Role of Calcium in an Experimental Breast Cancer Model Induced by Radiation and Estrogen
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Irradiation
2.3. Preparation of Fluorescence-Labeled Probes for the Analysis of Cell Lines
2.4. Examining Microarray Gene Expression with the Affymetrix HG-U133A Plus 2.0 GeneChip
2.5. Bioinformatic and Statistical Analysis
3. Results
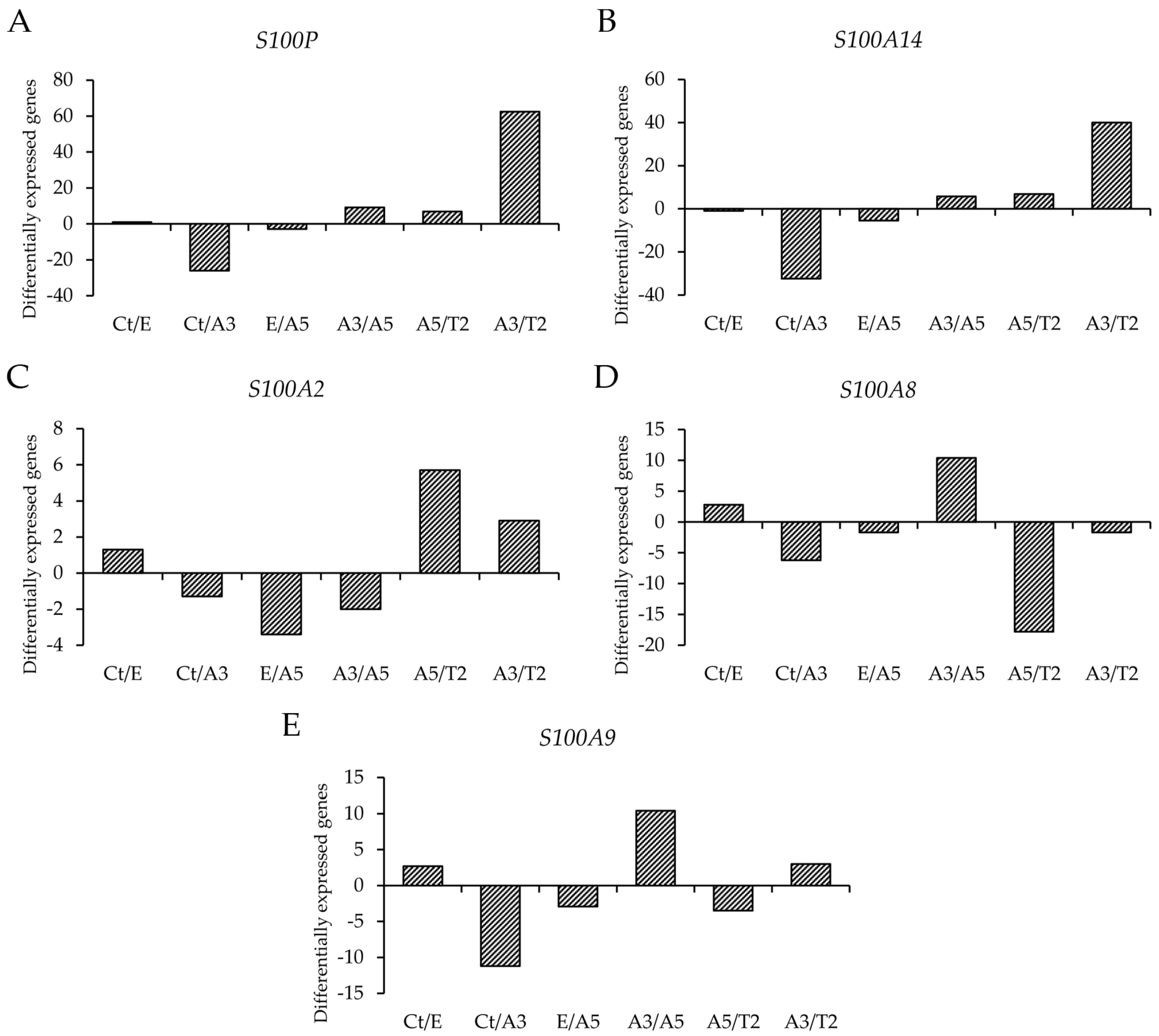
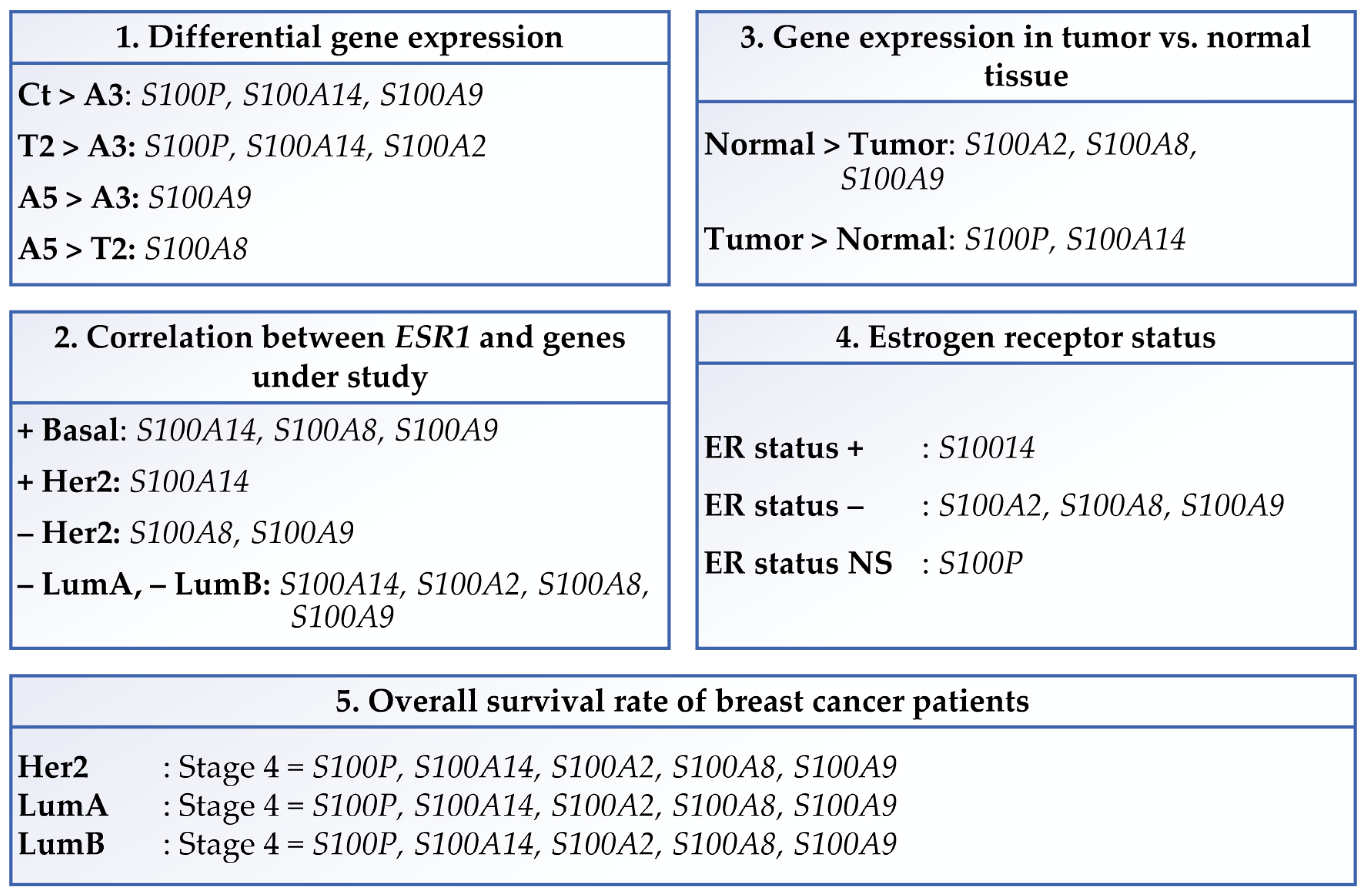
3.1. Differential Gene Expression of Genes That Code for the S100 Calcium-Binding Proteins
3.2. Clinical Significance and Gene Expression in Different Breast Cancer Subtypes
3.2.1. Comparison of the Genes in This Study with the Estrogen Receptor Alpha Gene
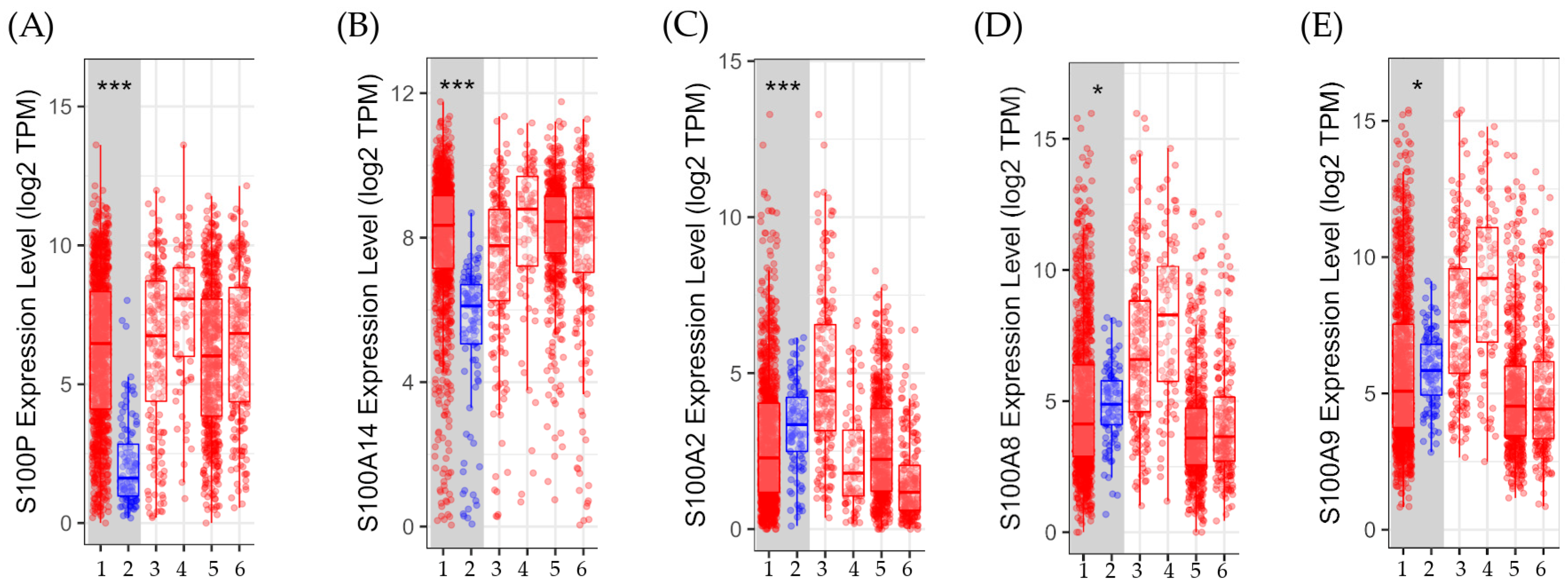
3.2.2. Gene Expression in Tumor Versus Normal Tissues
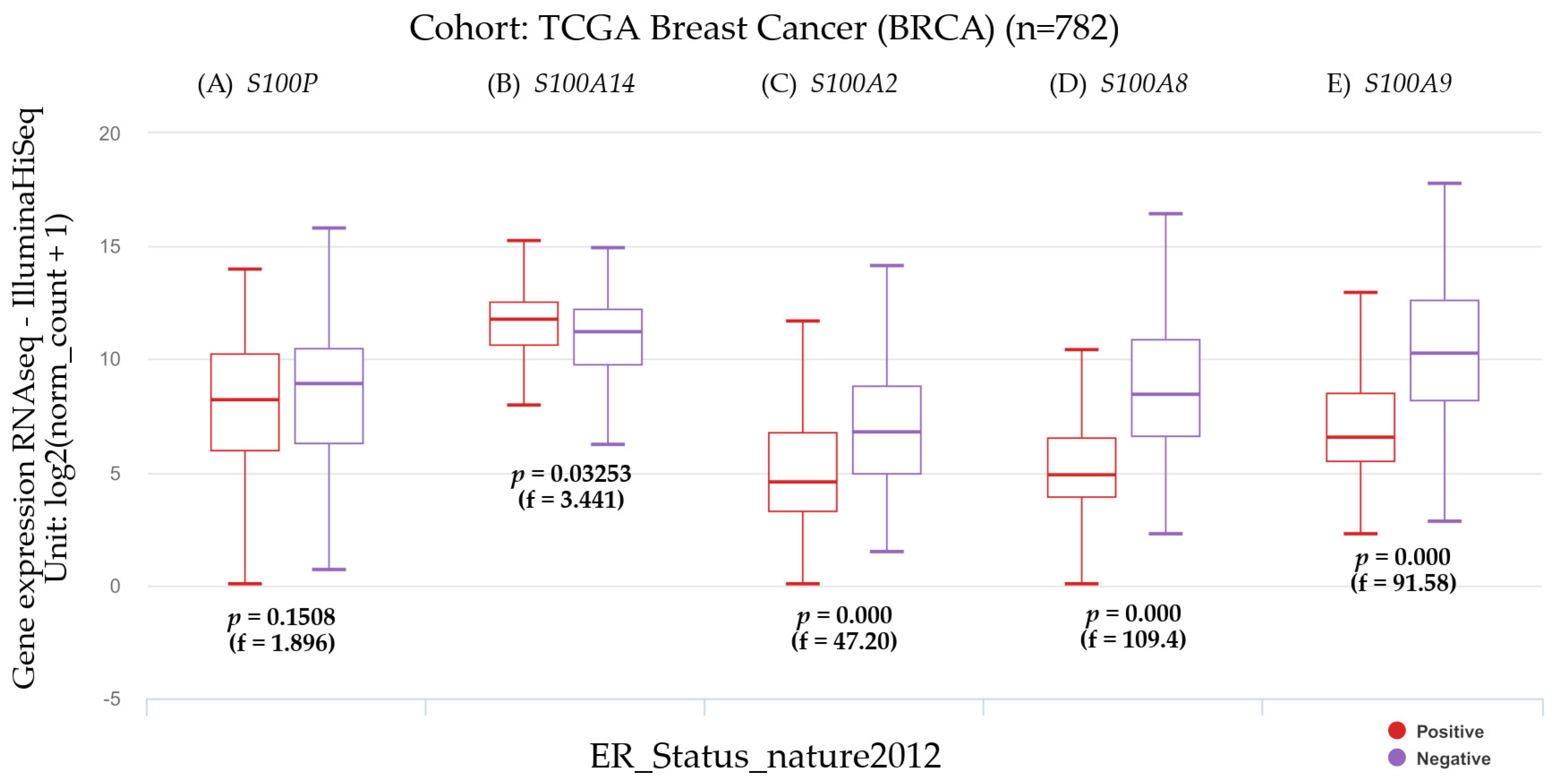
3.2.3. The Estrogen Receptor Status and Gene Expression
3.2.4. Gene Expression and the Disease Stage Factor
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Trapani, D.; Ginsburg, O.; Fadelu, T.; Lin, N.U.; Hassett, M.; Ilbawi, A.M.; Anderson, B.O.; Curigliano, G. Global challenges and policy solutions in breast cancer control. Cancer Treat. Rev. 2022, 104, 102339. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Zheng, R.; Zhang, S.; Wang, S.; Chen, R.; Sun, K.; Zeng, H.; Zhou, J.; Wei, W. Global patterns of breast cancer incidence and mortality: A population-based cancer registry data analysis from 2000 to 2020. Cancer Commun. 2021, 41, 1183–1194. [Google Scholar] [CrossRef] [PubMed]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Hu, H.; Tong, X.; Jiang, Q.; Zhu, H.; Zhang, S. Calcium-binding protein S100P and cancer: Mechanisms and clinical relevance. J. Cancer Res. Clin. Oncol. 2012, 138, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Fard, S.; Majidpoor, J.; Shoorei, H.; Hussen, B.M.; Hadayat Jamal, H.; Baniahmad, A.; Taheri, M.; Mokhtari, M. The Interaction Between Non-Coding RNAs and Calcium Binding Proteins. Front. Oncol. 2022, 12, 848376. [Google Scholar] [CrossRef]
- Rigiracciolo, D.C.; Nohata, N.; Lappano, R.; Cirillo, F.; Talia, M.; Adame-Garcia, S.R.; Arang, N.; Lubrano, S.; De Francesco, E.M.; Belfiore, A.; et al. Focal Adhesion Kinase (FAK)-Hippo/YAP transduction signaling mediates the stimulatory effects exerted by S100A8/A9-RAGE system in triple-negative breast cancer (TNBC). J. Exp. Clin. Cancer Res. 2022, 41, 193. [Google Scholar] [CrossRef]
- Donato, R.; Cannon, B.R.; Sorci, G.; Riuzzi, F.; Hsu, K.; Weber, D.J.; Geczy, C.L. Functions of S100 proteins. Curr. Mol. Med. 2013, 13, 24–57. [Google Scholar] [CrossRef]
- Allgower, C.; Kretz, A.L.; von Karstedt, S.; Wittau, M.; Henne-Bruns, D.; Lemke, J. Friend or Foe: S100 Proteins in Cancer. Cancers 2020, 12, 2037. [Google Scholar] [CrossRef]
- Schafer, B.W.; Heizmann, C.W. The S100 family of EF-hand calcium-binding proteins: Functions and pathology. Trends Biochem. Sci. 1996, 21, 134–140. [Google Scholar] [CrossRef]
- Hu, Y.; Han, Y.; He, M.; Zhang, Y.; Zou, X. S100 proteins in head and neck squamous cell carcinoma (Review). Oncol. Lett. 2023, 26, 362. [Google Scholar] [CrossRef] [PubMed]
- Russo, J.; Hu, Y.F.; Silva, I.D.; Russo, I.H. Cancer risk related to mammary gland structure and development. Microsc. Res. Tech. 2001, 52, 204–223. [Google Scholar] [CrossRef]
- Soule, H.D.; Maloney, T.M.; Wolman, S.R.; Peterson, W.D., Jr.; Brenz, R.; McGrath, C.M.; Russo, J.; Pauley, R.J.; Jones, R.F.; Brooks, S.C. Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF-10. Cancer Res. 1990, 50, 6075–6086. [Google Scholar] [PubMed]
- Russo, J.; Calaf, G.; Russo, I.H. A critical approach to the malignant transformation of human breast epithelial cells with chemical carcinogens. Crit. Rev. Oncog. 1993, 4, 403–417. [Google Scholar]
- Bond, J.A.; Wyllie, F.S.; Wynford-Thomas, D. Escape from senescence in human diploid fibroblasts induced directly by mutant p53. Oncogene 1994, 9, 1885–1889. [Google Scholar]
- Briand, P.; Petersen, O.W.; Van Deurs, B. A new diploid nontumorigenic human breast epithelial cell line isolated and propagated in chemically defined medium. In Vitro Cell Dev. Biol. 1987, 23, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Tait, L.; Soule, H.D.; Russo, J. Ultrastructural and immunocytochemical characterization of an immortalized human breast epithelial cell line, MCF-10. Cancer Res. 1990, 50, 6087–6094. [Google Scholar] [PubMed]
- Ochieng, J.; Tahin, Q.S.; Booth, C.C.; Russo, J. Buffering of intracellular calcium in response to increased extracellular levels in mortal, immortal, and transformed human breast epithelial cells. J. Cell Biochem. 1991, 46, 250–254. [Google Scholar] [CrossRef]
- Barnabas, N.; Moraes, R.; Calaf, G.; Estrada, S.; Russo, J. Role of p53 in mcf-10f cell immortalization and chemically-induced neoplastic transformation. Int. J. Oncol. 1995, 7, 1289–1296. [Google Scholar] [CrossRef]
- Higgy, N.A.; Salicioni, A.M.; Russo, I.H.; Zhang, P.L.; Russo, J. Differential expression of human ferritin H chain gene in immortal human breast epithelial MCF-10F cells. Mol. Carcinog. 1997, 20, 332–339. [Google Scholar] [CrossRef]
- Strynadka, N.C.; James, M.N. Crystal structures of the helix-loop-helix calcium-binding proteins. Annu. Rev. Biochem. 1989, 58, 951–998. [Google Scholar] [CrossRef] [PubMed]
- Gifford, J.L.; Walsh, M.P.; Vogel, H.J. Structures and metal-ion-binding properties of the Ca2+-binding helix-loop-helix EF-hand motifs. Biochem. J. 2007, 405, 199–221. [Google Scholar] [CrossRef] [PubMed]
- Bagur, R.; Hajnoczky, G. Intracellular Ca(2+) Sensing: Its Role in Calcium Homeostasis and Signaling. Mol. Cell 2017, 66, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Ogoma, Y.; Kobayashi, H.; Fujii, T.; Kondo, Y.; Hachimori, A.; Shimizu, T.; Hatano, M. Binding study of metal ions to S100 protein: 43Ca, 25Mg, 67Zn and 39K n.m.r. Int. J. Biol. Macromol. 1992, 14, 279–286. [Google Scholar] [CrossRef]
- Zhuang, H.; Chen, X.; Dong, F.; Zhang, Z.; Zhou, Z.; Ma, Z.; Huang, S.; Chen, B.; Zhang, C.; Hou, B. Prognostic values and immune suppression of the S100A family in pancreatic cancer. J. Cell Mol. Med. 2021, 25, 3006–3018. [Google Scholar] [CrossRef]
- Choi, J.; Kim, D.I.; Kim, J.; Kim, B.H.; Kim, A. Hornerin Is Involved in Breast Cancer Progression. J. Breast Cancer 2016, 19, 142–147. [Google Scholar] [CrossRef]
- Maderka, M.; Pilka, R.; Neubert, D.; Hambalek, J. New serum tumor markers S100, TFF3 and AIF-1 and their possible use in oncogynecology. Ceska Gynekol. 2019, 84, 303–308. [Google Scholar]
- Gibadulinova, A.; Tothova, V.; Pastorek, J.; Pastorekova, S. Transcriptional regulation and functional implication of S100P in cancer. Amino Acids 2011, 41, 885–892. [Google Scholar] [CrossRef]
- Wang, X.; Yang, J.; Qian, J.; Liu, Z.; Chen, H.; Cui, Z. S100A14, a mediator of epithelial-mesenchymal transition, regulates proliferation, migration and invasion of human cervical cancer cells. Am. J. Cancer Res. 2015, 5, 1484–1495. [Google Scholar]
- Wolf, S.; Haase-Kohn, C.; Pietzsch, J. S100A2 in cancerogenesis: A friend or a foe? Amino Acids 2011, 41, 849–861. [Google Scholar] [CrossRef]
- Wang, D.; Liu, G.; Wu, B.; Chen, L.; Zeng, L.; Pan, Y. Clinical Significance of Elevated S100A8 Expression in Breast Cancer Patients. Front. Oncol. 2018, 8, 496. [Google Scholar] [CrossRef] [PubMed]
- Calaf, G.M.; Hei, T.K. Establishment of a radiation- and estrogen-induced breast cancer model. Carcinogenesis 2000, 21, 769–776. [Google Scholar] [CrossRef]
- IARC. Ionizing radiation, Part I, X- and gamma (y)-radiation, and neutrons. In IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 2000; pp. 1–448. [Google Scholar]
- Calaf, G.; Russo, J. Transformation of human breast epithelial cells by chemical carcinogens. Carcinogenesis 1993, 14, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Calaf, G.M.; Roy, D. Gene and protein expressions induced by 17beta-estradiol and parathion in cultured breast epithelial cells. Mol. Med. 2007, 13, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Calaf, G.M.; Roy, D. Cell adhesion proteins altered by 17beta estradiol and parathion in breast epithelial cells. Oncol. Rep. 2008, 19, 165–169. [Google Scholar]
- Hei, T.K.; Piao, C.Q.; Willey, J.C.; Thomas, S.; Hall, E.J. Malignant transformation of human bronchial epithelial cells by radon-simulated alpha-particles. Carcinogenesis 1994, 15, 431–437. [Google Scholar] [CrossRef]
- Calaf, G.M.; Roy, D.; Narayan, G.; Balajee, A.S. Differential expression of cell adhesion molecules in an ionizing radiation-induced breast cancer model system. Oncol. Rep. 2013, 30, 285–291. [Google Scholar] [CrossRef]
- Calaf, G.M.; Crispin, L.A.; Munoz, J.P.; Aguayo, F.; Roy, D.; Narayan, G. Ionizing Radiation and Estrogen Affecting Growth Factor Genes in an Experimental Breast Cancer Model. Int. J. Mol. Sci. 2022, 23, 14284. [Google Scholar] [CrossRef]
- Li, T.; Fu, J.; Zeng, Z.; Cohen, D.; Li, J.; Chen, Q.; Li, B.; Liu, X.S. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020, 48, W509–W514. [Google Scholar] [CrossRef]
- Goldman, M.; Craft, B.; Hastie, M.; Repecka, K.; McDade, F.; Kamath, A.; Banerjee, A.; Luo, Y.; Rogers, D.; Brooks, A.N.; et al. The UCSC Xena platform for public and private cancer genomics data visualization and interpretation. bioRxiv 2019, 1–16. [Google Scholar] [CrossRef]
- Guerreiro Da Silva, I.D.; Hu, Y.F.; Russo, I.H.; Ao, X.; Salicioni, A.M.; Yang, X.; Russo, J. S100P calcium-binding protein overexpression is associated with immortalization of human breast epithelial cells in vitro and early stages of breast cancer development in vivo. Int. J. Oncol. 2000, 16, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Maciejczyk, A.; Lacko, A.; Ekiert, M.; Jagoda, E.; Wysocka, T.; Matkowski, R.; Halon, A.; Gyorffy, B.; Lage, H.; Surowiak, P. Elevated nuclear S100P expression is associated with poor survival in early breast cancer patients. Histol. Histopathol. 2013, 28, 513–524. [Google Scholar] [CrossRef]
- Peng, C.; Chen, H.; Wallwiener, M.; Modugno, C.; Cuk, K.; Madhavan, D.; Trumpp, A.; Heil, J.; Marme, F.; Nees, J.; et al. Plasma S100P level as a novel prognostic marker of metastatic breast cancer. Breast Cancer Res. Treat. 2016, 157, 329–338. [Google Scholar] [CrossRef]
- Yang, R.; Stocker, S.; Schott, S.; Heil, J.; Marme, F.; Cuk, K.; Chen, B.; Golatta, M.; Zhou, Y.; Sutter, C.; et al. The association between breast cancer and S100P methylation in peripheral blood by multicenter case-control studies. Carcinogenesis 2017, 38, 312–320. [Google Scholar] [CrossRef]
- Derventzi, A.; Rattan, S.I.; Gonos, E.S. Molecular links between cellular mortality and immortality (review). Anticancer. Res. 1996, 16, 2901–2910. [Google Scholar]
- Sato, N.; Hitomi, J. S100P expression in human esophageal epithelial cells: Human esophageal epithelial cells sequentially produce different S100 proteins in the process of differentiation. Anat. Rec. 2002, 267, 60–69. [Google Scholar] [CrossRef]
- Becker, T.; Gerke, V.; Kube, E.; Weber, K. S100P, a novel Ca(2+)-binding protein from human placenta. cDNA cloning, recombinant protein expression and Ca2+ binding properties. Eur. J. Biochem. 1992, 207, 541–547. [Google Scholar] [CrossRef]
- Emoto, Y.; Kobayashi, R.; Akatsuka, H.; Hidaka, H. Purification and characterization of a new member of the S-100 protein family from human placenta. Biochem. Biophys. Res. Commun. 1992, 182, 1246–1253. [Google Scholar] [CrossRef]
- Chen, H.; Yuan, Y.; Zhang, C.; Luo, A.; Ding, F.; Ma, J.; Yang, S.; Tian, Y.; Tong, T.; Zhan, Q.; et al. Involvement of S100A14 protein in cell invasion by affecting expression and function of matrix metalloproteinase (MMP)-2 via p53-dependent transcriptional regulation. J. Biol. Chem. 2012, 287, 17109–17119. [Google Scholar] [CrossRef]
- He, H.; Li, S.; Chen, H.; Li, L.; Xu, C.; Ding, F.; Zhan, Y.; Ma, J.; Zhang, S.; Shi, Y.; et al. 12-O-tetradecanoylphorbol-13-acetate promotes breast cancer cell motility by increasing S100A14 level in a Kruppel-like transcription factor 4 (KLF4)-dependent manner. J. Biol. Chem. 2014, 289, 9089–9099. [Google Scholar] [CrossRef]
- Tanaka, M.; Ichikawa-Tomikawa, N.; Shishito, N.; Nishiura, K.; Miura, T.; Hozumi, A.; Chiba, H.; Yoshida, S.; Ohtake, T.; Sugino, T. Co-expression of S100A14 and S100A16 correlates with a poor prognosis in human breast cancer and promotes cancer cell invasion. BMC Cancer 2015, 15, 53. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, M.; Gong, T.; Lei, X.; Hu, T.; Tian, M.; Ding, F.; Ma, F.; Chen, H.; Liu, Z. A S100A14-CCL2/CXCL5 signaling axis drives breast cancer metastasis. Theranostics 2020, 10, 5687–5703. [Google Scholar] [CrossRef] [PubMed]
- Al-Ashkar, N.; Zetoune, A.B. S100A14 serum level and its correlation with prognostic factors in breast cancer. J. Egypt. Natl. Cancer Inst. 2020, 32, 37. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Kong, F.; Pan, Y. Prognostic and clinicopathological significance of S100A14 expression in cancer patients: A meta-analysis. Medicine 2019, 98, e16356. [Google Scholar] [CrossRef]
- Ehmsen, S.; Hansen, L.T.; Bak, M.; Brasch-Andersen, C.; Ditzel, H.J.; Leth-Larsen, R. S100A14 is a novel independent prognostic biomarker in the triple-negative breast cancer subtype. Int. J. Cancer 2015, 137, 2093–2103. [Google Scholar] [CrossRef]
- Naba, A.; Clauser, K.R.; Lamar, J.M.; Carr, S.A.; Hynes, R.O. Extracellular matrix signatures of human mammary carcinoma identify novel metastasis promoters. eLife 2014, 3, e01308. [Google Scholar] [CrossRef]
- Liu, D.; Rudland, P.S.; Sibson, D.R.; Platt-Higgins, A.; Barraclough, R. Expression of calcium-binding protein S100A2 in breast lesions. Br. J. Cancer 2000, 83, 1473–1479. [Google Scholar] [CrossRef]
- Wicki, R.; Franz, C.; Scholl, F.A.; Heizmann, C.W.; Schafer, B.W. Repression of the candidate tumor suppressor gene S100A2 in breast cancer is mediated by site-specific hypermethylation. Cell Calcium 1997, 22, 243–254. [Google Scholar] [CrossRef]
- Miller, P.; Kidwell, K.M.; Thomas, D.; Sabel, M.; Rae, J.M.; Hayes, D.F.; Hudson, B.I.; El-Ashry, D.; Lippman, M.E. Elevated S100A8 protein expression in breast cancer cells and breast tumor stroma is prognostic of poor disease outcome. Breast Cancer Res. Treat. 2017, 166, 85–94. [Google Scholar] [CrossRef]
- Borkja, M.L.B.; Giambelluca, M.S.; Ytterhus, B.; Prestvik, W.S.; Bjorkoy, G.; Bofin, A.M. S100A8 gene copy number and protein expression in breast cancer: Associations with proliferation, histopathological grade and molecular subtypes. Breast Cancer Res. Treat. 2023, 201, 339–350. [Google Scholar] [CrossRef]
- Lim, H.; Koh, M.; Jin, H.; Bae, M.; Lee, S.Y.; Kim, K.M.; Jung, J.; Kim, H.J.; Park, S.Y.; Kim, H.S.; et al. Cancer-associated fibroblasts induce an aggressive phenotypic shift in non-malignant breast epithelial cells via interleukin-8 and S100A8. J. Cell Physiol. 2021, 236, 7014–7032. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.H.; Ortega-Rivera, O.A.; Volckaert, B.A.; Jung, E.; Zhao, Z.; Steinmetz, N.F. Viral nanoparticle vaccines against S100A9 reduce lung tumor seeding and metastasis. Proc. Natl. Acad. Sci. USA 2023, 120, e2221859120. [Google Scholar] [CrossRef] [PubMed]
- Gunaldi, M.; Okuturlar, Y.; Gedikbasi, A.; Akarsu, C.; Karabulut, M.; Kural, A. Diagnostic importance of S100A9 and S100A12 in breast cancer. Biomed. Pharmacother. 2015, 76, 52–56. [Google Scholar] [CrossRef]
- Chen, Y.; Ouyang, Y.; Li, Z.; Wang, X.; Ma, J. S100A8 and S100A9 in Cancer. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 188891. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, C.; Nemeth, J.; Angel, P.; Hess, J. S100A8 and S100A9 in inflammation and cancer. Biochem. Pharmacol. 2006, 72, 1622–1631. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, A.; Charafe-Jauffret, E.; Bertucci, F.; Audebert, S.; Toiron, Y.; Esterni, B.; Monville, F.; Tarpin, C.; Jacquemier, J.; Houvenaeghel, G.; et al. Protein profiling of human breast tumor cells identifies novel biomarkers associated with molecular subtypes. Mol. Cell Proteomics 2008, 7, 1420–1433. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Wang, S.; Song, R.; Wang, Z.; Jing, Z.; Wang, S.; Ma, J. S100A8/A9 in Inflammation. Front. Immunol. 2018, 9, 1298. [Google Scholar] [CrossRef]
- Li, Z.; McGinn, O.; Wu, Y.; Bahreini, A.; Priedigkeit, N.M.; Ding, K.; Onkar, S.; Lampenfeld, C.; Sartorius, C.A.; Miller, L.; et al. ESR1 mutant breast cancers show elevated basal cytokeratins and immune activation. Nat. Commun. 2022, 13, 2011. [Google Scholar] [CrossRef]
- Cormier, K.; Harquail, J.; Ouellette, R.J.; Tessier, P.A.; Guerrette, R.; Robichaud, G.A. Intracellular expression of inflammatory proteins S100A8 and S100A9 leads to epithelial-mesenchymal transition and attenuated aggressivity of breast cancer cells. Anticancer. Agents Med. Chem. 2014, 14, 35–45. [Google Scholar] [CrossRef]


| Cancer | S100P | S100A14 | S100A2 | S100A8 | S100A9 |
|---|---|---|---|---|---|
| All breast subtypes (n = 1100) | 3, 4 *** | 3, 4 *** | 3, 4 *** | 3, 4 *** | 3, 4 *** |
| Basal (n = 191) | N.S. | N.S. | N.S. | N.S. | N.S. |
| Her2 (n = 82) | 4 ** | 4 * | 4 ** | 4 * | 4 * |
| Luminal A (n = 568) | 4 *** | 4*** | 4 ** | 4 *** | 4 *** |
| Luminal B (n = 219) | 4 ** | 4 ** | 4 ** | 4 ** | 4 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calaf, G.M.; Ardiles, L.N.; Crispin, L.A. Role of Calcium in an Experimental Breast Cancer Model Induced by Radiation and Estrogen. Biomedicines 2024, 12, 2432. https://doi.org/10.3390/biomedicines12112432
Calaf GM, Ardiles LN, Crispin LA. Role of Calcium in an Experimental Breast Cancer Model Induced by Radiation and Estrogen. Biomedicines. 2024; 12(11):2432. https://doi.org/10.3390/biomedicines12112432
Chicago/Turabian StyleCalaf, Gloria M., Luis N. Ardiles, and Leodan A. Crispin. 2024. "Role of Calcium in an Experimental Breast Cancer Model Induced by Radiation and Estrogen" Biomedicines 12, no. 11: 2432. https://doi.org/10.3390/biomedicines12112432
APA StyleCalaf, G. M., Ardiles, L. N., & Crispin, L. A. (2024). Role of Calcium in an Experimental Breast Cancer Model Induced by Radiation and Estrogen. Biomedicines, 12(11), 2432. https://doi.org/10.3390/biomedicines12112432






