Luteinizing Hormone Surge-Induced Krüppel-like Factor 4 Inhibits Cyp17A1 Expression in Preovulatory Granulosa Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Reagents
2.2. Preparation of Granulosa Cells
2.3. Plasmid Constructs
2.4. Transient Transfection of Granulosa Cells
2.5. Real-Time Quantitative PCR
2.6. Western Blot Analysis
2.7. Assessment of Progesterone Production
2.8. Luciferase Assays
2.9. Data Analysis
3. Results
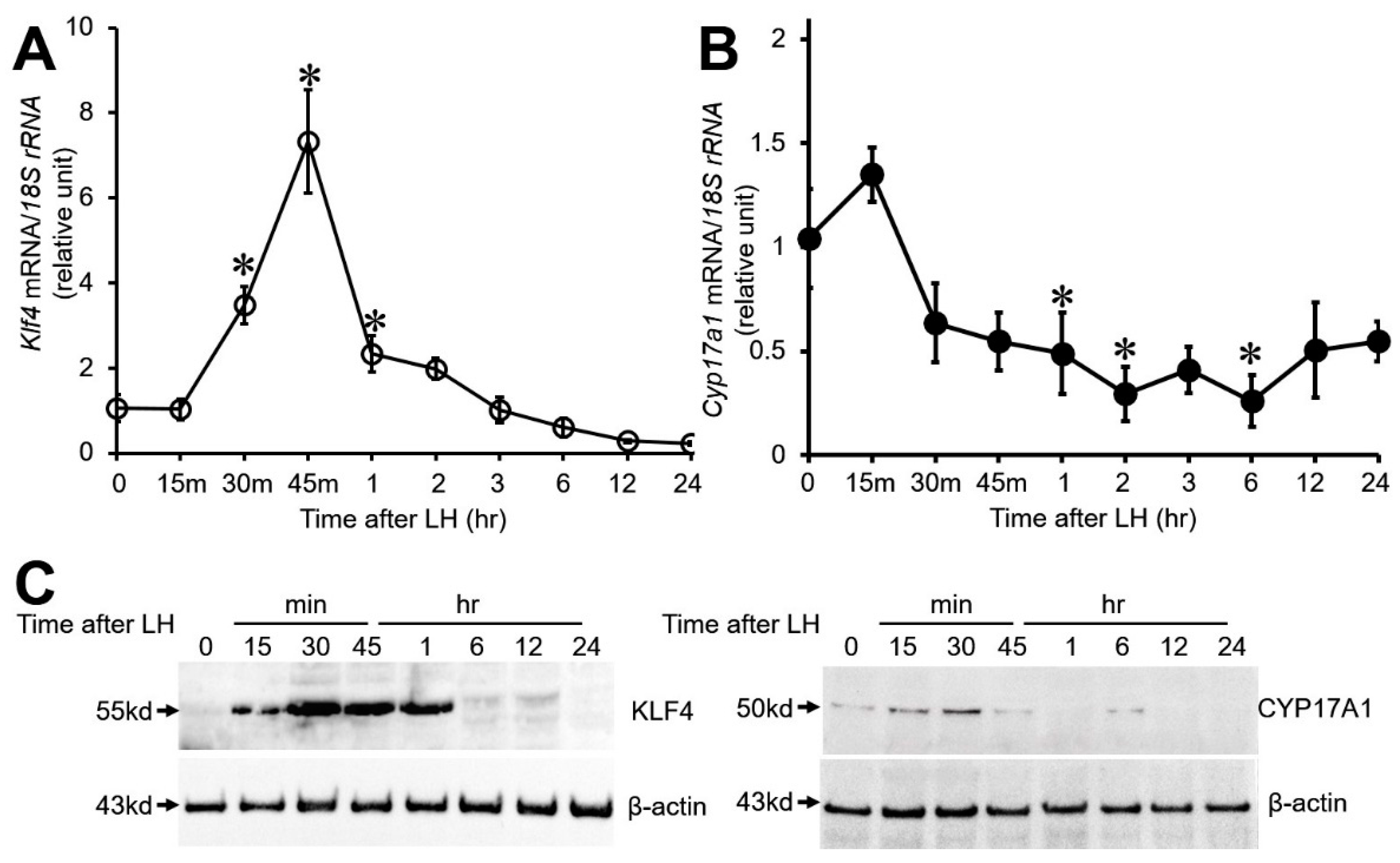
3.1. Effect of LH on Klf4 and Cyp17A1 Expression in Cultured Preovulatory GCs
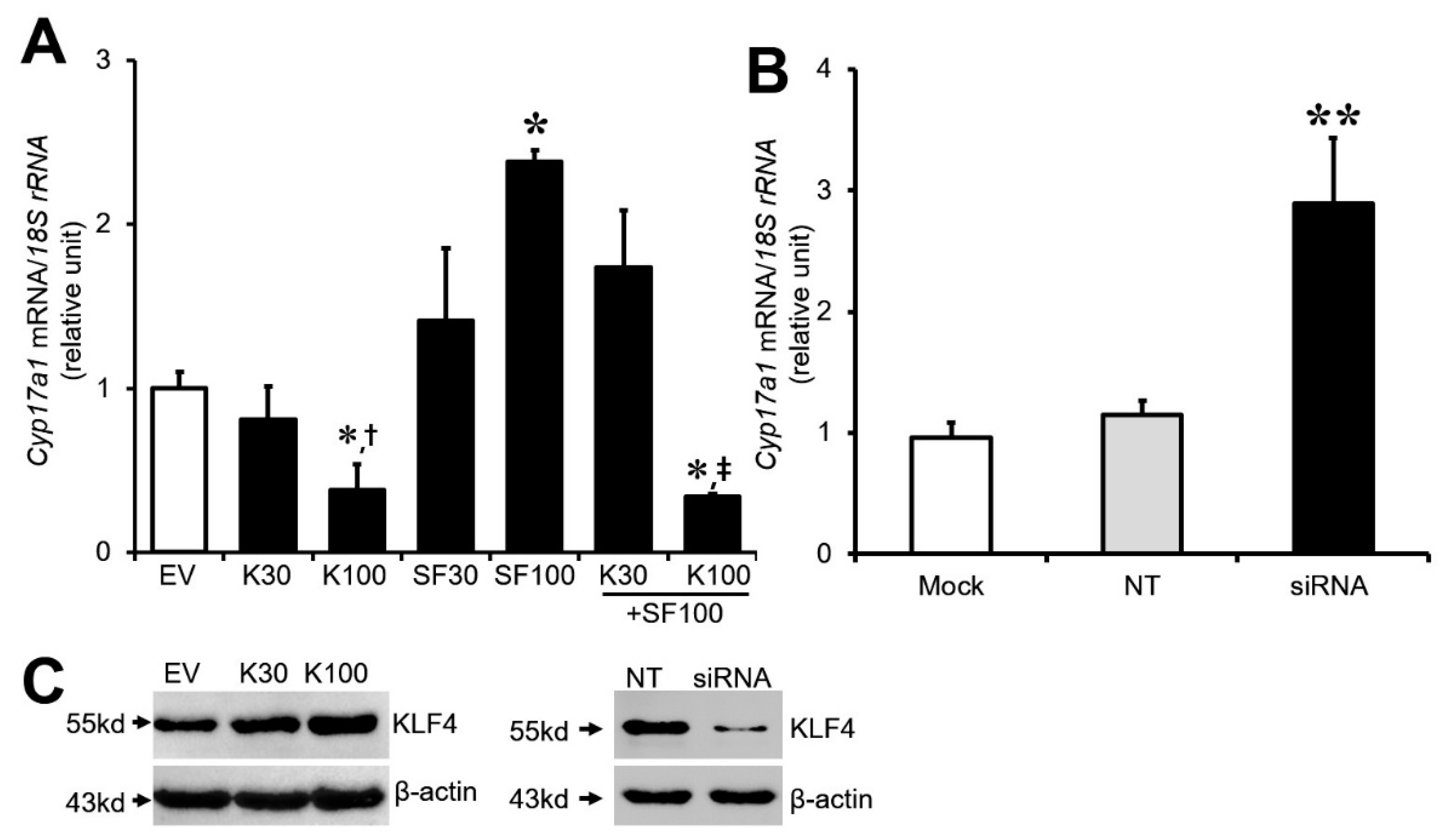
3.2. Effect of Klf4 on Basal and Sf1-Mediated Cyp17A1 Gene Expression in Cultured GCs
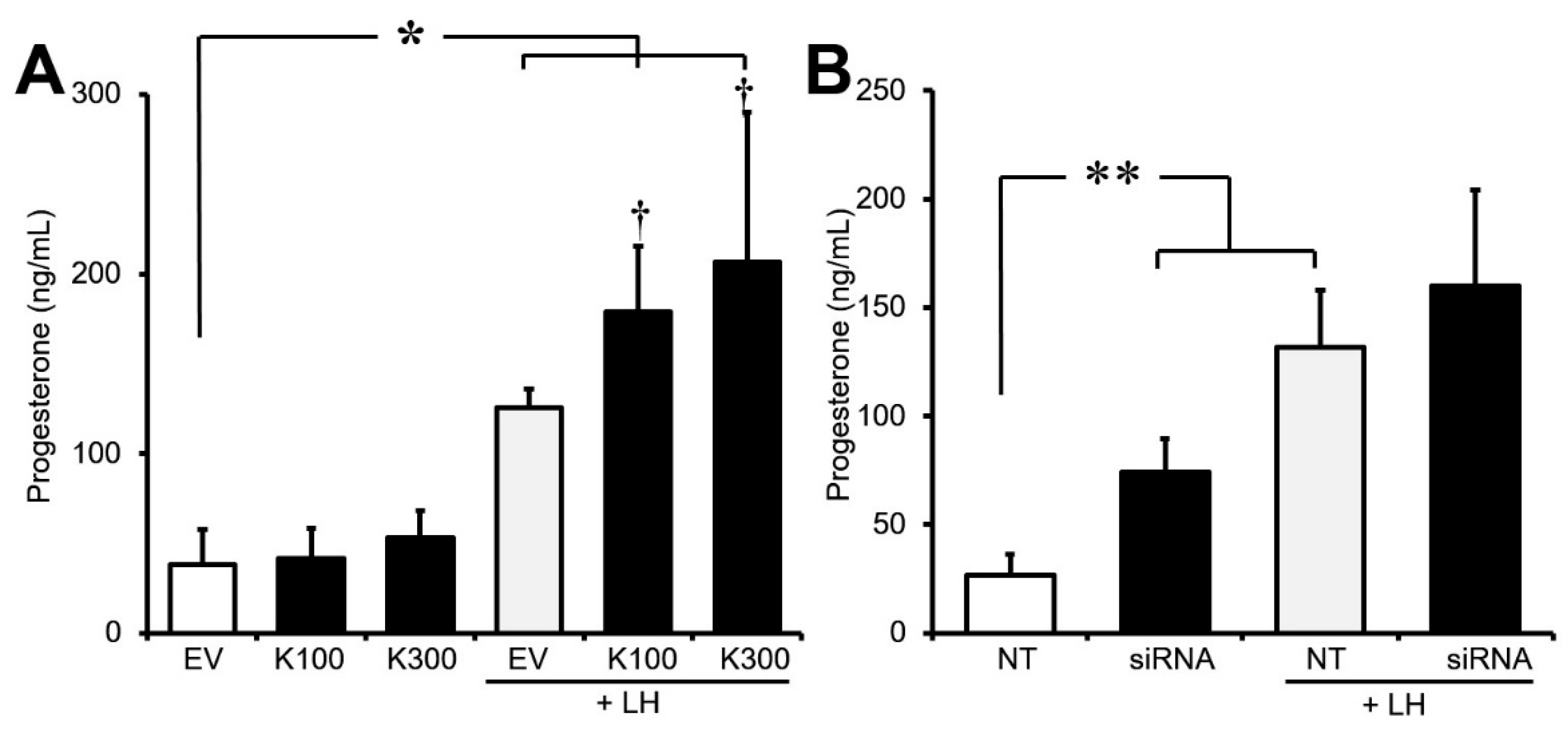
3.3. Effect of Klf4 on Basal and LH-Stimulated Progesterone Production in Cultured GCs
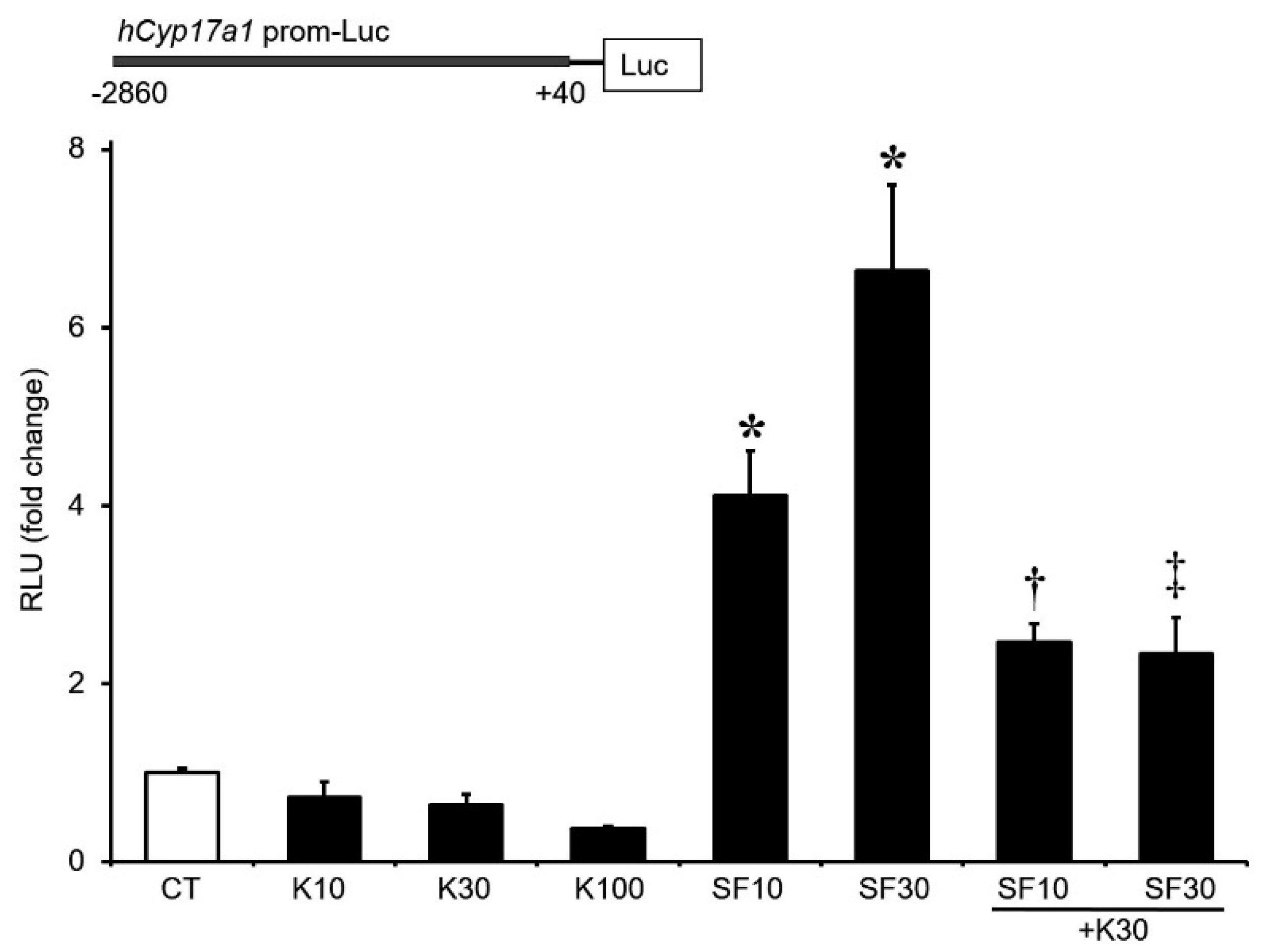
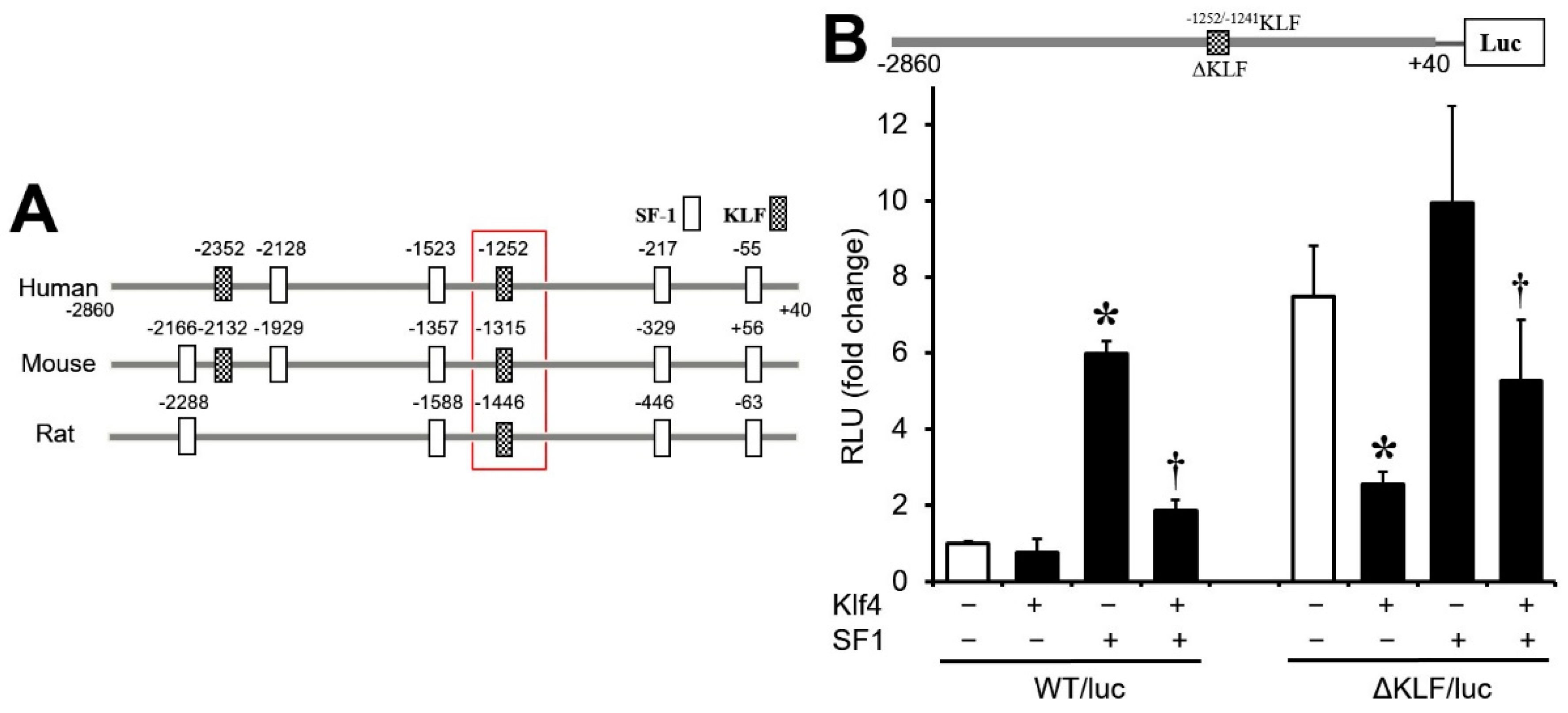
3.4. Regulation of Cyp17A1 Promoter Activity by Klf4
3.5. Sp1/Klf4-Binding Sequences Are Not Involved in Klf4 Repression of Cyp17A1
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hsueh, A.; Jones, P.; Adashi, E.; Wang, C.; Zhuang, L.-Z.; Welsh, T. Intraovarian mechanisms in the hormonal control of granulosa cell differentiation in rats. Reproduction 1983, 69, 325–342. [Google Scholar] [CrossRef]
- Murphy, B.D. Models of luteinization. Biol. Reprod. 2000, 63, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Christenson, L.K.; Gunewardena, S.; Hong, X.; Spitschak, M.; Baufeld, A.; Vanselow, J. Research resource: Preovulatory LH surge effects on follicular theca and granulosa transcriptomes. Mol. Endocrinol. 2013, 27, 1153–1171. [Google Scholar] [CrossRef] [PubMed]
- Espey, L.L.; Richards, J.S. Temporal and spatial patterns of ovarian gene transcription following an ovulatory dose of gonadotropin in the rat. Biol. Reprod. 2002, 67, 1662–1670. [Google Scholar] [CrossRef] [PubMed]
- Rajapaksha, W.; McBride, M.; Robertson, L.; O’shaughnessy, P. Sequence of the bovine HDL-receptor (SR-BI) cDNA and changes in receptor mRNA expression during granulosa cell luteinization in vivo and in vitro. Mol. Cell. Endocrinol. 1997, 134, 59–67. [Google Scholar] [CrossRef]
- Rodgers, R.; Waterman, M.; Simpson, E. Cytochromes P-450scc, P-45017 α, Adrenodoxin, and Reduced Nicotinamide Adenine Dinucleotide Phosphate-Cytochrome P-450 Reductase in Bovine Follicles and Corpora Lutea. Changes in Specific Contents during the Ovarian Cycle. Endocrinology 1986, 118, 1366–1374. [Google Scholar] [CrossRef]
- Fitzpatrick, S.L.; Carlone, D.L.; Robker, R.L.; Richards, J.S. Expression of aromatase in the ovary: Down-regulation of mRNA by the ovulatory luteinizing hormone surge. Steroids 1997, 62, 197–206. [Google Scholar] [CrossRef]
- Hickey, G.J.; Krasnow, J.S.; Beattie, W.G.; Richards, J.S. Aromatase cytochrome P450 in rat ovarian granulosa cells before and after luteinization: Adenosine 3′, 5′-monophosphate-dependent and independent regulation. Cloning and sequencing of rat aromatase cDNA and 5′ genomic DNA. Mol. Endocrinol. 1990, 4, 3–12. [Google Scholar] [CrossRef]
- Carletti, M.Z.; Christenson, L.K. Rapid effects of luteinizing hormone on gene expression in the mural granulosa cells of mouse preovulatory follicles. Reproduction 2009, 137, 843. [Google Scholar] [CrossRef]
- Aflalo, L.; Meidan, R. The hormonal regulation of cholesterol side-chain cleavage cytochrome P450, adrenodoxin, and their messenger ribonucleic acid expression in bovine small-like and large-like luteal cells: Relationship with progesterone production. Endocrinology 1993, 132, 410–416. [Google Scholar] [CrossRef]
- Conley, A.J.; Kaminski, M.A.; Dubowsky, S.A.; Ablonka-Shariff, A.; Redmer, D.A.; Reynolds, L.P. Immunohistochemical localization of 3β-hydroxysteroid dehydrogenase and P450 17α-hydroxylase during follicular and luteal development in pigs, sheep, and cows. Biol. Reprod. 1995, 52, 1081–1094. [Google Scholar] [CrossRef] [PubMed]
- Nimz, M.; Spitschak, M.; Fürbass, R.; Vanselow, J. The pre-ovulatory luteinizing hormone surge is followed by down-regulation of CYP19A1, HSD3B1, and CYP17A1 and chromatin condensation of the corresponding promoters in bovine follicles. Mol. Reprod. Dev. 2010, 77, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- Jo, M.; Gieske, M.C.; Payne, C.E.; Wheeler-Price, S.E.; Gieske, J.B.; Ignatius, I.V.; Curry, T.E.; Ko, C. Development and application of a rat ovarian gene expression database. Endocrinology 2004, 145, 5384–5396. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oktem, O.; Akin, N.; Bildik, G.; Yakin, K.; Alper, E.; Balaban, B.; Urman, B. FSH Stimulation promotes progesterone synthesis and output from human granulosa cells without luteinization. Hum. Reprod. 2017, 32, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Komar, C.; Berndtson, A.; Evans, A.; Fortune, J. Decline in circulating estradiol during the preovulatory period is correlated with decreases in estradiol and androgen, and in messenger RNA for P450 aromatase and P450 17α-hydroxylase, in bovine preovulatory follicles. Biol. Reprod. 2001, 64, 1797–1805. [Google Scholar] [CrossRef] [PubMed]
- Magoffin, D.A.; Weitsman, S.R. Differentiation of ovarian theca-interstitial cells in vitro: Regulation of 17 alpha-hydroxylase messenger ribonucleic acid expression by luteinizing hormone and insulin-like growth factor-I. Endocrinology 1993, 132, 1945–1951. [Google Scholar] [CrossRef]
- Garrett-Sinha, L.A.; Eberspaecher, H.; Seldin, M.F.; de Crombrugghe, B. A gene for a novel zinc-finger protein expressed in differentiated epithelial cells and transiently in certain mesenchymal cells. J. Biol. Chem. 1996, 271, 31384–31390. [Google Scholar] [CrossRef]
- Choi, H.; Roh, J. LH-induced transcriptional regulation of KLF4 expression in granulosa cells occurs via the camp/pka pathway and requires a putative sp1 binding site. Int. J. Mol. Sci. 2020, 21, 7385. [Google Scholar] [CrossRef]
- Choi, H.; Ryu, K.-Y.; Roh, J. Krüppel-like factor 4 plays a role in the luteal transition in steroidogenesis by downregulating Cyp19A1 expression. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E1071–E1080. [Google Scholar] [CrossRef]
- Jansen, E.; Laven, J.S.; Dommerholt, H.B.; Polman, J.; van Rijt, C.; van den Hurk, C.; Westland, J.; Mosselman, S.; Fauser, B.C. Abnormal gene expression profiles in human ovaries from polycystic ovary syndrome patients. Mol. Endocrinol. 2004, 18, 3050–3063. [Google Scholar] [CrossRef]
- Wickenheisser, J.K.; Quinn, P.G.; Nelson, V.L.; Legro, R.S.; Strauss, J.F., III; McAllister, J.M. Differential activity of the cytochrome P450 17α-hydroxylase and steroidogenic acute regulatory protein gene promoters in normal and polycystic ovary syndrome theca cells. J. Clin. Endocrinol. Metab. 2000, 85, 2304–2311. [Google Scholar] [CrossRef] [PubMed]
- Borroni, R.; Liu, Z.; Simpson, E.R.; Hinshelwood, M.M. A putative binding site for Sp1 is involved in transcriptional regulation of CYP17 gene expression in bovine ovary. Endocrinology 1997, 138, 2011–2020. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Natesampillai, S.; Kerkvliet, J.; Leung, P.C.; Veldhuis, J.D. Regulation of Kruppel-like factor 4, 9, and 13 genes and the steroidogenic genes LDLR, StAR, and CYP11A in ovarian granulosa cells. Am. J. Physiol.-Endocrinol. Metab. 2008, 294, E385–E391. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.S.; Beshay, V.E.; Escobar, J.C.; Carr, B.R. 17α-Hydroxylase (CYP17) expression and subsequent androstenedione production in the human ovary. Reprod. Sci. 2010, 17, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Shields, J.M.; Yang, V.W. Identification of the DNA sequence that interacts with the gut-enriched Krüppel-like factor. Nucleic Acids Res. 1998, 26, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Ikeda, Y.; Parker, K.L. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell 1994, 77, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Parker, K.L.; Rice, D.A.; Lala, D.S.; Ikeda, Y.; Luo, X.; Wong, M.; Bakke, M.; Zhao, L.; Frigeri, C.; Hanley, N.A.; et al. Steroidogenic factor 1: An essential mediator of endocrine development. Recent Prog. Horm. Res. 2002, 57, 19–36. [Google Scholar] [CrossRef] [PubMed]
- Falender, A.E.; Lanz, R.; Malenfant, D.; Belanger, L.; Richards, J.S. Differential expression of steroidogenic factor-1 and FTF/LRH-1 in the rodent ovary. Endocrinology 2003, 144, 3598–3610. [Google Scholar] [CrossRef]
- Lomberk, G.; Urrutia, R. The family feud: Turning off Sp1 by Sp1-like KLF proteins. Biochem. J. 2005, 392, 1–11. [Google Scholar] [CrossRef]
- Agarwal, P.; Peluso, J.J.; White, B.A. Steroidogenic factor-1 expression is transiently repressed and c-myc expression and deoxyribonucleic acid synthesis are induced in rat granulosa cells during the preovulatory period. Biol. Reprod. 1996, 55, 1271–1275. [Google Scholar] [CrossRef]
- Al-Omar, Z.; Ozbakir, B.; Tulay, P. Differential expression of genes involved in steroidogenesis pathway in human oocytes obtained from patients with polycystic ovaries. J. Reprod. Immunol. 2020, 142, 103191. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Feng, H.L. Extra-and intra-ovarian factors in polycystic ovary syndrome: Impact on oocyte maturation and embryo developmental competence. Hum. Reprod. Update 2011, 17, 17–33. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.; Lee, O.; Ryu, K.; Roh, J. Luteinizing Hormone Surge-Induced Krüppel-like Factor 4 Inhibits Cyp17A1 Expression in Preovulatory Granulosa Cells. Biomedicines 2024, 12, 71. https://doi.org/10.3390/biomedicines12010071
Choi Y, Lee O, Ryu K, Roh J. Luteinizing Hormone Surge-Induced Krüppel-like Factor 4 Inhibits Cyp17A1 Expression in Preovulatory Granulosa Cells. Biomedicines. 2024; 12(1):71. https://doi.org/10.3390/biomedicines12010071
Chicago/Turabian StyleChoi, Yuri, Okto Lee, Kiyoung Ryu, and Jaesook Roh. 2024. "Luteinizing Hormone Surge-Induced Krüppel-like Factor 4 Inhibits Cyp17A1 Expression in Preovulatory Granulosa Cells" Biomedicines 12, no. 1: 71. https://doi.org/10.3390/biomedicines12010071
APA StyleChoi, Y., Lee, O., Ryu, K., & Roh, J. (2024). Luteinizing Hormone Surge-Induced Krüppel-like Factor 4 Inhibits Cyp17A1 Expression in Preovulatory Granulosa Cells. Biomedicines, 12(1), 71. https://doi.org/10.3390/biomedicines12010071




