A Cross-Sectional Study: Systematic Quantification of Chemerin in Human Cerebrospinal Fluid
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Quantification of Serum and CSF Chemerin Levels
2.3. Statistical Analyses
3. Results
3.1. Study Cohort
3.2. Quantification of Chemerin Levels Among the Entire Study Cohort
3.3. Chemerin Levels in Patients Without Evidence of a Neurological Disease
3.4. Chemerin Levels in Patients with Neurological Diseases Compared to Matched Control Groups
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bovolini, A.; Garcia, J.; Andrade, M.A.; Duarte, J.A. Metabolic Syndrome Pathophysiology and Predisposing Factors. Int. J. Sports Med. 2021, 42, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef]
- Correale, J.; Marrodan, M. Multiple sclerosis and obesity: The role of adipokines. Front. Immunol. 2022, 13, 1038393. [Google Scholar] [CrossRef] [PubMed]
- Picone, P.; Di Carlo, M.; Nuzzo, D. Obesity and Alzheimer’s disease: Molecular bases. Eur. J. Neurosci. 2020, 52, 3944–3950. [Google Scholar] [CrossRef]
- Neto, A.; Fernandes, A.; Barateiro, A. The complex relationship between obesity and neurodegenerative diseases: An updated review. Front. Cell. Neurosci. 2023, 17, 1294420. [Google Scholar] [CrossRef]
- Smith, N.K.; Hackett, T.A.; Galli, A.; Flynn, C.R. GLP-1: Molecular mechanisms and outcomes of a complex signaling system. Neurochem. Int. 2019, 128, 94–105. [Google Scholar] [CrossRef]
- Del Prato, S.; Gallwitz, B.; Holst, J.J.; Meier, J.J. The incretin/glucagon system as a target for pharmacotherapy of obesity. Obes. Rev. 2022, 23, e13372. [Google Scholar] [CrossRef]
- Schwartz, M.W.; Peskind, E.; Raskind, M.; Boyko, E.J.; Porte, D. Cerebrospinal fluid leptin levels: Relationship to plasma levels and to adiposity in humans. Nat. Med. 1996, 2, 589–593. [Google Scholar] [CrossRef]
- Thomas, S.A.; Preston, J.E.; Wilson, M.R.; Farrell, C.L.; Segal, M.B. Leptin transport at the blood--cerebrospinal fluid barrier using the perfused sheep choroid plexus model. Brain Res. 2001, 895, 283–290. [Google Scholar] [CrossRef]
- Mazen, I.H.; El-Gammal, M.A.; Elaidy, A.A.; Anwar, G.M.; Ashaat, E.A.; Abdel-Ghafar, S.F.; Abdel-Hamid, M.S. Congenital leptin and leptin receptor deficiencies in nine new families: Identification of six novel variants and review of literature. Mol. Genet. Genom. 2023, 298, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.B. Leptin—Much more than a satiety signal. Annu. Rev. Nutr. 2000, 20, 45–75. [Google Scholar] [CrossRef] [PubMed]
- Berghoff, M.; Hochberg, A.; Schmid, A.; Schlegel, J.; Karrasch, T.; Kaps, M.; Schäffler, A. Quantification and regulation of the adipokines resistin and progranulin in human cerebrospinal fluid. Eur. J. Clin. Investig. 2016, 46, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Buechler, C.; Feder, S.; Haberl, E.M.; Aslanidis, C. Chemerin Isoforms and Activity in Obesity. Int. J. Mol. Sci. 2019, 20, 1128. [Google Scholar] [CrossRef]
- Macvanin, M.T.; Rizzo, M.; Radovanovic, J.; Sonmez, A.; Paneni, F.; Isenovic, E.R. Role of Chemerin in Cardiovascular Diseases. Biomedicines 2022, 10, 2970. [Google Scholar] [CrossRef]
- Zabel, B.A.; Zuniga, L.; Ohyama, T.; Allen, S.J.; Cichy, J.; Handel, T.M.; Butcher, E.C. Chemoattractants, extracellular proteases, and the integrated host defense response. Exp. Hematol. 2006, 34, 1021–1032. [Google Scholar] [CrossRef]
- Azamar-Llamas, D.; Hernández-Molina, G.; Ramos-Ávalos, B.; Furuzawa-Carballeda, J. Adipokine Contribution to the Pathogenesis of Osteoarthritis. Mediat. Inflamm. 2017, 2017, 5468023. [Google Scholar] [CrossRef]
- Lu, Z.; Liu, J.; Wan, Q.; Wu, Y.; Wu, W.; Chen, Y. Chemerin promotes invasion of oral squamous cell carcinoma by stimulating IL-6 and TNF-α production via STAT3 activation. Mol. Biol. Rep. 2024, 51, 436. [Google Scholar] [CrossRef]
- Schmid, A.; Roderfeld, M.; Karrasch, T.; Roeb, E.; Schäffler, A. Serum Chemerin Is Decreased by Roux-en-Y Gastric Bypass and Low Calorie-Formula Diet in Obese Individuals. Biomedicines 2023, 12, 33. [Google Scholar] [CrossRef]
- Ashtary-Larky, D.; Lamuchi-Deli, N.; Kashkooli, S.; Mombaini, D.; Alipour, M.; Khodadadi, F.; Bagheri, R.; Dutheil, F.; Wong, A. The effects of exercise training on serum concentrations of chemerin in individuals with overweight and obesity: A systematic review, meta-analysis, and meta-regression of 43 clinical trials. Arch. Physiol. Biochem. 2023, 129, 1012–1027. [Google Scholar] [CrossRef]
- Schmid, A.; Bala, M.; Leszczak, S.; Ober, I.; Buechler, C.; Karrasch, T. Pro-inflammatory chemokines CCL2, chemerin, IP-10 and RANTES in human serum during an oral lipid tolerance test. Cytokine 2016, 80, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Helfer, G.; Ross, A.W.; Thomson, L.M.; Mayer, C.D.; Stoney, P.N.; McCaffery, P.J.; Morgan, P.J. A neuroendocrine role for chemerin in hypothalamic remodelling and photoperiodic control of energy balance. Sci. Rep. 2016, 6, 26830. [Google Scholar] [CrossRef] [PubMed]
- Frigerio, F.; Pasqualini, G.; Craparotta, I.; Marchini, S.; van Vliet, E.A.; Foerch, P.; Vandenplas, C.; Leclercq, K.; Aronica, E.; Porcu, L.; et al. n-3 Docosapentaenoic acid-derived protectin D1 promotes resolution of neuroinflammation and arrests epileptogenesis. Brain 2018, 141, 3130–3143. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yamaguchi, Y.; Sharif, S.; Du, X.-Y.; Song, J.J.; Lee, D.M.; Recht, L.D.; Robinson, W.H.; Morser, J.; Leung, L.L.K. Chemerin158K protein is the dominant chemerin isoform in synovial and cerebrospinal fluids but not in plasma. J. Biol. Chem. 2011, 286, 39520–39527. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Du, X.-Y.; Zhao, L.; Morser, J.; Leung, L.L.K. Proteolytic cleavage of chemerin protein is necessary for activation to the active form, Chem157S, which functions as a signaling molecule in glioblastoma. J. Biol. Chem. 2011, 286, 39510–39519. [Google Scholar] [CrossRef]
- Graham, K.L.; Zabel, B.A.; Loghavi, S.; Zuniga, L.A.; Ho, P.P.; Sobel, R.A.; Butcher, E.C. Chemokine-like receptor-1 expression by central nervous system-infiltrating leukocytes and involvement in a model of autoimmune demyelinating disease. J. Immunol. 2009, 183, 6717–6723. [Google Scholar] [CrossRef]
- Tomalka-Kochanowska, J.; Baranowska, B.; Wolinska-Witort, E.; Uchman, D.; Litwiniuk, A.; Martynska, L.; Kalisz, M.; Bik, W.; Kochanowski, J. Plasma chemerin levels in patients with multiple sclerosis. Neuro Endocrinol. Lett. 2014, 35, 218–223. [Google Scholar]
- Wildemann, B.; Oschmann, P.; Reiber, H. Neurologische Labordiagnostik; Georg Thieme Verlag: Stuttgart, Germany, 2006; ISBN 9783131365910. [Google Scholar]
- Tan, L.; Lu, X.; Danser, A.H.J.; Verdonk, K. The Role of Chemerin in Metabolic and Cardiovascular Disease: A Literature Review of Its Physiology and Pathology from a Nutritional Perspective. Nutrients 2023, 15, 2878. [Google Scholar] [CrossRef]
- Schmid, A.; Berghoff, M.; Hochberg, A.; Schäffler, A.; Karrasch, T. CTRP-3 is permeable to the blood-brain barrier and is not regulated by glucose or lipids in vivo. Eur. J. Clin. Investig. 2017, 47, 203–212. [Google Scholar] [CrossRef]
- Kos, K.; Harte, A.L.; da Silva, N.F.; Tonchev, A.; Chaldakov, G.; James, S.; Snead, D.R.; Hoggart, B.; O’Hare, J.P.; McTernan, P.G.; et al. Adiponectin and resistin in human cerebrospinal fluid and expression of adiponectin receptors in the human hypothalamus. J. Clin. Endocrinol. Metab. 2007, 92, 1129–1136. [Google Scholar] [CrossRef]
- Dötsch, J.; Adelmann, M.; Englaro, P.; Dötsch, A.; Hänze, J.; Blum, W.F.; Kiess, W.; Rascher, W. Relation of leptin and neuropeptide Y in human blood and cerebrospinal fluid. J. Neurol. Sci. 1997, 151, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Berghoff, M.; Höpfinger, A.; Rajendran, R.; Karrasch, T.; Schmid, A.; Schäffler, A. Evidence of a Muscle-Brain Axis by Quantification of the Neurotrophic Myokine METRNL (Meteorin-Like Protein) in Human Cerebrospinal Fluid and Serum. J. Clin. Med. 2021, 10, 3271. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Inomata, S.; Okimura, Y.; Iguchi, G.; Fukuoka, H.; Miyake, K.; Koga, D.; Akamatsu, S.; Kasuga, M.; Takahashi, Y. Decreased serum chemerin levels in male Japanese patients with type 2 diabetes: Sex dimorphism. Endocr. J. 2013, 60, 37–44. [Google Scholar] [CrossRef]
- Li, L.; Ma, P.; Huang, C.; Liu, Y.; Zhang, Y.; Gao, C.; Xiao, T.; Ren, P.-G.; Zabel, B.A.; Zhang, J.V. Expression of chemerin and its receptors in rat testes and its action on testosterone secretion. J. Endocrinol. 2014, 220, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Bozaoglu, K.; Bolton, K.; McMillan, J.; Zimmet, P.; Jowett, J.; Collier, G.; Walder, K.; Segal, D. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology 2007, 148, 4687–4694. [Google Scholar] [CrossRef] [PubMed]
- Dranse, H.J.; Muruganandan, S.; Fawcett, J.P.; Sinal, C.J. Adipocyte-secreted chemerin is processed to a variety of isoforms and influences MMP3 and chemokine secretion through an NFkB-dependent mechanism. Mol. Cell. Endocrinol. 2016, 436, 114–129. [Google Scholar] [CrossRef]
- Smolinska, N.; Kiezun, M.; Dobrzyn, K.; Rytelewska, E.; Kisielewska, K.; Gudelska, M.; Zaobidna, E.; Bogus-Nowakowska, K.; Wyrebek, J.; Bors, K.; et al. Expression of Chemerin and Its Receptors in the Porcine Hypothalamus and Plasma Chemerin Levels during the Oestrous Cycle and Early Pregnancy. Int. J. Mol. Sci. 2019, 20, 3887. [Google Scholar] [CrossRef]
- Golden, P.L.; Maccagnan, T.J.; Pardridge, W.M. Human blood-brain barrier leptin receptor. Binding and endocytosis in isolated human brain microvessels. J. Clin. Investig. 1997, 99, 14–18. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Z.; Gong, P.; Zhang, H.; Chen, Y.; Yao, S.; Li, W.; Zhang, Y.; Yu, Y. The Chemerin/CMKLR1 Axis Is Involved in the Recruitment of Microglia to Aβ Deposition through p38 MAPK Pathway. Int. J. Mol. Sci. 2022, 23, 9041. [Google Scholar] [CrossRef]
- Żółkiewicz, J.; Stochmal, A.; Rudnicka, L. The role of adipokines in systemic sclerosis: A missing link? Arch. Dermatol. Res. 2019, 311, 251–263. [Google Scholar] [CrossRef]
- Gonzalez-Ponce, F.; Gamez-Nava, J.I.; Perez-Guerrero, E.E.; Saldaña-Cruz, A.M.; Vazquez-Villegas, M.L.; Ponce-Guarneros, J.M.; Huerta, M.; Trujillo, X.; Contreras-Haro, B.; Rocha-Muñoz, A.D.; et al. Serum chemerin levels: A potential biomarker of joint inflammation in women with rheumatoid arthritis. PLoS ONE 2021, 16, e0255854. [Google Scholar] [CrossRef] [PubMed]
- Graham, K.L.; Zhang, J.V.; Lewén, S.; Burke, T.M.; Dang, T.; Zoudilova, M.; Sobel, R.A.; Butcher, E.C.; Zabel, B.A. A novel CMKLR1 small molecule antagonist suppresses CNS autoimmune inflammatory disease. PLoS ONE 2014, 9, e112925. [Google Scholar] [CrossRef] [PubMed]
- Berg, V.; Sveinbjörnsson, B.; Bendiksen, S.; Brox, J.; Meknas, K.; Figenschau, Y. Human articular chondrocytes express ChemR23 and chemerin; ChemR23 promotes inflammatory signalling upon binding the ligand chemerin(21-157). Arthritis Res. Ther. 2010, 12, R228. [Google Scholar] [CrossRef] [PubMed]
- Zegar, A.; Godlewska, U.; Kozłowska-Chmielewska, D.; Majewski, P.; Zabel, B.A.; Cichy, J. Chemerin-Derived Peptide Val66-Pro85 Is Effective in Limiting Methicillin-Resistant S. aureus Skin Infection. Front. Microbiol. 2021, 12, 742610. [Google Scholar] [CrossRef]
- Suzuki, Y.; Hong, Y.H.; Song, S.H.; Ardiyanti, A.; Kato, D.; So, K.H.; Katoh, K.; Roh, S.G. The Regulation of Chemerin and CMKLR1 Genes Expression by TNF-α, Adiponectin, and Chemerin Analog in Bovine Differentiated Adipocytes. Asian-Australas. J. Anim. Sci. 2012, 25, 1316–1321. [Google Scholar] [CrossRef]
- Zabel, B.A.; Kwitniewski, M.; Banas, M.; Zabieglo, K.; Murzyn, K.; Cichy, J. Chemerin regulation and role in host defense. Am. J. Clin. Exp. Immunol. 2014, 3, 1–19. [Google Scholar]
- Bondue, B.; Vosters, O.; de Nadai, P.; Glineur, S.; de Henau, O.; Luangsay, S.; van Gool, F.; Communi, D.; de Vuyst, P.; Desmecht, D.; et al. ChemR23 dampens lung inflammation and enhances anti-viral immunity in a mouse model of acute viral pneumonia. PLoS Pathog. 2011, 7, e1002358. [Google Scholar] [CrossRef]
- Chen, Y.; Song, Y.; Wang, Z.; Lai, Y.; Yin, W.; Cai, Q.; Han, M.; Cai, Y.; Xue, Y.; Chen, Z.; et al. The chemerin-CMKLR1 axis in keratinocytes impairs innate host defense against cutaneous Staphylococcus aureus infection. Cell. Mol. Immunol. 2024, 21, 533–545. [Google Scholar] [CrossRef]
- Abareshi, A.; Momenabadi, S.; Vafaei, A.A.; Bandegi, A.R.; Vakili, A. Neuroprotective Effects of Chemerin on a Mouse Stroke Model: Behavioral and Molecular Dimensions. Neurochem. Res. 2021, 46, 3301–3313. [Google Scholar] [CrossRef]
- Huang, Y.; Li, S.; Yang, Y.; Li, K.; Wen, L.; Li, J. Chemerin 15 peptide reduces neuroinflammation via the ChemR23 receptor after ischemia-reperfusion injury. Neural Regen. Res. 2024; online ahead of print. [Google Scholar] [CrossRef]
- Yu, C.; Deng, X.-J.; Xu, D. Microglia in epilepsy. Neurobiol. Dis. 2023, 185, 106249. [Google Scholar] [CrossRef]
- Elhady, M.; Youness, E.R.; Gafar, H.S.; Abdel Aziz, A.; Mostafa, R.S.I. Circulating irisin and chemerin levels as predictors of seizure control in children with idiopathic epilepsy. Neurol. Sci. 2018, 39, 1453–1458. [Google Scholar] [CrossRef]
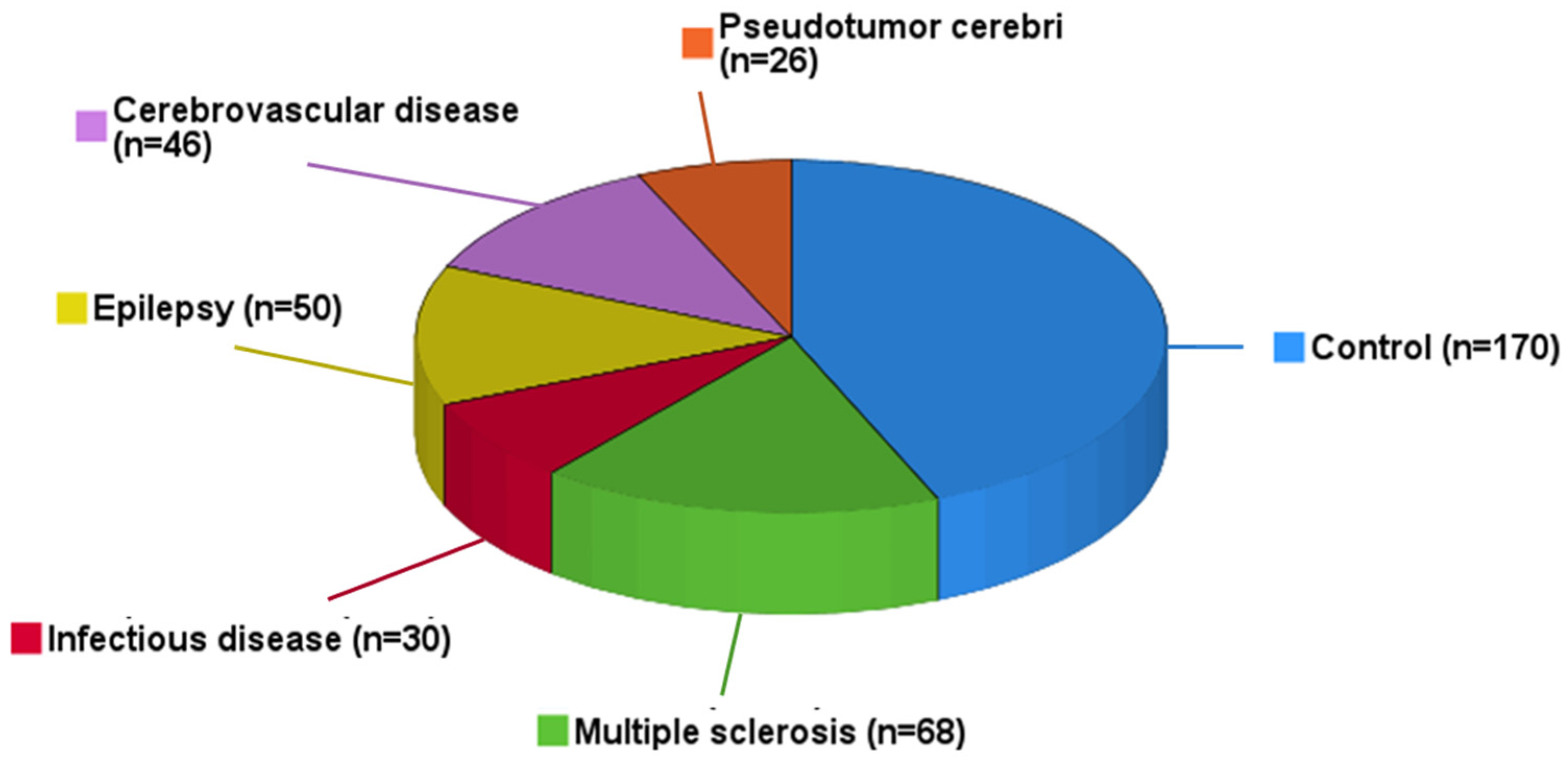

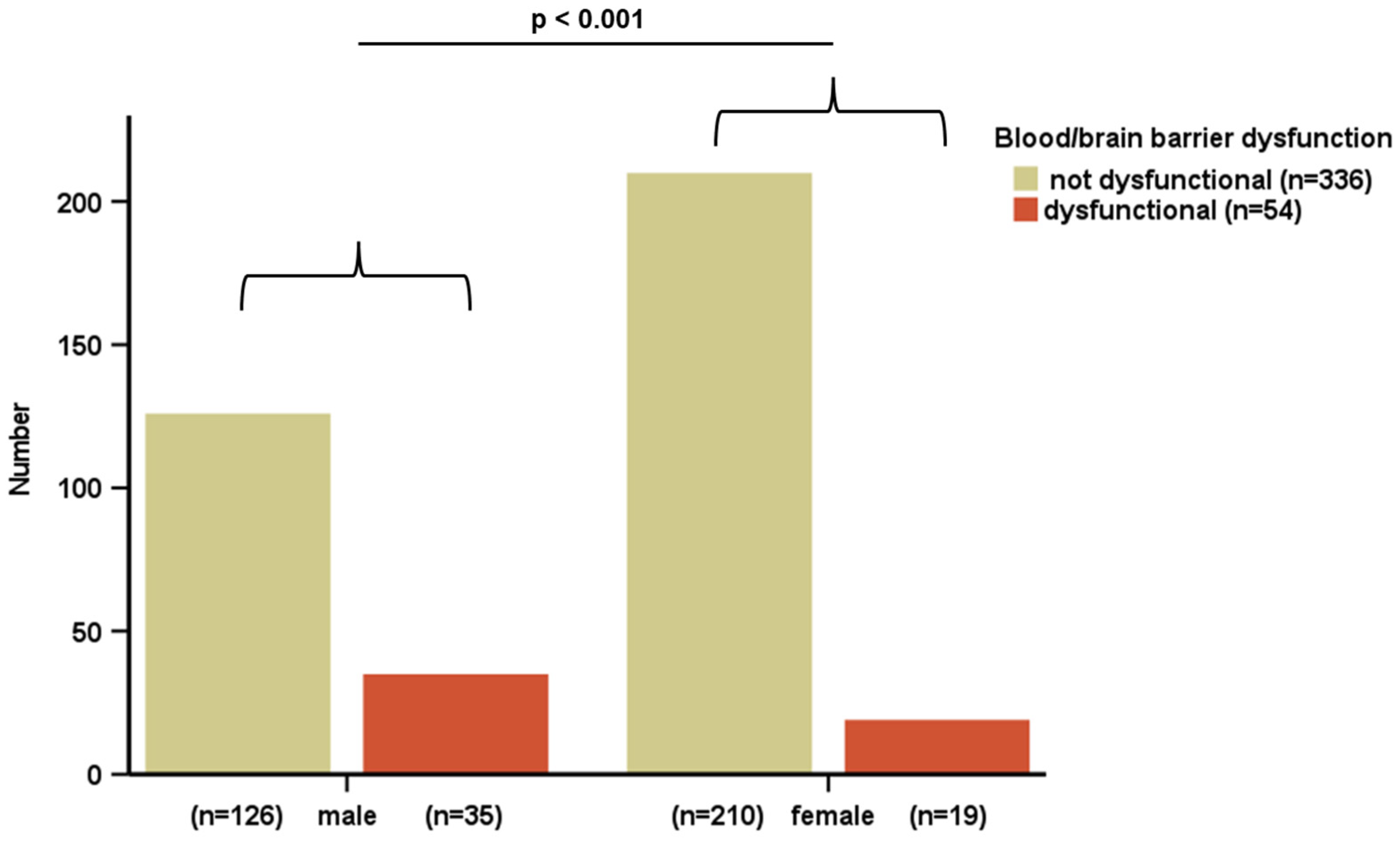

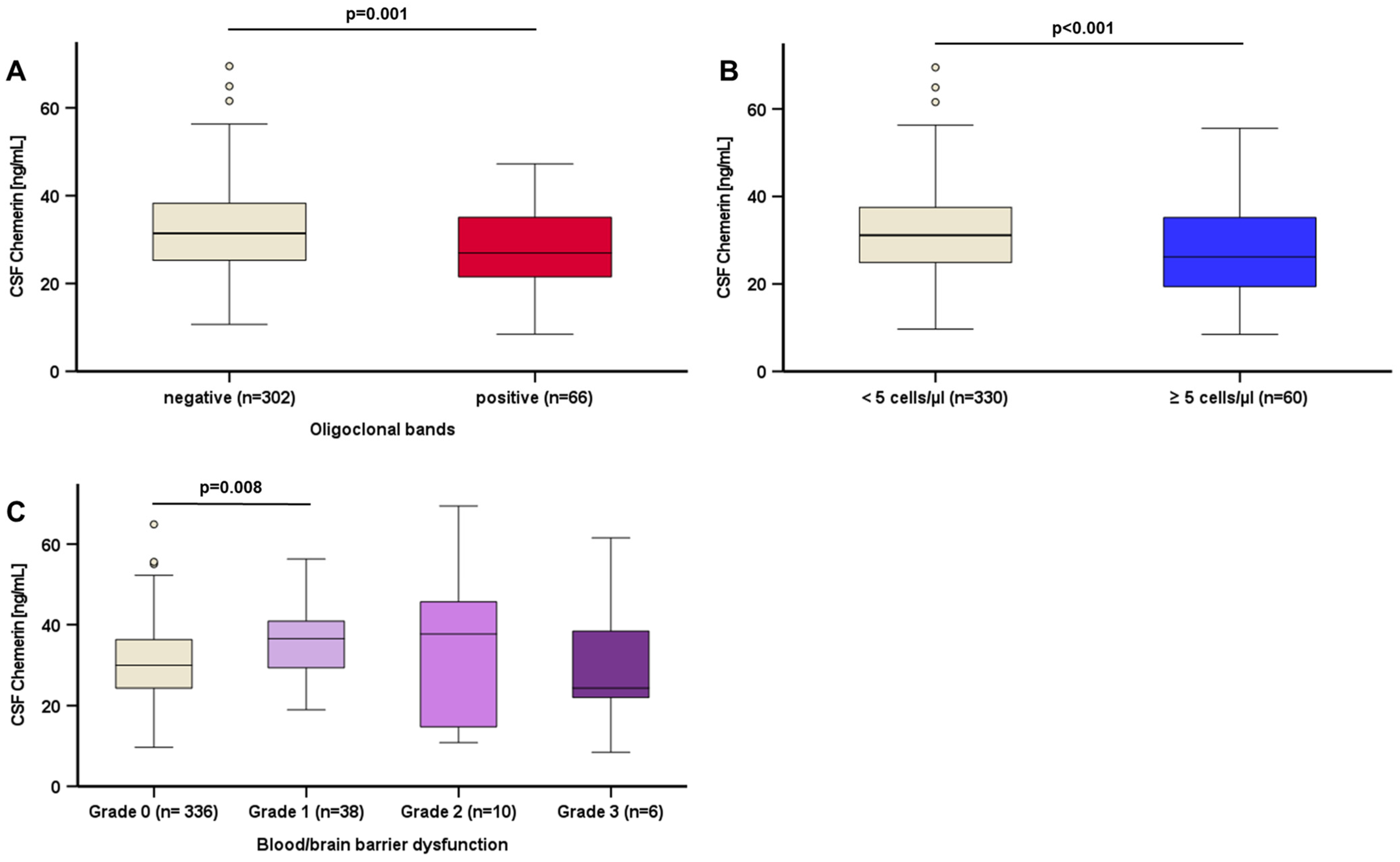
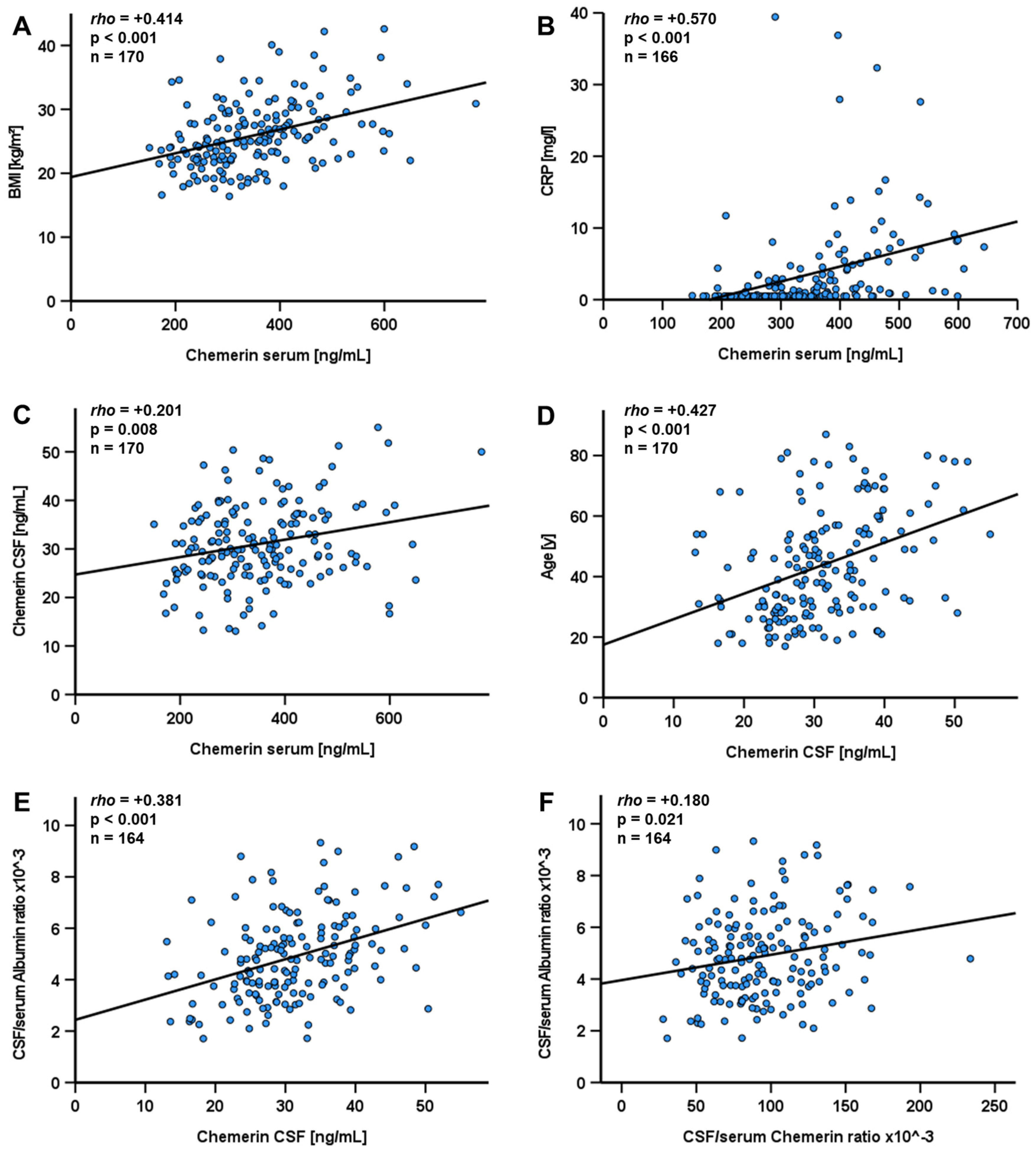
| Study Population (n = 390) | |
|---|---|
| Men, n (%) | 161 (41.3) |
| Women, n (%) | 229 (58.7) |
| Anthropometric parameters | |
| Age [y] | 45.6 ± 17.9 [17–87] |
| Weight [kg] | 79.9 ± 20.2 [44.0–190.0] |
| Height [cm] | 171.3 ± 8.9 [146–194] |
| Mean BMI (kg/m2) | 27.19 ± 6.31 [16.4–62.0] |
| BMI < 25 kg/m2, n (%) | 161 (41.3) |
| BMI ≥ 25 kg/m2, n (%) | 229 (58.7) |
| Serum parameters | |
| Carbohydrate metabolism | |
| Random plasma glucose [mg/dL] | 108.97 ± 35.43 [62–308] |
| HbA1c [%] | 5.72 ± 0.74 [2.0–8.3] |
| Diabetes mellitus type 2, n (%) | 27 (6.9) |
| Lipoprotein metabolism | |
| Total cholesterol [mg/dL] | 192 ± 48 [82–335] |
| LDL cholesterol [mg/dL] | 126 ± 47 [33–287] |
| HDL cholesterol [mg/dL] | 50 ± 16 [14–102] |
| Triglycerides [mg/dL] | 149 ± 91 [35–559] |
| Inflammation | |
| CRP [mg/L] | 6.09 ± 18.02 [0.50–284.11] |
| Leukocytes [giga/L] | 7.71 ± 2.73 [1.7–22.5] |
| Total protein [g/L] | 70.65 ± 6.13 [52–85] |
| Total albumin [g/L] | 42.42 ± 4.76 [22.8–55.3] |
| GOT [U/L] | 22.22 ± 13.31 [8–124] |
| GPT [U/L] | 28.06 ± 30.15 [7–445] |
| Bilirubin [mg/dL] | 0.66 ± 0.66 [0.1–9.0] |
| Creatinine [mg/dL] | 0.82 ± 0.66 [0.4–13.3] |
| Urea [mg/dL] | 27.89 ± 11.49 [9–129] |
| LDH [U/L] | 191.68 ± 44.44 [92–375] |
| CSF parameters | |
| Cell count [cells/µL] | 11.77 ± 55.35 [0–608] |
| Total protein [g/L] | 1.09 ± 13.30 [0.118–263] |
| Albumin [g/L] | 0.26 ± 0.17 [0.06–1.56] |
| Lactate [mmol/L] | 1.77 ± 0.41 [1.00–3.44] |
| IgG [g/L] | 0.037 ± 0.048 [0.007–0.733] |
| IgA [g/L] | 0.005 ± 0.022 [0.00027–0.34300] |
| IgM [g/L] | 0.001 ± 0.005 [0.00000–0.059700] |
| CSF/serum albumin ratio × 10−3 | 6.18 ± 4.25 [1.68–40.52] |
| Adipokines | |
| Chemerin in serum [ng/mL] | 348.02 ± 118.54 [92.34–864.27] |
| Chemerin in CSF [ng/mL] | 31.02 ± 9.80 [8.48–69.51] |
| CSF/serum chemerin ratio × 10−3 | 96.31 ± 36.78 [21.37–233.583] |
| Neurological diseases/Clinical subgroups | |
| Control, n (%) | 170 (43.6) |
| Multiple sclerosis, n (%) | 68 (17.4) |
| Infectious disease, n (%) | 30 (7.7) |
| Epilepsy, n (%) | 50 (12.8) |
| Cerebrovascular disease, n (%) | 46 (11.8) |
| Pseudotumor cerebri, n (%) | 26 (6.7) |
| (A) | MS (n = 68) | Matched Controls (n = 68) |
|---|---|---|
| Chemerin serum [ng/mL] | 297.71 ± 113.26 [92.34–707.25] * | 338.86 ± 101.58 [168.65–599.64] * |
| Chemerin CSF [ng/mL] | 27.80 ± 8.69 [10.70–46.92] | 30.26 ± 8.01 [13.27–50.39] |
| Chemerin CSF/serum ratio × 10−3 | 102.76 ± 40.17 [38.73–221.18] | 95.83 ± 32.74 [27.79–167.31] |
| (B) | ID (n = 30) | Matched Controls (n = 30) |
| Chemerin serum [ng/mL] | 335.06 ± 90.89 [189.66–550.76] | 364.57 ± 141.82 [150.22–775.28] |
| Chemerin CSF [ng/mL] | 26.74 ± 10.67 [8.48–55.59] * | 33.48 ± 8.28 [14.17–51.84] * |
| Chemerin CSF/serum ratio × 10−3 | 81.92 ± 31.05 [33.40–159.10] * | 101.83 ± 38.69 [39.82–233.58] * |
| (C) | Epilepsy (n = 50) | Matched Controls (n = 50) |
| Chemerin serum [ng/mL] | 346.03 ± 109.06 [146.26–669.26] | 354.20 ± 108.30 [168.65–649.70] |
| Chemerin CSF [ng/mL] | 34.77 ± 12.48 [9.69–69.51] * | 30.41 ± 8.84 [13.07–51.25] * |
| Chemerin CSF/serum ratio × 10−3 | 106.08 ± 38.49 [21.37–226.71] | 93.81 ± 37.73 [27.79–193.04] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Höpfinger, A.; Behrendt, M.; Schmid, A.; Karrasch, T.; Schäffler, A.; Berghoff, M. A Cross-Sectional Study: Systematic Quantification of Chemerin in Human Cerebrospinal Fluid. Biomedicines 2024, 12, 2508. https://doi.org/10.3390/biomedicines12112508
Höpfinger A, Behrendt M, Schmid A, Karrasch T, Schäffler A, Berghoff M. A Cross-Sectional Study: Systematic Quantification of Chemerin in Human Cerebrospinal Fluid. Biomedicines. 2024; 12(11):2508. https://doi.org/10.3390/biomedicines12112508
Chicago/Turabian StyleHöpfinger, Alexandra, Manuel Behrendt, Andreas Schmid, Thomas Karrasch, Andreas Schäffler, and Martin Berghoff. 2024. "A Cross-Sectional Study: Systematic Quantification of Chemerin in Human Cerebrospinal Fluid" Biomedicines 12, no. 11: 2508. https://doi.org/10.3390/biomedicines12112508
APA StyleHöpfinger, A., Behrendt, M., Schmid, A., Karrasch, T., Schäffler, A., & Berghoff, M. (2024). A Cross-Sectional Study: Systematic Quantification of Chemerin in Human Cerebrospinal Fluid. Biomedicines, 12(11), 2508. https://doi.org/10.3390/biomedicines12112508






