1. Introduction
Bladder cancer is one of the most significant neoplasms in contemporary urology. It is the most frequent cancer in the urinary tract and is notably more common in men than in women [
1,
2]. Its classical symptoms are hematuria and painful urination [
3]. The recognized risk factors are tobacco smoking and occupational exposure [
4]. Surprisingly, there are reports on the protective role of cardiovascular diseases [
4]. The most important examination, the gold standard, in the diagnostic process is cystoscopy with histopathological examination of samples from the suspicious lesions [
3]. Non-muscle-invasive bladder cancer (NMIBC) is a particular type of bladder cancer which is a tumor that is limited to the mucosa and submucosa and does not penetrate through the muscle layer. This type is most common among patients, and more than 70% are diagnosed at this stage [
2,
4]. The treatment of bladder cancer depends the grading and staging of the tumor, as well as the patient’s general condition and accompanying diseases. It comprises the transurethral resection of the bladder tumor (TURBT), cystectomy, BCG therapy, chemotherapy, or radiotherapy [
3,
5]. Unfortunately, the 5-year survival rate in localized bladder cancer is only 30%, which highlights the need for early detection and treatment [
6].
Semaphorins are a broad group of extracellular proteins which are engaged in various biological process. The common feature of all family members is the sema domain. They are involved in neural network and cardiovascular system development, immunity, bone homeostasis, and tumorigenesis [
7].
Sema3C is a member of class III semaphorins which are secretory proteins in humans. Sema3C’s role has been proved in immunological processes and nervous and cardiovascular system development [
8]. In tumorigenesis, sema3C seems to have a multifaced nature. There are reports on sema3C promotion in cases of prostate and gastric cancer, as well as glioma [
7], often associated with unfavorable prognosis [
9]. On the other hand, there is information about its suppressive influence, especially due to the inhibition of tumor-associated angiogenesis [
7]. So far, sema3C has never been evaluated in bladder cancer.
Sema4A is a member of class IV semaphorins which are transmembrane proteins. It is involved in inflammatory processes, nervous system development, and angiogenesis [
8]. In particular, its role in the latter is highlighted since it is a vital anti-angiogenic agent which inhibits the development of malignant tumors, whereas sema4A downregulation leads to neoplasm progression [
10]. Similar to sema3C, sema4A has never been studied in bladder cancer.
The aim of this study was to assess the potential role of sema4A and sema3C in NMIBC. We have already explored the role of sema6D in bladder cancer [
11] and proved it could be a potential urinary marker of this neoplasm. Encouraged by our previous results, we would like to determine the potential role of the other two semaphorins. Moreover, the design of this study makes it possible to easily translate the obtained observations into daily clinical practice and potentially apply the findings in cancer patients.
2. Materials and Methods
The study group involved 15 patients (10 men, 5 women) with an average age of 63.73 years. The following inclusion criteria were set: age over 18 years, and NMIBC diagnosed for the first time, confirmed by histopathological examination. The exclusion criteria were as follows: age under 18 years, pregnancy or breast-feeding, past history of malignancy (including NMIBC), muscle-invasive bladder cancer (MIBC), and immunosuppressive drug intake.
Following the administration of general anesthesia or a subarachnoid spinal block, all patients underwent a transurethral resection of a bladder tumor (TURBT). This procedure involved obtaining tumor tissue for a histopathological examination to verify the presence of bladder cancer and rule out patients with MIBC.
The control group of 5 patients (3 men, 2 women) without NMIBC (and any other malignancy) was matched with the study group in terms of gender and geographical location. The criteria for inclusion were individuals aged over 18 years who did not have NMIBC or MIBC as confirmed by cystoscopy, had no prior history of other malignancies, and were not taking immunosuppressants. In the control group, the absence of bladder tumors was verified during non-cancer-related procedures such as transurethral resection of the prostate (TURP), through ureterorenoscopic lithotripsy, or through a negative cystoscopy in patients suspected of having a bladder tumor.
Blood and urine samples were collected at the beginning of the study both from the study and control group. Fasting blood samples were collected using vacuum tubes. They were centrifugated for 10 min at 2000× g. Urine samples were collected as first morning specimens from mid-stream and were centrifugated for 10 min at 2000× g. The obtained plasma and urine were stored at −80 °C until the analysis. Sema3C and sema4A concentrations were measured by using an enzyme-linked immunosorbent assay (ELISA) with commercially available kits according to the manufacturer’s protocol. Each run was performed in duplicate, and the average was considered a single sample. Sema3C concentration was measured by the sema3C kit provided by Aviva Systems (OKD02877, San Diego, CA, USA). The detection range was estimated to be 78–5000 pg/mL, and the sensitivity was 6.7 pg/mL. Sema4A concentration was measured by the sema4A kit provided by Cloud Clone (SEL921Hu, Wuhan, China), the range was estimated to be 31.2–2000 pg/mL, and the sensitivity was 12.7 pg/mL. The absorbance was read at 450 nm. All experiments were conducted by the same investigator in the same laboratory setting.
The collected data underwent Shapiro–Wilk normality testing, and all of the data with Gaussian distribution are presented as the mean ± SE. The correlation results have been generated using the Pearson Correlation Coefficient or Spearman’s Rank Test, as appropriate. Values with p < 0.05 were considered statistically significant. The data were analyzed using GraphPad Prism 8.0 Software (La Jolla, CA, USA).
The research was conducted following the guidelines of the Helsinki Declaration [
12] and was approved by the Bioethics Committee of the Medical University of Lodz, Poland (no RNN/31/23/KE).
3. Results
For this pilot study, we recruited 15 patients and 5 volunteers as the control group, with their baseline parameters listed below (
Table 1).
There was no statistically significant difference between the patients and controls in terms of sex and kidney function; however, there was a weak difference in terms of the mean age.
The participants’ plasma and urine were tested twice by using an ELISA for sema3C and sema4A. Urinary sema4A concentration was below the detection level so it was not further analyzed.
There was no statistically significant difference between the patients and controls in terms of plasma sema4A and sema3C or urinary sema3C concentration (
p > 0.05,
Figure 1a–c).
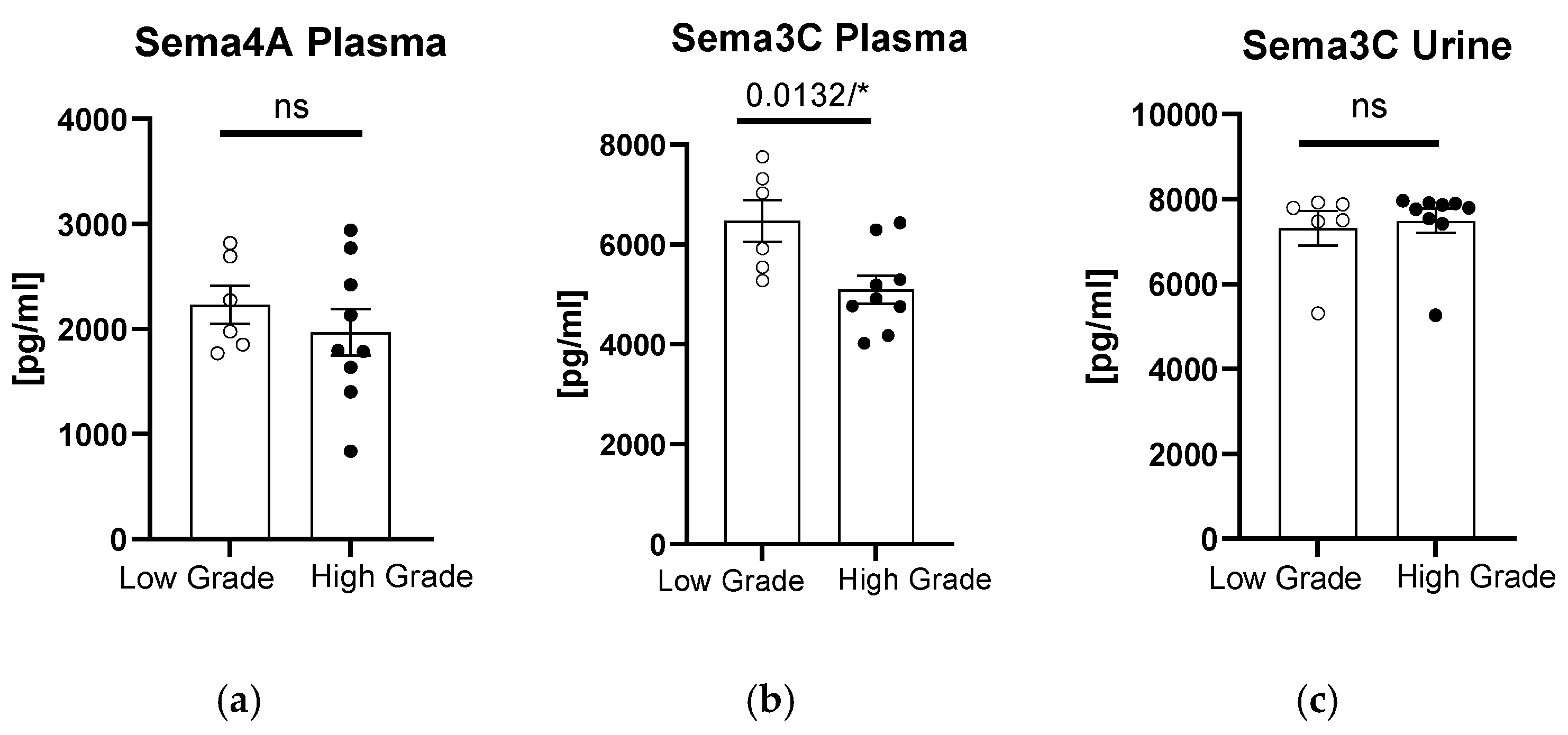
When we divided the patients according to the grade of bladder tumor, we observed significantly higher sema3C plasma concentrations in patients with low-grade tumors (
p = 0.0132,
Figure 2b). In terms of plasma sema4A or urinary sema3C concentrations, there were no significant differences between the patients and controls (
p > 0.05,
Figure 2a,c).
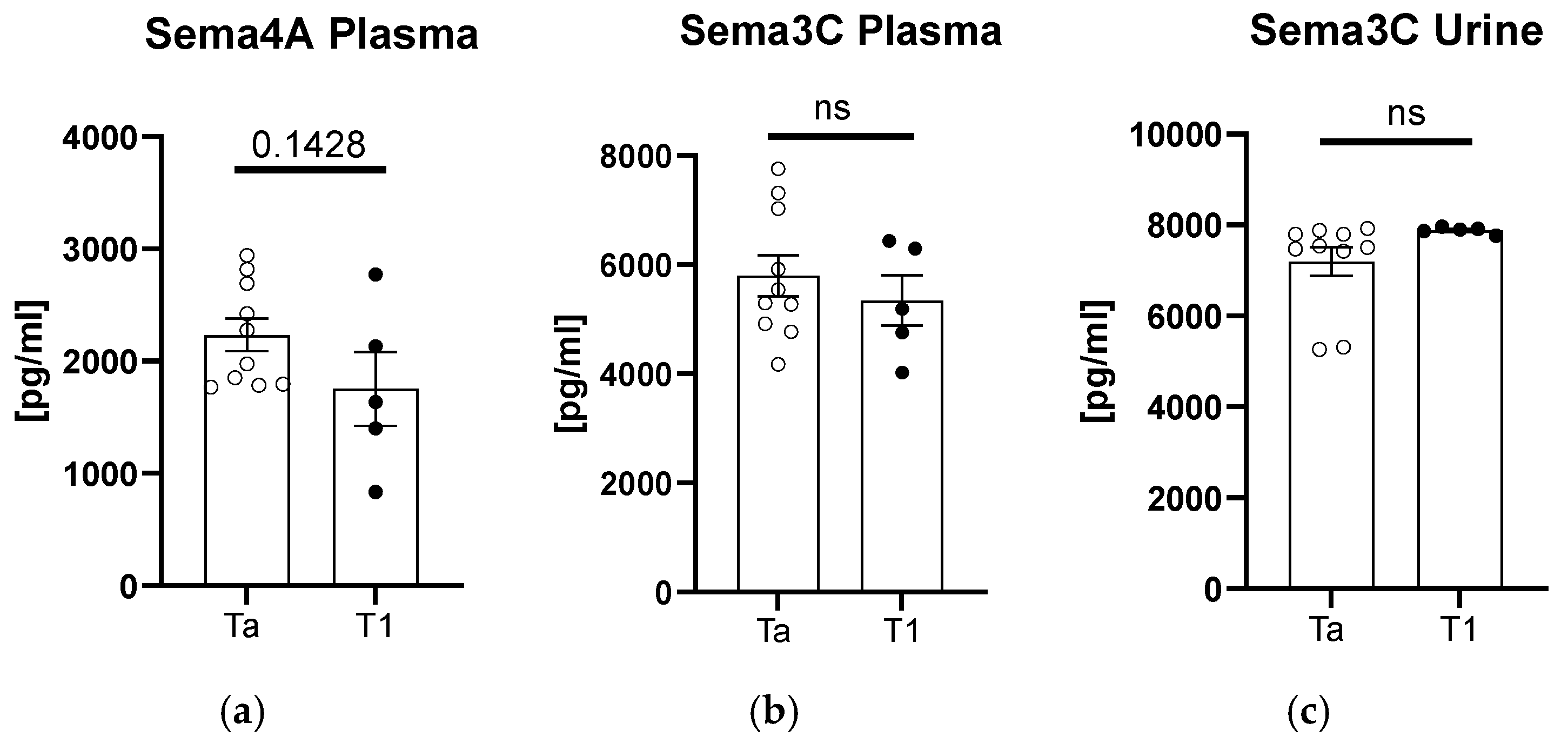
After the division of patients according to tumor staging, we found an upward trend in the sema4A plasma concentration for the subjects with Ta-stage tumor (
Figure 3a). In terms of plasma and urinary sema3C concentrations, there were no significant differences between the patients and controls (
p > 0.05,
Figure 3b,c).
We also searched for correlations between semaphorin concentrations and available clinical or laboratory parameters (
Figure 4). Urinary sema3C concentration positively correlated with tumor size (R = 0.57,
p = 0.03). There was also an upward trend for urinary sema3C and smoking (R = 0.48,
p = 0.07). Plasma sema3C concentration correlated negatively with tumor grade (R = −0.62,
p = 0.01). Plasma sema4A concentration positively correlated with the age of patients (R = 0.59,
p = 0.02).
We also attempted to search for predictors of sema3C or sema4A concentrations; however, we did not find any significant factor (
Table 2).
4. Discussion
In our previous experiment, we proved the potential utility of urinary sema6D as it was significantly higher in patients with NMIBC compared to controls [
11]. This study, on the other hand, is a preliminary investigation of the potential role of sema3C and sema4A in bladder cancer. However, we did not observe significant differences in semaphorin concentrations between the patients and controls, although we obtained some interesting findings that could be studied more deeply in the future to determine their clinical utility. The only non-invasive test that has gained its actual place in urological practice is urine cytology. However, it is characterized by high specificity, and its sensitivity is low [
13], so better tests are necessary.
To the best of our knowledge, sema3C and sema4A have never been studied among patients with bladder cancer, so we are the first to report on this matter.
Among all urological cancers, both sema3C and 4A have been studied in prostate cancer. Sema4A was documented to promote prostate cancer invasion. It was overexpressed in cancer cells and even correlated with Gleason score [
14]. Sema3C also seems to be involved in the progression of prostate cancer via the mutation of the transcription factor FOXA1 and even contributes to its resistance to therapy [
15,
16]. Sema3C could be considered a future therapeutic target [
16].
In our study, urinary sema4A concentration was below the detection threshold; therefore, it is unlikely to become useful as a marker of NMIBC. The lack of in-depth information regarding kidney metabolism of sema4A, leading to its urinary secretion, makes it difficult to interpret this observation at this point.
Plasma sema4A concentration and sema3C concentration in plasma and urine cannot be used as stand-alone markers of NMIBC at this time due to no statistically significant differences between the patients and controls.
Interesting observations were made about sema3C and sema4A correlations with clinical parameters; however, considering that neither of them was significantly different between the patients and controls, these findings must be approached with caution and studied in larger groups. Nevertheless, taking into account the nature of bladder cancer and its tendency to reoccur, measures must be taken to lower the number of invasive procedures related to assessing the diagnosis and further grading and staging of the tumor [
2]. So far, there have been several non-invasive tools proposed; however, they have not been very popular in the clinical setting. They are mainly based on the detection of gene mutations, chromosome abnormalities, or mRNA. Only a few comprise the detection of specific proteins [
2], which is easier to perform and thus cheaper to translate into clinical practice.
Considering that the plasma concentration of sema3C was significantly higher in patients with low-grade tumors and that it correlated negatively with this grade, sema3C can potentially be considered in the future as a marker for tumors of lower grades. It would have to be tested in larger cohorts; however, it is a potentially clinically useful observation, since lower sema3C concentrations could predict high-grade tumors. Interestingly, urinary sema3C concentration positively correlated at the same time with tumor size, so it seems to become higher in subjects with bigger lesions. It could be potentially explained in such a way that low-grade tumors grow slower, eventually reaching greater volumes which translate to higher amounts of released sema3C.
Plasma sema4A concentration could potentially be considered in the future as a marker for tumors of earlier stages. Similar to sema3C, more in-depth studies are required to establish its clinical application, but its lower concentrations could perhaps indicate higher NMIBC stages, and the other way round—higher concentrations could be predictive of earlier stages and better prognoses. It has an essential clinical application with prognostic purposes, since the 5-year relative survival rates for bladder cancer are 97% in cases of carcinoma in situ and 71% for localized bladder cancer [
17].
Our study lays the groundwork for further research to develop potential tests that could be used in daily practice to monitor and predict the course of cancer.
The main limitation of this study is the relatively small number of participants enrolled, especially in the control group. The preliminary nature of this study and the strict enrolling criteria are the main reasons for the occurrence of this limitation. Although the results lack of robust significance, the presented data deliver novel insights into the possible role of sema4A as a biomarker for early stages of tumors. Future extended studies could further explore whether distinct genomic subtypes of bladder cancer exhibit differential responses to semaphorin signaling. This could be particularly relevant for therapeutic targeting or understanding resistance mechanisms.
5. Conclusions
Urinary sema4A concentration, which is below the detection threshold, is unlikely to be useful as a marker of NMIBC. Plasma sema4A concentration and sema3C concentration in plasma and urine cannot be used as stand-alone markers of NMIBC at this point. The plasma concentration of sema3C can potentially be considered in the future as a marker for tumors of lower grades. Plasma sema4A concentration could potentially be considered in the future as a marker for tumors of earlier stages. All of these observations are preliminary, so they have to be assessed in larger cohorts to make reliable recommendations.
Author Contributions
P.P.: conceptualization, resources, data curation, project administration, writing—original draft preparation, writing—review and editing; Z.J.: writing—review and editing, data curation, supervision; W.K.: funding acquisition; A.K.: investigation; T.W.K.: formal analysis, software, visualization, writing—review and editing; Z.P.: supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Faculty Research Fund of the University of Warmia and Mazury in Olsztyn, Poland, No. 11.610.003-110.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Medical University of Lodz, Poland no RNN/31/23/KE.
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lobo, N.; Afferi, L.; Moschini, M.; Mostafid, H.; Porten, S.; Psutka, S.P.; Gupta, S.; Smith, A.B.; Williams, S.B.; Lotan, Y. Epidemiology, Screening, and Prevention of Bladder Cancer. Eur. Urol. Oncol. 2022, 5, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Schulz, A.; Loloi, J.; Pina Martina, L.; Sankin, A. The Development of Non-Invasive Diagnostic Tools in Bladder Cancer. Onco Targets Ther. 2022, 15, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Smolensky, D.; Rathore, K.; Cekanova, M. Molecular targets in urothelial cancer: Detection, treatment, and animal models of bladder cancer. Drug Des. Devel. Ther. 2016, 10, 3305–3322. [Google Scholar] [CrossRef] [PubMed]
- Barone, B.; Finati, M.; Cinelli, F.; Fanelli, A.; Del Giudice, F.; De Berardinis, E.; Sciarra, A.; Russo, G.; Mancini, V.; D’Altilia, N.; et al. Bladder Cancer and Risk Factors: Data from a Multi-Institutional Long-Term Analysis on Cardiovascular Disease and Cancer Incidence. J. Pers. Med. 2023, 13, 512. [Google Scholar] [CrossRef] [PubMed]
- European Association of Urology Guidelines on Muscle-Invasive and Metastatic Bladder Cancer. Available online: https://uroweb.org/guidelines/muscle-invasive-and-metastatic-bladder-cancer (accessed on 4 July 2024).
- Crocetto, F.; Buonerba, C.; Caputo, V.; Ferro, M.; Persico, F.; Trama, F.; Iliano, E.; Rapisarda, S.; Bada, M.; Facchini, G.; et al. Urologic malignancies: Advances in the analysis and interpretation of clinical findings. Future Sci. OA 2021, 7, FSO674. [Google Scholar] [CrossRef] [PubMed]
- Fard, D.; Tamagnone, L. Semaphorins in health and disease. Cytokine Growth Factor Rev. 2021, 57, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Gong, L.; Sun, Y.; Lin, J.; Hu, W.; Wei, J.; Miao, X.; Gao, T.; Suo, J.; Xu, J.; et al. Semaphorin3C identified as mediator of neuroinflammation and microglia polarization after spinal cord injury. iScience 2024, 27, 109649. [Google Scholar] [CrossRef] [PubMed]
- Peacock, J.W.; Takeuchi, A.; Hayashi, N.; Liu, L.; Tam, K.J.; Al Nakouzi, N.; Khazamipour, N.; Tombe, T.; Dejima, T.; Lee, K.C.; et al. SEMA3C drives cancer growth by transactivating multiple receptor tyrosine kinases via Plexin B1. EMBO Mol. Med. 2018, 10, 219–238. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.S.; Chapoval, S.P. Neuroimmune Semaphorin 4A in Cancer Angiogenesis and Inflammation: A Promoter or a Suppressor? Int. J. Mol. Sci. 2018, 20, 124. [Google Scholar] [CrossRef] [PubMed]
- Purpurowicz, P.; Kaminski, T.W.; Kordan, W.; Korzekwa, A.J.; Purpurowicz, Z.; Jabłonowski, Z. The Role of Semaphorin 6D (Sema6D) in Non-Muscle-Invasive Bladder Cancer—A Preliminary Study on Human Plasma and Urine. Biomedicines 2024, 12, 1426. [Google Scholar] [CrossRef] [PubMed]
- Helsinki Declaration. Available online: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ (accessed on 1 December 2022).
- Tomiyama, E.; Fujita, K.; Hashimoto, M.; Uemura, H.; Nonomura, N. Urinary markers for bladder cancer diagnosis: A review of current status and future challenges. Int. J. Urol. 2024, 31, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tan, W.; Wang, W.; Feng, T.; Wang, C.; Wang, L.; Zhou, W. SEMA4A promotes prostate cancer invasion: Involvement of tumor microenvironment. J. Cancer 2023, 14, 2633–2643. [Google Scholar] [CrossRef] [PubMed]
- Tam, K.J.; Liu, L.; Hsing, M.; Dalal, K.; Thaper, D.; McConeghy, B.; Yenki, P.; Bhasin, S.; Peacock, J.W.; Wang, Y.; et al. Clinically-observed FOXA1 mutations upregulate SEMA3C through transcriptional derepression in prostate cancer. Sci. Rep. 2024, 14, 7082. [Google Scholar] [CrossRef] [PubMed]
- Hui, D.H.F.; Tam, K.J.; Jiao, I.Z.F.; Ong, C.J. Semaphorin 3C as a Therapeutic Target in Prostate and Other Cancers. Int. J. Mol. Sci. 2019, 20, 774. [Google Scholar] [CrossRef]
- Statistics on Bladder Cancer from National Cancer Institute. Available online: https://www.cancer.gov/types/bladder/survival (accessed on 1 July 2024).
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).