 (80.6%) | |
| BMI | |||
| <25 | 12 (48%) | 17 (47.2%) | NS |
| >25 | 13 (52%) | 19 (52.8%) | |
| Age | |||
| <65 years old | 17 (68%) | 18 (50%) | NS |
| >65 years old | 8 (32%) | 18 (50%) | |
| Non-HGSOC (n = 18) | HGSOC (n = 31) | p-Value * | |
|---|---|---|---|
| Group of 0–1 BRIP-1 protein expression intensity | |||
| Grade | |||
| High | 6 (33.3%) | 31 (100%) | <0.0001 |
| Low | 12 (66.7%) | 0 (0%) | |
| Histological type | |||
| Serous | 7 (38.9%) | 31 (100%) | <0.0001 |
| Non-serous | 11 (61.1%) | 0 (0%) | |
| Menopausal status | |||
| Premenopausal | 9 (50%) | 7 (22.6%) | 0.04 |
| Postmenopausal | 9 (50%) | 24 (77.4%) | |
| FIGO stage | |||
| I–II | 9 (50%) | 5 (16.1%) | 0.01 |
| III–IV | 9 (50%) | 26 (83.9%) | |
| BMI | |||
| <25 | 7 (38.9%) | 13 (41.9%) | NS |
| >25 | 11 (61.1%) | 18 (58.1%) | |
| Age | |||
| <65 years old | 14 (77.8%) | 17 (54.8%) | NS |
| >65 years old | 4 (22.2%) | 14 (45.2%) | |
| Non-HGSOC (n = 10) | HGSOC (n = 9) | p-Value * | |
|---|---|---|---|
| Group of 2–3 BRIP-1 protein expression intensity | |||
| Grade | |||
| High | 4 (40%) | 9 (100%) | 0.005 |
| Low | 6 (60%) | 0 (0%) | |
| Histological type | |||
| Serous | 5 (50%) | 9 (100%) | 0.01 |
| Non-serous | 5 (50%) | 0 (0%) | |
| Menopausal status | |||
| Premenopausal | 3 (30%) | 3 (33.3%) | NS |
| Postmenopausal | 7 (70%) | 6 (66.7%) | |
| FIGO stage | |||
| I–II | 4 (40%) | 3 (33.3%) | NS |
| III–IV | 6 (60%) | 6 (66.7%) | |
| BMI | |||
| <25 | 6 (60%) | 5 (55.6%) | NS |
| >25 | 4 (40%) | 4 (44.4%) | |
| Age | |||
| <65 years old | 5 (50%) | 3 (33.3%) | NS |
| >65 years old | 5 (50%) | 6 (66.7%) | |
| Criterion | Sensitivity | 95% CI | Specificity | 95% CI | +LR | −LR |
|---|---|---|---|---|---|---|
| <0 | 0.00 | 0.0–16.1 | 100.00 | 92.3–100.0 | 1.00 | |
| ≤0 | 14.29 | 3.0–36.3 | 95.65 | 85.2–99.5 | 3.29 | 0.90 |
| ≤1 | 19.05 | 5.4–41.9 | 93.48 | 82.1–98.6 | 2.92 | 0.87 |
| ≤2 | 61.90 | 38.4–81.9 | 69.57 | 54.2–82.3 | 2.03 | 0.55 |
| ≤3 | 100.00 | 83.9–100.0 | 0.00 | 0.0–7.7 | 1.00 |
| Criterion | Sensitivity | 95% CI | Specificity | 95% CI | +LR | −LR |
|---|---|---|---|---|---|---|
| ≥0 | 100.00 | 92.7–100.0 | 0.00 | 0.0–18.5 | 1.00 | |
| >0 | 93.88 | 83.1–98.7 | 11.11 | 1.4–34.7 | 1.06 | 0.55 |
| >1 | 91.84 | 80.4–97.7 | 16.67 | 3.6–41.4 | 1.10 | 0.49 |
| >2 | 67.35 | 52.5–80.1 | 61.11 | 35.7–82.7 | 1.73 | 0.53 |
| >3 | 0.00 | 0.0–7.3 | 100.00 | 81.5–100.0 | 1.00 |
| Criterion | Sensitivity | 95% CI | Specificity | 95% CI | +LR | −LR |
|---|---|---|---|---|---|---|
| <0 | 0.00 | 0.0–9.7 | 100.00 | 88.8–100.0 | 1.00 | |
| ≤0 | 13.89 | 4.7–29.5 | 100.00 | 88.8–100.0 | 0.86 | |
| ≤1 | 13.89 | 4.7–29.5 | 93.55 | 78.6–99.2 | 2.15 | 0.92 |
| ≤2 | 52.78 | 35.5–69.6 | 74.19 | 55.4–88.1 | 2.05 | 0.64 |
| ≤3 | 100.00 | 90.3–100.0 | 0.00 | 0.0–11.2 | 1.00 |
| Criterion | Sensitivity | 95% CI | Specificity | 95% CI | +LR | −LR |
|---|---|---|---|---|---|---|
| <0 | 0.00 | 0.0–24.7 | 100.00 | 78.2–100.0 | 1.00 | |
| ≤1 | 23.08 | 5.0–53.8 | 100.00 | 78.2–100.0 | 0.77 | |
| ≤2 | 69.23 | 38.6–90.9 | 73.33 | 44.9–92.2 | 2.60 | 0.42 |
| ≤3 | 100.00 | 75.3–100.0 | 0.00 | 0.0–21.8 | 1.00 |
| Criterion | Sensitivity | 95% CI | Specificity | 95% CI | +LR | −LR |
|---|---|---|---|---|---|---|
| ≥0 | 100.00 | 69.2–100.0 | 0.00 | 0.0–18.5 | 1.00 | |
| >1 | 100.00 | 69.2–100.0 | 16.67 | 3.6–41.4 | 1.20 | 0.00 |
| >2 | 80.00 | 44.4–97.5 | 61.11 | 35.7–82.7 | 2.06 | 0.33 |
| >3 | 0.00 | 0.0–30.8 | 100.00 | 81.5–100.0 | 1.00 |
| Criterion | Sensitivity | 95% CI | Specificity | 95% CI | +LR | −LR |
|---|---|---|---|---|---|---|
| <0 | 0.00 | 0.0–26.5 | 100.00 | 79.4–100.0 | 1.00 | |
| ≤1 | 25.00 | 5.5–57.2 | 100.00 | 79.4–100.0 | 0.75 | |
| ≤2 | 66.67 | 34.9–90.1 | 68.75 | 41.3–89.0 | 2.13 | 0.48 |
| ≤3 | 100.00 | 73.5–100.0 | 0.00 | 0.0–20.6 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozłowski, M.; Borzyszkowska, D.; Golara, A.; Durys, D.; Piotrowska, K.; Kempińska-Podhorodecka, A.; Cymbaluk-Płoska, A. Evaluation of BRIP-1 (FANCJ) and FANCI Protein Expression in Ovarian Cancer Tissue. Biomedicines 2024, 12, 2652. https://doi.org/10.3390/biomedicines12122652
Kozłowski M, Borzyszkowska D, Golara A, Durys D, Piotrowska K, Kempińska-Podhorodecka A, Cymbaluk-Płoska A. Evaluation of BRIP-1 (FANCJ) and FANCI Protein Expression in Ovarian Cancer Tissue. Biomedicines. 2024; 12(12):2652. https://doi.org/10.3390/biomedicines12122652
Chicago/Turabian StyleKozłowski, Mateusz, Dominika Borzyszkowska, Anna Golara, Damian Durys, Katarzyna Piotrowska, Agnieszka Kempińska-Podhorodecka, and Aneta Cymbaluk-Płoska. 2024. "Evaluation of BRIP-1 (FANCJ) and FANCI Protein Expression in Ovarian Cancer Tissue" Biomedicines 12, no. 12: 2652. https://doi.org/10.3390/biomedicines12122652
APA StyleKozłowski, M., Borzyszkowska, D., Golara, A., Durys, D., Piotrowska, K., Kempińska-Podhorodecka, A., & Cymbaluk-Płoska, A. (2024). Evaluation of BRIP-1 (FANCJ) and FANCI Protein Expression in Ovarian Cancer Tissue. Biomedicines, 12(12), 2652. https://doi.org/10.3390/biomedicines12122652








