Comparative Analysis of Corneal Wound Healing: Differential Molecular Responses in Tears Following PRK, FS-LASIK, and SMILE Procedures
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. Characteristics of Patients Who Qualified for PRK Procedure
2.3. Characteristics of Patients Who Qualified for FS-LASIK Procedure
2.4. Characteristics of Patients Who Qualified for SMILE Procedure
2.5. Tear Collection
2.6. Molecular Assessment
2.6.1. Strategy to Find Genes and Their Encoded Proteins for Molecular Analysis
2.6.2. Isolation of Total Ribonucleic Acid (RNA)
2.6.3. RT-qPCR
2.6.4. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Statistical Analysis
3. Results
3.1. Analysis of Transcriptional Activity of Selected Genes Based on RT-qPCR
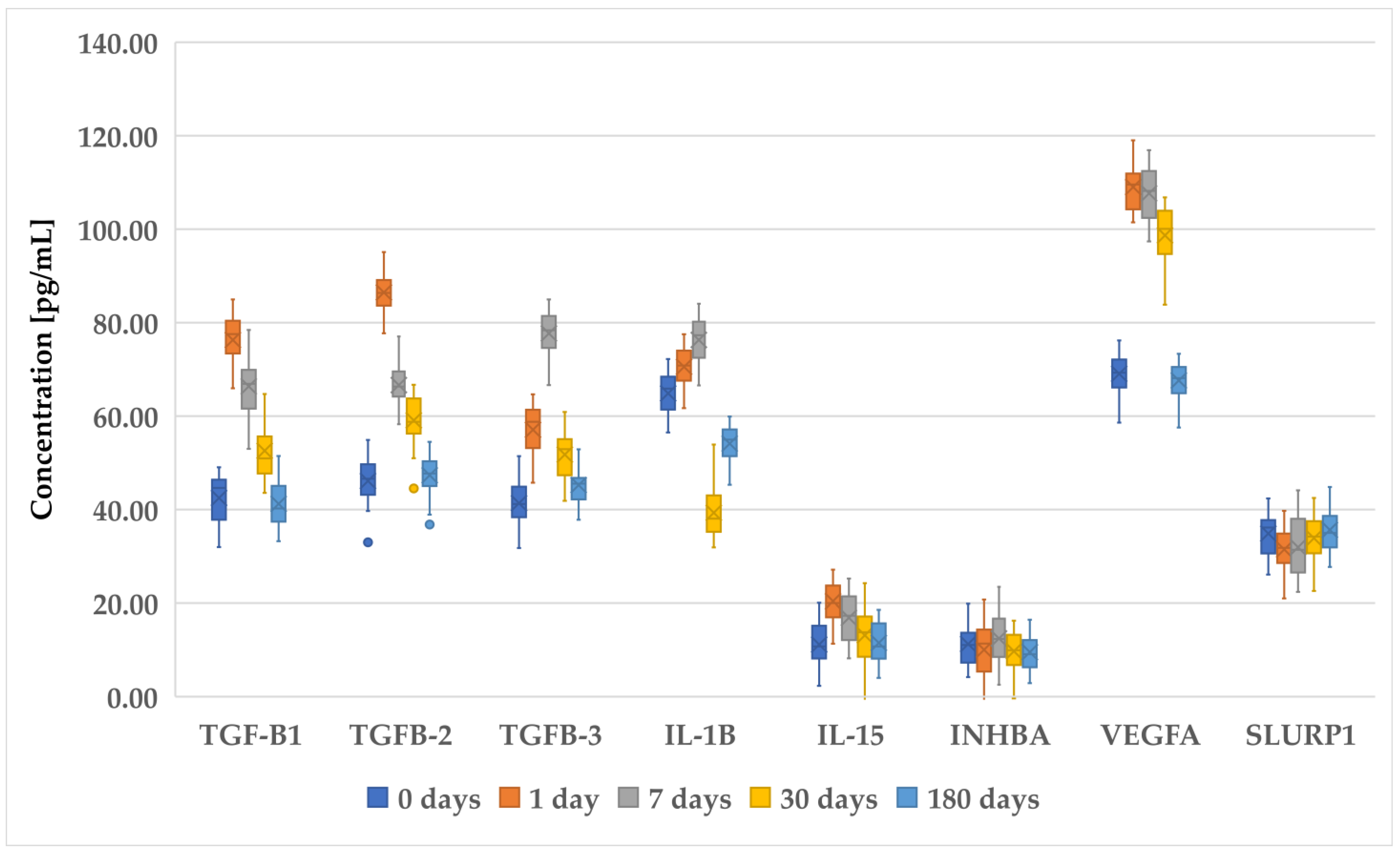
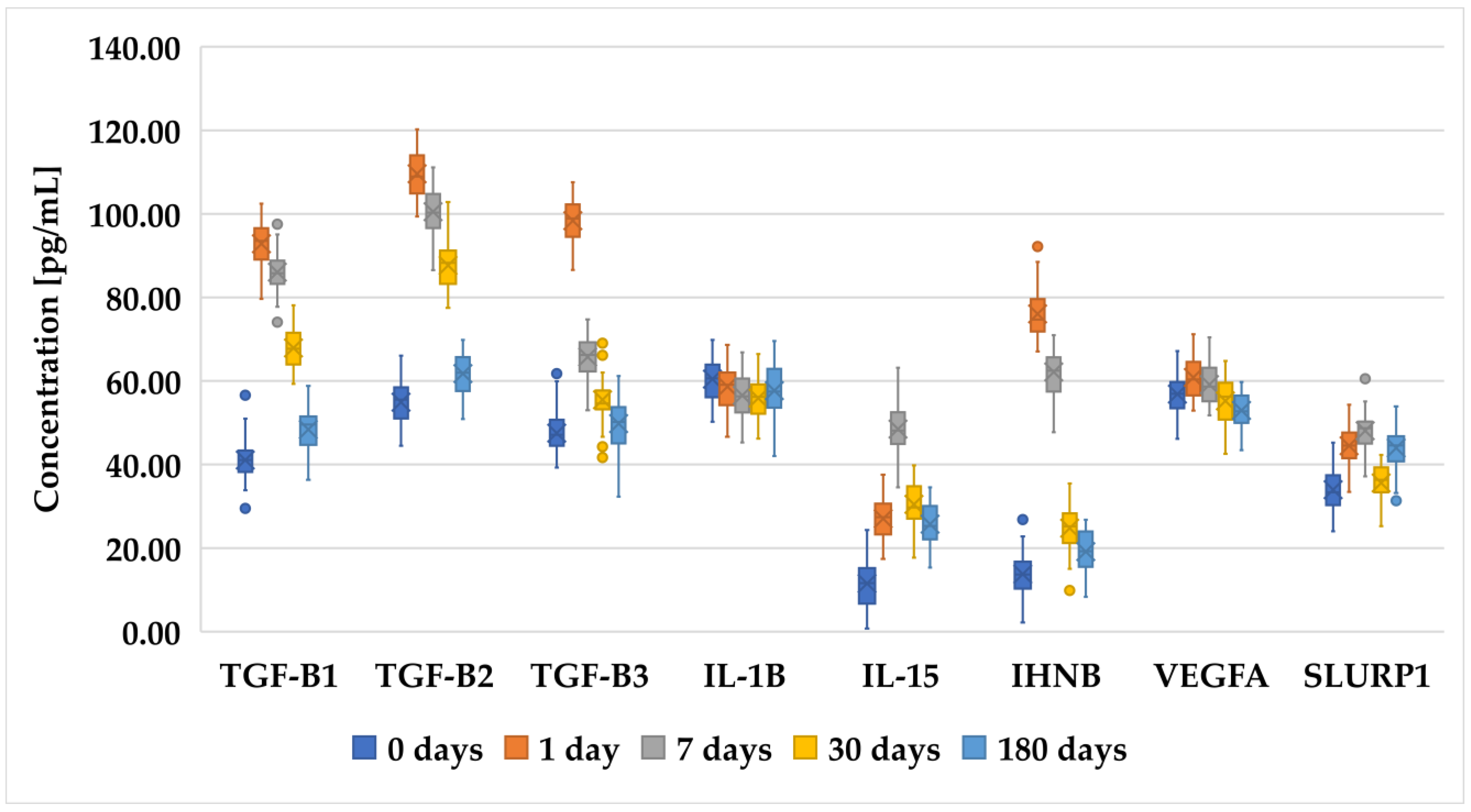
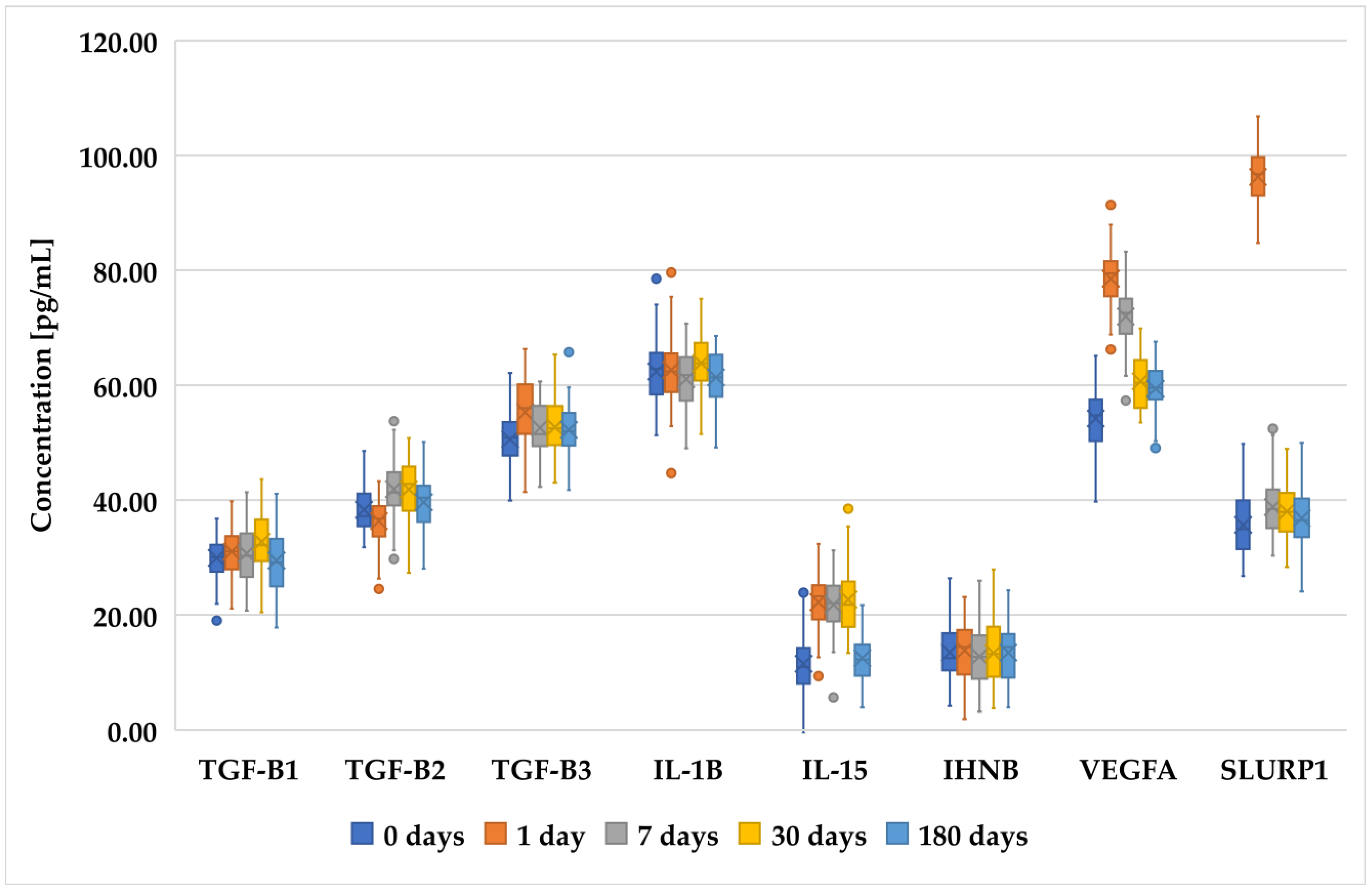
3.2. Changes in Concentration of Selected Proteins Determined with ELISA in Patients before and after PRK, FS-LASIK, and SMILE
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lim, E.W.L.; Lim, L. Review of Laser Vision Correction (LASIK, PRK and SMILE) with Simultaneous Accelerated Corneal Crosslinking–Long-Term Results. Curr. Eye Res. 2019, 44, 1171–1180. [Google Scholar] [CrossRef] [PubMed]
- Stonecipher, K.; Parrish, J.; Stonecipher, M. Comparing Wavefront-Optimized, Wavefront-Guided and Topography-Guided Laser Vision Correction: Clinical Outcomes Using an Objective Decision Tree. Curr. Opin. Ophthalmol. 2018, 29, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Hosseini-Moghaddam, S.M.; Hodge, W. Corneal Biomechanical Properties after SMILE versus FLEX, LASIK, LASEK, or PRK: A Systematic Review and Meta-Analysis. BMC Ophthalmol. 2019, 19, 167. [Google Scholar] [CrossRef]
- Spadea, L.; Paroli, M.P.; Spadea, L. No-Alcohol Laser Epithelial Keratomileusis (LASEK) and PhotoRefractive Keratectomy (PRK) for the Correction of Low to Medium Myopia: A Review of the Literature. J. Ophthal. Opto. 2021, 3, 10. [Google Scholar]
- Vetter, J.M.; Holzer, M.P.; Teping, C.; Weingärtner, W.E.; Gericke, A.; Stoffelns, B.; Pfeiffer, N.; Sekundo, W. Intraocular Pressure during Corneal Flap Preparation: Comparison among Four Femtosecond Lasers in Porcine Eyes. J. Refract. Surg. 2011, 27, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Reinstein, D.Z.; Archer, T.J.; Gobbe, M. The History of LASIK. J. Refract. Surg. 2012, 28, 291–298. [Google Scholar] [CrossRef]
- Solomon, K.D.; De Castro, L.E.F.; Sandoval, H.P.; Biber, J.M.; Groat, B.; Neff, K.D.; Ying, M.S.; French, J.W.; Donnenfeld, E.D.; Lindstrom, R.L. LASIK World Literature Review: Quality of Life and Patient Satisfaction. Ophthalmology 2009, 116, 691–701. [Google Scholar] [CrossRef]
- Blum, M.; Täubig, K.; Gruhn, C.; Sekundo, W.; Kunert, K.S. Five-Year Results of Small Incision Lenticule Extraction (ReLEx SMILE). Br. J. Ophthalmol. 2016, 100, 1192–1195. [Google Scholar] [CrossRef]
- Vatsa, S.; Dhawan, P. Shana Sood A to Z of ReLeX SMILE: ALL You Need to Know. Delhi J. Ophthalmol. 2021, 32, 25–31. [Google Scholar] [CrossRef]
- Ganesh, S.; Brar, S.; Arra, R.R. Refractive Lenticule Extraction Small Incision Lenticule Extraction: A New Refractive Surgery Paradigm. Indian J. Ophthalmol. 2018, 66, 10. [Google Scholar]
- Chen, H.; Lin, H.; Zheng, D.; Liu, Y.; Chen, W.; Liu, Y. Expression of Cytokines, Chmokines and Growth Factors in Patients Undergoing Cataract Surgery with Femtosecond Laser Pretreatment. PLoS ONE 2015, 10, e0137227. [Google Scholar] [CrossRef] [PubMed]
- Binz, N.; Graham, C.E.; Simpson, K.; Lai, Y.K.Y.; Shen, W.; Lai, C.; Speed, T.P.; Rakoczy, P.E. Long-term Effect of Therapeutic Laser Photocoagulation on Gene Expression in the Eye. FASEB J. 2006, 20, 383–385. [Google Scholar] [CrossRef] [PubMed]
- González-García, M.J.; Murillo, G.M.; Pinto-Fraga, J.; García, N.; Fernandez, I.; Maldonado, M.J.; Calonge, M.; Enriquez-de-Salamanca, A. Clinical and Tear Cytokine Profiles after Advanced Surface Ablation Refractive Surgery: A Six-Month Follow-Up. Exp. Eye Res. 2020, 193, 107976. [Google Scholar] [CrossRef] [PubMed]
- Resan, M.; Vukosavljevic, M.; Vojvodic, D.; Pajic-Eggspuehler, B.; Pajic, B. The Acute Phase of Inflammatory Response Involved in the Wound-Healing Process after Excimer Laser Treatment. Clin. Ophthalmol. 2016, 10, 993–1000. [Google Scholar] [CrossRef]
- Ang, M.; Gatinel, D.; Reinstein, D.Z.; Mertens, E.; Alió del Barrio, J.L.; Alió, J.L. Refractive Surgery beyond 2020. Eye 2021, 35, 362–382. [Google Scholar] [CrossRef]
- Khamar, P.; Nishtala, K.; Shetty, R.; Panigrahi, T.; Shetty, K.; Pahuja, N.; Deshpande, V.; Ghosh, A. Early Biological Responses in Ocular Tissue after SMILE and LASIK Surgery. Exp. Eye Res. 2020, 192, 107936. [Google Scholar] [CrossRef]
- Hou, C.; Li, J.; Li, J.; Peng, H.; Wang, Q. In Vivo Confocal Microscopy of Sub-Basal Corneal Nerves and Corneal Densitometry after Three Kinds of Refractive Procedures for High Myopia. Int. Ophthalmol. 2023, 43, 925–935. [Google Scholar] [CrossRef]
- Yagi-Yaguchi, Y.; Kojima, T.; Higa, K.; Dogru, M.; Ibrahim, O.M.; Shimizu, T.; Tsubota, K.; Shimazaki, J. The Effects of 3% Diquafosol Sodium Eye Drops on Tear Function and the Ocular Surface of Cu, Zn-Superoxide Dismutase-1 (Sod1) Knockout Mice Treated with Antiglaucoma Eye Medications. Diagnostics 2020, 10, 20. [Google Scholar] [CrossRef]
- Janiszewska-Bil, D.; Czarnota-Nowakowska, B.; Grabarek, B.O.; Dobrowolski, D.; Wylęgała, E.; Lyssek-Boroń, A. Comparison of Vision Correction and Corneal Thickness at 180-Day Follow-Up After Femtosecond Laser-Assisted In-Situ Keratomileusis (FS-LASIK), Photorefractive Keratectomy (PRK), and Small Incision Lenticule Extraction (SMILE): A Study from a Single Center in Poland of 120 Patients with Myopia. Med. Sci. Monit. 2023, 29, e939099-1–e939099-14. [Google Scholar] [CrossRef]
- Wilson, S.E. Corneal Wound Healing. Exp. Eye Res. 2020, 197, 108089. [Google Scholar] [CrossRef]
- Gao, S.; Li, S.; Liu, L.; Wang, Y.; Ding, H.; Li, L.; Zhong, X. Early Changes in Ocular Surface and Tear Inflammatory Mediators after Small-Incision Lenticule Extraction and Femtosecond Laser-Assisted Laser in Situ Keratomileusis. PLoS ONE 2014, 9, e107370. [Google Scholar] [CrossRef]
- Belinky, F.; Nativ, N.; Stelzer, G.; Zimmerman, S.; Iny Stein, T.; Safran, M.; Lancet, D. PathCards: Multi-Source Consolidation of Human Biological Pathways. Database 2015, 2015, bav006. [Google Scholar] [CrossRef] [PubMed]
- Mi, H.; Ebert, D.; Muruganujan, A.; Mills, C.; Albou, L.-P.; Mushayamaha, T.; Thomas, P.D. PANTHER Version 16: A Revised Family Classification, Tree-Based Classification Tool, Enhancer Regions and Extensive API. Nucleic Acids Res. 2021, 49, D394–D403. [Google Scholar] [CrossRef]
- Thomas, P.D.; Ebert, D.; Muruganujan, A.; Mushayahama, T.; Albou, L.-P.; Mi, H. PANTHER: Making Genome-Scale Phylogenetics Accessible to All. Protein Sci. 2022, 31, 8–22. [Google Scholar] [CrossRef]
- Bukowiecki, A.; Hos, D.; Cursiefen, C.; Eming, S.A. Wound-Healing Studies in Cornea and Skin: Parallels, Differences and Opportunities. Int. J. Mol. Sci. 2017, 18, 1257. [Google Scholar] [CrossRef]
- Huang, J.C.-C.; Sun, C.-C.; Chang, C.-K.; Ma, D.H.-K.; Lin, Y.-F. Effect of Hinge Position on Corneal Sensation and Dry Eye Parameters After Femtosecond Laser–Assisted LASIK. J. Refract. Surg. 2012, 28, 625–631. [Google Scholar] [CrossRef]
- Ganesh, S.; Brar, S.; Patel, U. Comparison of ReLEx SMILE and PRK in Terms of Visual and Refractive Outcomes for the Correction of Low Myopia. Int. Ophthalmol. 2018, 38, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, S.; Brar, S.; Pawar, A. Results of Intraoperative Manual Cyclotorsion Compensation for Myopic Astigmatism in Patients Undergoing Small Incision Lenticule Extraction (SMILE). J. Refract. Surg. 2017, 33, 506–512. [Google Scholar] [CrossRef]
- Shetty, R.; Francis, M.; Shroff, R.; Pahuja, N.; Khamar, P.; Girrish, M.; Nuijts, R.M.; Roy, A.S. Corneal Biomechanical Changes and Tissue Remodeling after SMILE and LASIK. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5703–5712. [Google Scholar] [CrossRef]
- Williams, G.P.; Wu, B.; Liu, Y.C.; Teo, E.; Nyein, C.L.; Peh, G.; Tan, D.T.; Mehta, J.S. Hyperopic Refractive Correction by LASIK, SMILE or Lenticule Reimplantation in a Non-Human Primate Model. PLoS ONE 2018, 13, e0194209. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Teo, E.P.W.; Lwin, N.C.; Yam, G.H.F.; Mehta, J.S. Early Corneal Wound Healing and Inflammatory Responses After SMILE: Comparison of the Effects of Different Refractive Corrections and Surgical Experiences. J. Refract. Surg. 2016, 32, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Ljubimov, A.V.; Saghizadeh, M. Progress in Corneal Wound Healing. Prog. Retin. Eye Res. 2015, 49, 17–45. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Reinach, P.S.; Kao, W.W. Corneal Epithelial Wound Healing. Exp. Biol. Med. 2001, 226, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Mohan, R.R.; Kempuraj, D.; D’Souza, S.; Ghosh, A. Corneal Stromal Repair and Regeneration. Prog. Retin. Eye Res. 2022, 91, 101090. [Google Scholar] [CrossRef] [PubMed]
- Fu-Shin, X.Y.; Lee, P.S.; Yang, L.; Gao, N.; Zhang, Y.; Ljubimov, A.V.; Yang, E.; Zhou, Q.; Xie, L. The Impact of Sensory Neuropathy and Inflammation on Epithelial Wound Healing in Diabetic Corneas. Prog. Retin. Eye Res. 2022, 89, 101039. [Google Scholar]
- Stepp, M.A.; Menko, A.S. Immune Responses to Injury and Their Links to Eye Disease. Transl. Res. 2021, 236, 52–71. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, Y.; Yuan, H.; Li, Y.; Wang, H.; An, Z.; Li, X. Influences of SMILE and FS-LASIK on Corneal Sub-Basal Nerves: A Systematic Review and Network Meta-Analysis. J. Refract. Surg. 2022, 38, 277–284. [Google Scholar] [CrossRef]
- Rathi, A.; Kaur, M.; Brar, A.S.; Sah, R.; Titiyal, J.S. SMILE versus Femtosecond LASIK: Weighing the Pros. In Small Incision Lenticule Extraction (SMILE): Surgical Technique and Challenges: Comprehensive Text and Video Guide; Jaypee Brothers Medical Publishers: New Delhi, India, 2017; Volume 148. [Google Scholar]
- De Stefano, V.S.; Dupps, W.J.; Wilson, S.E. Biomechanics and Wound Healing in the Cornea. In Albert and Jakobiec’s Principles and Practice of Ophthalmology; Albert, D., Miller, J., Azar, D., Young, L.H., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–22. ISBN 978-3-319-90495-5. [Google Scholar]
- Wilson, S.E.; Sampaio, L.P.; Shiju, T.M.; Hilgert, G.S.; de Oliveira, R.C. Corneal Opacity: Cell Biological Determinants of the Transition from Transparency to Transient Haze to Scarring Fibrosis, and Resolution, after Injury. Investig. Ophthalmol. Vis. Sci. 2022, 63, 22. [Google Scholar] [CrossRef]
- Wilson, S.E.; Kim, W.-J. Keratocyte Apoptosis: Implications on Corneal Wound Healing, Tissue Organization, and Disease. Investig. Ophthalmol. Vis. Sci. 1998, 39, 220–226. [Google Scholar]
- Chen, K.; Li, Y.; Zhang, X.; Ullah, R.; Tong, J.; Shen, Y. The Role of the PI3K/AKT Signalling Pathway in the Corneal Epithelium: Recent Updates. Cell Death Dis. 2022, 13, 513. [Google Scholar] [CrossRef]
- Wilson, S.E.; Li, Q.; Weng, J.; Barry-Lane, P.A.; Jester, J.V.; Liang, Q.; Wordinger, R.J. The Fas-Fas Ligand System and Other Modulators of Apoptosis in the Cornea. Investig. Ophthalmol. Vis. Sci. 1996, 37, 1582–1592. [Google Scholar]
- Kamil, S.; Mohan, R.R. Corneal Stromal Wound Healing: Major Regulators and Therapeutic Targets. Ocul. Surf. 2021, 19, 290–306. [Google Scholar] [CrossRef]
- Wilson, S.E.; He, Y.-G.; Weng, J.; Li, Q.; McDOWALL, A.W.; Vital, M.; Chwang, E.L. Epithelial Injury Induces Keratocyte Apoptosis: Hypothesized Role for the Interleukin-1 System in the Modulation of Corneal Tissue Organization and Wound Healing. Exp. Eye Res. 1996, 62, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.E. Interleukin-1 and Transforming Growth Factor Beta: Commonly Opposing, but Sometimes Supporting, Master Regulators of the Corneal Wound Healing Response to Injury. Investig. Ophthalmol. Vis. Sci. 2021, 62, 8. [Google Scholar] [CrossRef] [PubMed]
- Fortingo, N.; Melnyk, S.; Sutton, S.H.; Watsky, M.A.; Bollag, W.B. Innate Immune System Activation, Inflammation and Corneal Wound Healing. Int. J. Mol. Sci. 2022, 23, 14933. [Google Scholar] [CrossRef]
- MøSller-Pedersen, T.; Cavanagh, H.D.; Petroll, W.M.; Jester, J.V. Neutralizing Antibody to TGF β Modulates Stromal Fibrosis but Not Regression of Photoablative Effect Following PRK. Curr. Eye Res. 1998, 17, 736–747. [Google Scholar] [CrossRef]
- Wang, L.; Ko, C.-Y.; Meyers, E.E.; Pedroja, B.S.; Pelaez, N.; Bernstein, A.M. Concentration-Dependent Effects of Transforming Growth Factor Β1 on Corneal Wound Healing. Mol. Vis. 2011, 17, 2835. [Google Scholar]
- Liu, L.; Cheng, W.; Wu, D.; Chen, L.; Yu, S.; Zuo, T.; Zhang, L.; Yang, K.; Li, H.; Zhang, H.; et al. The Differential Expression of Cytokines and Growth Factors after SMILE Compared with FS-LASIK in Rabbits. Investig. Ophthalmol. Vis. Sci. 2020, 61, 55. [Google Scholar] [CrossRef]
- Homer, N.; Jurkunas, U.V. The Use of Femtosecond Laser in Refractive and Cataract Surgery. Int. Ophthalmol. Clin. 2017, 57, 1–10. [Google Scholar] [CrossRef]
- Maier, T.; Güell, M.; Serrano, L. Correlation of mRNA and Protein in Complex Biological Samples. FEBS Lett. 2009, 583, 3966–3973. [Google Scholar] [CrossRef]
- Cavalcante-Silva, J.; Koh, T.J. Role of NK Cells in Skin Wound Healing of Mice. J. Immunol. 2023, 210, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bai, Y.; Li, Y.; Liang, G.; Jiang, Y.; Liu, Z.; Liu, M.; Hao, J.; Zhang, X.; Hu, X.; et al. IL-15 Enhances Activation and IGF-1 Production of Dendritic Epidermal T Cells to Promote Wound Healing in Diabetic Mice. Front. Immunol. 2017, 8, 1557. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.; Crane, E.D.; Kuo, Y.; Kim, A.; Crane, J.D. The Exercise Cytokine Interleukin-15 Rescues Slow Wound Healing in Aged Mice. J. Biol. Chem. 2019, 294, 20024–20038. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M.; Griffiths, J.L.; Sanders, A.J.; Owen, S.; Ruge, F.; Harding, K.G.; Jiang, W.G. The Clinical Significance and Impact of Interleukin 15 on Keratinocyte Cell Growth and Migration. Int. J. Mol. Med. 2016, 38, 679–686. [Google Scholar] [CrossRef]
- Jeon, H.H.; Yu, Q.; Lu, Y.; Spencer, E.; Lu, C.; Milovanova, T.; Yang, Y.; Zhang, C.; Stepanchenko, O.; Vafa, R.P.; et al. FOXO1 Regulates VEGFA Expression and Promotes Angiogenesis in Healing Wounds. J. Pathol. 2018, 245, 258–264. [Google Scholar] [CrossRef]
- Eming, S.A.; Krieg, T. Molecular Mechanisms of VEGF-A Action during Tissue Repair. J. Investig. Dermatol. Symp. Proc. 2006, 11, 79–86. [Google Scholar] [CrossRef]
- Gan, L.; Fagerholm, P.; Palmblad, J. Vascular Endothelial Growth Factor (VEGF) and Its Receptor VEGFR-2 in the Regulation of Corneal Neovascularization and Wound Healing. Acta Ophthalmol. Scand. 2004, 82, 557–563. [Google Scholar] [CrossRef]
- Vogel, C.; Marcotte, E.M. Insights into the Regulation of Protein Abundance from Proteomic and Transcriptomic Analyses. Nat. Rev. Genet. 2012, 13, 227–232. [Google Scholar] [CrossRef]
- Walsh, C.T.; Garneau-Tsodikova, S.; Gatto, G.J. Protein Posttranslational Modifications: The Chemistry of Proteome Diversifications. Angew. Chem. Int. Ed. Engl. 2005, 44, 7342–7372. [Google Scholar] [CrossRef]
- Schwanhäusser, B.; Busse, D.; Li, N.; Dittmar, G.; Schuchhardt, J.; Wolf, J.; Chen, W.; Selbach, M. Global Quantification of Mammalian Gene Expression Control. Nature 2011, 473, 337–342. [Google Scholar] [CrossRef]
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in Wound Repair: Molecular and Cellular Mechanisms. J. Investig. Dermatol. 2007, 127, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Swamynathan, S.; Campbell, G.; Tiwari, A.; Swamynathan, S.K. Secreted Ly-6/uPAR-Related Protein-1 (SLURP1) Is a pro-Differentiation Factor That Stalls G1-S Transition during Corneal Epithelial Cell Cycle Progression. Ocul. Surf. 2022, 24, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ashcroft, G.S.; Mills, S.J. Androgen Receptor-Mediated Inhibition of Cutaneous Wound Healing. J. Clin. Investig. 2002, 110, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Gilliver, S.C.; Ashworth, J.J.; Ashcroft, G.S. The Hormonal Regulation of Cutaneous Wound Healing. Clin. Dermatol. 2007, 25, 56–62. [Google Scholar] [CrossRef]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Voluntary informed consent to participate in the study | Lack of informed voluntary consent |
| Aged 18 or older | Aged under 18 |
| Stable refraction within the year preceding the study | Opaque optical media |
| Myopia ≤ −6.0 D for femtoLASIK and ReLeX SMILE or ≤−2.0 D for PRK | Current and past uveitis |
| Astigmatism ≤ 5.0 Dcyl | History of eye injuries |
| CDVA ≥ 0.5 on the Snellen chart | Previous corneal laser treatment |
| Corneal epithelial thickness (CET) ≥ 490 µm | Previous eye surgery |
| Residual stromal thickness (RST) ≥ 250 µm | Autoimmune diseases |
| Normal corneal topography | Diabetes |
| Dry eye syndrome | |
| Pregnancy or breastfeeding |
| mRNA | Primer Sequences |
|---|---|
| TGF-β1 | Forward: 5′-GGCCAGATCCTGTCCAAGC-3′ Reverse 5′-GTGGGTTTCCACCATTAGCAC-3′ |
| TGF-β2 | Forward: 5′-CAGCACACTCGATATGGACCA-3′ Reverse 5′-CCTCGGGCTCAGGATAGTCT-3′ |
| TGF-β3 | Forward: 5′-CTGGATTGTGGTTCCATGCA-3′ Reverse 5′-TCCCCGAATGCCTCACAT-3′ |
| IL-1β | Forward: 5′-TTTAGGATTTGGATTTTTGTTTTTT-3′ Reverse 5′- ACATCTTCCTCAACTTATCCATAAC-3′ |
| IL-15 | Forward: 5′-GGTTTTATTGATTTTAAGAATGAAGT-3′ Reverse 5′-ATAACAAAAAATTTCCAACAACCAT-3′ |
| INHBA | Forward: 5′-AAATATGTTGTATTTGAAGAAGAGATT-3′ Reverse 5′-TTTAAAAAAAACCAAACTTCTACAC-3′ |
| VEGFA | Forward: 5′-GATAGGGGTAAAGTGAGTGATTTGT-3′ Reverse 5′-AAAACAACCCAAAAATTAAAC-3′ |
| SLURP1 | Forward: 5′-TTTGTTTTAGTTTTTGTGTGGTTAT-3′ Reverse 5′-AAAAAATACCTAAAAAACACCTTCC-3′ |
| GAPDH | Forward: 5′-GGTGAAGGTCGGAGTCAACGGA-3′ Reverse 5′-GAGGGATCTCGCTCCTGGAAGA-3′ |
| mRNA | PRK | FS-LASIK | SMILE | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 Day | 7 Days | 30 Days | 180 Days | 1 Day | 7 Days | 30 Days | 180 Days | 1 Day | 7 Days | 30 Days | 180 Days | |
| TGF-β1 | 2.42 ± 0.19 A,B,C | 1.76 ± 0.23 D,E | 1.27 ± 0.21 | 1.18 ± 0.44 | 3.45 ± 0.31 | 3.12 ± 0.23 E | 2.98 ± 0.11 F | 1.19 ± 0.12 | 1.27 ± 0.27 | 1.14 ± 0.12 | 1.21 ± 0.31 | 1.27 ± 0.17 |
| TGF-β2 | 3.45 ± 0.21 A,B,C | 2.14 ± 0.31 D,E | 1.09 ± 0.23 F | 1.45 ± 0.31 | 4.98 ± 0.35 | 4.56 ± 0.25 E | 4.12 ± 0.78 F | 1.12 ± 0.11 | 1.13 ± 0.16 | 1.02 ± 0.17 E | 1.05 ± 0.18 | 1.12 ± 032 |
| TGF-β3 | 1.13 ± 0.12 B,C | 1.03 ± 0.19 D,E | 0.88 ± 0.13 F | 0.78 ± 0.13 | 2.01 ± 0.19 | 2.13 ± 0.43 E | 2.34 ± 0.24 F | 1.09 ± 0.21 | 0.76 ± 0.12 | 0.65 ± 0.08 | 0.67 ± 0.17 | 0.87 ± 0.23 |
| IL-1β | 3.14 ± 0.19 B,C | 3.02 ± 0.76 D,E | 2.54 ± 0.24 | 1.98 ± 0.23 | 1.98 ± 0.24 A,B,C | 1.11 ± 0.18 | 0.97 ± 0.43 | 0.96 ± 0.37 | 1.04 ± 0.31 | 1.12 ± 0.25 | 1.13 ± 0.13 | 1.09 ± 0.07 |
| IL-15 | 4.65 ± 0.65 | 4.98 ± 0.46 E | 4.54 ± 0.76 F | 3.21 ± 0.41 | 3.55 ± 0.37 A,B,C | 4.99 ± 0.28 D,E | 1.98 ± 0.19 | 2.13 ± 0.24 | 2.09 ± 0.23 | 1.45 ± 0.13 D | 1.12 ± 0.08 | 1.32 ± 0.19 |
| INHBA | 0.65 ± 0.12 A,B,C | 1.09 ± 0.19 | 1.56 ± 0.21 F | 1.13 ± 0.34 | 0.56 ± 0.08 A,B,C | 1.01 ± 0.17 | 1.22 ± 0.12 | 1.23 ± 0.44 | 1.09 ± 0.08 | 1.15 ± 0.17 | 1.01 ± 0.04 | 1.01 ± 0.12 |
| VEGFA | 2.98 ± 0.23 | 2.17 ± 0.23 D,E | 3.01 ± 0.26 F | 1.16 ± 0.46 | 3.45 ± 1.09 C | 3.46 ± 0.41 D,E | 2.09 ± 0.87 F | 1.54 ± 0.32 | 1.87 ± 0.31 | 1.76 ± 0.23 | 1.31 ± 0.16 | 1.45 ± 0.44 |
| SLURP1 | 1.04 ± 0.41 | 1.07 ± 0.11 | 1.17 ± 0.12 | 1.02 ± 0.12 | 1.54 ± 0.32 | 1.21 ± 0.19 | 1.18 ± 0.17 | 1.09 ± 0.10 | 1.08 ± 0.29 | 1.15 ± 0.21 | 1.21 ± 0.21 | 1.03 ± 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janiszewska-Bil, D.; Grabarek, B.O.; Lyssek-Boroń, A.; Kiełbasińska, A.; Kuraszewska, B.; Wylęgała, E.; Krysik, K. Comparative Analysis of Corneal Wound Healing: Differential Molecular Responses in Tears Following PRK, FS-LASIK, and SMILE Procedures. Biomedicines 2024, 12, 2289. https://doi.org/10.3390/biomedicines12102289
Janiszewska-Bil D, Grabarek BO, Lyssek-Boroń A, Kiełbasińska A, Kuraszewska B, Wylęgała E, Krysik K. Comparative Analysis of Corneal Wound Healing: Differential Molecular Responses in Tears Following PRK, FS-LASIK, and SMILE Procedures. Biomedicines. 2024; 12(10):2289. https://doi.org/10.3390/biomedicines12102289
Chicago/Turabian StyleJaniszewska-Bil, Dominika, Beniamin Oskar Grabarek, Anita Lyssek-Boroń, Aleksandra Kiełbasińska, Bernadeta Kuraszewska, Edward Wylęgała, and Katarzyna Krysik. 2024. "Comparative Analysis of Corneal Wound Healing: Differential Molecular Responses in Tears Following PRK, FS-LASIK, and SMILE Procedures" Biomedicines 12, no. 10: 2289. https://doi.org/10.3390/biomedicines12102289
APA StyleJaniszewska-Bil, D., Grabarek, B. O., Lyssek-Boroń, A., Kiełbasińska, A., Kuraszewska, B., Wylęgała, E., & Krysik, K. (2024). Comparative Analysis of Corneal Wound Healing: Differential Molecular Responses in Tears Following PRK, FS-LASIK, and SMILE Procedures. Biomedicines, 12(10), 2289. https://doi.org/10.3390/biomedicines12102289






