miRNA in Machine-Learning-Based Diagnostics of Oral Cancer
Abstract
1. Introduction
2. Methods
2.1. Data Pre-Processing and Analysis
2.2. Generation of miRNA Attributes
2.3. Software and Database Utilization
2.4. Attribute Selection and Machine-Learning Analysis
2.5. Validation and Testing
3. Results
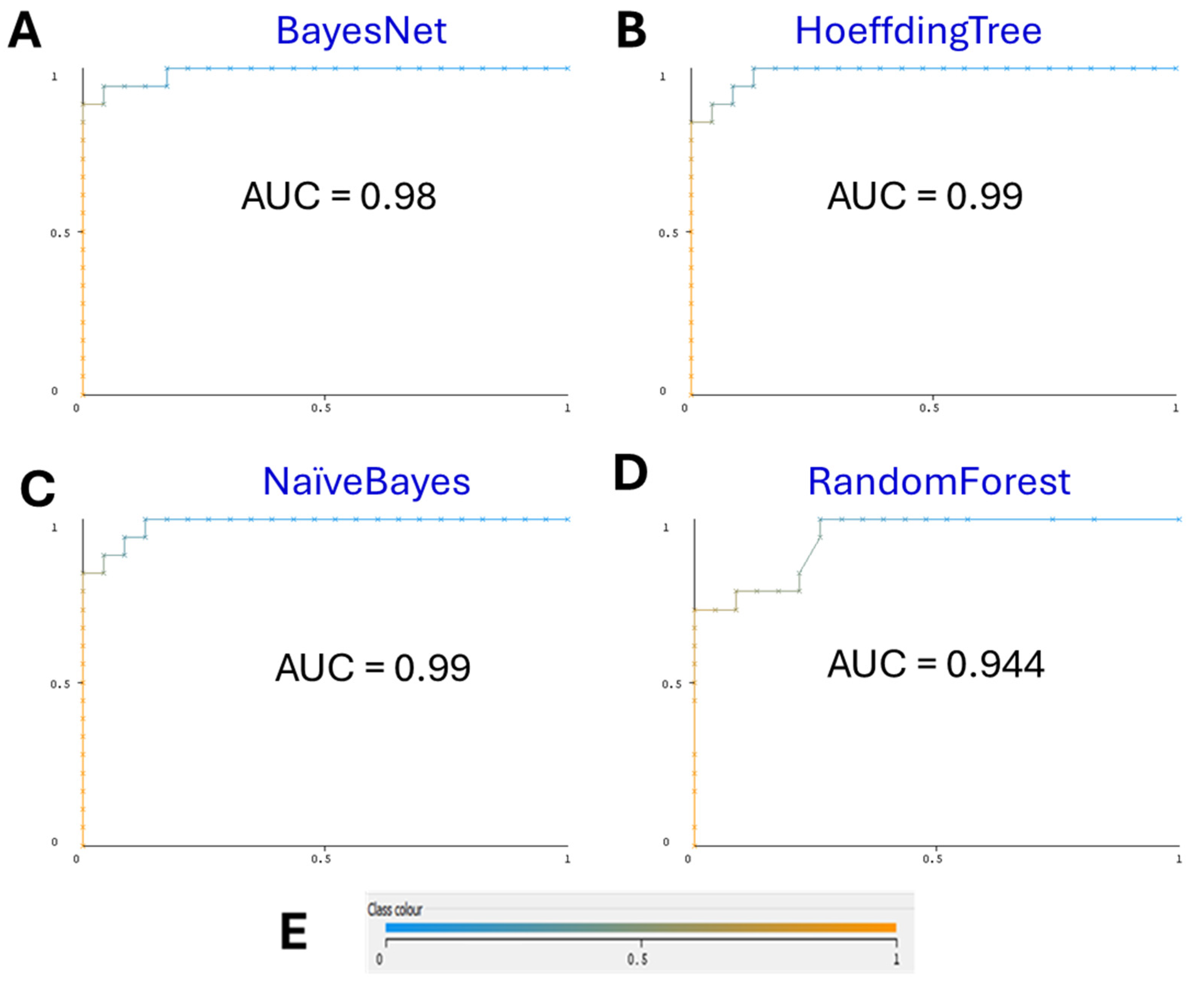
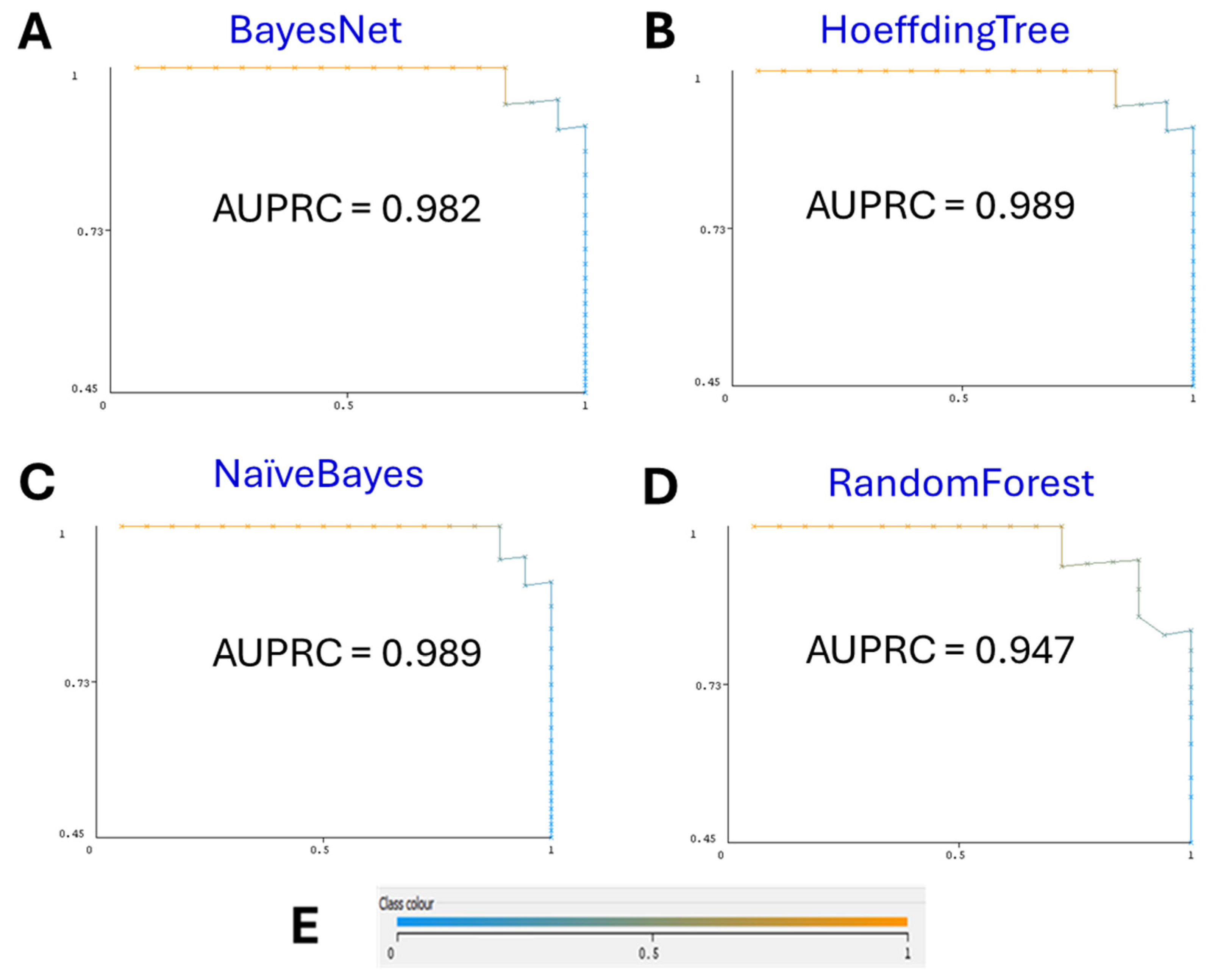
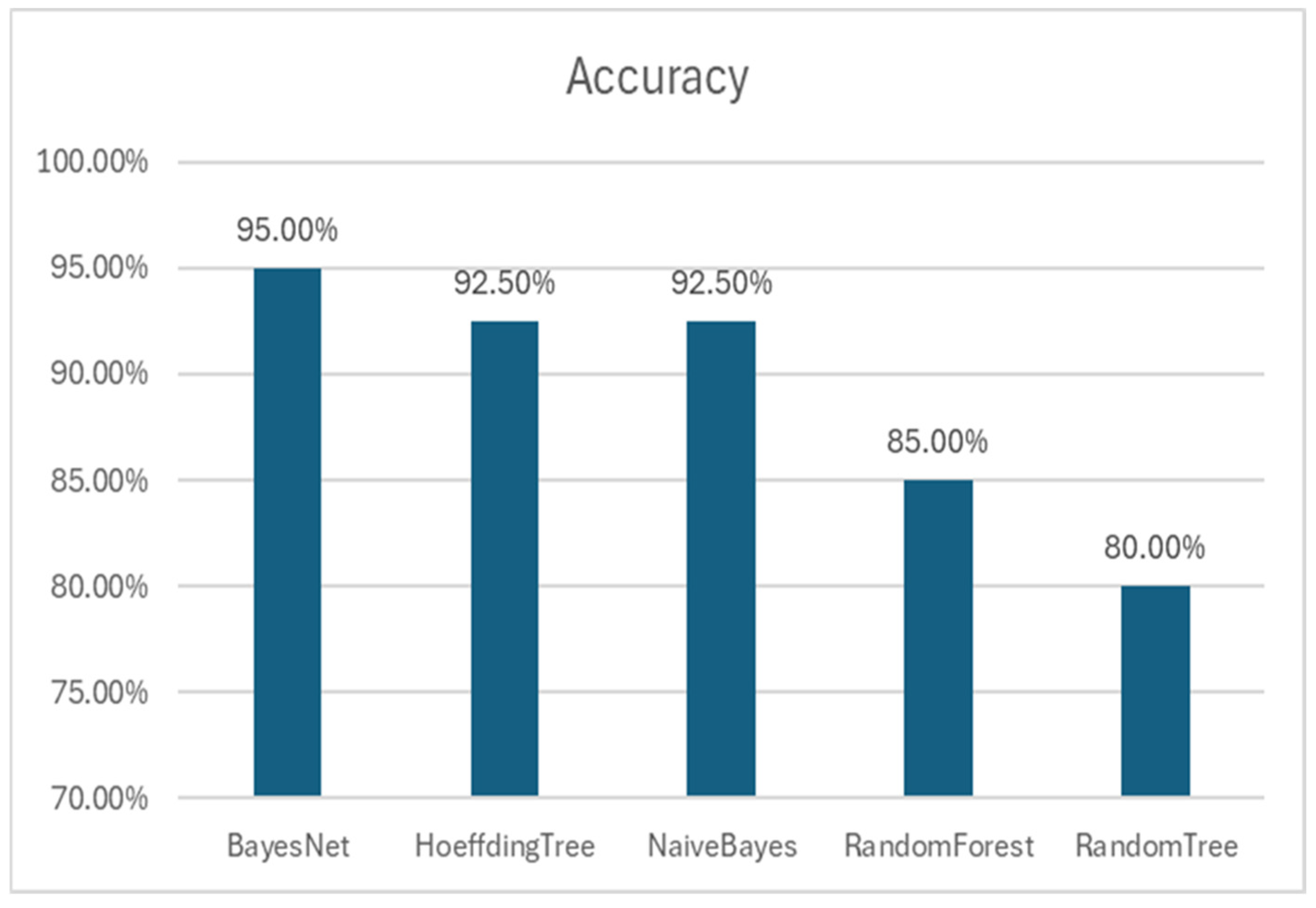
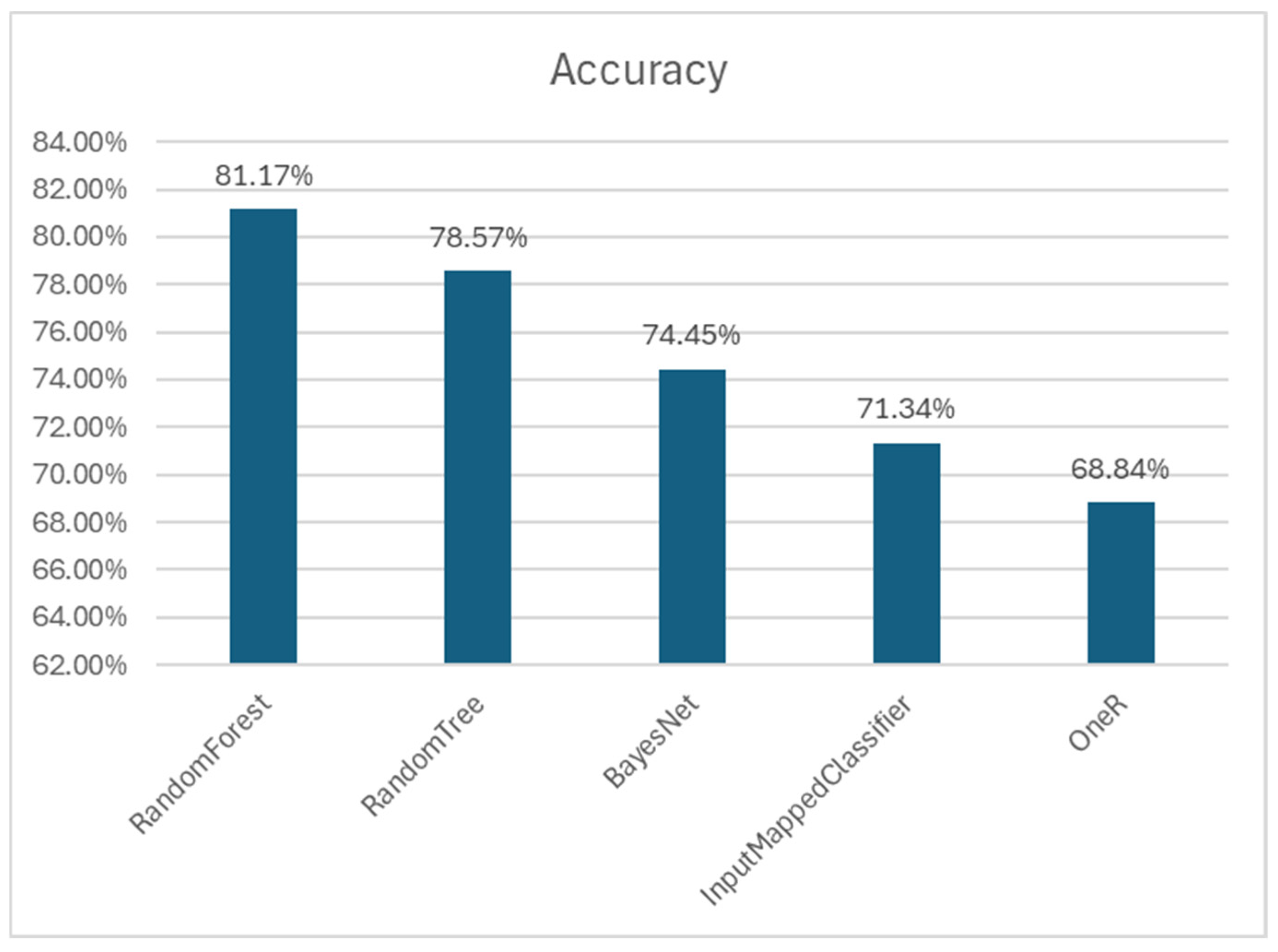
3.1. Machine-Learning Models
3.2. Comparison with Other Algorithms
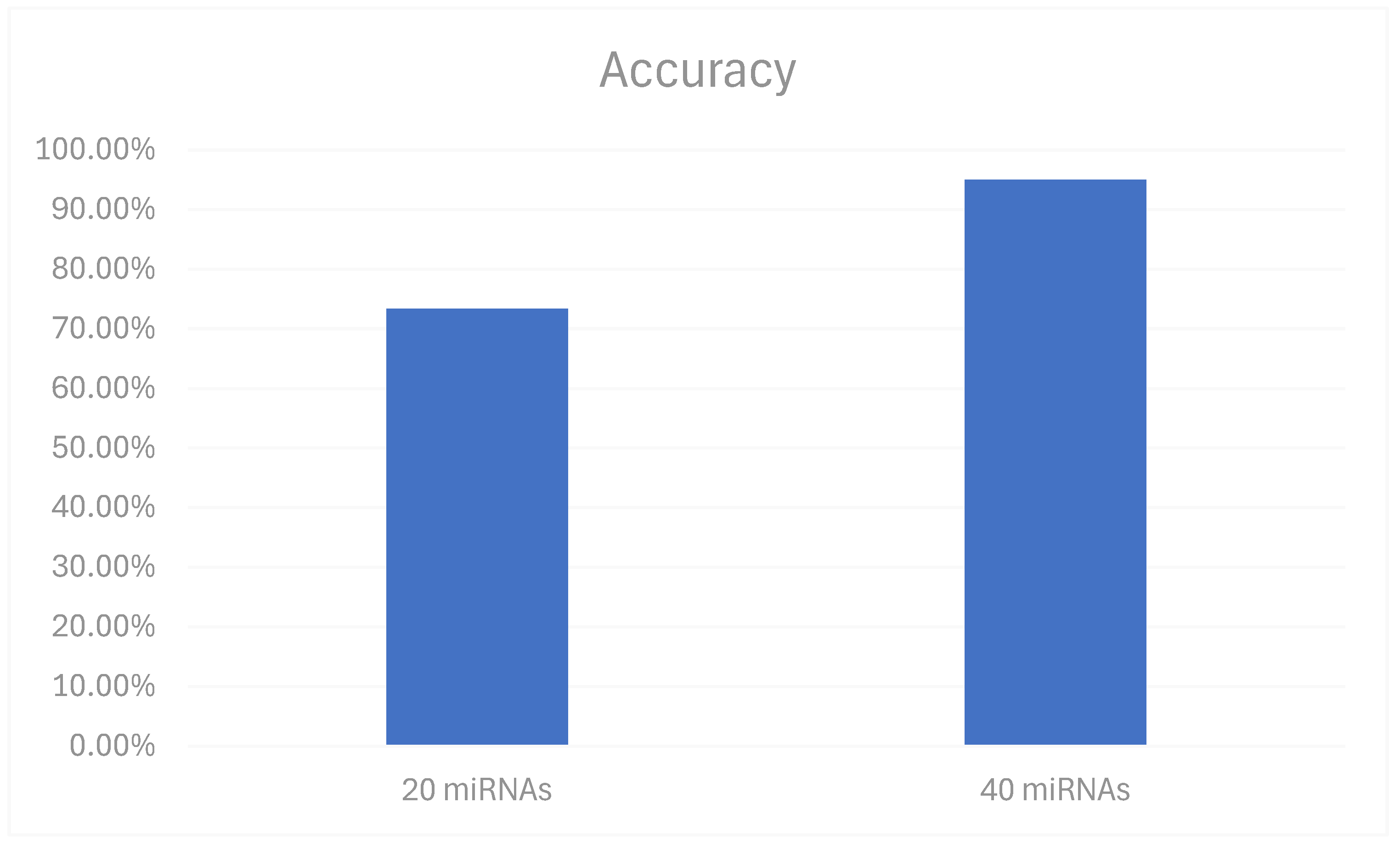
3.3. Optimization of Model Parameters
3.4. Validation of the Trained Model
3.5. Analysis of Model Attributes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Calin, G.A.; Croce, C.M. MicroRNA signatures in human cancers. Nat. Rev. Cancer 2006, 6, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.J.; Walsh, S.; McDermott, E.W.; Crown, J. Biomarkers in breast cancer: Where are we and where are we going? Adv. Clin. Chem. 2015, 71, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kwon, H. Going Deeper with Contextual CNN for Hyperspectral Image Classification. IEEE Trans. Image Process. 2017, 26, 4843–4855. [Google Scholar] [CrossRef]
- Hsu, C.W.; Chen, Y.T.; Hsieh, Y.J.; Chang, K.P.; Hsueh, P.C.; Chen, T.W.; Yu, J.S.; Chang, Y.S.; Li, L.; Wu, C.C. Integrated analyses utilizing metabolomics and transcriptomics reveal perturbation of the polyamine pathway in oral cavity squamous cell carcinoma. Anal. Chim. Acta 2019, 1050, 113–122. [Google Scholar] [CrossRef]
- Kouznetsova, V.L.; Li, J.; Romm, E.; Tsigelny, I.F. Finding distinctions between oral cancer and periodontitis using saliva metabolites and machine learning. Oral Dis. 2021, 27, 484–493. [Google Scholar] [CrossRef]
- Jiang, Q.; Wang, Y.; Hao, Y.; Juan, L.; Teng, M.; Zhang, X.; Li, M.; Wang, G.; Liu, Y. miR2Disease: A manually curated database for microRNA deregulation in human disease. Nucleic Acids Res. 2009, 37, D98–D104. [Google Scholar] [CrossRef]
- Hayes, J.; Peruzzi, P.P.; Lawler, S. MicroRNAs in cancer: Biomarkers, functions and therapy. Trends Mol. Med. 2014, 20, 460–469. [Google Scholar] [CrossRef]
- Aravind, V.A.; Kouznetsova, V.L.; Kesari, S.; Tsigelny, I.F. Using Machine Learning and miRNA for the Diagnosis of Esophageal Cancer. J Appl. Lab. Med. 2024, 9, 684–695. [Google Scholar] [CrossRef]
- Kumar, A.; Kouznetsova, V.L.; Kesari, S.; Tsigelny, I.F. Parkinson’s Disease Diagnosis Using miRNA Biomarkers and Deep Learning. Front. Biosci. 2024, 29, 4. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Li, Y. Prospective applications of microRNAs in oral cancer. Oncol. Lett. 2019, 18, 3974–3984. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kozomara, A.; Griffiths-Jones, S. miRBase: Annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014, 42, D68–D73. [Google Scholar] [CrossRef] [PubMed]
- Bild, A.H.; Yao, G.; Chang, J.T.; Wang, Q.; Potti, A.; Chasse, D.; Joshi, M.B.; Harpole, D.; Lancaster, J.M.; Berchuck, A.; et al. Oncogenic pathway signatures in human cancers as a guide to targeted therapies. Nature 2006, 439, 353–357. [Google Scholar] [CrossRef]
- Griffiths-Jones, S.; Grocock, R.J.; van Dongen, S.; Bateman, A.; Enright, A.J. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006, 34, D140–D144. [Google Scholar] [CrossRef]
- Ambros, V. The functions of animal microRNAs. Nature 2004, 431, 350–355. [Google Scholar] [CrossRef]
- Wong, N.; Wang, X. miRDB: An online resource for microRNA target prediction and functional annotations. Nucleic Acids Res. 2015, 43, D146–D152. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Frank, E.; Hall, M.A.; Witten, I.H. Appendix B: The WEKA Workbench. In Data Mining: Practical Machine Learning Tools and Techniques, 4th ed.; Witten, I.H., Frank, E., Hall, M.A., Pal, C.J., Eds.; Morgan Kaufmann: Cambridge, MA, USA, 2016; pp. 573–600. [Google Scholar]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Kohavi, R. A study of cross-validation and bootstrap for accuracy estimation and model selection. IJCAI 1995, 14, 1137–1143. [Google Scholar]
- Cancer Genome Atlas Research Network; Weinstein, J.N.; Collisson, E.A.; Mills, G.B.; Shaw, K.R.; Ozenberger, B.A.; Ellrott, K.; Shmulevich, I.; Sander, C.; Stuart, J.M. The Cancer Genome Atlas Pan-Cancer analysis project. Nat. Genet. 2013, 45, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, T. An introduction to ROC analysis. Pattern Recognit. Lett. 2006, 27, 861–874. [Google Scholar] [CrossRef]
- Díaz-Uriarte, R.; Alvarez de Andrés, S. Gene selection and classification of microarray data using random forest. BMC Bioinform. 2006, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Falzone, L.; Lupo, G.; La Rosa, G.R.M.; Crimi, S.; Anfuso, C.D.; Salemi, R.; Rapisarda, E.; Libra, M.; Candido, S. Identification of novel microRNAs and their diagnostic and prognostic significance in oral cancer. Cancers 2019, 11, 610. [Google Scholar] [CrossRef] [PubMed]
- Guyon, I.; Elisseeff, A. An introduction to variable and feature selection. J. Mach. Learn. Res. 2003, 3, 1157–1182. [Google Scholar] [CrossRef][Green Version]
- Hand, D.J.; Yu, K. Idiot’s Bayes—Not so stupid after all? Int. Stat. Rev. 2001, 69, 385–398. [Google Scholar] [CrossRef]
- Friedman, N.; Geiger, D.; Goldszmidt, M. Bayesian network classifiers. Mach. Learn. 1997, 29, 131–163. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef]
- Alpaydin, E. Introduction to Machine Learning, 4th ed.; Adaptive Computation and Machine Learning Series; MIT Press: Boston, MA, USA, 2020; 712p. [Google Scholar]
- Wheeler, H.E.; Kim, S.K. Genetics and genomics of human ageing. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011, 366, 43–50. [Google Scholar] [CrossRef]
- Calin, G.A.; Ferracin, M.; Cimmino, A.; Di Leva, G.; Shimizu, M.; Wojcik, S.E.; Iorio, M.V.; Visone, R.; Sever, N.I.; Fabbri, M.; et al. A MicroRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. N. Engl. J. Med. 2005, 353, 1793–1801, Erratum in: N. Engl. J. Med. 2006, 355, 533. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Kohavi, R.; Provost, F. Glossary of terms. Mach. Learn. 1998, 30, 271–274. [Google Scholar]
| miRNAs | Genes | |||||||
|---|---|---|---|---|---|---|---|---|
| hsa-miR-301a-5p | MAB21L2 | PPM1L | KLHL42 | STK39 | TNRC6B | SMAD1 | EPC2 | ERLIN1 |
| hsa-miR-3160-5p | C5orf24 | ELMOD2 | RPS6KA6 | LSM12 | UBN2 | FBXO28 | NLK | LOXL2 |
| hsa-miR-1297 | SLC8A1 | SERINC5 | CDK8 | LARP1 | NAP1L5 | CAMSAP1 | PLEKHH1 | IQCJ |
| hsa-miR-3677-5p | MBNL2 | SLC5A3 | ETNK1 | LEF1 | ULK2 | FAM136A | COL10A1 | ZIC5 |
| hsa-miR-4535 | RGL1 | TNFAIP1 | POLR3G | ATP11C | BAG4 | TMC7 | RSPRY1 | PARPBP |
| hsa-miR-6732-5p | CDH11 | DIP2A | SLC7A11 | PEX13 | BLOC1S2 | REEP3 | PFKFB2 | MSMO1 |
| hsa-miR-6717-5p | ESR1 | STRADB | RHOQ | E2F7 | SLC25A36 | FBXL19 | ULK1 | KDM1B |
| hsa-miR-3619-5p | MGA | SLC2A13 | OTUD4 | NHS | MAB21L1 | PLOD2 | UBR3 | ITGB1BP1 |
| hsa-miR-4746-3p | ZNF236 | CIPC | NAB1 | NUS1 | USP3 | ZNF608 | CDK6 | RC3H1 |
| hsa-miR-4746-3p | TADA2B | TET2 | CHORDC1 | STRBP | NAA15 | CEP350 | UBE2G1 | MED19 |
| hsa-miR-4746-3p | BNC2 | CASZ1 | CREBRF | SLC25A16 | ALDH5A1 | SULF1 | CREB1 | MFN2 |
| hsa-miR-1304-3p | DHX9 | USP9X | TET1 | CLASP2 | MTM1 | BRWD1 | ADM | AMMECR1L |
| hsa-miR-570-3p | NR2C2 | FAM98A | B3GNT5 | TET3 | GNA13 | ZSWIM6 | SLC45A4 | ZFAND3 |
| hsa-miR-21-3p | ZFHX4 | TP53INP1 | PHF3 | PITPNC1 | SYT10 | EIF2S1 | BBX | MAPK1 |
| hsa-miR-32-3p | TSC22D2 | PTEN | KCNJ2 | OSBPL11 | GSK3B | FRMD4B | MTDH | NEO1 |
| hsa-miR-4763-3p | CTNND2 | KLHL42 | STK39 | TNRC6B | SMAD1 | EPC2 | ERLIN1 | DOLPP1 |
| hsa-miR-6796-3p | PPM1L | RPS6KA6 | LSM12 | UBN2 | FBXO28 | NLK | LOXL2 | CBL |
| hsa-miR-486-3p | ELMOD2 | CDK8 | LARP1 | NAP1L5 | CAMSAP1 | PLEKHH1 | IQCJ | SEC24C |
| hsa-miR-3605-3p | SERINC5 | ETNK1 | LEF1 | ULK2 | FAM136A | COL10A1 | ZIC5 | DMXL2 |
| hsa-miR-548ae-3p | SLC5A3 | POLR3G | ATP11C | BAG4 | TMC7 | RSPRY1 | PARPBP | C17orf49 |
| hsa-miR-525-3p | EIF5A2 | FAM160B1 | COL19A1 | SLC30A7 | HOOK3 | NR3C1 | HPSE2 | C17orf49 |
| hsa-let-7a-3p | TMTC2 | C21orf91 | 44991 | PLEKHM3 | KIAA0232 | KIAA1217 | BAZ2A | PIM1 |
| Pathways | |
|---|---|
| Endocrine and other factor-regulated calcium reabsorption | Oxytocin signaling pathway |
| mTOR signaling pathway | Adrenergic signaling in cardiomyocytes |
| Prostate cancer | Progesterone-mediated oocyte maturation |
| Insulin resistance | MAPK signaling pathway |
| AMPK signaling pathway | MicroRNAs in cancer |
| Autophagy—animal | TGF-beta signaling pathway |
| Breast cancer | Oocyte meiosis |
| Endometrial cancer | Signaling pathways regulating pluripotency of stem cells |
| Hepatocellular carcinoma | Hippo signaling pathway |
| Kaposi sarcoma-associated herpesvirus infection | JAK–STAT signaling pathway |
| Human cytomegalovirus infection | Endocytosis |
| Longevity regulating pathway | Measles |
| Choline metabolism in cancer | Alcoholic liver disease |
| Phospholipase D signaling pathway | Hepatitis B |
| African trypanosomiasis | Tuberculosis |
| Allograft rejection | Pathogenic Escherichia coli infection |
| Type I diabetes mellitus | Epstein–Barr virus infection |
| Pathways in cancer | Human immunodeficiency virus 1 infection |
| Pertussis | Lipid and atherosclerosis |
| Leishmaniasis | B cell receptor signaling pathway |
| Chagas disease | C-type lectin receptor signaling pathway |
| Toll-like receptor signaling pathway | T cell receptor signaling pathway |
| Ras signaling pathway | Proteoglycans in cancer |
| Melanoma | Long-term depression |
| p53 signaling pathway | Fc epsilon RI signaling pathway |
| NF-kappa B signaling pathway | Gastric cancer |
| Toxoplasmosis | Melanogenesis |
| Classifier | Accuracy | Precision | Recall | F-Measure | AUC | AUPRC |
|---|---|---|---|---|---|---|
| BayesNet | 0.950 | 0.954 | 0.950 | 0.950 | 0.980 | 0.982 |
| HoeffdingTree | 0.925 | 0.934 | 0.925 | 0.924 | 0.990 | 0.989 |
| NaïveBayes | 0.925 | 0.934 | 0.925 | 0.924 | 0.990 | 0.989 |
| RandomForest | 0.850 | 0.852 | 0.850 | 0.849 | 0.944 | 0.947 |
| RandomTree | 0.850 | 0.800 | 0.800 | 0.800 | 0.798 | 0.738 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Kouznetsova, V.L.; Tsigelny, I.F. miRNA in Machine-Learning-Based Diagnostics of Oral Cancer. Biomedicines 2024, 12, 2404. https://doi.org/10.3390/biomedicines12102404
Li X, Kouznetsova VL, Tsigelny IF. miRNA in Machine-Learning-Based Diagnostics of Oral Cancer. Biomedicines. 2024; 12(10):2404. https://doi.org/10.3390/biomedicines12102404
Chicago/Turabian StyleLi, Xinghang, Valentina L. Kouznetsova, and Igor F. Tsigelny. 2024. "miRNA in Machine-Learning-Based Diagnostics of Oral Cancer" Biomedicines 12, no. 10: 2404. https://doi.org/10.3390/biomedicines12102404
APA StyleLi, X., Kouznetsova, V. L., & Tsigelny, I. F. (2024). miRNA in Machine-Learning-Based Diagnostics of Oral Cancer. Biomedicines, 12(10), 2404. https://doi.org/10.3390/biomedicines12102404






