Oncological Outcomes of Partial Gland Ablation Using High-Intensity Focused Ultrasound After Additional Confirmatory Transperineal Mapping Biopsy in Men with Prostate Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.3. mpMRI Protocol and Data Interpretation
2.4. Protocol for TRUS-Guided Biopsy
2.5. Protocol for TPMB
2.6. Histopathologic Analysis
2.7. Protocol for HIFU
2.8. Follow-Up Protocol
2.9. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Wilt, T.J.; Brawer, M.K.; Jones, K.M.; Barry, M.J.; Aronson, W.J.; Fox, S.; Gingrich, J.R.; Wei, J.T.; Gilhooly, P.; Grob, B.M.; et al. Radical prostatectomy versus observation for localized prostate cancer. N. Engl. J. Med. 2012, 367, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Wilt, T.J.; Vo, T.N.; Langsetmo, L.; Dahm, P.; Wheeler, T.; Aronson, W.J.; Cooperberg, M.R.; Taylor, B.C.; Brawer, M.K. Radical Prostatectomy or Observation for Clinically Localized Prostate Cancer: Extended Follow-up of the Prostate Cancer Intervention Versus Observation Trial (PIVOT). Eur. Urol. 2020, 77, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, F.C.; Donovan, J.L.; Lane, J.A.; Mason, M.; Metcalfe, C.; Holding, P.; Davis, M.; Peters, T.J.; Turner, E.L.; Martin, R.M.; et al. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N. Engl. J. Med. 2016, 375, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Boorjian, S.A.; Eastham, J.A.; Graefen, M.; Guillonneau, B.; Karnes, R.J.; Moul, J.W.; Schaeffer, E.M.; Stief, C.; Zorn, K.C. A critical analysis of the long-term impact of radical prostatectomy on cancer control and function outcomes. Eur. Urol. 2012, 61, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Sanda, M.G.; Dunn, R.L.; Michalski, J.; Sandler, H.M.; Northouse, L.; Hembroff, L.; Lin, X.; Greenfield, T.K.; Litwin, M.S.; Saigal, C.S.; et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N. Engl. J. Med. 2008, 358, 1250–1261. [Google Scholar] [CrossRef]
- Donovan, J.L.; Hamdy, F.C.; Lane, J.A.; Mason, M.; Metcalfe, C.; Walsh, E.; Blazeby, J.M.; Peters, T.J.; Holding, P.; Bonnington, S.; et al. Patient-Reported Outcomes after Monitoring, Surgery, or Radiotherapy for Prostate Cancer. N. Engl. J. Med. 2016, 375, 1425–1437. [Google Scholar] [CrossRef]
- van den Bergh, R.C.; Korfage, I.J.; Bangma, C.H. Psychological aspects of active surveillance. Curr. Opin. Urol. 2012, 22, 237–242. [Google Scholar] [CrossRef]
- Ahmed, H.U.; Zacharakis, E.; Dudderidge, T.; Armitage, J.N.; Scott, R.; Calleary, J.; Illing, R.; Kirkham, A.; Freeman, A.; Ogden, C.; et al. High-intensity-focused ultrasound in the treatment of primary prostate cancer: The first UK series. Br. J. Cancer 2009, 101, 19–26. [Google Scholar] [CrossRef]
- Ong, S.; Chen, K.; Grummet, J.; Yaxley, J.; Scheltema, M.J.; Stricker, P.; Tay, K.J.; Lawrentschuk, N. Guidelines of guidelines: Focal therapy for prostate cancer, is it time for consensus? BJU Int. 2023, 131, 20–31. [Google Scholar] [CrossRef]
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer—2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef] [PubMed]
- Barentsz, J.O.; Choyke, P.L.; Cornud, F.; Haider, M.A.; Macura, K.J.; Margolis, D.; Shtern, F.; Padhani, A.R.; Tempany, C.M.; Thoeny, H.C.; et al. Reply to Erik Rud and Eduard Baco’s Letter to the Editor re: Re: Jeffrey C. Weinreb, Jelle O. Barentsz, Peter L. Choyke; et al. PI-RADS Prostate Imaging—Reporting and Data System: 2015, Version 2. Eur. Urol. 2016;69:16–40. Eur. Urol. 2016, 70, e137–e138. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Kang, M.; Jeong, B.C.; Seo, S.I.; Jeon, S.S.; Lee, H.M.; Jeon, H.G. The clinical utility of transperineal template-guided saturation prostate biopsy for risk stratification after transrectal ultrasound-guided biopsy. Investig. Clin. Urol. 2019, 60, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Loeb, S.; Vellekoop, A.; Ahmed, H.U.; Catto, J.; Emberton, M.; Nam, R.; Rosario, D.J.; Scattoni, V.; Lotan, Y. Systematic review of complications of prostate biopsy. Eur. Urol. 2013, 64, 876–892. [Google Scholar] [CrossRef]
- Oerther, B.; Engel, H.; Bamberg, F.; Sigle, A.; Gratzke, C.; Benndorf, M. Cancer detection rates of the PI-RADSv2.1 assessment categories: Systematic review and meta-analysis on lesion level and patient level. Prostate Cancer Prostatic Dis. 2022, 25, 256–263. [Google Scholar] [CrossRef]
- Hong, S.K.; Lee, H. Outcomes of partial gland ablation using high intensity focused ultrasound for prostate cancer. Urol. Oncol. 2022, 40, 193.e1–193.e5. [Google Scholar] [CrossRef]
- Park, S.Y.; Jung, D.C.; Oh, Y.T.; Cho, N.H.; Choi, Y.D.; Rha, K.H.; Hong, S.J.; Han, K. Prostate Cancer: PI-RADS Version 2 Helps Preoperatively Predict Clinically Significant Cancers. Radiology 2016, 280, 108–116. [Google Scholar] [CrossRef]
- Taira, A.V.; Merrick, G.S.; Galbreath, R.W.; Andreini, H.; Taubenslag, W.; Curtis, R.; Butler, W.M.; Adamovich, E.; Wallner, K.E. Performance of transperineal template-guided mapping biopsy in detecting prostate cancer in the initial and repeat biopsy setting. Prostate Cancer Prostatic Dis. 2010, 13, 71–77. [Google Scholar] [CrossRef]
- Voss, J.; Pal, R.; Ahmed, S.; Hannah, M.; Jaulim, A.; Walton, T. Utility of early transperineal template-guided prostate biopsy for risk stratification in men undergoing active surveillance for prostate cancer. BJU Int. 2018, 121, 863–870. [Google Scholar] [CrossRef]
- Barentsz, J.O.; Richenberg, J.; Clements, R.; Choyke, P.; Verma, S.; Villeirs, G.; Rouviere, O.; Logager, V.; Futterer, J.J.; European Society of Urogenital Radiology. ESUR prostate MR guidelines 2012. Eur. Radiol. 2012, 22, 746–757. [Google Scholar] [CrossRef]
- Hansen, N.; Patruno, G.; Wadhwa, K.; Gaziev, G.; Miano, R.; Barrett, T.; Gnanapragasam, V.; Doble, A.; Warren, A.; Bratt, O.; et al. Magnetic Resonance and Ultrasound Image Fusion Supported Transperineal Prostate Biopsy Using the Ginsburg Protocol: Technique, Learning Points, and Biopsy Results. Eur. Urol. 2016, 70, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Crawford, E.D.; Wilson, S.S.; Torkko, K.C.; Hirano, D.; Stewart, J.S.; Brammell, C.; Wilson, R.S.; Kawata, N.; Sullivan, H.; Lucia, M.S.; et al. Clinical staging of prostate cancer: A computer-simulated study of transperineal prostate biopsy. BJU Int. 2005, 96, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.I.; Egevad, L.; Amin, M.B.; Delahunt, B.; Srigley, J.R.; Humphrey, P.A.; Grading, C. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am. J. Surg. Pathol. 2016, 40, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Bjurlin, M.A.; Mendhiratta, N.; Wysock, J.S.; Taneja, S.S. Multiparametric MRI and targeted prostate biopsy: Improvements in cancer detection, localization, and risk assessment. Cent. Eur. J. Urol. 2016, 69, 9–18. [Google Scholar] [CrossRef]
- Nafie, S.; Wanis, M.; Khan, M. The Efficacy of Transrectal Ultrasound Guided Biopsy Versus Transperineal Template Biopsy of the Prostate in Diagnosing Prostate Cancer in Men with Previous Negative Transrectal Ultrasound Guided Biopsy. Urol. J. 2017, 14, 3008–3012. [Google Scholar]
- Rischmann, P.; Gelet, A.; Riche, B.; Villers, A.; Pasticier, G.; Bondil, P.; Jung, J.L.; Bugel, H.; Petit, J.; Toledano, H.; et al. Focal High Intensity Focused Ultrasound of Unilateral Localized Prostate Cancer: A Prospective Multicentric Hemiablation Study of 111 Patients. Eur. Urol. 2017, 71, 267–273. [Google Scholar] [CrossRef]
- Bass, R.; Fleshner, N.; Finelli, A.; Barkin, J.; Zhang, L.; Klotz, L. Oncologic and Functional Outcomes of Partial Gland Ablation with High Intensity Focused Ultrasound for Localized Prostate Cancer. J. Urol. 2019, 201, 113–119. [Google Scholar] [CrossRef]
| Variables | Value |
|---|---|
| Number of patients | 96 (100) |
| Age, years | |
| Median (IQR) | 65.0 (60.0–72.0) |
| Mean (SD) | 65.3 (8.7) |
| Body mass index, kg/m2 | |
| Median (IQR) | 24.8 (22.8–26.8) |
| Mean (SD) | 24.9 (2.6) |
| Hypertension, n (%) | 48 (50.0) |
| Diabetes mellitus, n (%) | 21 (21.9) |
| PSA, ng/dL | |
| Median (IQR) | 5.20 (3.71–7.81) |
| Mean (SD) | 6.69 (5.60) |
| Prostate volume, mL | |
| Median (IQR) | 34.6 (25.5–46.1) |
| Mean (SD) | 38.1 (18.6) |
| PSA density | |
| Median (IQR) | 0.12 (0.10–0.21) |
| Mean (SD) | 0.16 (0.19) |
| PI-RADS score in mpMRI | |
| 1–2 | 25 (26.0) |
| 3 | 57 (59.4) |
| 4 | 14 (14.6) |
| Clinical stage | |
| cT1 | 25 (26.0) |
| cT2a | 32 (33.3) |
| cT2b | 22 (22.9) |
| cT2c | 17 (17.8) |
| Variables | TRUS-Guided Biopsy | Transperineal Mapping Biopsy |
|---|---|---|
| Number of total cores, n (%) | ||
| Median (IQR) | 12.0 (12.0–12.0) | 26.0 (24.0–36.0) |
| Mean (SD) | 12.2 (1.7) | 29.2 (5.8) |
| Number of positive cores, n (%) | ||
| Median (IQR) | 1.0 (1.0–2.0) | 2.0 (1.0–3.0) |
| Mean (SD) | 1.8 (1.5) | 2.1 (2.1) |
| Number of target cores, n (%) | ||
| Median (IQR) | 2.0 (2.0–3.0) | |
| Mean (SD) | 2.2 (2.1) | |
| Number of positive target cores, n (%) | ||
| Median (IQR) | 1.0 (1–2) | |
| Mean (SD) | 1.5 (1.4) | |
| Gleason grade, n (%) | ||
| Benign | 25 (26.0) | |
| Gleason grade 1 | 86 (89.6) | 60 (62.5) |
| Gleason grade 2 | 8 (8.3) | 9 (9.4) |
| Gleason grade 3 | 2 (2.1) | 2 (2.1) |
| Maximal tumor involvement, % | ||
| Median (IQR) | 10 (5.0–25.0) | 10.0 (2.5–25.0) |
| Mean (SD) | 17.3 (18.6) | 18.5 (24.9) |
| No. | Age | PSA | Prostate Volume | PSAD | TRUS-Guided Biopsy | Transperineal Mapping Biopsy | Treatment Plan | |
|---|---|---|---|---|---|---|---|---|
| From | To | |||||||
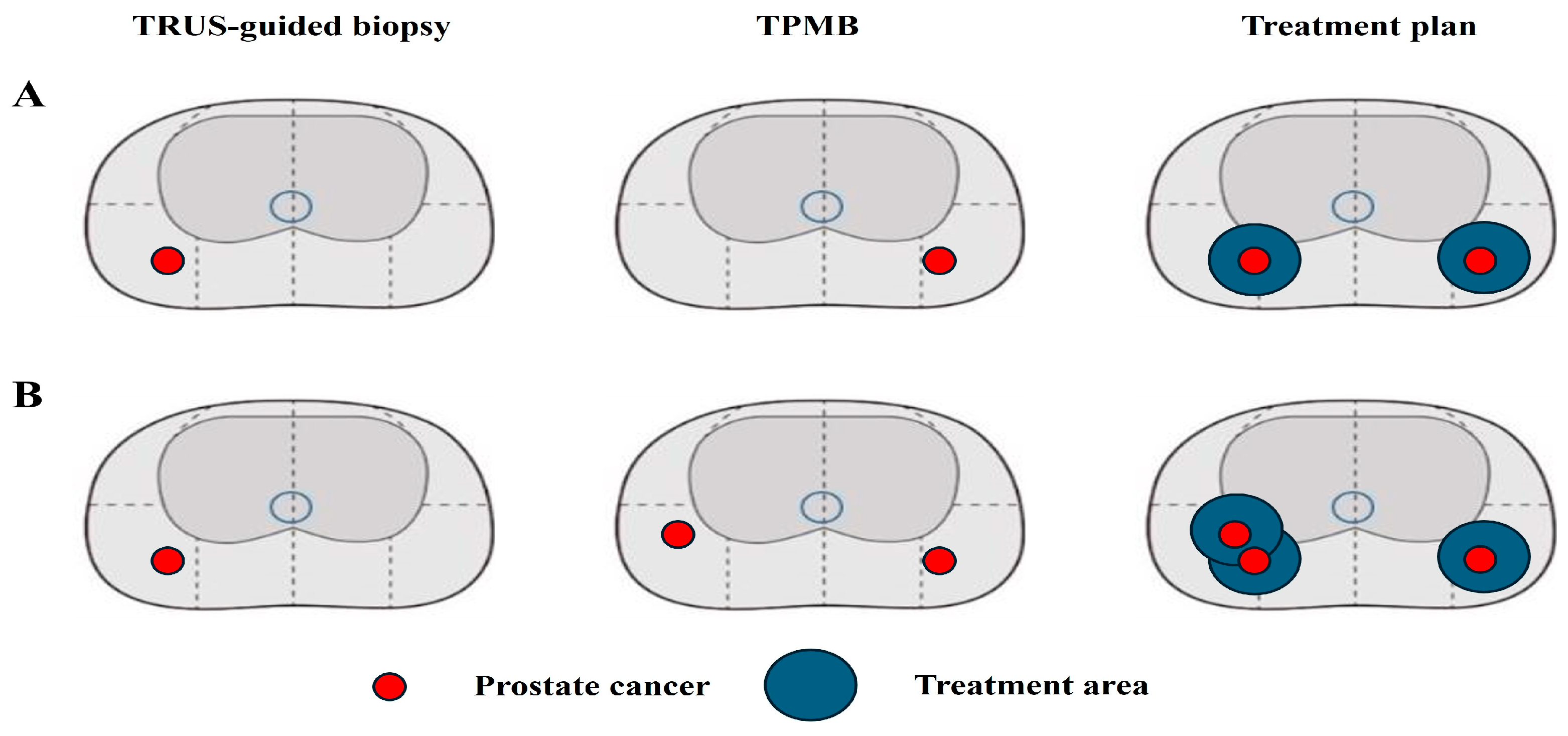
| 1 | 45 | 5.24 | 28.2 | 0.19 | 1/12(+), Left, 3 + 3 | 1/26(+), Right, 3 + 3 | Left PGA | Bilateral PGA |
| 2 | 61 | 5.10 | 35.4 | 0.14 | 2/12(+), Right, 3 + 3 | 1/24(+), Left, 3 + 3 | Right PGA | Bilateral PGA |
| 3 | 63 | 6.36 | 33.4 | 0.19 | 1/12(+), Right, 3 + 3 | 3/24(+), Left, 3 + 3 | Right PGA | Bilateral PGA |
| 4 | 72 | 3.70 | 45.7 | 0.08 | 2/10(+), Right, 3 + 3 | 3/36, bilateral, 3 + 3 | Right PGA | Bilateral PGA |
| 5 | 70 | 4.90 | 35.4 | 0.14 | 2/12(+), Right, 3 + 3 | 1/24(+), Left, 3 + 3 | Right PGA | Bilateral PGA |
| 6 | 61 | 3.48 | 20.4 | 0.17 | 1/12(+), Right, 3 + 3 | 2/24(+), Left, 3 + 3 | Right PGA | Bilateral PGA |
| 7 | 55 | 5.78 | 61.9 | 0.09 | 1/12(+), Right, 3 + 3 | 4/36(+), bilateral, 3 + 3 | Right PGA | Bilateral PGA |
| 8 | 62 | 6.42 | 39.4 | 0.16 | 1/12(+), Left, 3 + 3 | 2/24(+), bilateral,3 + 3 | Left PGA | Bilateral PGA |
| 9 | 71 | 3.86 | 44.2 | 0.09 | 1/12(+), Right, 3 + 4 | 2/36, bilateral, 3 + 3 | Right PGA | Bilateral PGA |
| 10 | 59 | 2.95 | 26.1 | 0.11 | 2/12(+), Right, 3 + 3 | 2/24(+), bilateral,3 + 3 | Right PGA | Bilateral PGA |
| 11 | 68 | 4.62 | 23.0 | 0.20 | 1/12(+), Left, 3 + 4 | 1/24(+), Right, 3 + 3 | Left PGA | Bilateral PGA |
| 12 | 73 | 7.36 | 53.6 | 0.14 | 1/12(+), Left, 3 + 3 | 2/36, bilateral, 3 + 3 | Left PGA | Bilateral PGA |
| 13 | 74 | 8.02 | 62.5 | 0.13 | 1/12(+), Right, 3 + 3 | 2/36, bilateral, 3 + 3 | Right PGA | Bilateral PGA |
| n (%) | |
|---|---|
| Any PCa-positive biopsy after PGA using HIFU | 13 (13.5) |
| Infield-positive | 8 (8.3) |
| Outfield-positive | 3 (3.1) |
| Both positive | 2 (2.1) |
| csPCa-positive biopsy after PGA using HIFU | 7 (7.3) |
| Infield-positive | 3 (3.1) |
| Outfield-positive | 2 (2.1) |
| Both positive | 2 (2.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Song, W. Oncological Outcomes of Partial Gland Ablation Using High-Intensity Focused Ultrasound After Additional Confirmatory Transperineal Mapping Biopsy in Men with Prostate Cancer. Biomedicines 2024, 12, 2487. https://doi.org/10.3390/biomedicines12112487
Lee J, Song W. Oncological Outcomes of Partial Gland Ablation Using High-Intensity Focused Ultrasound After Additional Confirmatory Transperineal Mapping Biopsy in Men with Prostate Cancer. Biomedicines. 2024; 12(11):2487. https://doi.org/10.3390/biomedicines12112487
Chicago/Turabian StyleLee, Jihwan, and Wan Song. 2024. "Oncological Outcomes of Partial Gland Ablation Using High-Intensity Focused Ultrasound After Additional Confirmatory Transperineal Mapping Biopsy in Men with Prostate Cancer" Biomedicines 12, no. 11: 2487. https://doi.org/10.3390/biomedicines12112487
APA StyleLee, J., & Song, W. (2024). Oncological Outcomes of Partial Gland Ablation Using High-Intensity Focused Ultrasound After Additional Confirmatory Transperineal Mapping Biopsy in Men with Prostate Cancer. Biomedicines, 12(11), 2487. https://doi.org/10.3390/biomedicines12112487





