How Co-Stimulatory/Inhibitory Molecules Vary Across Immune Cell Subtypes in the Severity of Systemic Lupus Erythematosus Compared to Controls
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Ethical Approval
2.3. Isolate Peripheral Blood Mononuclear Cells
2.4. Cell Culture
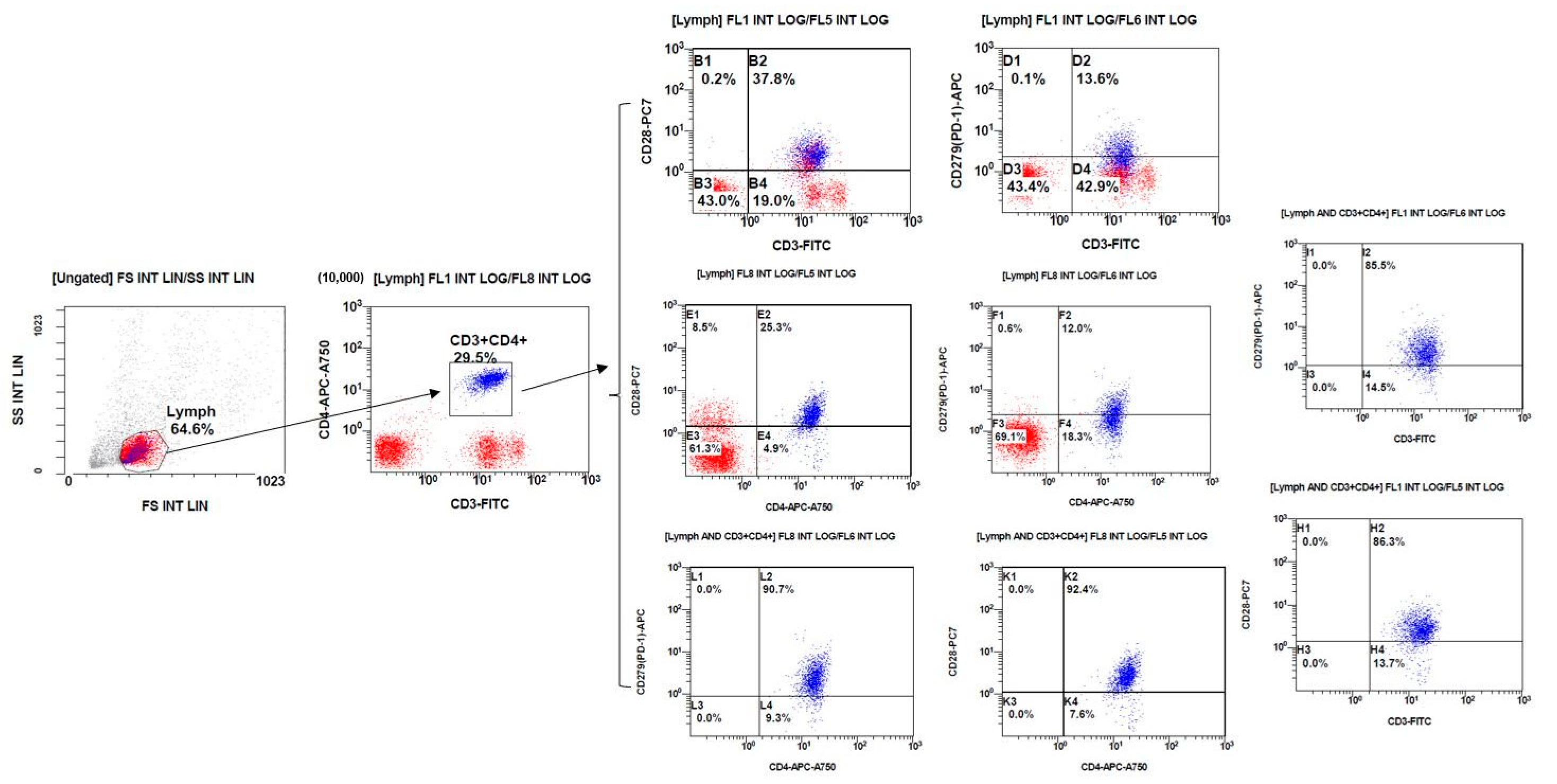
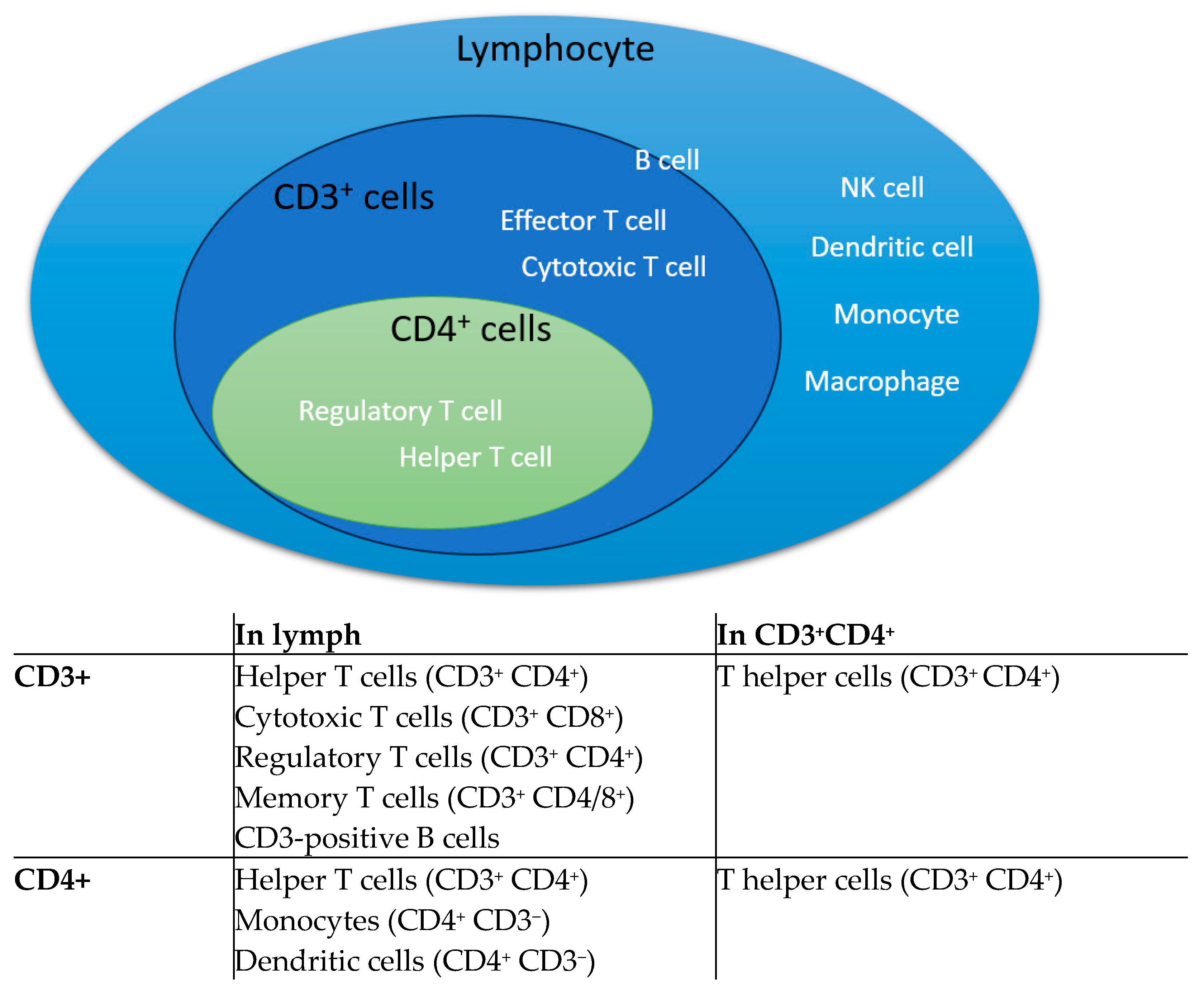
2.5. Analyze by Flow Cytometry
2.6. Statistics
3. Results
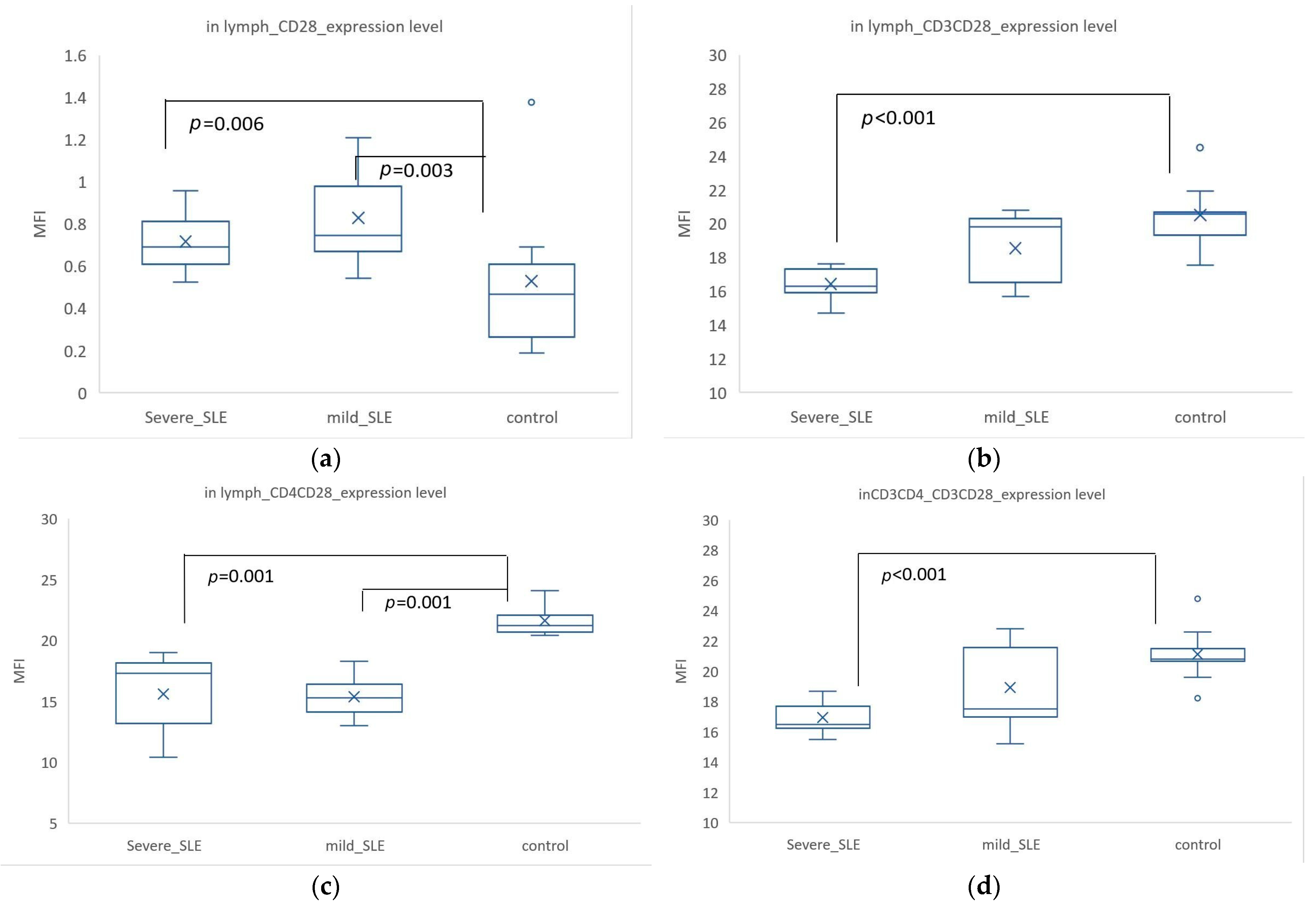
3.1. CD28 Expression Was Higher in SLE, but When Co-Expressed with CD3+ or CD4+, Its Expression Was Higher in Controls
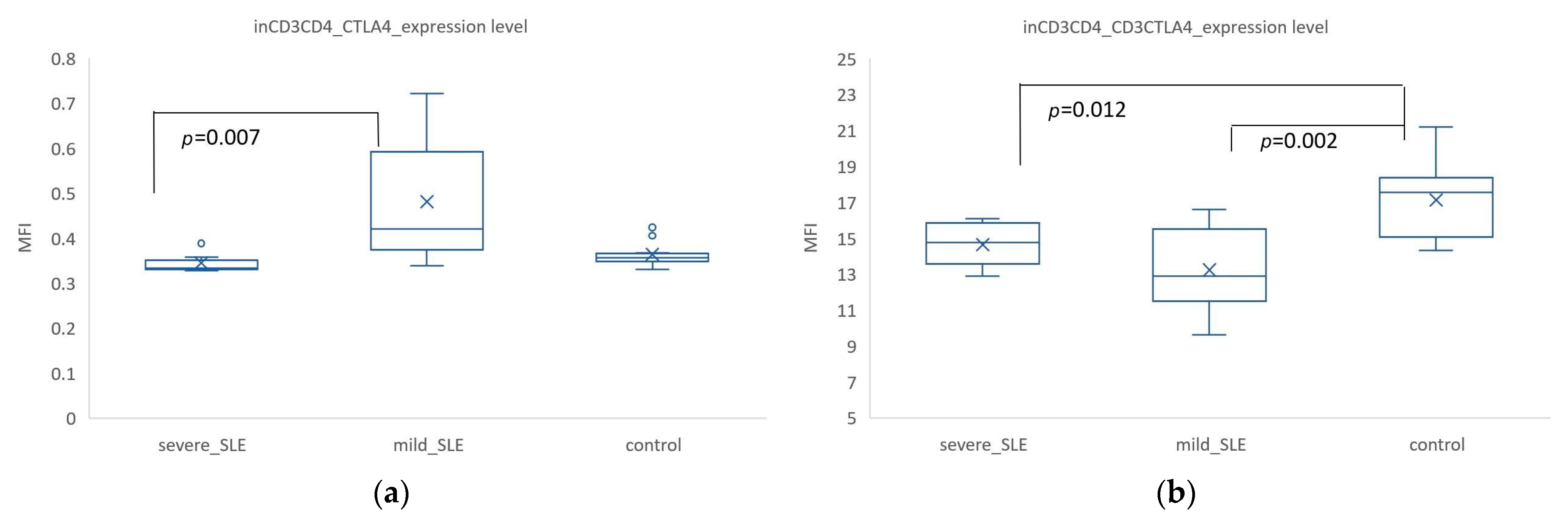
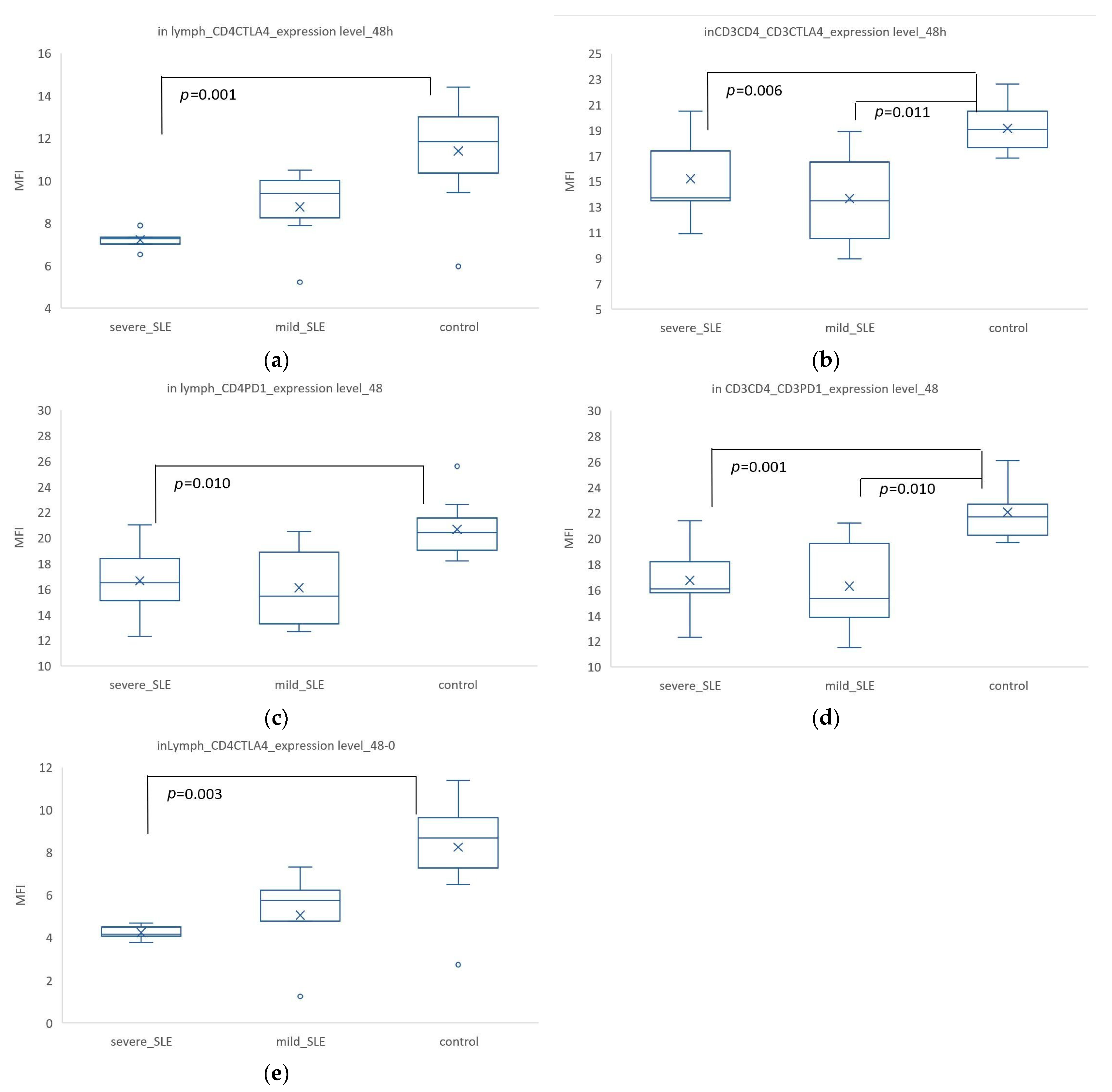
3.2. Mild SLE Showed Significantly Higher Expression of CTLA-4. In CD3+ CD4+ Cells, Co-Expression of CD3+ CTLA4+ Was Significantly Higher in the Control Group Compared to SLE
3.3. The Expression of PD-1 Was Significantly Lower in SLE. However, in CD3+ CD4+ Cells, PD-1 Expression Was Highest in Mild SLE
3.4. Induced CTLA-4 and PD-1 Expression Was Significantly Increased in T Cells from Patients Compared with SLE
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bolouri, N.; Akhtari, M.; Farhadi, E.; Mansouri, R.; Faezi, S.T.; Jamshidi, A.; Mahmoudi, M. Role of the innate and adaptive immune responses in the pathogenesis of systemic lupus erythematosus. Inflamm. Res. 2022, 71, 537–554. [Google Scholar] [CrossRef] [PubMed]
- Janeway, C.A., Jr.; Travers, P.; Walport, M.; Shlomchik, M. Immunobiology: The Immune System in Health and Disease, 5th ed.; Garland Science: New York, NY, USA, 2001. [Google Scholar]
- Suárez-Fueyo, A.; Bradley, S.J.; Tsokos, G.C. T cells in Systemic Lupus Erythematosus. Curr. Opin. Immunol. 2016, 43, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Gardner, D.; Jeffery, L.E.; Sansom, D.M. Understanding the CD28/CTLA-4 (CD152) pathway and its implications for costimulatory blockade. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2014, 14, 1985–1991. [Google Scholar] [CrossRef] [PubMed]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef]
- Hossen, M.M.; Ma, Y.; Yin, Z.; Xia, Y.; Du, J.; Huang, J.Y.; Huang, J.J.; Zou, L.; Ye, Z.; Huang, Z. Current understanding of CTLA-4: From mechanism to autoimmune diseases. Front. Immunol. 2023, 14, 1198365. [Google Scholar] [CrossRef]
- He, W.; Wang, B.; Li, Q.; Yao, Q.; Jia, X.; Song, R.; Li, S.; Zhang, J.A. Aberrant Expressions of Co-stimulatory and Co-inhibitory Molecules in Autoimmune Diseases. Front. Immunol. 2019, 10, 261. [Google Scholar] [CrossRef]
- Chen, D.P.; Lin, W.T.; Yu, K.H. Investigation of the association between the genetic polymorphisms of the co-stimulatory system and systemic lupus erythematosus. Front. Immunol. 2022, 13, 946456. [Google Scholar] [CrossRef]
- Brambila-Tapia, A.J.; Gámez-Nava, J.I.; Salazar-Páramo, M.; Munoz-Valle, J.F.; González-López, L.; Llamas-Covarrubias, M.A.; Gutiérrez-Urena, S.R.; Vázquez-Del Mercado, M.; Dávalos-Rodríguez, I.P. Increased CD28 serum levels are not associated with specific clinical activity in systemic lupus erythematosus. Rheumatol. Int. 2011, 31, 1321–1324. [Google Scholar] [CrossRef]
- Gong, J.Q.; Chen, Z.Q.; Hu, Z.J.; Li, W.Z. Expression of CD28 mRNA in peripheral blood mononuclear cells of patients with systemic lupus erythematosus. Chin. J. Rheumat 2001, 5, 12–14. [Google Scholar]
- AlFadhli, S. Overexpression and secretion of the soluble CTLA-4 splice variant in various autoimmune diseases and in cases with overlapping autoimmunity. Genet. Test. Mol. Biomark. 2013, 17, 336–341. [Google Scholar] [CrossRef]
- Bassiouni, S.; Abdeen, H.; Morsi, H.; Zaki, M.; Abdelsalam, M.; Gharbia, O. Programmed death 1 (PD-1) serum level and gene expression in recent onset systemic lupus erythematosus patients. Egypt. Rheumatol. 2021, 43, 213–218. [Google Scholar] [CrossRef]
- Wong, C.K.; Lit, L.C.; Tam, L.S.; Li, E.K.; Lam, C.W. Aberrant production of soluble costimulatory molecules CTLA-4, CD28, CD80 and CD86 in patients with systemic lupus erythematosus. Rheumatology 2005, 44, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Cancro, M.P.; D’Cruz, D.P.; Khamashta, M.A. The role of B lymphocyte stimulator (BLyS) in systemic lupus erythematosus. J. Clin. Investig. 2009, 119, 1066–1073. [Google Scholar] [CrossRef]
- Zagales, R.; Tarvin, S.E.; Rodriguez, M. Clinical Characteristics of Pre-pubescent Patients with Systemic Lupus Erythematosus (SLE). Proc. IMPRS 2023, 6. [Google Scholar] [CrossRef]
- López-Domínguez, R.; Villatoro-García, J.A.; Marañón, C.; Goldman, D.; Petri, M.; Carmona-Sáez, P.; Alarcón-Riquelme, M.; Toro-Dominguez, D. Immune and molecular landscape behind non-response to Mycophenolate Mofetil and Azathioprine in lupus nephritis therapy. Res. Sq. 2024; preprint. [Google Scholar] [CrossRef]
- Milone, A.; Parlati, C.; Patrone, M.; Riccio, F.; Annunziata, M.; Brancaccio, M.; Marchesano, M.; Fasano, S.; Mauro, D.; Ciccia, F. AB1094 predictive factors of response to mycophenolate mofetil treatment in a single-center cohort of patients with systemic lupus erythematosus. Ann. Rheum. Dis. 2024, 83, 1875–1876. [Google Scholar]
- Steiner, K.; Waase, I.; Rau, T.; Dietrich, M.; Fleischer, B.; Bröker, B.M. Enhanced expression of CTLA-4 (CD152) on CD4+ T cells in HIV infection. Clin. Exp. Immunol. 1999, 115, 451–457. [Google Scholar] [CrossRef]
- Molvi, Z. Activation of Human T Cells with Phytohaemagglutinin (PHA); Spring: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Cossarizza, A.; Chang, H.D.; Radbruch, A.; Acs, A.; Adam, D.; Adam-Klages, S.; Agace, W.W.; Aghaeepour, N.; Akdis, M.; Allez, M.; et al. Guidelines for the use of flow cytometry and cell sorting in immunological studies (second edition). Eur. J. Immunol. 2019, 49, 1457–1973. [Google Scholar] [CrossRef]
- Pradet-Balade, B.; Boulmé, F.; Beug, H.; Müllner, E.W.; Garcia-Sanz, J.A. Translation control: Bridging the gap between genomics and proteomics? Trends Biochem. Sci. 2001, 26, 225–229. [Google Scholar] [CrossRef]
- Nagel, A.; Möbs, C.; Raifer, H.; Wiendl, H.; Hertl, M.; Eming, R. CD3-positive B cells: A storage-dependent phenomenon. PLoS ONE 2014, 9, e110138. [Google Scholar] [CrossRef]
- Sun, L.; Su, Y.; Jiao, A.; Wang, X.; Zhang, B. T cells in health and disease. Signal Transduct. Target. Ther. 2023, 8, 235. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Long, E.O. CD28 Homolog Is a Strong Activator of Natural Killer Cells for Lysis of B7H7(+) Tumor Cells. Cancer Immunol. Res. 2019, 7, 939–951. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wei, L.; Meng, S.; Song, W.; Chen, Y.; Li, H.; Zhao, Q.; Jiang, Z.; Liu, D.; Ren, H.; et al. Coordinated Priming of NKG2D Pathway by IL-15 Enhanced Functional Properties of Cytotoxic CD4+CD28− T Cells Expanded in Systemic Lupus Erythematosus. Inflammation 2023, 46, 1587–1601. [Google Scholar] [CrossRef]
- Li, H.; Yang, P. Identification of biomarkers related to neutrophils and two molecular subtypes of systemic lupus erythematosus. BMC Med. Genom. 2022, 15, 162. [Google Scholar] [CrossRef]
- Cui, M.; Li, T.; Yan, X.; Wang, C.; Shen, Q.; Ren, H.; Li, L.; Zhang, R. Blood Genomics Identifies Three Subtypes of Systemic Lupus Erythematosus: “IFN-High,” “NE-High,” and “Mixed”. Mediat. Inflamm. 2021, 2021, 6660164. [Google Scholar] [CrossRef]
- Rogers, J.L.; Eudy, A.M.; Pisetsky, D.; Criscione-Schreiber, L.G.; Sun, K.; Doss, J.; Clowse, M.E.B. Using Clinical Characteristics and Patient-Reported Outcome Measures to Categorize Systemic Lupus Erythematosus Subtypes. Arthritis Care Res. 2021, 73, 386–393. [Google Scholar] [CrossRef]
- Blank, C.U.; Haining, W.N.; Held, W.; Hogan, P.G.; Kallies, A.; Lugli, E.; Lynn, R.C.; Philip, M.; Rao, A.; Restifo, N.P.; et al. Defining ‘T cell exhaustion’. Nat. Rev. Immunol. 2019, 19, 665–674. [Google Scholar] [CrossRef]
- Żabińska, M.; Krajewska, M.; Kościelska-Kasprzak, K.; Klinger, M. CD3(+)CD8(+)CD28(-) T Lymphocytes in Patients with Lupus Nephritis. J. Immunol. Res. 2016, 2016, 1058165. [Google Scholar] [CrossRef]
- Takeuchi, A.; Saito, T. CD4 CTL, a Cytotoxic Subset of CD4(+) T Cells, Their Differentiation and Function. Front. Immunol. 2017, 8, 194. [Google Scholar] [CrossRef]
- Kosmaczewska, A.; Ciszak, L.; Stosio, M.; Szteblich, A.; Madej, M.; Frydecka, I.; Wiland, P.; Szmyrka, M. CD4+CD28null T cells are expanded in moderately active systemic lupus erythematosus and secrete pro-inflammatory interferon gamma, depending on the Disease Activity Index. Lupus 2020, 29, 705–714. [Google Scholar] [CrossRef]
- Gofur, N.R.P.; Handono, K.; Kalim, H.; Wahono, C.S.; Poeranto, S.; Barlianto, W. Association of Th-Tc protein CD28+ and Periodontal Inflammation among Indonesian Women with SLE Disease. Syst. Rev. Pharm. 2020, 11, 580. [Google Scholar]
- Hu, S.; Tao, D.; He, P. Expression of costimulatory molecules B7/CD28 in systemic lupus erythematosus. J. Huazhong Univ. Sci. Technol. Med. Sci. 2004, 24, 245–246, 265. [Google Scholar] [CrossRef]
- Yuan, S.; Zeng, Y.; Li, J.; Wang, C.; Li, W.; He, Z.; Ye, J.; Li, F.; Chen, Y.; Lin, X.; et al. Phenotypical changes and clinical significance of CD4+/CD8+ T cells in SLE. Lupus Sci. Med. 2022, 9, e000660. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.-R.; Choi, J.-M. Current Understanding of Cytotoxic T Lymphocyte Antigen-4 (CTLA-4) Signaling in T-Cell Biology and Disease Therapy. Mol. Cells 2022, 45, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Oyewole-Said, D.; Konduri, V.; Vazquez-Perez, J.; Weldon, S.A.; Levitt, J.M.; Decker, W.K. Beyond T-Cells: Functional Characterization of CTLA-4 Expression in Immune and Non-Immune Cell Types. Front. Immunol. 2020, 11, 608024. [Google Scholar] [CrossRef]
- Mitsuiki, N.; Schwab, C.; Grimbacher, B. What did we learn from CTLA-4 insufficiency on the human immune system? Immunol. Rev. 2019, 287, 33–49. [Google Scholar] [CrossRef]
- Mohamed, E.; Safaa, I.; Manal, Y.; Ibrahim, I. Expression of cytotoxic T-lymphocyte-associated antigen-4 [CTLA-4; CD152] on peripheral blood T lymphocytes in systemic lupus erythematosus children. Egypt. J. Med. Microbiol. 2007, 16, 491–501. [Google Scholar]
- Zhao, L.; Zhou, X.; Zhou, X.; Wang, H.; Gu, L.; Ke, Y.; Zhang, M.; Ji, X.; Yang, X. Low expressions of PD-L1 and CTLA-4 by induced CD4(+)CD25(+) Foxp3(+) Tregs in patients with SLE and their correlation with the disease activity. Cytokine 2020, 133, 155119. [Google Scholar] [CrossRef]
- Petrelli, A.; Mijnheer, G.; van Konijnenburg, D.P.H.; Van Der Wal, M.M.; Giovannone, B.; Mocholi, E.; Vazirpanah, N.; Broen, J.C.; Hijnen, D.; Oldenburg, B.; et al. PD-1+ CD8+ T cells are clonally expanding effectors in human chronic inflammation. J. Clin. Investig. 2018, 128, 4669–4681. [Google Scholar] [CrossRef]
- Park, H.J.; Park, J.S.; Jeong, Y.H.; Son, J.; Ban, Y.H.; Lee, B.-H.; Chen, L.; Chang, J.; Chung, D.H.; Choi, I.; et al. Correction: PD-1 upregulated on regulatory T cells during chronic virus infection enhances the suppression of CD8+ T cell immune response via the interaction with PD-L1 expressed on CD8+ T cells. J. Immunol. 2015, 195, 5841–5842. [Google Scholar] [CrossRef]
- Piantoni, S.; Regola, F.; Zanola, A.; Andreoli, L.; Dall’Ara, F.; Tincani, A.; Airo, P. Effector T-cells are expanded in systemic lupus erythematosus patients with high disease activity and damage indexes. Lupus 2018, 27, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Saito, N.; Tomita, M.; Ishihama, Y. Unbiased Quantitation of Escherichia coli Membrane Proteome Using Phase Transfer Surfactants. Mol. Cell. Proteom. 2009, 8, 2770–2777. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.Y.; Francois, A.; McGray, A.R.; Miliotto, A.; Odunsi, K. Compensatory upregulation of PD-1, LAG-3, and CTLA-4 limits the efficacy of single-agent checkpoint blockade in metastatic ovarian cancer. Oncoimmunology 2017, 6, e1249561. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Yu, Y.; Ma, J.; Ren, C.; Chen, W. PD-1+CXCR5-CD4+T cells are correlated with the severity of systemic lupus erythematosus. Rheumatology 2019, 58, 2188–2192. [Google Scholar] [CrossRef]
- Ahmadzadeh, M.; Johnson, L.A.; Heemskerk, B.; Wunderlich, J.R.; Dudley, M.E.; White, D.E.; Rosenberg, S.A. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood 2009, 114, 1537–1544. [Google Scholar] [CrossRef]
- Luo, Q.; Kong, Y.; Fu, B.; Li, X.; Huang, Q.; Huang, Z.; Li, J. Increased TIM-3(+)PD-1(+) NK cells are associated with the disease activity and severity of systemic lupus erythematosus. Clin. Exp. Med. 2022, 22, 47–56. [Google Scholar] [CrossRef]
- Yeo, J.; Yaung, K.; Law, A.; Wasser, M.; Arkachaisri, T.; Thumboo, J.; Low, A.H.L.; Albani, S. OP0214 a multi-dimensional approach reveals a dysregulated systemic lupus erythematosus immune rheostat with an abnormal immunoregulatory response and reduced ctla4 expression in effector T cells. Ann. Rheum. Dis. 2023, 82, 140–141. [Google Scholar] [CrossRef]
- Francisco, L.M.; Sage, P.T.; Sharpe, A.H. The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 2010, 236, 219–242. [Google Scholar] [CrossRef]
- Filion, L.G.; Izaguirre, C.A.; Garber, G.E.; Huebsh, L.; Aye, M.T. Detection of surface and cytoplasmic CD4 on blood monocytes from normal and HIV-1 infected individuals. J. Immunol. Methods 1990, 135, 59–69. [Google Scholar] [CrossRef]
- Jago, C.B.; Yates, J.; Câmara, N.O.; Lechler, R.I.; Lombardi, G. Differential expression of CTLA-4 among T cell subsets. Clin. Exp. Immunol. 2004, 136, 463–471. [Google Scholar] [CrossRef]
- Li, H.; Boulougoura, A.; Endo, Y.; Tsokos, G.C. Abnormalities of T cells in systemic lupus erythematosus: New insights in pathogenesis and therapeutic strategies. J. Autoimmun. 2022, 132, 102870. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Feng, Y.; Xu, J.; Liang, J. T-cell exhaustion in immune-mediated inflammatory diseases: New implications for immunotherapy. Front. Immunol. 2022, 13, 977394. [Google Scholar] [CrossRef] [PubMed]
- Miggelbrink, A.M.; Jackson, J.D.; Lorrey, S.J.; Srinivasan, E.S.; Waibl-Polania, J.; Wilkinson, D.S.; Fecci, P.E. CD4 T-Cell Exhaustion: Does It Exist and What Are Its Roles in Cancer? Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021, 27, 5742–5752. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, K.-H.; Lin, W.-T.; Chen, D.-P. How Co-Stimulatory/Inhibitory Molecules Vary Across Immune Cell Subtypes in the Severity of Systemic Lupus Erythematosus Compared to Controls. Biomedicines 2024, 12, 2444. https://doi.org/10.3390/biomedicines12112444
Yu K-H, Lin W-T, Chen D-P. How Co-Stimulatory/Inhibitory Molecules Vary Across Immune Cell Subtypes in the Severity of Systemic Lupus Erythematosus Compared to Controls. Biomedicines. 2024; 12(11):2444. https://doi.org/10.3390/biomedicines12112444
Chicago/Turabian StyleYu, Kuang-Hui, Wei-Tzu Lin, and Ding-Ping Chen. 2024. "How Co-Stimulatory/Inhibitory Molecules Vary Across Immune Cell Subtypes in the Severity of Systemic Lupus Erythematosus Compared to Controls" Biomedicines 12, no. 11: 2444. https://doi.org/10.3390/biomedicines12112444
APA StyleYu, K.-H., Lin, W.-T., & Chen, D.-P. (2024). How Co-Stimulatory/Inhibitory Molecules Vary Across Immune Cell Subtypes in the Severity of Systemic Lupus Erythematosus Compared to Controls. Biomedicines, 12(11), 2444. https://doi.org/10.3390/biomedicines12112444





