Individualized and Controlled Exercise Training Improves Fatigue and Exercise Capacity in Patients with Long-COVID
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval, Recruitment, and Study Cohort
2.2. Study Design and Intervention
2.3. Testing Protocol
2.4. Exercise Intervention
2.5. Data Analysis
3. Results
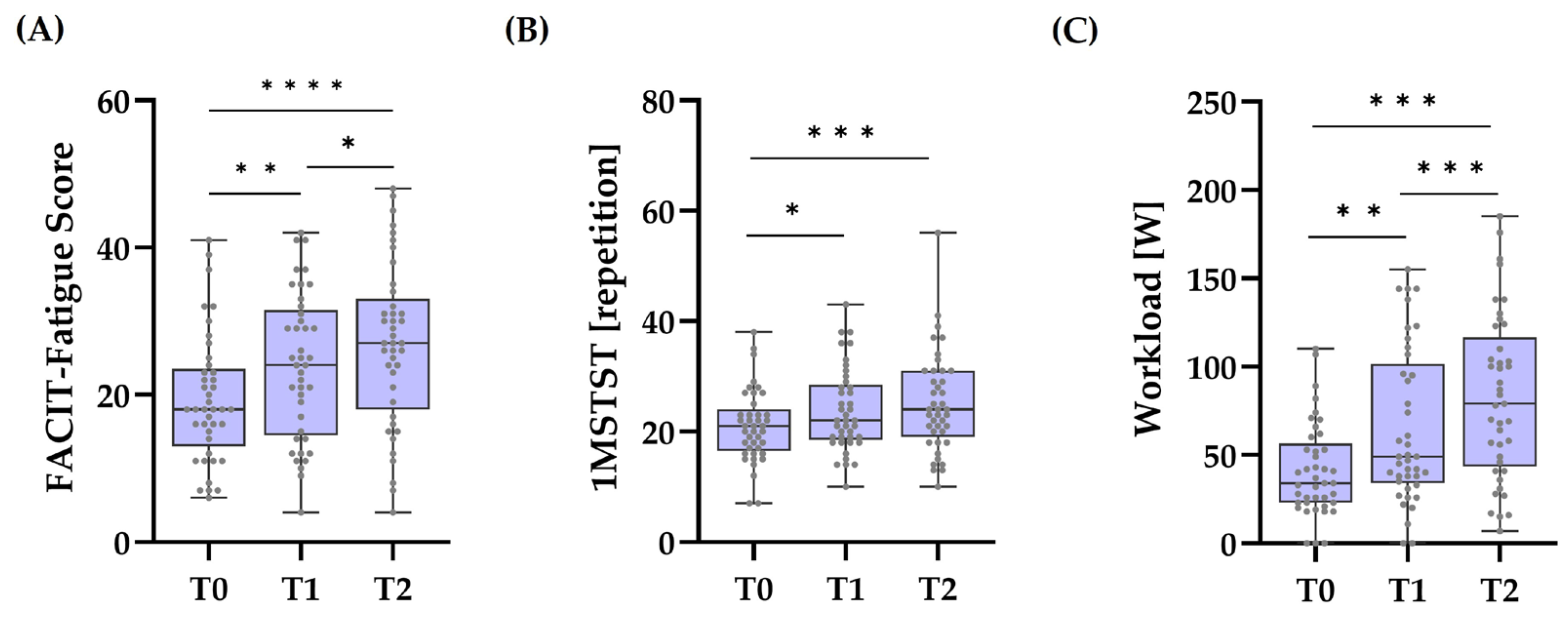
3.1. Effects of Time
3.2. Effects of Sex
3.3. Effects of Training Sessions
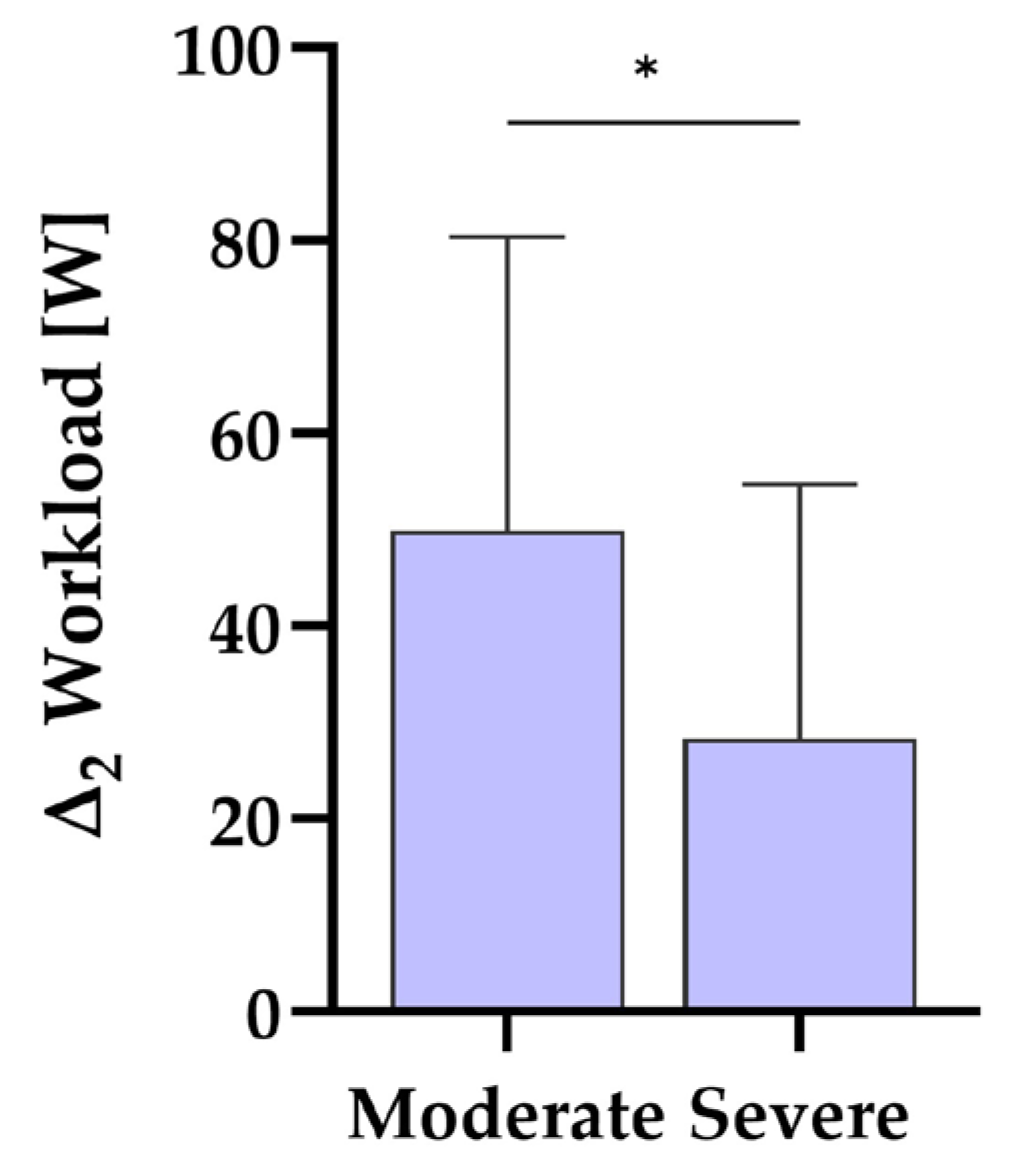
3.4. Effects of Symptom Severity
3.4.1. Change in PCFS Score After 12 Weeks of Intervention
3.4.2. Change in Workload, Fatigue Score, and 1MSTST After 12 Weeks Related to Initial PCS Score
3.5. Correlation Analyses
4. Discussion
4.1. Main Effect of Time
4.2. The Effect of Sex
4.3. The Influence of Symptom Severity
4.4. The Influence of Completed Training Sessions
4.5. Relationships of Outcomes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. Interaction Effects of Time and Sex
Appendix A.2. Completed Training Sessions (CTS) and Change Scores
Appendix A.2.1. Relationship Analyses
Appendix A.2.2. Difference Analyses Related to Number of CTS
Appendix A.3. Interaction Effects of PCS Score and Change Scores
Appendix A.4. Relationships between Outcomes
Appendix B
| PCFS | Fatigue | 1MSTST | WL | |
|---|---|---|---|---|
| T0 | 3 | 19.39 (8.72) **,*** | 20.98 (6.59) *,*** | 41.29 (26.47) **,*** |
| T1 | 3 | 23.95 (9.92) **,* | 23.83 (7.59) * | 64.98 (43.88) **,*** |
| T2 | 3 | 26.80 (11.11) ***,* | 25.07 (9.02) *** | 82.76 (46.97) ***,*** |
| n | Fatigue | 1MSTST | WL | |
|---|---|---|---|---|
| CTS, Group 1 | 13 | 7.62 (12.57) | 5.69 (6.57) | 29.15 (21.57) * |
| CTS, Group 2 | 17 | 6.41 (7.48) | 4.00 (5.61) | 38.12 (24.46) |
| CTS, Group 3 | 11 | 8.73 (7.59) | 2.36 (3.85) | 61.18 (39.67) * |
| PCS, moderate | 24 | 7.50 (8.68) | 3.96 (7.06) | 51.25 (30.38) * |
| PCS, severe | 17 | 7.29 (10.22) | 4.29 (2.39) | 27.65 (25.71) * |
References
- Al-Aly, Z.; Davis, H.; McCorkell, L.; Soares, L.; Wulf-Hanson, S.; Iwasaki, A.; Topol, E.J. Long COVID Science, Research and Policy. Nat. Med. 2024, 30, 2148–2164. [Google Scholar] [CrossRef] [PubMed]
- Gloeckl, R.; Leitl, D.; Schneeberger, T.; Jarosch, I.; Koczulla, A.R. Rehabilitative Interventions in Patients with Persistent Post COVID-19 Symptoms—A Review of Recent Advances and Future Perspectives. Eur. Arch. Psychiatry Clin. Neurosci. 2023. [Google Scholar] [CrossRef] [PubMed]
- Robert Koch Institut. Long COVID. 2023. Available online: https://www.rki.de/SharedDocs/FAQ/NCOV2019/FAQ_Liste_Gesundheitliche_Langzeitfolgen.html (accessed on 22 August 2023).
- Peter, R.S.; Nieters, A.; Kräusslich, H.-G.; Brockmann, S.O.; Göpel, S.; Kindle, G.; Merle, U.; Steinacker, J.M.; Rothenbacher, D.; Kern, W.V. Post-Acute Sequelae of COVID-19 Six to 12 Months after Infection: Population Based Study. BMJ 2022, 379, e071050. [Google Scholar] [CrossRef]
- Alkodaymi, M.S.; Omrani, O.A.; Fawzy, N.A.; Shaar, B.A.; Almamlouk, R.; Riaz, M.; Obeidat, M.; Obeidat, Y.; Gerberi, D.; Taha, R.M.; et al. Prevalence of Post-Acute COVID-19 Syndrome Symptoms at Different Follow-up Periods: A Systematic Review and Meta-Analysis. Clin. Microbiol. Infect. 2022, 28, 657–666. [Google Scholar] [CrossRef]
- Michelen, M.; Manoharan, L.; Elkheir, N.; Cheng, V.; Dagens, A.; Hastie, C.; O’Hara, M.; Suett, J.; Dahmash, D.; Bugaeva, P.; et al. Characterising Long COVID: A Living Systematic Review. BMJ Glob. Health 2021, 6, e005427. [Google Scholar] [CrossRef]
- Bahmer, T.; Borzikowsky, C.; Lieb, W.; Horn, A.; Krist, L.; Fricke, J.; Scheibenbogen, C.; Rabe, K.F.; Maetzler, W.; Maetzler, C.; et al. Severity, Predictors and Clinical Correlates of Post-COVID Syndrome (PCS) in Germany: A Prospective, Multi-Centre, Population-Based Cohort Study. eClinicalMedicine 2022, 51, 101549. [Google Scholar] [CrossRef]
- Durstenfeld, M.S.; Peluso, M.J.; Kaveti, P.; Hill, C.; Li, D.; Sander, E.; Swaminathan, S.; Arechiga, V.M.; Lu, S.; Goldberg, S.A.; et al. Reduced Exercise Capacity, Chronotropic Incompetence, and Early Systemic Inflammation in Cardiopulmonary Phenotype Long Coronavirus Disease 2019. J. Infect. Dis. 2023, 228, 542–554. [Google Scholar] [CrossRef]
- Yelin, D.; Levi, R.; Babu, C.; Moshe, R.; Shitenberg, D.; Atamna, A.; Tishler, O.; Babich, T.; Shapira-Lichter, I.; Abecasis, D.; et al. Assessment of Exercise Capacity of Individuals with Long COVID: A Cross-Sectional Study. Isr. Med. Assoc. J. 2023, 25, 83–87. [Google Scholar]
- Wright, J.; Astill, S.; Sivan, M. The Relationship between Physical Activity and Long COVID: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2022, 19, 5093. [Google Scholar] [CrossRef]
- Koczulla, A.R.; Ankermann, T.; Behrends, U.; Berlit, P.; Berner, R.; Böing, S.; Brinkmann, F.; Frank, U.; Franke, C.; Glöckl, R.; et al. S1-Leitlinie Long-/Post-COVID. Pneumologie 2022, 76, 855–907. [Google Scholar] [CrossRef]
- Walter, N.; Rupp, M.; Lang, S.; Leinberger, B.; Alt, V.; Hinterberger, T.; Loew, T. A Comprehensive Report of German Nationwide Inpatient Data on the Post-COVID-19 Syndrome Including Annual Direct Healthcare Costs. Viruses 2022, 14, 2600. [Google Scholar] [CrossRef] [PubMed]
- Kluge, H.H.P.; Muscat, N.A.; Mishra, S.; Nielsen, S.; Tille, F.; Pfeifer, D.; COVID Europe, L.; Sivan, M. Call for Action: Health Services in the European Region Must Adopt Integrated Care Models to Manage Post-COVID-19 Condition. Lancet Reg. Health Eur. 2022, 18, 100435. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19. [NICE Guideline No. 188]. 2024. Available online: https://www.nice.org.uk/guidance/ng188 (accessed on 18 October 2024).
- Dong, B.; Qi, Y.; Lin, L.; Liu, T.; Wang, S.; Zhang, Y.; Yuan, Y.; Cheng, H.; Chen, Q.; Fang, Q.; et al. Which Exercise Approaches Work for Relieving Cancer-Related Fatigue? A Network Meta-Analysis. J. Orthop. Sports Phys. Ther. 2023, 53, 343–352. [Google Scholar] [CrossRef]
- Posadzki, P.; Pieper, D.; Bajpai, R.; Makaruk, H.; Könsgen, N.; Neuhaus, A.L.; Semwal, M. Exercise/Physical Activity and Health Outcomes: An Overview of Cochrane Systematic Reviews. BMC Public. Health 2020, 20, 1724. [Google Scholar] [CrossRef]
- Torres-Costoso, A.; Martínez-Vizcaíno, V.; Reina-Gutiérrez, S.; Álvarez-Bueno, C.; Guzmán-Pavón, M.J.; Pozuelo-Carrascosa, D.P.; Fernández-Rodríguez, R.; Sanchez-López, M.; Cavero-Redondo, I. Effect of Exercise on Fatigue in Multiple Sclerosis: A Network Meta-Analysis Comparing Different Types of Exercise. Arch. Phys. Med. Rehabil. 2022, 103, 970–987.e18. [Google Scholar] [CrossRef]
- Sick, J.; König, D. Exercise Training in Non-Hospitalized Patients with Post-COVID-19 Syndrome—A Narrative Review. Healthcare 2023, 11, 2277. [Google Scholar] [CrossRef]
- Klok, F.A.; Boon, G.J.A.M.; Barco, S.; Endres, M.; Geelhoed, J.J.M.; Knauss, S.; Rezek, S.A.; Spruit, M.A.; Vehreschild, J.; Siegerink, B. The Post-COVID-19 Functional Status Scale: A Tool to Measure Functional Status over Time after COVID-19. Eur. Respir. J. 2020, 56, 2001494. [Google Scholar] [CrossRef]
- FACIT-Group. (n.d.). FACIT-Fatigue. Assessment of Chronic Illness Therapy-Fatigue Scale. Available online: https://www.facit.org/measures/facit-fatigue (accessed on 25 January 2024).
- Gradidge, P.J.-L.; Torres, G.; Constantinou, D.; Zanwar, P.P.; Pinto, S.M.; Negm, A.; Heyn, P.C. Exercise Reporting Template for Long COVID Patients: A Rehabilitation Practitioner Guide. Arch. Phys. Med. Rehabil. 2023, 104, 991–995. [Google Scholar] [CrossRef]
- Greenhouse, S.W.; Geisser, S. On Methods in the Analysis of Profile Data. Psychometrika 1959, 24, 95–112. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis. Curr. Dir. Psychol. Sci. 1992, 1, 98–101. [Google Scholar] [CrossRef]
- Sawilowsky, S.S. New Effect Size Rules of Thumb. J. Mod. App. Stat. Meth. 2009, 8, 597–599. [Google Scholar] [CrossRef]
- Tomczak, M.; Tomczak, E. The Need to Report Effect Size Estimates Revisited. An Overview of Some Recommended Measures of Effect Si. Trends Sport Sci. 2014, 1, 19–25. [Google Scholar]
- Mooren, J.M.; Garbsch, R.; Schäfer, H.; Kotewitsch, M.; Waranski, M.; Teschler, M.; Schmitz, B.; Mooren, F.C. Medical Rehabilitation of Patients with Post-COVID-19 Syn-Drome—A Comparison of Aerobic Interval and Continuous Training. J. Clin. Med. 2023, 12, 6739. [Google Scholar] [CrossRef] [PubMed]
- Hartung, T.J.; Neumann, C.; Bahmer, T.; Chaplinskaya-Sobol, I.; Endres, M.; Geritz, J.; Haeusler, K.G.; Heuschmann, P.U.; Hildesheim, H.; Hinz, A.; et al. Fatigue and Cognitive Impairment after COVID-19: A Prospective Multicentre Study. eClinicalMedicine 2022, 53, 101651. [Google Scholar] [CrossRef]
- Piper, B.F.; Cella, D. Cancer-Related Fatigue: Definitions and Clinical Subtypes. J. Natl. Compr. Canc. Netw. 2010, 8, 958–966. [Google Scholar] [CrossRef]
- Cella, D.; Johansson, P.; Ueda, Y.; Tomazos, I.; Gustovic, P.; Wang, A.; Patel, A.S.; Schrezenmeier, H. Clinically Important Change for the FACIT-Fatigue Scale in Paroxysmal Nocturnal Hemoglobinuria: A Derivation from International PNH Registry Patient Data. J. Patient Rep. Outcomes 2023, 7, 63. [Google Scholar] [CrossRef]
- Jimeno-Almazán, A.; Franco-López, F.; Buendía-Romero, Á.; Martínez-Cava, A.; Sánchez-Agar, J.A.; Sánchez-Alcaraz Martínez, B.J.; Courel-Ibáñez, J.; Pallarés, J.G. Rehabilitation for Post-COVID-19 Condition through a Supervised Exercise Intervention: A Randomized Controlled Trial. Scand. Med. Sci. Sports 2022, 32, 1791–1801. [Google Scholar] [CrossRef]
- Jimeno-Almazán, A.; Buendía-Romero, Á.; Martínez-Cava, A.; Franco-López, F.; Sánchez-Alcaraz, B.J.; Courel-Ibáñez, J.; Pallarés, J.G. Effects of a Concurrent Training, Respiratory Muscle Exercise, and Self-Management Recommendations on Recovery from Post-COVID-19 Conditions: The RECOVE Trial. J. Appl. Physiol. 2023, 134, 95–104. [Google Scholar] [CrossRef]
- Nopp, S.; Moik, F.; Klok, F.A.; Gattinger, D.; Petrovic, M.; Vonbank, K.; Koczulla, A.R.; Ay, C.; Zwick, R.H. Outpatient Pulmonary Rehabilitation in Patients with Long COVID Improves Exercise Capacity, Functional Status, Dyspnea, Fatigue, and Quality of Life. Respiration 2022, 101, 593–601. [Google Scholar] [CrossRef]
- Vaidya, T.; De Bisschop, C.; Beaumont, M.; Ouksel, H.; Jean, V.; Dessables, F.; Chambellan, A. Is the 1-Minute Sit-to-Stand Test a Good Tool for the Evaluation of the Impact of Pulmonary Rehabilitation? Determination of the Minimal Important Difference in COPD. COPD 2016, 11, 2609–2616. [Google Scholar] [CrossRef]
- Kasper, K. Sports Training Principles. Curr. Sports Med. Rep. 2019, 18, 95–96. [Google Scholar] [CrossRef] [PubMed]
- Renz-Polster, H.; Scheibenbogen, C. Post-COVID-Syndrom mit Fatigue und Belastungsintoleranz: Myalgische Enzephalomyelitis bzw. Chronisches Fatigue-Syndrom. Inn. Med. 2022, 63, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Vernon, S.D.; Hartle, M.; Sullivan, K.; Bell, J.; Abbaszadeh, S.; Unutmaz, D.; Bateman, L. Post-Exertional Malaise among People with Long COVID Compared to Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). WOR 2023, 74, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Kostev, K.; Smith, L.; Koyanagi, A.; Jacob, L. Prevalence of and Factors Associated with Post-Coronavirus Disease 2019 (COVID-19) Condition in the 12 Months After the Diagnosis of COVID-19 in Adults Followed in General Practices in Germany. Open Forum Infect. Dis. 2022, 9, ofac333. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.R.; Thomas, R.J.; Bonikowske, A.R.; Hammer, S.M.; Olson, T.P. Sex Differences in Cardiac Rehabilitation Outcomes. Circ. Res. 2022, 130, 552–565. [Google Scholar] [CrossRef]
- Machado, F.V.C.; Meys, R.; Delbressine, J.M.; Vaes, A.W.; Goërtz, Y.M.J.; Van Herck, M.; Houben-Wilke, S.; Boon, G.J.A.M.; Barco, S.; Burtin, C.; et al. Construct Validity of the Post-COVID-19 Functional Status Scale in Adult Subjects with COVID-19. Health Qual. Life Outcomes 2021, 19, 40. [Google Scholar] [CrossRef]

| Variable | Value |
|---|---|
| Age [years] | 48.05 ± 15.15 |
| BMI [kg/m2] | 26.44 ± 5.36 |
| Sex [women] | 24 (58.5%) |
| Comorbidities | |
| Reported comorbidities [yes] | 30 (73.2%) |
| Endocrine, nutritional, or metabolic diseases | 15 (36.6%) |
| Diseases of the musculoskeletal system and connective tissue | 12 (29.3%) |
| Diseases of the cardiovascular system | 9 (21.9%) |
| Diseases of the respiratory system | 8 (19.5%) |
| Pain disorders/chronic pain syndrome | 5 (12.2%) |
| Neurological diseases | 3 (7.3%) |
| Psychiatric disorders | 3 (7.3%) |
| Others 1 | 4 (9.7%) |
| Medication | |
| Analgesics | 21 (51.2%) |
| Thyroid medication | 9 (21.9%) |
| Antidepressants | 9 (21.9%) |
| Antihypertensives | 8 (19.5%) |
| Corticosteroids | 7 (17.1%) |
| Anticoagulants | 5 (12.2%) |
| Bronchodilators | 4 (9.7%) |
| Others 2 | 9 (21.9%) |
| SARS-CoV-2 specific information | |
| Duration between infection and training start [weeks] | 51.54 ± 43.10 |
| Vaccination status [yes] | 41 (100%) |
| Received two SARS-CoV-2 vaccinations | 7 (17.1%) |
| Received three SARS-CoV-2 vaccinations | 29 (70.7%) |
| Received four SARS-CoV-2 vaccinations | 5 (12.2%) |
| Inpatient treatment since SARS-CoV-2 infection [yes] | 14 (34.1%) |
| Post COVID-19 specific information | |
| Number of symptom clusters 3 per patient, median | 4 |
| Fatigue | 41 (100%) |
| Cognitive/neurological system | 36 (87.8%) |
| Chest symptoms | 26 (63.4%) |
| Musculoskeletal system | 24 (58.5%) |
| Anxiety, depression, or sleep disorders | 16 (39%) |
| Tachycardia | 8 (19.5%) |
| Gastrointestinal symptoms | 6 (14.6%) |
| Flu-like symptoms, chills, or fever | 6 (14.6%) |
| Others 4 | 3 (7.3%) |
| PCS score 5 | 23.59 ± 7.33 |
| None/mild | 0 |
| Moderate | 24 (58.5%) |
| Severe | 17 (41.5%) |
| Variables | 1. | 2. | 3. | 4. |
|---|---|---|---|---|
| 1. PCS | - | - | - | - |
| 2. PCFS | 0.08 | - | - | - |
| 3. Fatigue | −0.23 | −0.47 ** | - | - |
| 4. 1MSTST | −0.29 | −0.27 | 0.13 | - |
| 5. WL | −0.25 | −0.30 | 0.42 ** | 0.50 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kieffer, S.; Krüger, A.-L.; Haiduk, B.; Grau, M. Individualized and Controlled Exercise Training Improves Fatigue and Exercise Capacity in Patients with Long-COVID. Biomedicines 2024, 12, 2445. https://doi.org/10.3390/biomedicines12112445
Kieffer S, Krüger A-L, Haiduk B, Grau M. Individualized and Controlled Exercise Training Improves Fatigue and Exercise Capacity in Patients with Long-COVID. Biomedicines. 2024; 12(11):2445. https://doi.org/10.3390/biomedicines12112445
Chicago/Turabian StyleKieffer, Simon, Anna-Lena Krüger, Björn Haiduk, and Marijke Grau. 2024. "Individualized and Controlled Exercise Training Improves Fatigue and Exercise Capacity in Patients with Long-COVID" Biomedicines 12, no. 11: 2445. https://doi.org/10.3390/biomedicines12112445
APA StyleKieffer, S., Krüger, A.-L., Haiduk, B., & Grau, M. (2024). Individualized and Controlled Exercise Training Improves Fatigue and Exercise Capacity in Patients with Long-COVID. Biomedicines, 12(11), 2445. https://doi.org/10.3390/biomedicines12112445






