Proton Pump Inhibitors and Oral–Gut Microbiota: From Mechanism to Clinical Significance
Abstract
1. Introduction
2. Impact of PPIs on Oral and Gut Microbiota
2.1. PPIs and Oral Microbiota
| Study | Study Design | Patient Characteristic | Confounders | Sample | Sequencing Methods | Alpha Diversity | Beta Diversity | Taxonomic Changes |
|---|---|---|---|---|---|---|---|---|
| Tsuda et al. (2015) [21] | Cross-sectional study | Total patients (n = 45), including 12 functional dyspepsia and six GERD patients taking a PPI for more than 2 years, 27 of 45 healthy volunteers. | Matching for age | Saliva | Barcoded 454 pyrosequencing, 16S rDNA, V1-V2 | OTU number (ns) | Unweighted UniFrac distance (sig.) PCoA analysis (ns) | ns |
| Rosen et al. (2015) [24] | Cross-sectional study | Children undergoing bronchoscopy and gastrointestinal endoscopy for chronic cough (n = 116), 59 were receiving a PPI dose within 24 h of endoscopy. | - | Oropharyngeal swabs | Illumina Miseq sequencing, 16S rDNA, - | Shannon index (ns) | - | Increased prevalence: Butyrivibrio Increased abundance: Allobaculum, Bifidobacterium, Cloacibacterium, Janthinobacterium, Ralstonia, Rhodobacter, Rhodoferax, Streptococcus, Yersinia, and Zoogloea. |
| Mishiro et al. (2018) [22] | Prospective self-controlled trial | Healthy adults (n = 10) before and after four weeks of 20 mg esomeprazole once daily. | - | Saliva, periodontal pocket fluid | Illumina Miseq sequencing, 16S rDNA, V3-V4 | Chao1 (ns) Shannon index (sig. in saliva) | UniFrac distance (ns) PCoA analysis (sig. in saliva) | Increase: Fusobacterium and Leptotrichia in periodontal pocket fluid Decrease: Neisseria, Veillonella, and Haemophilus in saliva |
| Kawar et al. (2021) [23] | Cross-sectional study | Total patients (n = 128), including 20 patients with GERD who used PPIs, and 16 who had GERD but did not use medication, 102 negative control subjects. | Matching for age, periodontal status, and edentualism. | Saliva | Illumina Miseq sequencing, 16S rDNA, V1-V3 | Chao1 (ns) Shannon index (ns) | PCoA analysis (ns) | Decrease: Prevotella melaninogenica, Prevotella pallens, Solobacterium moorei and Leptotrichia in the GERD patients not using PPIs compared to negative controls. |
2.2. PPIs and Gut Microbiota
3. Influence of Oral–Gut Translocation on PPI-Induced Gut Microbiota Alteration
4. Oral–Gut Microbiota and PPI-Related Digestive System Complications
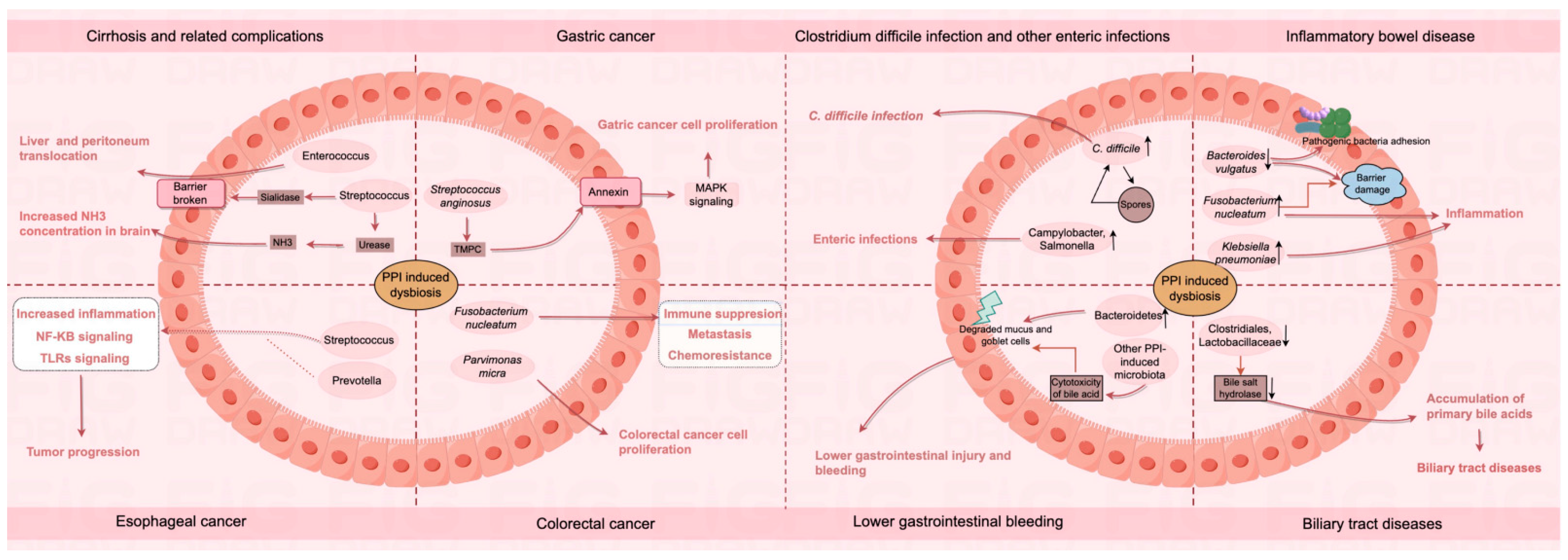
4.1. Cirrhosis and Related Complications
4.2. Gastric Cancer
4.3. Esophageal Cancer
4.4. Colorectal Cancer
4.5. Clostridium Difficile Infection and Other Enteric Infections
4.6. Inflammatory Bowel Disease
4.7. Lower Gastrointestinal Bleeding
4.8. Biliary Tract Diseases
5. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Freedberg, D.E.; Kim, L.S.; Yang, Y.X. The Risks and Benefits of Long-term Use of Proton Pump Inhibitors: Expert Review and Best Practice Advice From the American Gastroenterological Association. Gastroenterology 2017, 152, 706–715. [Google Scholar] [CrossRef]
- Targownik, L.E.; Fisher, D.A.; Saini, S.D. AGA Clinical Practice Update on De-Prescribing of Proton Pump Inhibitors: Expert Review. Gastroenterology 2022, 162, 1334–1342. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.M.; Sachs, G. Pharmacology of proton pump inhibitors. Curr. Gastroenterol. Rep. 2008, 10, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Kurlander, J.E.; Laine, L.; Kim, H.M.; Roberts, C.B.; Saffar, D.; Myers, A.; Holleman, R.; Gao, Y.; Shank, M.; Nelson, R.; et al. Impact of large scale, multicomponent intervention to reduce proton pump inhibitor overuse in integrated healthcare system: Difference-in-difference study. BMJ 2024, 385, e076484. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Kandulski, A.; Venerito, M. Proton-pump inhibitors: Understanding the complications and risks. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 697–710. [Google Scholar] [CrossRef] [PubMed]
- Perry, I.E.; Sonu, I.; Scarpignato, C.; Akiyama, J.; Hongo, M.; Vega, K.J. Potential proton pump inhibitor-related adverse effects. Ann. N. Y. Acad. Sci. 2020, 1481, 43–58. [Google Scholar] [CrossRef]
- Abrahami, D.; Pradhan, R.; Yin, H.; Yanofsky, R.; McDonald, E.G.; Bitton, A.; Azoulay, L. Proton pump inhibitors and the risk of inflammatory bowel disease: Population-based cohort study. Gut 2023, 72, 1288–1295. [Google Scholar] [CrossRef]
- Bavishi, C.; Dupont, H.L. Systematic review: The use of proton pump inhibitors and increased susceptibility to enteric infection. Aliment. Pharmacol. Ther. 2011, 34, 1269–1281. [Google Scholar] [CrossRef]
- Lee, J.K.; Merchant, S.A.; Schneider, J.L.; Jensen, C.D.; Fireman, B.H.; Quesenberry, C.P.; Corley, D.A. Proton Pump Inhibitor Use and Risk of Gastric, Colorectal, Liver, and Pancreatic Cancers in a Community-Based Population. Am. J. Gastroenterol. 2020, 115, 706–715. [Google Scholar] [CrossRef]
- Laine, L.; Ahnen, D.; McClain, C.; Solcia, E.; Walsh, J.H. Review article: Potential gastrointestinal effects of long-term acid suppression with proton pump inhibitors. Aliment. Pharmacol. Ther. 2000, 14, 651–668. [Google Scholar] [CrossRef]
- Imhann, F.; Bonder, M.J.; Vich Vila, A.; Fu, J.; Mujagic, Z.; Vork, L.; Tigchelaar, E.F.; Jankipersadsing, S.A.; Cenit, M.C.; Harmsen, H.J.; et al. Proton pump inhibitors affect the gut microbiome. Gut 2016, 65, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.A.; Goodrich, J.K.; Maxan, M.E.; Freedberg, D.E.; Abrams, J.A.; Poole, A.C.; Sutter, J.L.; Welter, D.; Ley, R.E.; Bell, J.T.; et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut 2016, 65, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Gómez Garre, D.; Modrego, J. Special Issue: Gut Microbiota in Disease and Health 2.0. Int. J. Mol. Sci. 2024, 25, 4344. [Google Scholar] [CrossRef] [PubMed]
- Escapa, I.F.; Chen, T.; Huang, Y.; Gajare, P.; Dewhirst, F.E.; Lemon, K.P. New Insights into Human Nostril Microbiome from the Expanded Human Oral Microbiome Database (eHOMD): A Resource for the Microbiome of the Human Aerodigestive Tract. mSystems 2018, 3, e00187-18. [Google Scholar] [CrossRef]
- Kitamoto, S.; Nagao-Kitamoto, H.; Hein, R.; Schmidt, T.M.; Kamada, N. The Bacterial Connection between the Oral Cavity and the Gut Diseases. J. Dent. Res. 2020, 99, 1021–1029. [Google Scholar] [CrossRef]
- Yamazaki, K.; Kamada, N. Exploring the oral-gut linkage: Interrelationship between oral and systemic diseases. Mucosal Immunol. 2024, 17, 147–153. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, X.; Wang, J.; Liu, Y.; Yan, H.; Xing, X.; Yang, J. Proton pump inhibitors alter gut microbiota by promoting oral microbiota translocation: A prospective interventional study. Gut 2024, 73, 1098–1109. [Google Scholar] [CrossRef]
- Lam, G.A.; Albarrak, H.; McColl, C.J.; Pizarro, A.; Sanaka, H.; Gomez-Nguyen, A.; Cominelli, F.; Paes Batista da Silva, A. The Oral-Gut Axis: Periodontal Diseases and Gastrointestinal Disorders. Inflamm. Bowel Dis. 2023, 29, 1153–1164. [Google Scholar] [CrossRef]
- Park, S.Y.; Hwang, B.O.; Lim, M.; Ok, S.H.; Lee, S.K.; Chun, K.S.; Park, K.K.; Hu, Y.; Chung, W.Y.; Song, N.Y. Oral-Gut Microbiome Axis in Gastrointestinal Disease and Cancer. Cancers 2021, 13, 2124. [Google Scholar] [CrossRef]
- Segata, N.; Haake, S.K.; Mannon, P.; Lemon, K.P.; Waldron, L.; Gevers, D.; Huttenhower, C.; Izard, J. Composition of the adult digestive tract bacterial microbiome based on seven mouth surfaces, tonsils, throat and stool samples. Genome Biol. 2012, 13, R42. [Google Scholar] [CrossRef]
- Tsuda, A.; Suda, W.; Morita, H.; Takanashi, K.; Takagi, A.; Koga, Y.; Hattori, M. Influence of Proton-Pump Inhibitors on the Luminal Microbiota in the Gastrointestinal Tract. Clin. Transl. Gastroenterol. 2015, 6, e89. [Google Scholar] [CrossRef] [PubMed]
- Mishiro, T.; Oka, K.; Kuroki, Y.; Takahashi, M.; Tatsumi, K.; Saitoh, T.; Tobita, H.; Ishimura, N.; Sato, S.; Ishihara, S.; et al. Oral microbiome alterations of healthy volunteers with proton pump inhibitor. J. Gastroenterol. Hepatol. 2018, 33, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Kawar, N.; Park, S.G.; Schwartz, J.L.; Callahan, N.; Obrez, A.; Yang, B.; Chen, Z.; Adami, G.R. Salivary microbiome with gastroesophageal reflux disease and treatment. Sci. Rep. 2021, 11, 188. [Google Scholar] [CrossRef]
- Rosen, R.; Hu, L.; Amirault, J.; Khatwa, U.; Ward, D.V.; Onderdonk, A. 16S community profiling identifies proton pump inhibitor related differences in gastric, lung, and oropharyngeal microflora. J. Pediatr. 2015, 166, 917–923. [Google Scholar] [CrossRef]
- Clooney, A.G.; Bernstein, C.N.; Leslie, W.D.; Vagianos, K.; Sargent, M.; Laserna-Mendieta, E.J.; Claesson, M.J.; Targownik, L.E. A comparison of the gut microbiome between long-term users and non-users of proton pump inhibitors. Aliment. Pharmacol. Ther. 2016, 43, 974–984. [Google Scholar] [CrossRef] [PubMed]
- Takagi, T.; Naito, Y.; Inoue, R.; Kashiwagi, S.; Uchiyama, K.; Mizushima, K.; Tsuchiya, S.; Okayama, T.; Dohi, O.; Yoshida, N.; et al. The influence of long-term use of proton pump inhibitors on the gut microbiota: An age-sex-matched case-control study. J. Clin. Biochem. Nutr. 2018, 62, 100–105. [Google Scholar] [CrossRef]
- Hojo, M.; Asahara, T.; Nagahara, A.; Takeda, T.; Matsumoto, K.; Ueyama, H.; Matsumoto, K.; Asaoka, D.; Takahashi, T.; Nomoto, K.; et al. Gut Microbiota Composition before and after Use of Proton Pump Inhibitors. Dig. Dis. Sci. 2018, 63, 2940–2949. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, C.; Zhang, Q.; Yu, L.; Chen, W.; Xue, Y.; Zhai, Q. Meta-analysis of the effects of proton pump inhibitors on the human gut microbiota. BMC Microbiol. 2023, 23, 171. [Google Scholar] [CrossRef]
- Freedberg, D.E.; Toussaint, N.C.; Chen, S.P.; Ratner, A.J.; Whittier, S.; Wang, T.C.; Wang, H.H.; Abrams, J.A. Proton Pump Inhibitors Alter Specific Taxa in the Human Gastrointestinal Microbiome: A Crossover Trial. Gastroenterology 2015, 149, 883–885.e9. [Google Scholar] [CrossRef]
- Reveles, K.R.; Ryan, C.N.; Chan, L.; Cosimi, R.A.; Haynes, W.L. Proton pump inhibitor use associated with changes in gut microbiota composition. Gut 2018, 67, 1369–1370. [Google Scholar] [CrossRef]
- Koo, S.H.; Deng, J.; Ang, D.S.W.; Hsiang, J.C.; Lee, L.S.; Aazmi, S.; Mohamed, E.H.M.; Yang, H.; Yap, S.Y.; Teh, L.K.; et al. Effects of proton pump inhibitor on the human gut microbiome profile in multi-ethnic groups in Singapore. Singap. Med. J. 2019, 60, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Li, J.; Cai, S.; Zhao, H.; Zhao, H.; Sun, G.; Yang, Y. Proton pump inhibitors induced fungal dysbiosis in patients with gastroesophageal reflux disease. Front. Cell. Infect. Microbiol. 2023, 13, 1205348. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Sun, C.; Li, M.; Hu, G.; Zhao, X.M.; Chen, W.H. Compared to histamine-2 receptor antagonist, proton pump inhibitor induces stronger oral-to-gut microbial transmission and gut microbiome alterations: A randomised controlled trial. Gut 2024, 73, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, T.; Sugimoto, M.; Inoue, R.; Ohno, M.; Ban, H.; Nishida, A.; Inatomi, O.; Takahashi, S.; Naito, Y.; Andoh, A. Influence of potassium-competitive acid blocker on the gut microbiome of Helicobacter pylori-negative healthy individuals. Gut 2017, 66, 1723–1725. [Google Scholar] [CrossRef] [PubMed]
- Maier, L.; Pruteanu, M.; Kuhn, M.; Zeller, G.; Telzerow, A.; Anderson, E.E.; Brochado, A.R.; Fernandez, K.C.; Dose, H.; Mori, H.; et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 2018, 555, 623–628. [Google Scholar] [CrossRef]
- Iwauchi, M.; Horigome, A.; Ishikawa, K.; Mikuni, A.; Nakano, M.; Xiao, J.Z.; Odamaki, T.; Hironaka, S. Relationship between oral and gut microbiota in elderly people. Immun. Inflamm. Dis. 2019, 7, 229–236. [Google Scholar] [CrossRef]
- Perler, B.K.; Friedman, E.S.; Wu, G.D. The Role of the Gut Microbiota in the Relationship Between Diet and Human Health. Annu. Rev. Physiol. 2023, 85, 449–468. [Google Scholar] [CrossRef]
- Lin, L.; Cui, B.; Deng, Y.; Jiang, X.; Liu, W.; Sun, C. The Efficacy of Proton Pump Inhibitor in Cirrhotics with Variceal Bleeding: A Systemic Review and Meta-Analysis. Digestion 2021, 102, 117–127. [Google Scholar] [CrossRef]
- Wong, Z.Y.; Koh, J.H.; Muthiah, M.; Koh, B.; Ong, E.Y.H.; Ong, C.E.Y.; Ou, K.Q.; Lim, W.H.; Tan, D.J.H.; Chee, D.; et al. Proton Pump Inhibitors Increases Longitudinal Risk of Mortality, Decompensation, and Infection in Cirrhosis: A Meta-Analysis. Dig. Dis. Sci. 2024, 69, 289–297. [Google Scholar] [CrossRef]
- Dever, J.B.; Sheikh, M.Y. Review article: Spontaneous bacterial peritonitis—Bacteriology, diagnosis, treatment, risk factors and prevention. Aliment. Pharmacol. Ther. 2015, 41, 1116–1131. [Google Scholar] [CrossRef]
- Janka, T.; Tornai, T.; Borbély, B.; Tornai, D.; Altorjay, I.; Papp, M.; Vitális, Z. Deleterious effect of proton pump inhibitors on the disease course of cirrhosis. Eur. J. Gastroenterol. Hepatol. 2020, 32, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Dam, G.; Vilstrup, H.; Watson, H.; Jepsen, P. Proton pump inhibitors as a risk factor for hepatic encephalopathy and spontaneous bacterial peritonitis in patients with cirrhosis with ascites. Hepatology 2016, 64, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.F.; Chen, M.H.; Wang, Y.P.; Chu, C.J.; Huang, Y.H.; Lin, H.C.; Hou, M.C.; Lee, F.Y.; Su, T.P.; Lu, C.L. Proton Pump Inhibitors Increase Risk for Hepatic Encephalopathy in Patients with Cirrhosis in A Population Study. Gastroenterology 2017, 152, 134–141. [Google Scholar] [CrossRef] [PubMed]
- China, L.; Tittanegro, T.; Crocombe, D.; Forrest, E.; Kallis, Y.; Ryder, S.D.; Wright, G.; Freemantle, N.; O’Brien, A. Investigating potential confounding by indication when considering the association between proton pump inhibitor use, infection, hepatic encephalopathy and mortality in hospitalised decompensated cirrhosis: A post-hoc analysis of the ATTIRE trial. EClinicalMedicine 2023, 58, 101924. [Google Scholar] [CrossRef]
- Shao, Y.J.; Chan, T.S.; Tsai, K.; Wu, S.Y. Association between proton pump inhibitors and the risk of hepatocellular carcinoma. Aliment. Pharmacol. Ther. 2018, 48, 460–468. [Google Scholar] [CrossRef]
- Li, D.K.; Yan, P.; Abou-Samra, A.B.; Chung, R.T.; Butt, A.A. Proton pump inhibitors are associated with accelerated development of cirrhosis, hepatic decompensation and hepatocellular carcinoma in noncirrhotic patients with chronic hepatitis C infection: Results from ERCHIVES. Aliment. Pharmacol. Ther. 2018, 47, 246–258. [Google Scholar] [CrossRef]
- Kao, W.Y.; Su, C.W.; Chia-Hui Tan, E.; Lee, P.C.; Chen, P.H.; Tang, J.H.; Huang, Y.H.; Huo, T.I.; Chang, C.C.; Hou, M.C.; et al. Proton Pump Inhibitors and Risk of Hepatocellular Carcinoma in Patients with Chronic Hepatitis B or C. Hepatology 2019, 69, 1151–1164. [Google Scholar] [CrossRef]
- Lai, S.W.; Liao, K.F.; Lai, H.C.; Lin, C.L.; Sung, F.C. Proton pump inhibitors and risk of hepatocellular carcinoma: A case-control study in Taiwan. Acta Gastro-Enterol. Belg. 2013, 76, 348–350. [Google Scholar]
- Kim, S.; Jeong, S.; Park, S.J.; Chang, J.; Choi, S.; Cho, Y.; Ahn, J.C.; Lee, G.; Son, J.S.; Park, S.M. Association between Proton Pump Inhibitor Use and Risk of Hepatocellular Carcinoma: A Korean Nationally Representative Cohort Study. J. Clin. Med. 2022, 11, 2865. [Google Scholar] [CrossRef]
- Tran, K.T.; McMenamin, Ú.C.; Hicks, B.; Murchie, P.; Thrift, A.P.; Coleman, H.G.; Iversen, L.; Johnston, B.T.; Lee, A.J.; Cardwell, C.R. Proton pump inhibitor and histamine-2 receptor antagonist use and risk of liver cancer in two population-based studies. Aliment. Pharmacol. Ther. 2018, 48, 55–64. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Acharya, C.; Fagan, A.; White, M.B.; Gavis, E.; Heuman, D.M.; Hylemon, P.B.; Fuchs, M.; Puri, P.; Schubert, M.L.; et al. Proton Pump Inhibitor Initiation and Withdrawal affects Gut Microbiota and Readmission Risk in Cirrhosis. Am. J. Gastroenterol. 2018, 113, 1177–1186. [Google Scholar] [CrossRef]
- Yamamoto, K.; Ishigami, M.; Honda, T.; Takeyama, T.; Ito, T.; Ishizu, Y.; Kuzuya, T.; Hayashi, K.; Goto, H.; Hirooka, Y. Influence of proton pump inhibitors on microbiota in chronic liver disease patients. Hepatol. Int. 2019, 13, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; Sikaroodi, M.; Shamsaddini, A.; Henseler, Z.; Santiago-Rodriguez, T.; Acharya, C.; Fagan, A.; Hylemon, P.B.; Fuchs, M.; Gavis, E.; et al. Interaction of bacterial metagenome and virome in patients with cirrhosis and hepatic encephalopathy. Gut 2021, 70, 1162–1173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhai, H.; Geng, J.; Yu, R.; Ren, H.; Fan, H.; Shi, P. Large-scale survey of gut microbiota associated with MHE via 16S rRNA-based pyrosequencing. Am. J. Gastroenterol. 2013, 108, 1601–1611. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.C.; Lee, S.; McPhail, M.J.W.; Da Silva, K.; Guilly, S.; Zamalloa, A.; Witherden, E.; Støy, S.; Manakkat Vijay, G.K.; Pons, N.; et al. Rifaximin-α reduces gut-derived inflammation and mucin degradation in cirrhosis and encephalopathy: RIFSYS randomised controlled trial. J. Hepatol. 2022, 76, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Vallianou, N.; Christodoulatos, G.S.; Karampela, I.; Tsilingiris, D.; Magkos, F.; Stratigou, T.; Kounatidis, D.; Dalamaga, M. Understanding the Role of the Gut Microbiome and Microbial Metabolites in Non-Alcoholic Fatty Liver Disease: Current Evidence and Perspectives. Biomolecules 2021, 12, 56. [Google Scholar] [CrossRef]
- Jin, M.; Kalainy, S.; Baskota, N.; Chiang, D.; Deehan, E.C.; McDougall, C.; Tandon, P.; Martínez, I.; Cervera, C.; Walter, J.; et al. Faecal microbiota from patients with cirrhosis has a low capacity to ferment non-digestible carbohydrates into short-chain fatty acids. Liver Int. 2019, 39, 1437–1447. [Google Scholar] [CrossRef]
- Llorente, C.; Jepsen, P.; Inamine, T.; Wang, L.; Bluemel, S.; Wang, H.J.; Loomba, R.; Bajaj, J.S.; Schubert, M.L.; Sikaroodi, M.; et al. Gastric acid suppression promotes alcoholic liver disease by inducing overgrowth of intestinal Enterococcus. Nat. Commun. 2017, 8, 837. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, R.; Zhang, P.; Chen, Z.; Hua, Y.; Huang, X.; Li, X. Association of proton pump inhibitors with gastric and colorectal cancer risk: A systematic review and meta-analysis. Front. Pharmacol. 2023, 14, 1129948. [Google Scholar] [CrossRef]
- Pan, S.; Thrift, A.P.; Akhdar, G.; El-Serag, H.B. Gastric Cancer Risk in Patients with Long-Term Use of Proton Pump Inhibitors: A Systematic Review and Meta-Analysis of Observational and Interventional Studies. Dig. Dis. Sci. 2023, 68, 3732–3744. [Google Scholar] [CrossRef]
- Peng, T.R.; Wu, T.W.; Li, C.H. Association between proton-pump inhibitors and the risk of gastric cancer: A systematic review and meta-analysis. Int. J. Clin. Oncol. 2023, 28, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Moayyedi, P.; Eikelboom, J.W.; Bosch, J.; Connolly, S.J.; Dyal, L.; Shestakovska, O.; Leong, D.; Anand, S.S.; Störk, S.; Branch, K.R.H.; et al. Safety of Proton Pump Inhibitors Based on a Large, Multi-Year, Randomized Trial of Patients Receiving Rivaroxaban or Aspirin. Gastroenterology 2019, 157, 682–691.e2. [Google Scholar] [CrossRef]
- Zheng, Z.; Lu, Z.; Song, Y. Long-term proton pump inhibitors use and its association with premalignant gastric lesions: A systematic review and meta-analysis. Front. Pharmacol. 2023, 14, 1244400. [Google Scholar] [CrossRef] [PubMed]
- Rais, R.; Trikalinos, N.A.; Liu, J.; Chatterjee, D. Enterochromaffin-like Cell Hyperplasia-Associated Gastric Neuroendocrine Tumors May Arise in the Setting of Proton Pump Inhibitor Use. Arch. Pathol. Lab. Med. 2022, 146, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, L.; Lin, L.; Jin, D.; Du, Y.; Lyu, J. Research progress on gut microbiota in patients with gastric cancer, esophageal cancer, and small intestine cancer. Appl. Microbiol. Biotechnol. 2021, 105, 4415–4425. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Bisseling, T.M.; van der Post, R.S.; Boleij, A. The influence of Helicobacter pylori, proton pump inhibitor, and obesity on the gastric microbiome in relation to gastric cancer development. Comput. Struct. Biotechnol. J. 2024, 23, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Paroni Sterbini, F.; Palladini, A.; Masucci, L.; Cannistraci, C.V.; Pastorino, R.; Ianiro, G.; Bugli, F.; Martini, C.; Ricciardi, W.; Gasbarrini, A.; et al. Effects of Proton Pump Inhibitors on the Gastric Mucosa-Associated Microbiota in Dyspeptic Patients. Appl. Environ. Microbiol. 2016, 82, 6633–6644. [Google Scholar] [CrossRef]
- Fu, K.; Cheung, A.H.K.; Wong, C.C.; Liu, W.; Zhou, Y.; Wang, F.; Huang, P.; Yuan, K.; Coker, O.O.; Pan, Y.; et al. Streptococcus anginosus promotes gastric inflammation, atrophy, and tumorigenesis in mice. Cell 2024, 187, 882–896.e17. [Google Scholar] [CrossRef]
- Yao, H.; Wang, L.; Li, H.; Xu, S.; Bai, Z.; Wu, Y.; Chen, H.; Goyal, H.; Qi, X. Proton pump inhibitors may reduce the risk of high-grade dysplasia and/or esophageal adenocarcinoma in Barrett’s esophagus: A systematic review and meta-analysis. Expert Rev. Clin. Pharmacol. 2022, 15, 79–88. [Google Scholar] [CrossRef]
- Lai, S.W.; Liao, K.F.; Lai, H.C.; Muo, C.H.; Sung, F.C. Atorvastatin correlates with decreased risk of esophageal cancer: A population-based case-control study from Taiwan. Libyan J. Med. 2012, 7. [Google Scholar] [CrossRef]
- Choi, H.G.; Lee, H.K.; Kang, H.S.; Lim, H.; Kim, J.H.; Kim, J.H.; Kim, N.Y.; Cho, S.J.; Nam, E.S.; Min, K.W.; et al. Possible Association between the Use of Proton Pump Inhibitors and H2 Receptor Antagonists, and Esophageal Cancer: A Nested Case-Control Study Using a Korean National Health Screening Cohort. Pharmaceuticals 2022, 15, 517. [Google Scholar] [CrossRef] [PubMed]
- Muszyński, D.; Kudra, A.; Sobocki, B.K.; Folwarski, M.; Vitale, E.; Filetti, V.; Dudzic, W.; Kaźmierczak-Siedlecka, K.; Połom, K. Esophageal cancer and bacterial part of gut microbiota—A multidisciplinary point of view. Front. Cell. Infect. Microbiol. 2022, 12, 1057668. [Google Scholar] [CrossRef] [PubMed]
- Amir, I.; Konikoff, F.M.; Oppenheim, M.; Gophna, U.; Half, E.E. Gastric microbiota is altered in oesophagitis and Barrett’s oesophagus and further modified by proton pump inhibitors. Environ. Microbiol. 2014, 16, 2905–2914. [Google Scholar] [CrossRef] [PubMed]
- Rajagopala, S.V.; Shilts, M.H.; Correa, H.; Das, S.R.; Choksi, Y.A.; Jacobse, J.; Goettel, J.A.; Hiremath, G. Proton Pump Inhibitors Modulate Gene Expression Profile in Esophageal Mucosa and Microbiome. J. Pediatr. Pharmacol. Ther. 2023, 28, 504–508. [Google Scholar] [CrossRef]
- Zhang, J.W.; Zhang, D.; Yin, H.S.; Zhang, H.; Hong, K.Q.; Yuan, J.P.; Yu, B.P. Fusobacterium nucleatum promotes esophageal squamous cell carcinoma progression and chemoresistance by enhancing the secretion of chemotherapy-induced senescence-associated secretory phenotype via activation of DNA damage response pathway. Gut Microbes 2023, 15, 2197836. [Google Scholar] [CrossRef]
- Sharma, T.; Gupta, A.; Chauhan, R.; Bhat, A.A.; Nisar, S.; Hashem, S.; Akhtar, S.; Ahmad, A.; Haris, M.; Singh, M.; et al. Cross-talk between the microbiome and chronic inflammation in esophageal cancer: Potential driver of oncogenesis. Cancer Metastasis Rev. 2022, 41, 281–299. [Google Scholar] [CrossRef]
- Liu, K.; Wang, Y.H.; Wang, J.; Chen, B.; Luo, N.; Gong, J. Meta-analysis of proton pump inhibitor use and the risk of developing gastric cancer or colorectal cancer. Anticancer Drugs 2023, 34, 971–978. [Google Scholar] [CrossRef]
- Kwon, M.J.; Han, K.M.; Kim, J.H.; Kim, J.H.; Kim, M.J.; Kim, N.Y.; Choi, H.G.; Kang, H.S. Proton Pump Inhibitors and Likelihood of Colorectal Cancer in the Korean Population: Insights from a Nested Case-Control Study Using National Health Insurance Data. Cancers 2023, 15, 5606. [Google Scholar] [CrossRef]
- Hwang, I.C.; Chang, J.; Park, S.M. Emerging hazard effects of proton pump inhibitor on the risk of colorectal cancer in low-risk populations: A Korean nationwide prospective cohort study. PLoS ONE 2017, 12, e0189114. [Google Scholar] [CrossRef]
- Lai, S.W.; Liao, K.F.; Lai, H.C.; Lin, C.L.; Sung, F.C. Use of proton pump inhibitors correlates with increased risk of colorectal cancer in Taiwan. Asia Pac. J. Clin. Oncol. 2013, 9, 192–193. [Google Scholar] [CrossRef]
- Lei, W.Y.; Wang, J.H.; Yi, C.H.; Liu, T.T.; Hung, J.S.; Wong, M.W.; Bair, M.J.; Vaezi, M.F.; Orr, W.C.; Chen, C.L. Association between use of proton pump inhibitors and colorectal cancer: A nationwide population-based study. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101397. [Google Scholar] [CrossRef] [PubMed]
- Babic, A.; Zhang, X.; Morales-Oyarvide, V.; Yuan, C.; Khalaf, N.; Khalili, H.; Lochhead, P.; Chan, A.T.; Ogino, S.; Wolpin, B.M.; et al. Acid-suppressive medications and risk of colorectal cancer: Results from three large prospective cohort studies. Br. J. Cancer 2020, 123, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.Y.; Wang, S.S.; Kang, Y.N.; Porpiglia, A.S.; Chang, Y.; Huang, C.H.; Bhimani, R.; Abdul-Lattif, E.; Azmat, M.; Wang, T.H.; et al. Do proton pump inhibitors affect the effectiveness of chemotherapy in colorectal cancer patients? A systematic review with meta-analysis. Front. Pharmacol. 2022, 13, 1048980. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.M.; Hahm, K.B.; Park, J.M.; Hong, S.P.; Kim, E.H. Paradoxically augmented anti-tumorigenic action of proton pump inhibitor and GastrininAPCMin/+ intestinal polyposis model. Neoplasia 2014, 16, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, Y.; Li, Y.; Zhang, X.; Li, C.; Long, N.; Chen, X.; Bao, L.; Zhou, J.; Xie, Y. Gastrin-17 induces gastric cancer cell epithelial-mesenchymal transition via the Wnt/β-catenin signaling pathway. J. Physiol. Biochem. 2021, 77, 93–104. [Google Scholar] [CrossRef]
- Mu, G.; Ding, Q.; Li, H.; Zhang, L.; Zhang, L.; He, K.; Wu, L.; Deng, Y.; Yang, D.; Wu, L.; et al. Gastrin stimulates pancreatic cancer cell directional migration by activating the Gα12/13-RhoA-ROCK signaling pathway. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef]
- Madka, V.; Kumar, G.; Pathuri, G.; Panneerselvam, J.; Zhang, Y.; Ganta, V.; Lightfoot, S.; Lubet, R.; Suen, C.S.; Steele, V.E.; et al. Proton Pump Inhibitor Omeprazole Suppresses Carcinogen-induced Colonic Adenoma Progression to Adenocarcinoma in F344 Rat. Cancer Prev. Res. (Phila.) 2021, 14, 1009–1020. [Google Scholar] [CrossRef]
- Flemer, B.; Warren, R.D.; Barrett, M.P.; Cisek, K.; Das, A.; Jeffery, I.B.; Hurley, E.; O’Riordain, M.; Shanahan, F.; O’Toole, P.W. The oral microbiota in colorectal cancer is distinctive and predictive. Gut 2018, 67, 1454–1463. [Google Scholar] [CrossRef]
- Koliarakis, I.; Messaritakis, I.; Nikolouzakis, T.K.; Hamilos, G.; Souglakos, J.; Tsiaoussis, J. Oral Bacteria and Intestinal Dysbiosis in Colorectal Cancer. Int. J. Mol. Sci. 2019, 20, 4146. [Google Scholar] [CrossRef]
- Mo, S.; Ru, H.; Huang, M.; Cheng, L.; Mo, X.; Yan, L. Oral-Intestinal Microbiota in Colorectal Cancer: Inflammation and Immunosuppression. J. Inflamm. Res. 2022, 15, 747–759. [Google Scholar] [CrossRef]
- Wang, N.; Fang, J.Y. Fusobacterium nucleatum, a key pathogenic factor and microbial biomarker for colorectal cancer. Trends Microbiol. 2023, 31, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Zepeda-Rivera, M.; Minot, S.S.; Bouzek, H.; Wu, H.; Blanco-Míguez, A.; Manghi, P.; Jones, D.S.; LaCourse, K.D.; Wu, Y.; McMahon, E.F.; et al. A distinct Fusobacterium nucleatum clade dominates the colorectal cancer niche. Nature 2024, 628, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Huang, Z.; Hou, F.; Liu, Y.; Wang, L.; Wang, Z.; Sun, Y.; Pan, Z.; Tan, Y.; Ding, L.; et al. Parvimonas micra activates the Ras/ERK/c-Fos pathway by upregulating miR-218-5p to promote colorectal cancer progression. J. Exp. Clin. Cancer Res. 2023, 42, 13. [Google Scholar] [CrossRef] [PubMed]
- Bergsten, E.; Mestivier, D.; Donnadieu, F.; Pedron, T.; Barau, C.; Meda, L.T.; Mettouchi, A.; Lemichez, E.; Gorgette, O.; Chamaillard, M.; et al. Parvimonas micra, an oral pathobiont associated with colorectal cancer, epigenetically reprograms human colonocytes. Gut Microbes 2023, 15, 2265138. [Google Scholar] [CrossRef]
- Kichenadasse, G.; Miners, J.O.; Mangoni, A.A.; Karapetis, C.S.; Hopkins, A.M.; Sorich, M.J. Proton Pump Inhibitors and Survival in Patients with Colorectal Cancer Receiving Fluoropyrimidine-Based Chemotherapy. J. Natl. Compr. Cancer Netw. 2021, 19, 1037–1044. [Google Scholar] [CrossRef]
- Huang, C.H.; Tseng, Y.H.; Tsai, W.S.; Su, C.C.; Cheng, C.L.; Kao Yang, Y.H.; Chang, Y.C.; Liu, Y.H. Association between Risk of Clostridium difficile Infection and Duration of Proton Pump Inhibitor or H2-Receptor Antagonist Use in Hospitalized Patients. Infect. Dis. Ther. 2024, 13, 373–383. [Google Scholar] [CrossRef]
- Oshima, T.; Wu, L.; Li, M.; Fukui, H.; Watari, J.; Miwa, H. Magnitude and direction of the association between Clostridium difficile infection and proton pump inhibitors in adults and pediatric patients: A systematic review and meta-analysis. J. Gastroenterol. 2018, 53, 84–94. [Google Scholar] [CrossRef]
- Inghammar, M.; Svanström, H.; Voldstedlund, M.; Melbye, M.; Hviid, A.; Mølbak, K.; Pasternak, B. Proton-Pump Inhibitor Use and the Risk of Community-Associated Clostridium difficile Infection. Clin. Infect. Dis. 2021, 72, e1084–e1089. [Google Scholar] [CrossRef]
- Berenson, C.S.; Lashner, B.; Korman, L.Y.; Hohmann, E.; Deshpande, A.; Louie, T.J.; Sims, M.; Pardi, D.; Kraft, C.S.; Wang, E.E.L.; et al. Prevalence of Comorbid Factors in Patients with Recurrent Clostridioides difficile Infection in ECOSPOR III, a Randomized Trial of an Oral Microbiota-Based Therapeutic. Clin. Infect. Dis. 2023, 77, 1504–1510. [Google Scholar] [CrossRef]
- Hafiz, R.A.; Wong, C.; Paynter, S.; David, M.; Peeters, G. The Risk of Community-Acquired Enteric Infection in Proton Pump Inhibitor Therapy: Systematic Review and Meta-analysis. Ann. Pharmacother. 2018, 52, 613–622. [Google Scholar] [CrossRef]
- Willems, R.P.J.; van Dijk, K.; Ket, J.C.F.; Vandenbroucke-Grauls, C. Evaluation of the Association Between Gastric Acid Suppression and Risk of Intestinal Colonization with Multidrug-Resistant Microorganisms: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2020, 180, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Macke, L.; Schulz, C.; Koletzko, L.; Malfertheiner, P. Systematic review: The effects of proton pump inhibitors on the microbiome of the digestive tract-evidence from next-generation sequencing studies. Aliment. Pharmacol. Ther. 2020, 51, 505–526. [Google Scholar] [CrossRef] [PubMed]
- Hvas, C.L.; Dahl Jørgensen, S.M.; Jørgensen, S.P.; Storgaard, M.; Lemming, L.; Hansen, M.M.; Erikstrup, C.; Dahlerup, J.F. Fecal Microbiota Transplantation Is Superior to Fidaxomicin for Treatment of Recurrent Clostridium difficile Infection. Gastroenterology 2019, 156, 1324–1332.e3. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, U.; Silverberg, M.S. Inflammatory Bowel Disease-Gastroenterology Diamond Jubilee Review. Gastroenterology 2018, 154, 1555–1558. [Google Scholar] [CrossRef] [PubMed]
- Shastri, S.A.; Kantamneni, R.; Rashid, M.; Chandran, V.P.; Suhita, R.; Begum, I.; Nair, S.; Thunga, G. Proton pump inhibitors use and risk of inflammatory bowel diseases: A meta-analysis of observational studies. Med. Pharm. Rep. 2022, 95, 357–369. [Google Scholar] [CrossRef]
- Xia, B.; Yang, M.; Nguyen, L.H.; He, Q.; Zhen, J.; Yu, Y.; Di, M.; Qin, X.; Lu, K.; Kuo, Z.C.; et al. Regular Use of Proton Pump Inhibitor and the Risk of Inflammatory Bowel Disease: Pooled Analysis of 3 Prospective Cohorts. Gastroenterology 2021, 161, 1842–1852.e10. [Google Scholar] [CrossRef]
- Schwartz, N.R.M.; Hutfless, S.; Herrinton, L.J.; Amsden, L.B.; Fevrier, H.B.; Giefer, M.; Lee, D.; Suskind, D.L.; Delaney, J.A.C.; Phipps, A.I. Proton Pump Inhibitors, H2 Blocker Use, and Risk of Inflammatory Bowel Disease in Children. J. Pediatr. Pharmacol. Ther. 2019, 24, 489–496. [Google Scholar] [CrossRef]
- Onwuzo, S.; Boustany, A.; Khaled Abou Zeid, H.; Hitawala, A.; Almomani, A.; Onwuzo, C.; Lawrence, F.; Mascarenhas Monteiro, J.; Ndubueze, C.; Asaad, I. Prevalence and Risk Factors Associated with Inflammatory Bowel Disease in Patients Using Proton-Pump Inhibitors: A Population-Based Study. Cureus 2023, 15, e34088. [Google Scholar] [CrossRef]
- Juillerat, P.; Schneeweiss, S.; Cook, E.F.; Ananthakrishnan, A.N.; Mogun, H.; Korzenik, J.R. Drugs that inhibit gastric acid secretion may alter the course of inflammatory bowel disease. Aliment. Pharmacol. Ther. 2012, 36, 239–247. [Google Scholar] [CrossRef]
- Shah, R.; Richardson, P.; Yu, H.; Kramer, J.; Hou, J.K. Gastric Acid Suppression Is Associated with an Increased Risk of Adverse Outcomes in Inflammatory Bowel Disease. Digestion 2017, 95, 188–193. [Google Scholar] [CrossRef]
- Lu, T.X.; Dapas, M.; Lin, E.; Peters, T.; Sakuraba, A. The influence of proton pump inhibitor therapy on the outcome of infliximab therapy in inflammatory bowel disease: A patient-level meta-analysis of randomised controlled studies. Gut 2021, 70, 2076–2084. [Google Scholar] [CrossRef] [PubMed]
- Son, M.; Park, I.S.; Kim, S.; Ma, H.W.; Kim, J.H.; Kim, T.I.; Kim, W.H.; Han, J.; Kim, S.W.; Cheon, J.H. Novel Potassium-Competitive Acid Blocker, Tegoprazan, Protects against Colitis by Improving Gut Barrier Function. Front. Immunol. 2022, 13, 870817. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, Y.; Cao, P.; Su, W.; Zhan, N.; Dong, W. Fusobacterium nucleatum facilitates ulcerative colitis through activating IL-17F signaling to NF-κB via the upregulation of CARD3 expression. J. Pathol. 2020, 250, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liang, L.; Liang, H.; Wang, M.; Lu, B.; Xue, M.; Deng, J.; Chen, Y. Fusobacterium nucleatum Aggravates the Progression of Colitis by Regulating M1 Macrophage Polarization via AKT2 Pathway. Front. Immunol. 2019, 10, 1324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xiao, X.; Chen, P.R.; Li, Y.N.; Lv, X.H.; Yang, J.L. Proton Pump Inhibitors Increase the Risk of Nonsteroidal Anti-inflammatory Drug-Related Small-Bowel Injury: A Systematic Review with Meta-analysis. Clin. Transl. Gastroenterol. 2023, 14, e00588. [Google Scholar] [CrossRef]
- Jung, Y.S.; Park, J.H.; Park, C.H. Impact of proton pump inhibitors on the risk of small bowel or colorectal bleeding: A systematic review and meta-analysis. United Eur. Gastroenterol. J. 2023, 11, 861–873. [Google Scholar] [CrossRef]
- Wallace, J.L.; Syer, S.; Denou, E.; de Palma, G.; Vong, L.; McKnight, W.; Jury, J.; Bolla, M.; Bercik, P.; Collins, S.M.; et al. Proton pump inhibitors exacerbate NSAID-induced small intestinal injury by inducing dysbiosis. Gastroenterology 2011, 141, 1314–1322.e5. [Google Scholar] [CrossRef]
- Yoshihara, T.; Oikawa, Y.; Kato, T.; Kessoku, T.; Kobayashi, T.; Kato, S.; Misawa, N.; Ashikari, K.; Fuyuki, A.; Ohkubo, H.; et al. The protective effect of Bifidobacterium bifidum G9-1 against mucus degradation by Akkermansia muciniphila following small intestine injury caused by a proton pump inhibitor and aspirin. Gut Microbes 2020, 11, 1385–1404. [Google Scholar] [CrossRef]
- Blackler, R.W.; De Palma, G.; Manko, A.; Da Silva, G.J.; Flannigan, K.L.; Bercik, P.; Surette, M.G.; Buret, A.G.; Wallace, J.L. Deciphering the pathogenesis of NSAID enteropathy using proton pump inhibitors and a hydrogen sulfide-releasing NSAID. Am. J. Physiol.-Gastrointest. Liver Physiol. 2015, 308, G994–G1003. [Google Scholar] [CrossRef]
- Yang, M.; Xia, B.; Lu, Y.; He, Q.; Lin, Y.; Yue, P.; Bai, B.; Dong, C.; Meng, W.; Qi, J.; et al. Association between Regular Use of Gastric Acid Suppressants and Subsequent Risk of Cholelithiasis: A Prospective Cohort Study of 0.47 Million Participants. Front. Pharmacol. 2021, 12, 813587. [Google Scholar] [CrossRef]
- Fukuba, N.; Ishihara, S.; Sonoyama, H.; Yamashita, N.; Aimi, M.; Mishima, Y.; Mishiro, T.; Tobita, H.; Shibagaki, K.; Oshima, N.; et al. Proton pump inhibitor is a risk factor for recurrence of common bile duct stones after endoscopic sphincterotomy—Propensity score matching analysis. Endosc. Int. Open 2017, 5, E291–E296. [Google Scholar] [CrossRef]
- Chuang, S.C.; Lin, C.C.; Peng, C.Y.; Huang, W.H.; Su, W.P.; Lai, S.W.; Lai, H.C. Proton pump inhibitors increase the risk of cholecystitis: A population-based case-control study. Gut 2019, 68, 1337–1339. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.W.; Kang, D.; Shin, J.Y.; Kang, M.; Park, J.K.; Lee, K.H.; Lee, J.K.; Lee, K.T.; Rhee, P.L.; Kim, J.J.; et al. Use of proton pump inhibitors and the risk of cholangitis: A nationwide cohort study. Aliment. Pharmacol. Ther. 2019, 50, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Hakuta, R.; Nakai, Y.; Hamada, T.; Nomura, Y.; Saito, T.; Takahara, N.; Mizuno, S.; Kogure, H.; Moriya, K.; Koike, K. Use of proton pump inhibitors and cholangitis complicated with multi-drug resistant bacteria. J. Hepato-Biliary Pancreat. Sci. 2022, 29, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Sbeit, W.; Abukaes, H.; Said Ahmad, H.; Sbeit, M.; Kalisky, I.; Katz, L.; Mari, A.; Khoury, T. The possible association of proton pump inhibitor use with acute cholangitis in patients with choledocholithiasis: A multi-center study. Scand. J. Gastroenterol. 2023, 58, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Kamal, H.; Sadr-Azodi, O.; Engstrand, L.; Brusselaers, N. Association between Proton Pump Inhibitor Use and Biliary Tract Cancer Risk: A Swedish Population-Based Cohort Study. Hepatology 2021, 74, 2021–2031. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Wang, Y.; Xu, W.; Liu, Z.; Wang, H.; Zhang, Z.; Han, Y.; Yin, C.; Cao, S.; Yang, Z.; et al. Proton pump inhibitors and odds of cholangiocarcinoma: A retrospective case-control study. Liver Int. 2020, 40, 2848–2857. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Wang, Y.; Chen, G.; Jin, L. Proton pump inhibitors and the risk of gallbladder cancer: A hospital-based case-control study. Gut 2020, 69, 2265–2267. [Google Scholar] [CrossRef]
- He, Q.; Huang, C.; Qin, X.; Yu, Y.; Tang, D.; Huang, J.; Kuo, Z.C.; Ling, Y.; Mao, D.; Xia, B.; et al. Confounded association between proton pump inhibitor use and risk of biliary tract cancer: Result from three cohorts. Int. J. Cancer 2023, 153, 942–949. [Google Scholar] [CrossRef]
- Yang, Y.S.H.; Chang, H.W.; Lin, I.H.; Chien, L.N.; Wu, M.J.; Liu, Y.R.; Chu, P.G.; Xie, G.; Dong, F.; Jia, W.; et al. Long-term Proton Pump Inhibitor Administration Caused Physiological and Microbiota Changes in Rats. Sci. Rep. 2020, 10, 866. [Google Scholar] [CrossRef]
- He, Q.; Xia, B.; Yang, M.; Lu, K.; Fan, D.; Li, W.; Liu, Y.; Pan, Y.; Yuan, J. Alterations in gut microbiota and bile acids by proton-pump inhibitor use and possible mediating effects on elevated glucose levels and insulin resistance. FASEB J. 2024, 38, e23541. [Google Scholar] [CrossRef] [PubMed]
| Study | Study Design | Patient Characteristic | Sample | Methods | Translocated Oral Microbes in the Gut | Major Findings | |
|---|---|---|---|---|---|---|---|
| Genus | Species | ||||||
| Imhann et al. (2016) [11] | Cross-sectional study | A total of 1815 individuals (211 PPI users) in three independent cohorts from the Netherlands: LifeLines-DEEP study, IBD cohort, IBS case-control study. | Oral cavity mucus samples and Feces | Illumina Miseq sequencing, 16S rDNA, V4 | Rothia, Streptococcus, Actinomyces, Alloscardovia, Lactobacillus, Oribacterium, Granulicatella, Scardovia, Staphylococcus, Atopobium, Corynebacterium | Rothia mucilaginosa, Rothia dentocariosa, Lactobacillus salivarius, Streptococcus sobrinus, Streptococcus anginosus, Staphylococcus aureus, Staphylococcus epidermidis, Lactobacillus reuteri, Lactobacillus vaginalis, Lactobacillus delbrueckii, Streptococcus infantis, Atopobium rimae, Staphylococcus haemolyticus. | Multiple oral bacteria were over represented in the fecal microbiome of PPI users. |
| Jackson et al. (2016) [12] | Cross-sectional study | 1827 healthy twins from the TwinsUK cohort. | Feces | Illumina Miseq sequencing, 16S rDNA, V4 | Rothia, Streptococcus, Scardovia, Granulicatella, Oribacterium, Lactobacillus, Corynebacterium | Rothia mucilaginosa, Streptococcus anginosus | PPI use was associated with a significant increase in the abundance of oral and upper gastrointestinal tract commensals in gut commensals. |
| Otsuka et al. (2016) [34] | Prospective interventional trial | Helicobacter pylori IgG-negative healthy individuals (n = 20) taking four-week of 30 mg lansoprazole (n = 11) or 20 mg vonoprazan daily (n = 9). | Feces | Illumina Miseq sequencing, 16S rDNA, - | PPI group: Streptococcus, Carnobacterium, Oribacterium Vonoprazan group: Actinomyces, Rothia, Granulicatella, Streptococcus | - | Oral microbiome is more abundant in the gut microbiome after vonoprazan treatment as compared with lansoprazole treatment. |
| Xiao et al. (2024) [17] | Prospective self-controlled trial | Healthy adults (n = 16) taking 7-day course of 40 mg esomeprazole once daily. | Saliva and Feces | Illumina Miseq sequencing, 16S rDNA, V3-V4 | Streptococcus, Gemella | Streptococcus anginosus, Streptococcus parasanguinis clade 411, Streptococcus salivarius, Streptococcus vestibularis, Streptococcus mitis, Streptococcus sp. HMT 061, Streptococcus oralis subsp. dentisani clade 398, Streptococcus oralis subsp. dentisani clade 058, Lactococcus lactis | PPI administration increased Streptococcus abundance in gut microbiota, and the increased species of Streptococcus were found to be from the oral site or oral/nasal sites, in which Streptococcus anginosus was identified as the significantly changed species. |
| Zhu et al. (2024) [33] | Prospective randomized controlled trial | Healthy adults (n = 49) before and after 7-day course of 20 mg omeprazole (n = 23) or 20 mg famotidine daily (n = 26). | Saliva and Feces | Shotgun metagenomic sequencing | PPI group: Streptococcus, Rothia H2RA group: Streptococcus | PPI group: Actinomyces bouchesdurhonensis, Actinomyces oris, Actinomyces SGB17168, Actinomyces sp ICM58, Actinomyces sp S6 Spd3, Trueperella pyogenes, Rothia mucilaginosa, Isoptericola variabilis, Gemella sanguinis, Abiotrophia defective, Streptococcus constellatus, Streptococcus cristatus, Streptococcus mitis, Streptococcus sanguinis, Streptococcus sp 263 SSPC, Mogibacterium diversum, Solobacterium SGB6833, Megasphaera micronuciformis, Parvimonas micra, Fusobacterium nucleatum, Fusobacterium pseudoperiodonticum, Haemophilus sputorum H2RA group: Actinomyces, Bouchesdurhonensis, Rothia mucilaginosa, Isoptericola variabilis, Gemella sanguinis, Solobacterium SGB6833 | PPI usage led to a significantly higher extent of oral-to-gut transmission and promoted the growth of specific oral microbes in the gut than H2RA usage. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Li, Q.; Xia, S.; He, Y.; Liu, Y.; Yang, J.; Xiao, X. Proton Pump Inhibitors and Oral–Gut Microbiota: From Mechanism to Clinical Significance. Biomedicines 2024, 12, 2271. https://doi.org/10.3390/biomedicines12102271
Zhang X, Li Q, Xia S, He Y, Liu Y, Yang J, Xiao X. Proton Pump Inhibitors and Oral–Gut Microbiota: From Mechanism to Clinical Significance. Biomedicines. 2024; 12(10):2271. https://doi.org/10.3390/biomedicines12102271
Chicago/Turabian StyleZhang, Xian, Qing Li, Siyuan Xia, Yan He, Yuqiang Liu, Jinlin Yang, and Xue Xiao. 2024. "Proton Pump Inhibitors and Oral–Gut Microbiota: From Mechanism to Clinical Significance" Biomedicines 12, no. 10: 2271. https://doi.org/10.3390/biomedicines12102271
APA StyleZhang, X., Li, Q., Xia, S., He, Y., Liu, Y., Yang, J., & Xiao, X. (2024). Proton Pump Inhibitors and Oral–Gut Microbiota: From Mechanism to Clinical Significance. Biomedicines, 12(10), 2271. https://doi.org/10.3390/biomedicines12102271






