Antibacterial, Antifungal, and Cytotoxic Effects of Endophytic Streptomyces Species Isolated from the Himalayan Regions of Nepal and Their Metabolite Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Source of Microbes
2.2. Isolation of Streptomyces Species
2.3. Molecular Identification
2.4. Shake Flask Fermentation
2.5. Antibacterial Assays
2.6. Antifungal Assay
2.7. Cytotoxicity Assay
2.8. Liquid Chromatography-Mass Spectrometric Analysis
2.9. GNPS-Based Molecular Networking
2.10. Gas Chromatography–Mass Spectrometric Analysis
2.11. Metabolomics Data Analysis
3. Results
3.1. Isolation and Morphological Characterization of Isolates
3.2. Molecular Characterization and Phylogenetic Analysis
3.3. Antibacterial Assays of Streptomyces Isolates
3.4. Antifungal Potency of Streptomyces Isolates
3.5. Cytotoxicity Assay
3.6. Liquid Chromatography–Mass Spectrometric Analysis
3.7. Global Natural Product Social Molecular Networking (GNPS) Analysis
3.8. Gas Chromatography–Mass Spectrometry Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verma, S.; Verma, S.; Abbas, M.; Mahdi, F. Combating the Antimicrobial Resistance by Personalized Medicine: A Mini-Review. Era’s J. Med. Res. 2023, 10, 88–92. [Google Scholar] [CrossRef]
- Bertagnolio, S.; Suthar, A.B.; Tosas, O.; Weezenbeek, K.V. Antimicrobial Resistance: Strengthening Surveillance for Public Health Action. PLOS Med. 2023, 20, e1004265. [Google Scholar] [CrossRef]
- Tang, K.W.K.; Millar, B.C.; Moore, J.E. Antimicrobial Resistance (AMR). Br. J. Biomed. Sci. 2023, 80, 11387. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Staff, T.A.P. Global Cancer Burden May Be Growing Amidst Mounting Need for Cancer Services. Available online: https://ascopost.com/news/february-2024/global-cancer-burden-may-be-growing-amidst-mounting-need-for-cancer-services/ (accessed on 6 July 2024).
- Gao, Q.; Deng, S.; Jiang, T. Recent Developments in the Identification and Biosynthesis of Antitumor Drugs Derived from Microorganisms. Eng. Microbiol. 2022, 2, 100047. [Google Scholar] [CrossRef]
- Fernandes, E.S.; da Silva Figueiredo, I.F.; Monteiro, C.R.; Monteiro-Neto, V. Antimicrobial and Anti-Infective Activity of Natural Products—Gaining Knowledge from Novel Studies. Antibiotics 2023, 12, 1051. [Google Scholar] [CrossRef]
- Ullah, R.; Rehman, N.U.; Jamshidi-Adegani, F.; Bari, A. Medicinal Plants and Marine-Derived Natural Products as Cancer Chemopreventive Agents. Front. Pharmacol. 2022, 13, 900275. [Google Scholar] [CrossRef]
- Kustrimovic, N.; Bombelli, R.; Baci, D.; Mortara, L. Microbiome and Prostate Cancer: A Novel Target for Prevention and Treatment. Int. J. Mol. Sci. 2023, 24, 1511. [Google Scholar] [CrossRef]
- Jaiswal, S.; Ojha, A.; Thakur, P.; Mishra, S.K. Functional Importance of Endophytic Microorganisms in Plant Growth Promotion Bioactive Compound Production for Sustainable Agriculture. Def. Life Sci. J. 2023, 8, 93–108. [Google Scholar] [CrossRef]
- Veilumuthu, P.; Nagarajan, T.; Magar, S.; Sundaresan, S.; Moses, L.J.; Theodore, T.; Christopher, J.G. Genomic Insights into an Endophytic Streptomyces sp. VITGV156 for Antimicrobial Compounds. Front. Microbiol. 2024, 15, 1407289. [Google Scholar] [CrossRef]
- Li, W.; Yang, X.; Yang, Y.; Zhao, L.; Xu, L.; Ding, Z. A New Anthracycline from Endophytic Streptomyces sp. YIM66403. J. Antibiot. 2015, 68, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Taechowisan, T.; Lu, C.; Shen, Y.; Lumyong, S. Antitumor activity of 4-arylcoumarins from endophytic Streptomyces aureofaciens CMUAc130. J. Cancer Res. Ther. 2007, 3, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Devi, S.; Sharma, M.; Manhas, R.K. Purification and Biological Analysis of Antimicrobial Compound Produced by an Endophytic Streptomyces sp. Sci. Rep. 2023, 13, 15248. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Yang, A.; Zhang, N.; Li, S.; Yuan, T.; Ding, N.; Zhang, S.; Bao, S.; Wang, C.; Zhang, Y.; et al. Insecticidal Endostemonines A–J Produced by Endophytic Streptomyces from Stemona Sessilifolia. J. Agric. Food Chem. 2020, 68, 1588–1595. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Kumaravel, K.; Xiong, Q.; Liang, Y.; Ju, Z.; Jiang, Y.; Zhang, J. Actinomycins Produced by Endophyte Streptomyces sp. GLL-9 from Navel Orange Plant Exhibit High Antimicrobial Effect against Xanthomonas citri Susp. Citri and Penicillium italicum. Pest. Manag. Sci. 2023, 79, 4679–4693. [Google Scholar] [CrossRef]
- Allard, P.-M.; Péresse, T.; Bisson, J.; Gindro, K.; Marcourt, L.; Pham, V.C.; Roussi, F.; Litaudon, M.; Wolfender, J.-L. Integration of Molecular Networking and In-Silico MS/MS Fragmentation for Natural Products Dereplication. Anal. Chem. 2016, 88, 3317–3323. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T. Sharing and Community Curation of Mass Spectrometry Data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef]
- Rana, S.; Salam, M.D. Antimicrobial Potential of Actinomycetes Isolated from Soil Samples of Punjab, India. J. Microbiol. Exp. 2014, 1, 00010. [Google Scholar]
- Kumar, N.; Singh, R.; Mishra, S.; Singh, A.; Pachouri, U.C. Isolation and Screening of Soil Actinomycetes as Source of Antibiotics Active against Bacteria. Int. J. Microbiol. Res. 2010, 2, 12–16. [Google Scholar] [CrossRef]
- Sripreechasak, P.; Athipornchai, A. Potential Antimicrobial Properties of Streptomyces Isolated from Sammuk Mountain Soil, Chonburi Province, Thailand. J. Adv. Pharm. Technol. Res. 2019, 10, 195. [Google Scholar] [CrossRef]
- Singh, V.; Haque, S.; Singh, H.; Verma, J.; Vibha, K.; Singh, R.; Jawed, A.; Tripathi, C.K.M. Isolation, Screening, and Identification of Novel Isolates of Actinomycetes from India for Antimicrobial Applications. Front. Microbiol. 2016, 7, 1921. [Google Scholar] [CrossRef] [PubMed]
- Bergey, D.H.; Holt, J.G.; Krieg, N.R. Bergey’s Manual of Systematic Bacteriology; Bergey’s Manual of Systematic Bacteriology; Williams & Wilkins: Philadelphia, PA, USA, 1984; ISBN 978-0-683-07908-1. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Hindra; Yang, D.; Luo, J.; Huang, T.; Yan, X.; Adhikari, A.; Teijaro, C.N.; Ge, H.; Shen, B. Submerged Fermentation of Streptomyces Uncialis Providing a Biotechnology Platform for Uncialamycin Biosynthesis, Engineering, and Production. J. Ind. Microbiol. Biotechnol. 2021, 48, kuab025. [Google Scholar] [CrossRef] [PubMed]
- Ibnouf, E.O.; Aldawsari, M.F.; Ali Waggiallah, H. Isolation and Extraction of Some Compounds That Act as Antimicrobials from Actinomycetes. Saudi J. Biol. Sci. 2022, 29, 103352. [Google Scholar] [CrossRef] [PubMed]
- Valgas, C.; de Souza, S.M.; Smânia, E.F.A.; Smânia, A., Jr. Screening Methods to Determine Antibacterial Activity of Natural Products. Braz. J. Microbiol. 2007, 38, 369–380. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and Broth Dilution Methods to Determine the Minimal Inhibitory Concentration (MIC) of Antimicrobial Substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Wei, Y.; Zhao, Y.; Zhou, D.; Qi, D.; Li, K.; Tang, W.; Chen, Y.; Jing, T.; Zang, X.; Xie, J.; et al. A Newly Isolated Streptomyces sp. YYS-7 With a Broad-Spectrum Antifungal Activity Improves the Banana Plant Resistance to Fusarium oxysporum f. sp. Cubense Tropical Race 4. Front. Microbiol. 2020, 11, 1712. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays. In Assay Guidance Manual; Markossian, S., Grossman, A., Arkin, M., Auld, D., Austin, C., Baell, J., Brimacombe, K., Chung, T.D.Y., Coussens, N.P., Dahlin, J.L., et al., Eds.; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2004. [Google Scholar]
- Yu, J.S.; Jeong, S.Y.; Li, C.; Oh, T.; Kwon, M.; Ahn, J.S.; Ko, S.-K.; Ko, Y.-J.; Cao, S.; Kim, K.H. New phenalenone derivatives from the Hawaiian volcanic soil-associated fungus Penicillium herquei FT729 and their inhibitory effects on indoleamine 2, 3-dioxygenase 1 (IDO1). Arch. Pharm. Res. 2022, 45, 105–113. [Google Scholar] [CrossRef]
- Green, M.R.; Sambrook, J. Molecular Cloning: A Laboratory Manual, 4th ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2012; ISBN 978-1-936113-41-5. [Google Scholar]
- Paudel, B.; Maharjan, R.; Rajbhandari, P.; Aryal, N.; Aziz, S.; Bhattarai, K.; Baral, B.; Malla, R.; Bhattarai, H.D. Maculosin, a Non-Toxic Antioxidant Compound Isolated from Streptomyces sp. KTM18. Pharm. Biol. 2021, 59, 931–934. [Google Scholar] [CrossRef]
- Kwon, O.S.; Park, S.H.; Yun, B.S.; Pyun, Y.R.; Kim, C.J. Cyclo(D-Pro-L-Val), a specific β-glucosidase inhibitor produced by Aspergillus sp. F70609. J. Antibiot. 2001, 54, 179–181. [Google Scholar] [CrossRef]
- Li, B.; Chen, G.; Bai, J.; Jing, Y.-K.; Pei, Y.-H. A Bisamide and Four Diketopiperazines from a Marine-Derived Streptomyces sp. J. Asian Nat. Prod. Res. 2011, 13, 1146–1150. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, S.; Bhattarai, B.R.; Adhikari, A.; Aryal, B.; Shrestha, A.; Aryal, N.; Lamichhane, U.; Thapa, R.; Thapa, B.B.; Yadav, R.P.; et al. Characterization of Streptomyces Species and Validation of Antimicrobial Activity of Their Metabolites through Molecular Docking. Processes 2022, 10, 2149. [Google Scholar] [CrossRef]
- Ravi, L.; Ragunathan, A.; Krishnan, K. Antidiabetic and Antioxidant Potential of GancidinW from Streptomyces Paradoxus VITALK03. Open Bioact. Compd. J. 2017, 5, 31–42. [Google Scholar] [CrossRef]
- Yu, H.; Wang, J.; Li, X.; Quan, C. Effect of the Environmental Factors on Diketopiperazine Cyclo(Pro-Phe) Production and Antifungal Activity of Bacillus Amyloliquefaciens Q-426. Biologia 2021, 76, 1789–1795. [Google Scholar] [CrossRef]
- Li, S.-Q.; Yang, Y.-B.; Yang, X.-Q.; Jiang, Y.; Li, Z.-J.; Li, X.-Z.; Chen, X.; Li, Q.-L.; Qin, S.-H.; Ding, Z.-T. Two New Cyclic Tetrapeptides of Streptomyces Rutgersensis T009 Isolated from Elaphodus Davidianus Excrement. Helv. Chim. Acta 2016, 99, 210–214. [Google Scholar] [CrossRef]
- Tangerina, M.M.P.; Furtado, L.C.; Leite, V.M.B.; Bauermeister, A.; Velasco-Alzate, K.; Jimenez, P.C.; Garrido, L.M.; Padilla, G.; Lopes, N.P.; Costa-Lotufo, L.V.; et al. Metabolomic Study of Marine Streptomyces sp.: Secondary Metabolites and the Production of Potential Anticancer Compounds. PLoS ONE 2020, 15, e0244385. [Google Scholar] [CrossRef]
- Liu, Z.G.; Tang, M.Y.; Meng, Q.H.; Zhang, C.; Sun, Y. Secondary metabolites of Streptomyces sp. A1693. Zhongguo Zhong yao za zhi = Zhongguo Zhongyao Zazhi = China J. Chin. Mater. Medica 2018, 43, 3301–3306. [Google Scholar] [CrossRef]
- Kimura, Y.; Sawada, A.; Kuramata, M.; Kusano, M.; Fujioka, S.; Kawano, T.; Shimada, A. Brevicompanine C, Cyclo-(d-Ile-l-Trp), and Cyclo-(d-Leu-l-Trp), Plant Growth Regulators from Penicillium Brevi-Compactum. J. Nat. Prod. 2005, 68, 237–239. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Lai, T.K.; Saha, A.; Selvin, J.; Mukherjee, J. Structural Elucidation and Antimicrobial Activity of a Diketopiperazine Isolated from a Bacillus sp. Assoc. Mar. Sponge Spongia Officinalis. Nat. Prod. Res. 2021, 35, 2315–2323. [Google Scholar] [CrossRef]
- Díaz-Cárdenas, C.; Rojas, L.Y.; Fiorentino, S.; Cala, M.P.; Díaz, J.I.; Ramos, F.A.; Armengaud, J.; Restrepo, S.; Baena, S. Bioactive Potential of Extracts of Labrenzia Aggregata Strain USBA 371, a Halophilic Bacterium Isolated from a Terrestrial Source. Molecules 2020, 25, 2546. [Google Scholar] [CrossRef]
- Pahl, I.; Pahl, A.; Hauk, A.; Budde, D.; Sievers, S.; Fruth, L.; Menzel, R. Assessing Biologic/Toxicologic Effects of Extractables from Plastic Contact Materials for Advanced Therapy Manufacturing Using Cell Painting Assay and Cytotoxicity Screening. Sci. Rep. 2024, 14, 5933. [Google Scholar] [CrossRef]
- Driche, E.H.; Badji, B.; Bijani, C.; Belghit, S.; Pont, F.; Mathieu, F.; Zitouni, A. A new saharan strain of Streptomyces sp. GSB-11 produces maculosin and N-acetyltyramine active against multidrug-resistant pathogenic bacteria. Curr. Microbiol. 2022, 79, 298. [Google Scholar] [CrossRef] [PubMed]
- Stelmasiewicz, M.; Świątek, Ł.; Ludwiczuk, A. Chemical and Biological Studies of Endophytes Isolated from Marchantia Polymorpha. Molecules 2023, 28, 2202. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. Genome Mining-Directed Discovery of Novel 2,5-Diketopiperazines from Actinobacteria. Available online: https://archiv.ub.uni-marburg.de/diss/z2021/0102 (accessed on 29 August 2024).
- Menéndez, N.; Nur-e-Alam, M.; Braña, A.F.; Rohr, J.; Salas, J.A.; Méndez, C. Tailoring Modification of Deoxysugars during Biosynthesis of the Antitumour Drug Chromomycin A3 by Streptomyces griseus Ssp. Griseus. Mol. Microbiol. 2004, 53, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Kalinovskaya, N.I.; Romanenko, L.A.; Kalinovsky, A.I.; Ermakova, S.P.; Dmitrenok, P.S.; Afiyatullov, S.S. The Antitumor Antibiotics Complex of Aureolic Acids from the Marine Sediment-Associated Strain of Streptomyces sp. KMM 9048. Nat. Prod. Commun. 2017, 12, 1934578X1701200. [Google Scholar] [CrossRef]
- Thapa, B.B.; Huo, C.; Budhathoki, R.; Chaudhary, P.; Joshi, S.; Poudel, P.B.; Magar, R.T.; Parajuli, N.; Kim, K.H.; Sohng, J.K. Metabolic Comparison and Molecular Networking of Antimicrobials in Streptomyces Species. Int. J. Mol. Sci. 2024, 25, 4193. [Google Scholar] [CrossRef]
- Zhang, F.; Li, B.; Wen, Y.; Liu, Y.; Liu, R.; Liu, J.; Liu, S.; Jiang, Y. An Integrated Strategy for the Comprehensive Profiling of the Chemical Constituents of Aspongopus Chinensis Using UPLC-QTOF-MS Combined with Molecular Networking. Pharm. Biol. 2022, 60, 1349–1364. [Google Scholar] [CrossRef]
- Koenuma, M.; Uchida, N.; Yamaguchi, K.; Kawamura, Y.; Matsumoto, K. New aureolic acid antibiotics i. Screening, isolation, characterization and biological propertieS. J. Antibiot. 1988, 41, 45–52. [Google Scholar] [CrossRef]
- Chhetri, G.; Kim, M.J.; Kim, I.; Tran, D.V.H.; Kim, Y.-W.; Kim, H.W.; Seo, T. Streptomyces tagetis sp. Nov., a Chromomycin Producing Bacteria Isolated from the Roots of Tagetes Patula. Front. Microbiol. 2024, 15, 1361583. [Google Scholar] [CrossRef]
- Roy, R.N.; Laskar, S.; Sen, S.K. Dibutyl Phthalate, the Bioactive Compound Produced by Streptomyces Albidoflavus 321.2. Microbiol. Res. 2006, 161, 121–126. [Google Scholar] [CrossRef]
- Zhu, Y.; Han, S.; Li, J.; Gao, H.; Dong, B. Aqueous Extract of Sea Squirt (Halocynthia roretzi) with Potent Activity against Human Cancer Cells Acts Synergistically with Doxorubicin. Mar. Drugs 2022, 20, 284. [Google Scholar] [CrossRef] [PubMed]
- Barale, S.S.; Ghane, S.G.; Sonawane, K.D. Purification and Characterization of Antibacterial Surfactin Isoforms Produced by Bacillus Velezensis SK. AMB Express 2022, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, B.R.; Khadayat, K.; Aryal, N.; Aryal, B.; Lamichhane, U.; Bhattarai, K.; Rana, N.; Regmi, B.P.; Adhikari, A.; Thapa, S.; et al. Untargeted Metabolomics of Streptomyces Species Isolated from Soils of Nepal. Processes 2022, 10, 1173. [Google Scholar] [CrossRef]
- Lautru, S.; Gondry, M.; Genet, R.; Pernodet, J.-L. The Albonoursin Gene Cluster of S. Noursei: Biosynthesis of Diketopiperazine Metabolites Independent of Nonribosomal Peptide Synthetases. Chem. Biol. 2002, 9, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- de Fretes, C.E.; Sembiring, L.; Purwestri, Y.A. Characterization of Streptomyces Spp. Producing Indole-3-Acetic Acid as Biostimulant Agent. Indones. J. Biotechnol. 2013, 18, 83–91. [Google Scholar] [CrossRef]
- Cimmino, A.; Puopolo, G.; Perazzolli, M.; Andolfi, A.; Melck, D.; Pertot, I.; Evidente, A. Cyclo(L-PRO-L-TYR), The Fungicide Isolated From Lysobacter Capsici AZ78: A Structure–Activity Relationship Study. Chem. Heterocycl. Comp. 2014, 50, 290–295. [Google Scholar] [CrossRef]
- Pagano, C.; Navarra, G.; Coppola, L.; Avilia, G.; Pastorino, O.; Della Monica, R.; Buonaiuto, M.; Torelli, G.; Caiazzo, P.; Bifulco, M.; et al. N6-Isopentenyladenosine Induces Cell Death through Necroptosis in Human Glioblastoma Cells. Cell Death Discov. 2022, 8, 1–11. [Google Scholar] [CrossRef]
- Hamed, A.; Abdel-Razek, A.S.; Araby, M.; Abu-Elghait, M.; El-Hosari, D.G.; Frese, M.; Soliman, H.S.M.; Stammler, H.G.; Sewald, N.; Shaaban, M. Meleagrin from Marine Fungus Emericella Dentata Nq45: Crystal Structure and Diverse Biological Activity Studies. Nat. Prod. Res. 2021, 35, 3830–3838. [Google Scholar] [CrossRef]
- Deng, X.; Liang, K.; Tong, X.; Ding, M.; Li, D.; Xia, C. Copper-Catalyzed Radical Cyclization To Access 3-Hydroxypyrroloindoline: Biomimetic Synthesis of Protubonine A. Org. Lett. 2014, 16, 3276–3279. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Xu, X.-Y.; Peng, J.; Ma, C.-F.; Nong, X.-H.; Bao, J.; Zhang, G.-Z.; Qi, S.-H. Antifouling Potentials of Eight Deep-Sea-Derived Fungi from the South China Sea. J. Ind. Microbiol. Biotechnol. 2014, 41, 741–748. [Google Scholar] [CrossRef]
- Ahsan, T.; Chen, J.; Zhao, X.; Irfan, M.; Wu, Y. Extraction and Identification of Bioactive Compounds (Eicosane and Dibutyl Phthalate) Produced by Streptomyces Strain KX852460 for the Biological Control of Rhizoctonia Solani AG-3 Strain KX852461 to Control Target Spot Disease in Tobacco Leaf. AMB Express 2017, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yang, Y.; Yang, X.; Li, W.; Xiong, Z.; Zhao, L.; Xu, L.; Ding, Z. A New Cyclic Tetrapeptide from an Endophytic Streptomyces sp. YIM67005. Nat. Prod. Res. 2014, 28, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Danh, C.D.; Dao, P.T.; Huong, D.T.M.; Thach, T.D.; Anh, N.M.; Minh, L.T.H.; Anh, T.T.; Van Cuong, P. Cyclopeptides from Marine Actinomycete Streptomyces sp. G261. Vietnam. J. Chem. 2018, 56, 570–573. [Google Scholar] [CrossRef]
- Yi, W.; Li, Q.; Song, T.; Chen, L.; Li, X.-C.; Zhang, Z.; Lian, X.-Y. Isolation, Structure Elucidation, and Antibacterial Evaluation of the Metabolites Produced by the Marine-Sourced Streptomyces sp. ZZ820. Tetrahedron 2019, 75, 1186–1193. [Google Scholar] [CrossRef]
- Mehdi-Ben Ameur, R.; Mellouli, L.; Chabchoub, F.; Fotso, S.; Bejar, S. Purification and structure elucidation of two biologically active molecules from a new isolated Streptomyces sp. US 24 strain. Chem. Nat. Compd. 2004, 40, 510–513. [Google Scholar] [CrossRef]
- Jankowitsch, F.; Schwarz, J.; Rückert, C.; Gust, B.; Szczepanowski, R.; Blom, J.; Pelzer, S.; Kalinowski, J.; Mack, M. Genome Sequence of the Bacterium Streptomyces Davawensis JCM 4913 and Heterologous Production of the Unique Antibiotic Roseoflavin. J. Bacteriol. 2012, 194, 6818–6827. [Google Scholar] [CrossRef]
- Lin, L.P.; Wu, M.; Jiang, N.; Wang, W.; Tan, R.X. Carbon-Nitrogen Bond Formation to Construct Novel Polyketide-Indole Hybrids from the Indole-3-Carbinol Exposed Culture of Daldinia eschscholzii. Synth. Syst. Biotechnol. 2022, 7, 750–755. [Google Scholar] [CrossRef]
- Menéndez, N.; Nur-e-Alam, M.; Braña, A.F.; Rohr, J.; Salas, J.A.; Méndez, C. Biosynthesis of the Antitumor Chromomycin A3 in Streptomyces Griseus: Analysis of the Gene Cluster and Rational Design of Novel Chromomycin Analogs. Chem. Biol. 2004, 11, 21–32. [Google Scholar] [CrossRef]
- Jiang, J.; Gao, L.; Bie, X.; Lu, Z.; Liu, H.; Zhang, C.; Lu, F.; Zhao, H. Identification of Novel Surfactin Derivatives from NRPS Modification of Bacillus Subtilis and Its Antifungal Activity against Fusarium Moniliforme. BMC Microbiol. 2016, 16, 31. [Google Scholar] [CrossRef]
- Chai, Y.; Chen, H.; Gao, G.; Liu, X.; Lu, C. Identification of New Interferences Leached from Plastic Microcentrifuge Tubes in Electrospray Ionization Mass Spectrometry. Rapid Commun. Mass. Spectrom. 2019, 33, 969–977. [Google Scholar] [CrossRef]
- Coulon, D.; Faure, L.; Salmon, M.; Wattelet, V.; Bessoule, J.-J. N-Acylethanolamines and Related Compounds: Aspects of Metabolism and Functions. Plant Sci. 2012, 184, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Çelik, M.S.; Aksu, A.; Yenidünya, A.F.; Çetinkaya, S. Tromethamine and Dodecanol Appear to Be the Major Secondary Metabolites of Streptomyces Decoyicus M*. Arch. Microbiol. 2022, 204, 456. [Google Scholar] [CrossRef] [PubMed]
- Redlich, S.; Ribes, S.; Schütze, S.; Nau, R. Palmitoylethanolamide Stimulates Phagocytosis of Escherichia Coli K1 by Macrophages and Increases the Resistance of Mice against Infections. J. Neuroinflammation 2014, 11, 108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chen, Y.; Zhu, J.; Lu, Q.; Cryle, M.J.; Zhang, Y.; Yan, F. Structural Diversity, Biosynthesis, and Biological Functions of Lipopeptides from Streptomyces. Nat. Prod. Rep. 2023, 40, 557–594. [Google Scholar] [CrossRef]
- Chevrette, M.G.; Carlson, C.M.; Ortega, H.E.; Thomas, C.; Ananiev, G.E.; Barns, K.J.; Book, A.J.; Cagnazzo, J.; Carlos, C.; Flanigan, W.; et al. The Antimicrobial Potential of Streptomyces from Insect Microbiomes. Nat. Commun. 2019, 10, 516. [Google Scholar] [CrossRef]
- Mohan, C.D.; Rangappa, S.; Nayak, S.C.; Jadimurthy, R.; Wang, L.; Sethi, G.; Garg, M.; Rangappa, K.S. Bacteria as a Treasure House of Secondary Metabolites with Anticancer Potential. Semin. Cancer Biol. 2022, 86, 998–1013. [Google Scholar] [CrossRef]
- Masuku, M.; Mozirandi, W.; Mukanganyama, S. Evaluation of the Antibacterial and Antibiofilm Effects of Ethyl Acetate Root Extracts from Vernonia Adoensis (Asteraceae) against Pseudomonas Aeruginosa. Sci. World J. 2023, 2023, 5782656. [Google Scholar] [CrossRef]
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 4, 481–511. [Google Scholar] [CrossRef]
- Nishanth Kumar, S.; Mohandas, C.; Nambisan, B. Purification, Structural Elucidation and Bioactivity of Tryptophan Containing Diketopiperazines, from Comamonas Testosteroni Associated with a Rhabditid Entomopathogenic Nematode against Major Human-Pathogenic Bacteria. Peptides 2014, 53, 48–58. [Google Scholar] [CrossRef]
- Cimmino, A.; Bejarano, A.; Masi, M.; Puopolo, G.; Evidente, A. Isolation of 2,5-Diketopiperazines from Lysobacter Capsici AZ78 with Activity against Rhodococcus Fascians. Nat. Prod. Res. 2021, 35, 4969–4977. [Google Scholar] [CrossRef]
- Ben Ameur Mehdi, R.; Shaaban, K.A.; Rebai, I.K.; Smaoui, S.; Bejar, S.; Mellouli, L. Five Naturally Bioactive Molecules Including Two Rhamnopyranoside Derivatives Isolated from the Streptomyces sp. Strain TN58. Nat. Prod. Res. 2009, 23, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Jamal, Q.; Cho, J.-Y.; Moon, J.-H.; Kim, K.Y. Purification and Antifungal Characterization of Cyclo (D-Pro-L- Val) from Bacillus amyloliquefaciens Y1 against Fusarium graminearum to Control Head Blight in Wheat. Biocatal. Agric. Biotechnol. 2017, 10, 141–147. [Google Scholar] [CrossRef]
- Bojarska, J.; Mieczkowski, A.; Ziora, Z.M.; Skwarczynski, M.; Toth, I.; Shalash, A.O.; Parang, K.; El-Mowafi, S.A.; Mohammed, E.H.M.; Elnagdy, S.; et al. Cyclic Dipeptides: The Biological and Structural Landscape with Special Focus on the Anti-Cancer Proline-Based Scaffold. Biomolecules 2021, 11, 1515. [Google Scholar] [CrossRef] [PubMed]
- P de Carvalho, M.; Abraham, W.-R. Antimicrobial and Biofilm Inhibiting Diketopiperazines. Curr. Med. Chem. 2012, 19, 3564–3577. [Google Scholar] [CrossRef]
- Huang, P.; Xie, F.; Ren, B.; Wang, Q.; Wang, J.; Wang, Q.; Abdel-Mageed, W.M.; Liu, M.; Han, J.; Oyeleye, A.; et al. Anti-MRSA and Anti-TB Metabolites from Marine-Derived Verrucosispora sp. MS100047. Appl. Microbiol. Biotechnol. 2016, 100, 7437–7447. [Google Scholar] [CrossRef]
- Youssef, F.S.; Simal-Gandara, J. Comprehensive Overview on the Chemistry and Biological Activities of Selected Alkaloid Producing Marine-Derived Fungi as a Valuable Reservoir of Drug Entities. Biomedicines 2021, 9, 485. [Google Scholar] [CrossRef]
- Cho, E.; Kwon, O.-S.; Chung, B.; Lee, J.; Sun, J.; Shin, J.; Oh, K.-B. Antibacterial Activity of Chromomycins from a Marine-Derived Streptomyces Microflavus. Mar. Drugs 2020, 18, 522. [Google Scholar] [CrossRef]
- Murase, H.; Noguchi, T.; Sasaki, S. Evaluation of Simultaneous Binding of Chromomycin A3 to the Multiple Sites of DNA by the New Restriction Enzyme Assay. Bioorganic Med. Chem. Lett. 2018, 28, 1832–1835. [Google Scholar] [CrossRef]
- Guimarães, L.A.; Jimenez, P.C.; Sousa, T.D.S.; Freitas, H.P.S.; Rocha, D.D.; Wilke, D.V.; Martín, J.; Reyes, F.; Deusdênia Loiola Pessoa, O.; Costa-Lotufo, L.V. Chromomycin A2 Induces Autophagy in Melanoma Cells. Mar. Drugs 2014, 12, 5839–5855. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Z.; Zhang, Z.; Liu, J.; Hong, L. β-Sitosterol as a Promising Anticancer Agent for Chemoprevention and Chemotherapy: Mechanisms of Action and Future Prospects. Adv. Nutr. 2023, 14, 1085–1110. [Google Scholar] [CrossRef]
- Rather, S.A.; Shah, A.M.; Ali, S.A.; Dar, R.A.; Rah, B.; Ali, A.; Hassan, Q.P. Isolation and Characterization of Streptomyces Tauricus from Thajiwas Glacier—A New Source of Actinomycin-D. Med. Chem. Res. 2017, 26, 1897–1902. [Google Scholar] [CrossRef]
- Lee, M.J.; Kim, G.J.; Shin, M.-S.; Moon, J.; Kim, S.; Nam, J.-W.; Kang, K.S.; Choi, H. Chemical Investigation of Diketopiperazines and N-Phenethylacetamide Isolated from Aquimarina sp. MC085 and Their Effect on TGF-β-Induced Epithelial–Mesenchymal Transition. Appl. Sci. 2021, 11, 8866. [Google Scholar] [CrossRef]
- Pirri, G.; Giuliani, A.; Nicoletto, S.; Pizzuto, L.; Rinaldi, A. Lipopeptides as Anti-Infectives: A Practical Perspective. Open Life Sci. 2009, 4, 258–273. [Google Scholar] [CrossRef]
- Sen, R. Surfactin: Biosynthesis, Genetics and Potential Applications. In Biosurfactants; Sen, R., Ed.; Springer: New York, NY, USA, 2010; pp. 316–323. ISBN 978-1-4419-5979-9. [Google Scholar]
- Carrillo, C.; Teruel, J.A.; Aranda, F.J.; Ortiz, A. Molecular Mechanism of Membrane Permeabilization by the Peptide Antibiotic Surfactin. Biochim. Et. Biophys. Acta (BBA)—Biomembr. 2003, 1611, 91–97. [Google Scholar] [CrossRef]
- Liu, X.; Tao, X.; Zou, A.; Yang, S.; Zhang, L.; Mu, B. Effect of Themicrobial Lipopeptide on Tumor Cell Lines: Apoptosis Induced by Disturbing the Fatty Acid Composition of Cell Membrane. Protein Cell 2010, 1, 584–594. [Google Scholar] [CrossRef]
- Tripathi, N.; Kumar, S.; Singh, R.; Singh, C.J.; Singh, P.; Varshney, V.K. Isolation and Identification of γ- Sitosterol by GC-MS from the Leaves of Girardinia Heterophylla (Decne). Open Bioact. Compd. J. 2013, 4, 25–27. [Google Scholar] [CrossRef]
- Toumatia, O.; Yekkour, A.; Goudjal, Y.; Riba, A.; Coppel, Y.; Mathieu, F.; Sabaou, N.; Zitouni, A. Antifungal Properties of an Actinomycin D-Producing Strain, Streptomyces sp. IA1, Isolated from a Saharan Soil. J. Basic. Microbiol. 2015, 55, 221–228. [Google Scholar] [CrossRef]





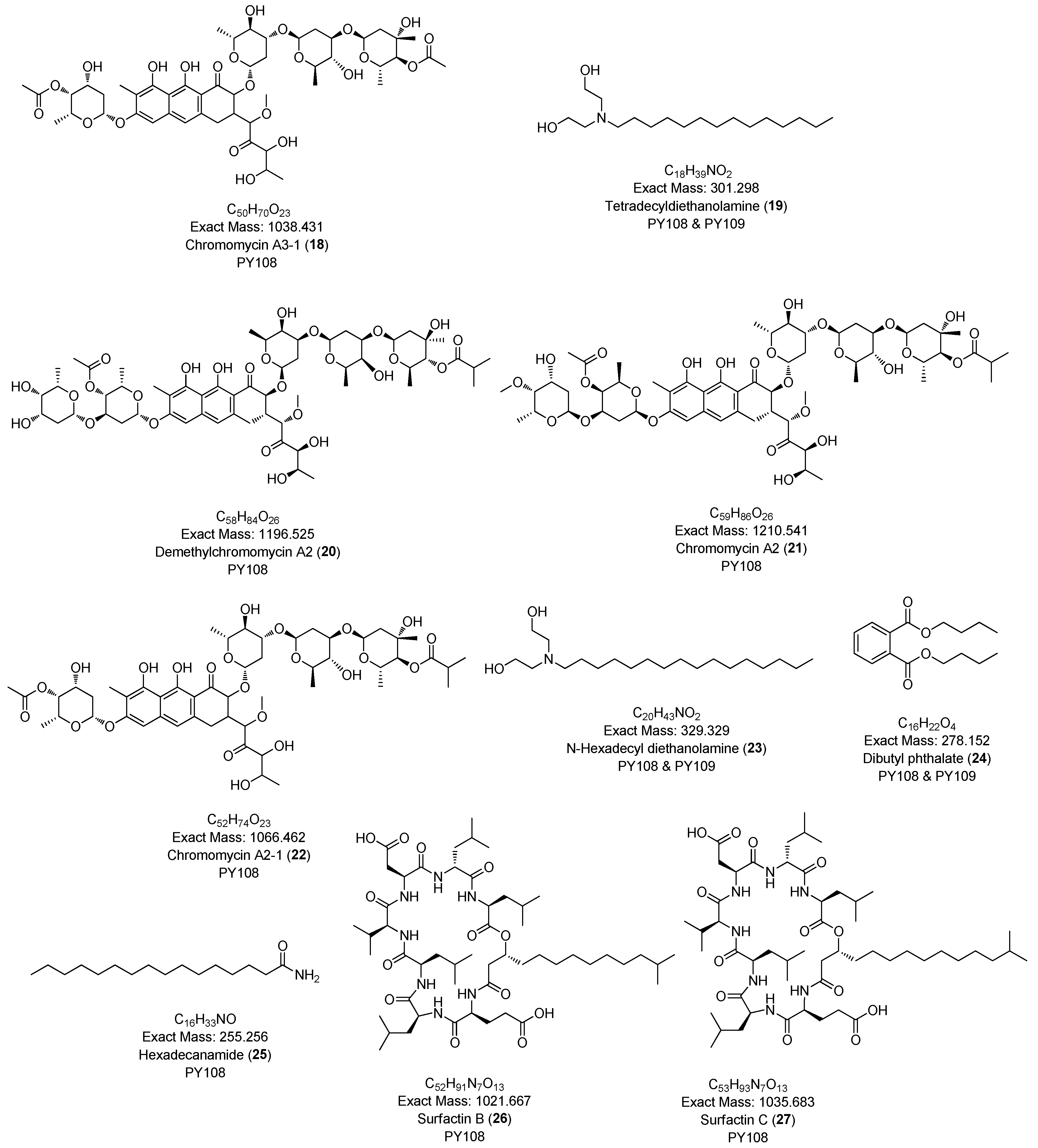
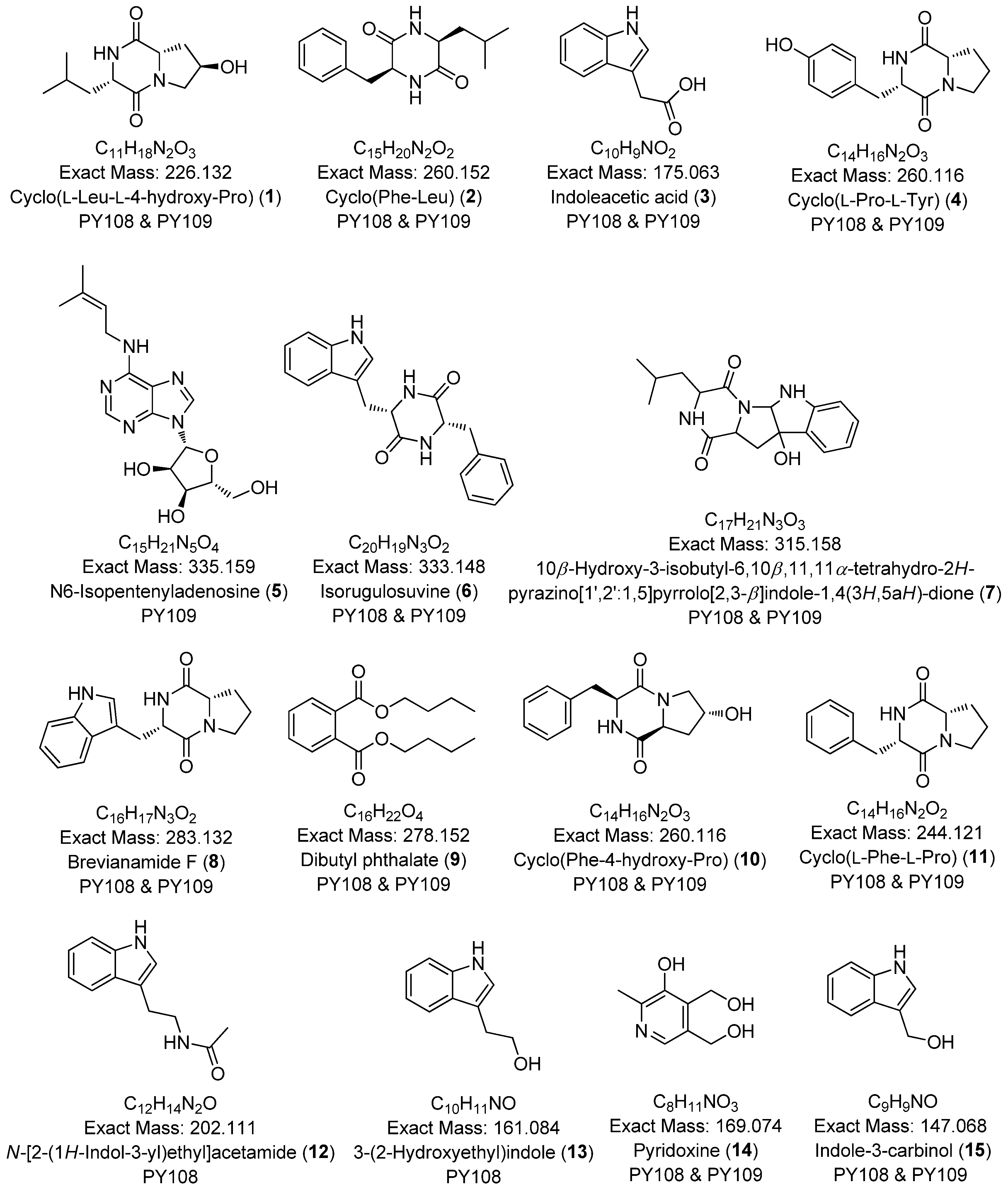

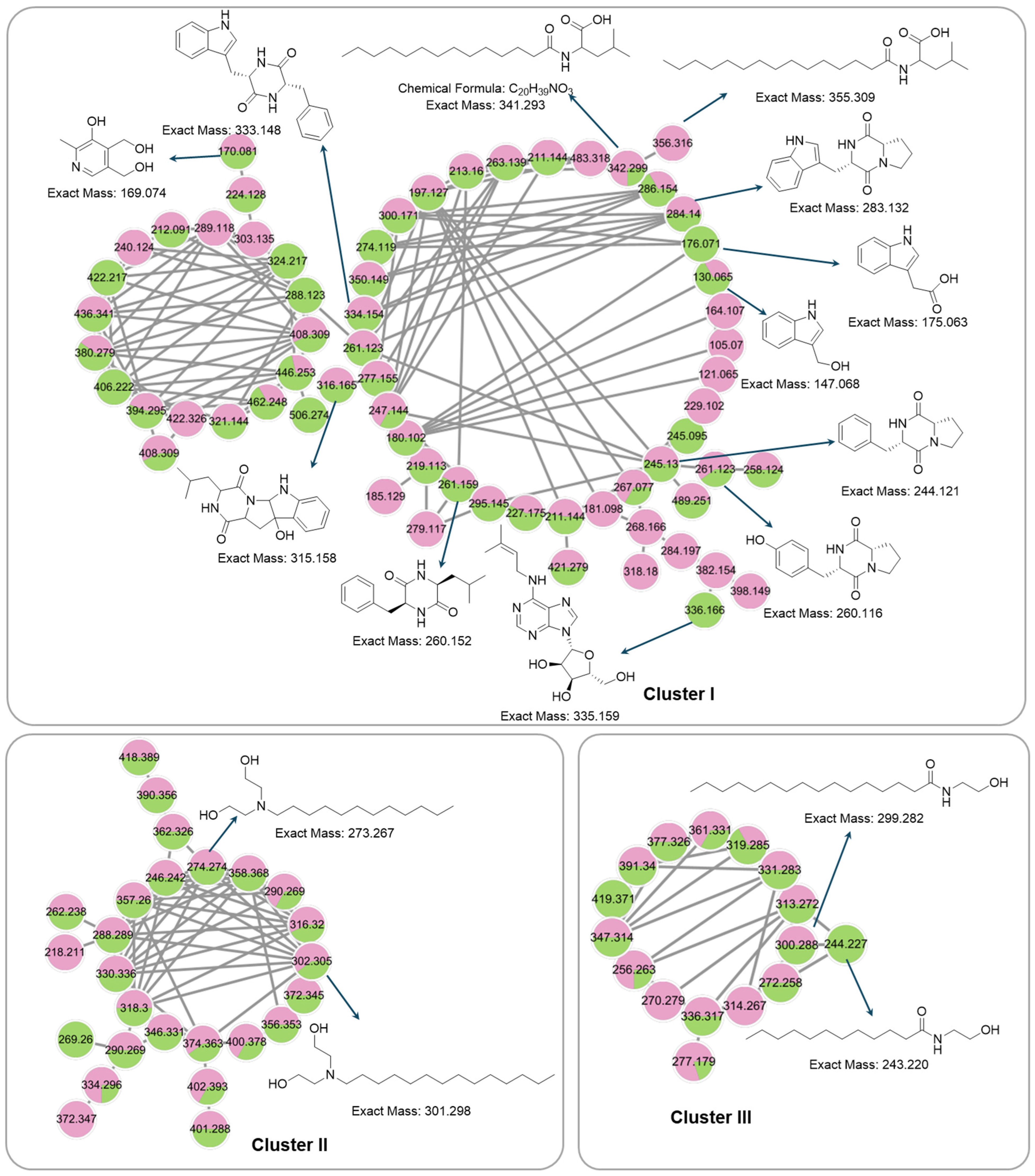
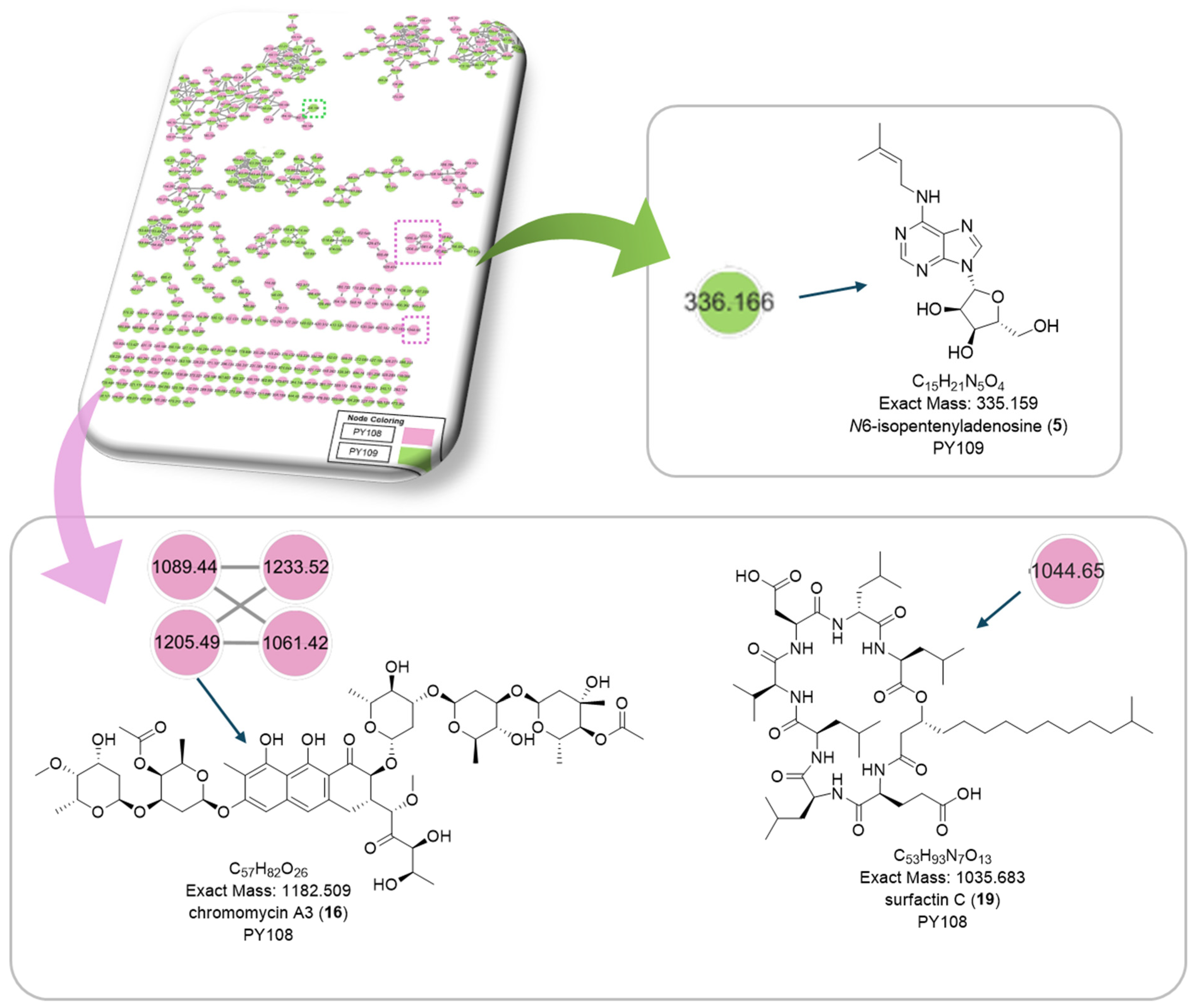
| C.N | Annotated Compound | Exact Mass m/z | Observed Mass m/z | Detected Ion | Molecular Formula | RDBE | Absolute Error (ppm) | Retention Time (min) | Sources | CSI:FingerID Score (%) | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Maculosin | 260.115 | 261.124 | [M+H]+ | C14H16N2O3 | 8.0 | 3.25 | 3.49 | PY109 PY108 (3.50) | 92.41 | [33] |
| 2 | Cyclo-(d-Pro-l-Val) | 196.121 | 197.129 | [M+H]+ | C10H16N2O2 | 4.0 | 4.06 | 3.76 | PY109 PY108 (3.38) | 95.52 | [34] |
| 3 | Cyclo(2-hydroxy-Pro-R-Leu) | 226.131 | 227.139 | [M+H]+ | C11H18N2O3 | 4.0 | 2.04 | 3.98 | PY109 | - | [35] |
| 4 | Cyclo(Tyr-Leu) | 276.147 | 277.155 | [M+H]+ | C15H20N2O3 | 7.0 | 2.98 | 4.25 | PY109 PY108 (4.21) | 78.98 | [36] |
| 5 | GancidinW Or, Cyclo-(d-Leu- l-Pro) | 210.136 | 211.145 | [M+H]+ | C11H18N2O2 | 4.0 | 3.90 | 4.43 | PY109 PY108 (4.41) | 98.69 | [37] |
| 6 | Cyclo-(d-Phe-l-Pro) | 244.120 | 245.129 | [M+H]+ | C14H16N2O2 | 8.0 | 2.05 | 4.93 | PY109 PY108 (4.98) | 100 | [38] |
| 7 | Cyclo-(l-Val-l-Leu) | 212.152 | 213.161 | [M+H]+ | C11H20N2O2 | 3.0 | 4.35 | 5.52 | PY109 PY108 (5.50) | 75.92 | [39] |
| 8 | Brevianamide F | 283.131 | 284.139 | [M+H]+ | C16H17N3O2 | 10.0 | 0.47 | 5.56 | PY109 PY108 (5.57) | 100 | [40] |
| 9 | Cyclo-(l-Valyl-Phenylalanyl) Or, Cyclo-(Phe-Val) | 246.136 | 247.144 | [M+H]+ | C14H18N2O2 | 7.0 | 1.46 | 6.02 | PY109 PY108 (5.98) | 88.89 | [41] |
| 10 | Cyclo-(d-Leu-l-Trp) | 299.163 | 300.170 | [M+H]+ | C17H21N3O2 | 9.0 | 0.58 | 6.43 | PY109 | 86.94 | [42] |
| 11 | Cyclo-(l-Leucyl-l-Leucyl) | 226.167 | 227.176 | [M+H]+ | C12H22N2O2 | 3.0 | 2.06 | 6.47 | PY109 PY108 (6.42) | 77.84 | [43,44] |
| 12 | Cyclo-(l-Leucyl-l-Phenylalanyl) | 260.152 | 261.160 | [M+H]+ | C15H20N2O2 | 7.0 | 0.83 | 6.84 | PY109 | 94.47 | [44] |
| 13 | N-Lauryldiethanolamine | 273.26 | 274.274 | [M+H]+ | C16H35NO2 | 0.0 | 1.63 | 10.28 | PY109 | 99.17 | [45] |
| 14 | N-Acetyltyramine | 179.094 | 180.102 | [M+H]+ | C10H13NO2 | 5.0 | 2.23 | 3.86 | PY108 | 69.47 | [46] |
| 15 | N-Phenethylacetamide | 163.099 | 164.107 | [M+H]+ | C10H13NO | 5.0 | 2.72 | 5.93 | PY108 | 86.52 | [47] |
| 16 | Cyclo-(l-Trp-l-Phe) | 333.147 | 334.155 | [M+H]+ | C20H19N3O2 | 13.0 | 0.42 | 7.20 | PY108 | 96.26 | [48] |
| 17 | Chromomycin A3 (Aburamycin B) | 1182.529 | 1205.497 | [M+Na]+ | C57H82O26 | 17.0 | 1.40 | 11.46 | PY108 | - | [49] |
| 18 | Chromomycin A3-1 | 1038.431 | 1061.418 | [M+Na]+ | C50H70O23 | 16.0 | 1.65 | 11.55 | PY108 | - | [50] |
| 19 | Tetradecyldiethanolamine | 301.298 | 302.306 | [M+H]+ | C18H39NO2 | 0.0 | 1.26 | 12.00 | PY108 PY109 (12.00) | 95.83 | [51,52] |
| 20 | Demethlychromomycin A2 | 1196.525 | 1219.513 | [M+Na]+ | C58H84O26 | 17.0 | 1.37 | 12.18 | PY108 | - | [53] |
| 21 | Chromomycin A2 (Aburamycin A) | 1210.541 | 1233.526 | [M+Na]+ | C59H85O26 | 17.0 | 2.99 | 12.86 | PY108 | - | [54] |
| 22 | Chromomycin A2-1 | 1066.462 | 1067.470 | [M+H]+ | C52H74O23 | 16.0 | 0.76 | 12.86 | PY108 | - | [50] |
| 23 | N-Hexadecyl diethanolamine | 329.329 | 330.337 | [M+H]+ | C20H43NO2 | 0.0 | 0.30 | 13.59 | PY108 PY109 (13.58) | 98.33 | [52] |
| 24 | Dibutyl Phthalate | 278.152 | 279.160 | [M+H]+ | C16H22O4 | 6.0 | 2.73 | 15.81 | PY108 PY109 (15.77) | 93.68 | [55] |
| 25 | Hexadecanamide | 255.256 | 256.263 | [M+H]+ | C16H33NO | 1.0 | 2.51 | 18.44 | PY108 | 95.69 | [56] |
| 26 | Surfactin B | 1021.667 | 1022.673 | [M+H]+ | C52H91N7O13 | 11.0 | 1.24 | 20.25 | PY108 | - | [57] |
| 27 | Surfactin C | 1035.683 | 1036.688 | [M+H]+ | C53H93N7O13 | 11.0 | 2.77 | 20.79 | PY108 | - | [57] |
| C.N. | Annotated Compound | Accurate Mass (Da) | Precursor Ion | Adduct Type | MS2 Fragmentation Pattern | Molecular Formula | Retention Time (min) | Sources | Error (ppm) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Diketopiperazines derivatives | ||||||||||
| 1 | Cyclo(L-Leu-L-4-hydroxy-Pro) | 226.132 | 227.139 | [M+H]+ | 136.113, 86.060 | C11H18N2O3 | 3.78 | PY108 PY109 | 0.0 | [58] |
| 2 | Cyclo(Phe-Leu) | 260.152 | 261.159 | [M+H]+ | 120.080 | C15H20N2O2 | 3.46 | PY108 PY109 | 0.0 | [59] |
| 3 | Indoleacetic acid | 175.063 | 176.071 | [M+H]+ | 130.065, 103.054, 77.038 | C10H9NO2 | 6.33 | PY109 | 0.0 | [60] |
| 4 | Cyclo(L-Pro-L-Tyr) | 260.116 | 261.123 | [M+H]+ | 261.123, 107.049 | C14H16N2O3 | 6.76 | PY108 PY109 | 0.0 | [61] |
| 5 | N6-Isopentenyladenosine | 335.1594 | 336.167 | [M+H]+ | 204.124, 148.061, 136.062 | C15H21N5O4 | 5.11 | PY109 | 2.9 | [62] |
| 6 | Isorugulosuvine | 333.147 | 334.155 | [M+H]+ | 130.064 | C20H19N3O2 | 7.19 | PY108 PY109 | 3.0 | [63] |
| 7 | 10β-Hydroxy-3-isobutyl-6,10β,11,11a-tetrahydro-2H-pyrazino[1′,2′:1,5]pyrrolo[2,3-b]indole-1,4(3H,5aH)-dione | 315.158 | 316.166 | [M+H]+ | 298.155, 130.064 | C17H21N3O3 | 6.20 | PY108 PY109 | 3.0 | [64] |
| 8 | Brevianamide F | 283.132 | 284.139 | [M+H]+ | 130.064 | C16H17N3O2 | 5.36 | PY108 PY109 | 3.5 | [65] |
| 9 | Dibutyl phthalate | 278.152 | 279.159 | [M+H]+ | 149.023, 121.028, 65.038 | C16H22O4 | 15.76 | PY108 PY109 | 3.6 | [66] |
| 10 | Cyclo(Phe-4-hydroxy-Pro) | 260.116 | 261.124 | [M+H]+ | 170.077, 120.080, 86.060, 68.049 | C14H16N2O3 | 4.23 | PY108 PY109 | 3.8 | [67] |
| 11 | Cyclo(L-Phe-L-Pro) | 244.121 | 245.130 | [M+H]+ | 154.072, 120.080, 98.059, 70.065 | C14H16N2O2 | 5.22 | PY108 PY109 | 8.1 | [68] |
| 12 | N-[2-(1H-Indol-3-yl)ethyl]acetamide | 202.111 | 203.118 | [M+H]+ | 144.081 | C12H14N2O | 6.32 | PY108 | 4.8 | [69] |
| 13 | 3-(2-Hydroxyethyl)indole | 161.084 | 144.081 | [M+H-H2O]+ | 155.060, 143.072, 115.054, 91.054 | C10H11NO | 6.33 | PY108 | 6.8 | [70] |
| 14 | Pyridoxine | 169.074 | 170.081 | [M+H]+ | 134.060, 106.065, 77.038, 65.038 | C8H11NO3 | 1.09 | PY108 PY109 | 0.0 | [71] |
| 15 | Indole-3-carbinol | 147.068 | 130.066 | [M+H-H2O]+ | 103.056, 95.050, 77.038, 51.045 | C9H9NO | 5.88 | PY108 PY109 | 7.62 | [72] |
| Aureolic acid derivatives | ||||||||||
| 16 | Chromomycin A3 | 1182.509 | 1205.5 | [M+Na]+ | 889.346, 469.204, 357.151 | C57H82O26 | 11.49 | PY108 | 4.15 | [73] |
| Lipopeptides and N-Acylethanolamines | ||||||||||
| 17 | Surfactin C | 1035.683 | 1036.690 | [M+H]+ | 685.449, 596.426, 554.354, 441.270 | C53H93N7O13 | 20.20 | PY108 | 8.6 | [74] |
| 18 | N-Lauryldiethanolamine | 273.266 | 274.274 | [M+H]+ | 88.075, 70.065, 57.069 | C16H35NO2 | 10.27 | PY108 PY109 | 0.0 | [75] |
| 19 | N-Tetradecyldiethanolamine | 301.298 | 302.305 | [M+H]+ | 88.075, 70.065, 57.069 | C18H39NO2 | 10.01 | PY108 PY109 | 0.0 | [52] |
| 20 | N-Lauroylethanolamine | 243.219 | 244.227 | [M+H]+ | 81.069, 67.054, 62.059, 57.069 | C14H29NO2 | 12.95 | PY109 | 0.0 | [76] |
| 21 | 13-Docosenamide | 337.3345 | 338.342 | [M+H]+ | 149.132, 121.101, 97.101, 69.070 | C22H43NO | 11.22 | PY108 PY109 | 2.9 | [77] |
| 22 | Palmitoyl ethanolamide | 299.2824 | 300.289 | [M+H]+ | 300.288, 283.264, 62.060 | C18H37NO2 | 17.94 | PY108 PY109 | 3.3 | [78] |
| 23 | Leu-C14:0 | 341.293 | 342.299 | [M+H]+ | 132.101, 86.096 | C20H39NO3 | 17.48 | PY108 PY109 | 2.85 | [79] |
| 24 | Leu-C15:0 | 355.309 | 356.316 | [M+H]+ | 132.101, 86.096 | C20H39NO3 | 19.02 | PY108 PY109 | 0.0 | [79] |
| C.N | Name of Volatile Compounds | Retention Time (min) | Molecular Formula | Sources |
|---|---|---|---|---|
| 1 | 2-Propenoic acid | 7.92 | C17H32O2 | PY108 |
| 2 | Benz[e]azulene-3,8-dione | 10.00 | C19H24O6 | PY108 |
| 3 | Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-(2-methyl propyl)- | 11.07 | C11H18N2O2 | PY108 |
| 4 | Actinomycin C2 Or Actinomycin D | 11.69 | C63H88N12O16 | PY108 |
| 5 | 1,9-Dioxacyclohexadeca-4,13-diene-2-10-dione, 7,8,15,16-tetramethyl- | 13.39 | C18H28O4 | PY108 |
| 6 | 1-Phenyl-3,6-diazahomoadamantane | 17.63 | C15H20N2 | PY108 |
| 7 | Ergotaman-3′,6′,18-trione, | 18.61 | C33H35N5O5 | PY108 |
| 8 | Ethyl iso-allocholate | 21.90 | C26H44O5 | PY108 |
| 9 | γ-Sitosterol | 25.25 | C29H50O | PY108 |
| 10 | 1H-Cyclopropa[3,4]benz[1,2-e]azulene-4a,5,7b,9,9a(1aH)-pentol | 26.82 | C28H38O10 | PY108 |
| 11 | 3′H-Cycloprop(1,2)-5-cholest-1-en-3-one | 27.31 | C26H44O5 | PY108 |
| 12 | 3′H-Cycloprop(1,2)-5α-cholest-1-en-3-one,1′,1′-dicarboethoxy-1β,2β-dihydro | 27.49 | C34H54O5 | PY108 |
| 13 | 7aH-Cyclopenta[a]cyclopropa[f]cycloundecene-2,4,7,7a,10,11-hexol | 29.69 | C30H44O11 | PY108 |
| 14 | Carda-16,20(22)-dienolide | 31.09 | C30H36O11 | PY108 |
| 15 | D-Homo-24-nor-17-oxachola-20,22-dien16-one, | 32.39 | C32H42O10 | PY108 |
| 16 | Pyrrolizin-1-one, 7-propyl- | 14.17 | C10H17NO | PY109 |
| 17 | 2,4-Di-tert-butylphenol | 15.29 | C14H22O | PY109 |
| 18 | Cetene | 17.12 | C16H32 | PY109 |
| 19 | Benzophenone | 17.91 | C13H10O | PY109 |
| 20 | 8-Pentadecanone | 18.91 | C15H30O | PY109 |
| 21 | 1,4-diazabicyclo[4.3.0]nonan-2,5-dione, 3-methyl | 19.61 | C8H12N2O2 | PY109 |
| 22 | Uric Acid | 20.12 | C5H4N4O3 | PY109 |
| 23 | Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro- | 20.52 | C7H10N2O2 | PY109 |
| 24 | Imidazole-4-carboxylic acid, 2-fluoro-1-methoxymethyl-, ethyl ester | 20.89 | C8H11N2 | PY109 |
| 25 | 3,4-Methylenedioxyamphetamine | 21.06 | C10H13NO2 | PY109 |
| 26 | E-15-Heptadecenal | 21.46 | C17H32O | PY109 |
| 27 | Cyclo(L-Pro-L-Val) | 21.94 | C10H16N2O2 | PY109 |
| 28 | 7-Ethylpentadecane-4,6-dione | 22.74 | C17H32O2 | PY109 |
| 29 | 10-Nonadecanone | 23.35 | C19H38O | PY109 |
| 30 | Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-(2-methylpropyl)- | 24.33 | C11H18N2O2 | PY109 |
| 31 | Dibutyl Phthalate | 25.83 | C16H22O4 | PY109 |
| 32 | 1-Henicosyl formate | 26.66 | C22H44O2 | PY109 |
| 33 | dl-Alanyl-dl-phenylalanine | 30.09 | C12H16N2O3 | PY109 |
| 34 | 2,5-Piperazinedione, 3,6-bis(2-methylpropyl)- | 30.60 | C12H22N2O2 | PY109 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, R.P.; Huo, C.; Budhathoki, R.; Budthapa, P.; Bhattarai, B.R.; Rana, M.; Kim, K.H.; Parajuli, N. Antibacterial, Antifungal, and Cytotoxic Effects of Endophytic Streptomyces Species Isolated from the Himalayan Regions of Nepal and Their Metabolite Study. Biomedicines 2024, 12, 2192. https://doi.org/10.3390/biomedicines12102192
Yadav RP, Huo C, Budhathoki R, Budthapa P, Bhattarai BR, Rana M, Kim KH, Parajuli N. Antibacterial, Antifungal, and Cytotoxic Effects of Endophytic Streptomyces Species Isolated from the Himalayan Regions of Nepal and Their Metabolite Study. Biomedicines. 2024; 12(10):2192. https://doi.org/10.3390/biomedicines12102192
Chicago/Turabian StyleYadav, Ram Prabodh, Chen Huo, Rabin Budhathoki, Padamlal Budthapa, Bibek Raj Bhattarai, Monika Rana, Ki Hyun Kim, and Niranjan Parajuli. 2024. "Antibacterial, Antifungal, and Cytotoxic Effects of Endophytic Streptomyces Species Isolated from the Himalayan Regions of Nepal and Their Metabolite Study" Biomedicines 12, no. 10: 2192. https://doi.org/10.3390/biomedicines12102192
APA StyleYadav, R. P., Huo, C., Budhathoki, R., Budthapa, P., Bhattarai, B. R., Rana, M., Kim, K. H., & Parajuli, N. (2024). Antibacterial, Antifungal, and Cytotoxic Effects of Endophytic Streptomyces Species Isolated from the Himalayan Regions of Nepal and Their Metabolite Study. Biomedicines, 12(10), 2192. https://doi.org/10.3390/biomedicines12102192









