1. Introduction
Multiple myeloma (MM) is hematological malignancy characterized by indefinite proliferation of plasma cells in bone marrow. It accounts for 1% of all cancers and approximately 13% of all hematologic malignancies [
1,
2]. MM patients are commonly treated with proteosome inhibitors such as bortezomib, immunomodulatory agents such as lenalidomide, and monoclonal antibodies such as daratumumab [
3]. Among these, bortezomib is approved by the FDA for multiple myeloma. Bortezomib therapy had a significant impact on clinical activity in the treatment of relapsed/refractory multiple myeloma. [
4]. Bortezomib reversibly inhibits chymotrypsin-like activity at the β5-subunit and, to a lesser extent, inhibits trypsin-like activity at the β1-subunit. It can also inhibit the post-glutamyl peptide-hydrolyzing activities of the 26S proteasome. Since its introduction, bortezomib has improved the survival and quality of life for patients with newly diagnosed MM. However, most patients eventually relapse [
5]. Therefore, more effective therapies are highly needed.
Immunotherapy has recently revolutionized cancer treatment. It constitutes the fourth cornerstone of cancer therapy after surgery, radiation, and chemotherapy. Current immunotherapy explores and harnesses every aspect of the immune system. Immunotherapy using immune checkpoint inhibitors (ICIs) and adoptive cell therapy (ACT) using chimeric antigen receptor (CAR) immune cells are among the most successful immunotherapies [
6]. As a result, chimeric antigen receptor T (CAR-T) cells have shown clinical efficacy against numerous hematological malignancies. So far, five CAR T cells have been approved by the FDA [
7]. However, many factors contribute to the failure of CAR T-cell therapy, and CAR NK-cell therapy is emerging.
One major advantage of CAR NK cells over CAR T cells is the source of immune cells. CAR NK therapy utilizes “off-the-shelf” ready-to-use CAR NK cells that can be manufactured through mass production and infused to patients at any time. The second major advantage of using CAR NK cells over CAR T cells is that activation of CAR T cells can lead to massive release of inflammatory cytokines which can cause cytokine release syndrome (CRS) and neurotoxicity, whereas the activation of CAR NK cells releases inflammatory cytokines but does not cause CRS [
5].
Natural killer (NK) cells play a key role in immune responses to tumors by producing immunoregulatory cytokines and chemokines [
8,
9]. Recently, chemokines, a family of low-molecular-weight proteins that can act as potent mediators of inflammation, have attracted much interest as they are considered as critical factors [
10].
The NK92 cell line is an interleukin-2 (IL-2)-dependent immortalized cell line that has the characteristics of highly active blood natural killer (NK) cells. It is being used in immunology research related to cancer. [
11]. Additionally, the safety and efficacy of NK92 cells have been established in clinical trials through clinical responses observed in some cancer patients treated with NK92 [
12]. CAR-engineered NK92 cell therapy has various theoretical advantages, including it being an ”Off the shelf” product and immediate availability and lower treatment cost.
In recent years, immune-based therapies such as chimeric antigen receptor (CAR)-engineered immune cells have emerged as a new treatment for MM. One potential target in CAR cell therapy for treatment of MM is B-cell maturation antigen (BCMA) [
13]. BCMA is known to play an important role in regulating the survival, proliferation, differentiation, and maturity of B cells in plasma cells. BCMA is a marker for the identification of hematologic malignancies, including multiple myeloma (MM), non-Hodgkin lymphoma (NHL), Hodgkin lymphoma, chronic lymphocytic leukemia, and acute B-lymphoblastic leukemia [
14].
So, in this study, we generated CAR NK92 cells armed with an anti-BCMA scFv with high antigen specificity and affinity, which was able to target BCMA. We then investigated the potential of this targeted therapy for the treatment of MM.
2. Materials and Methods
2.1. Generation and Production of BCMA CAR Lentiviral Construct
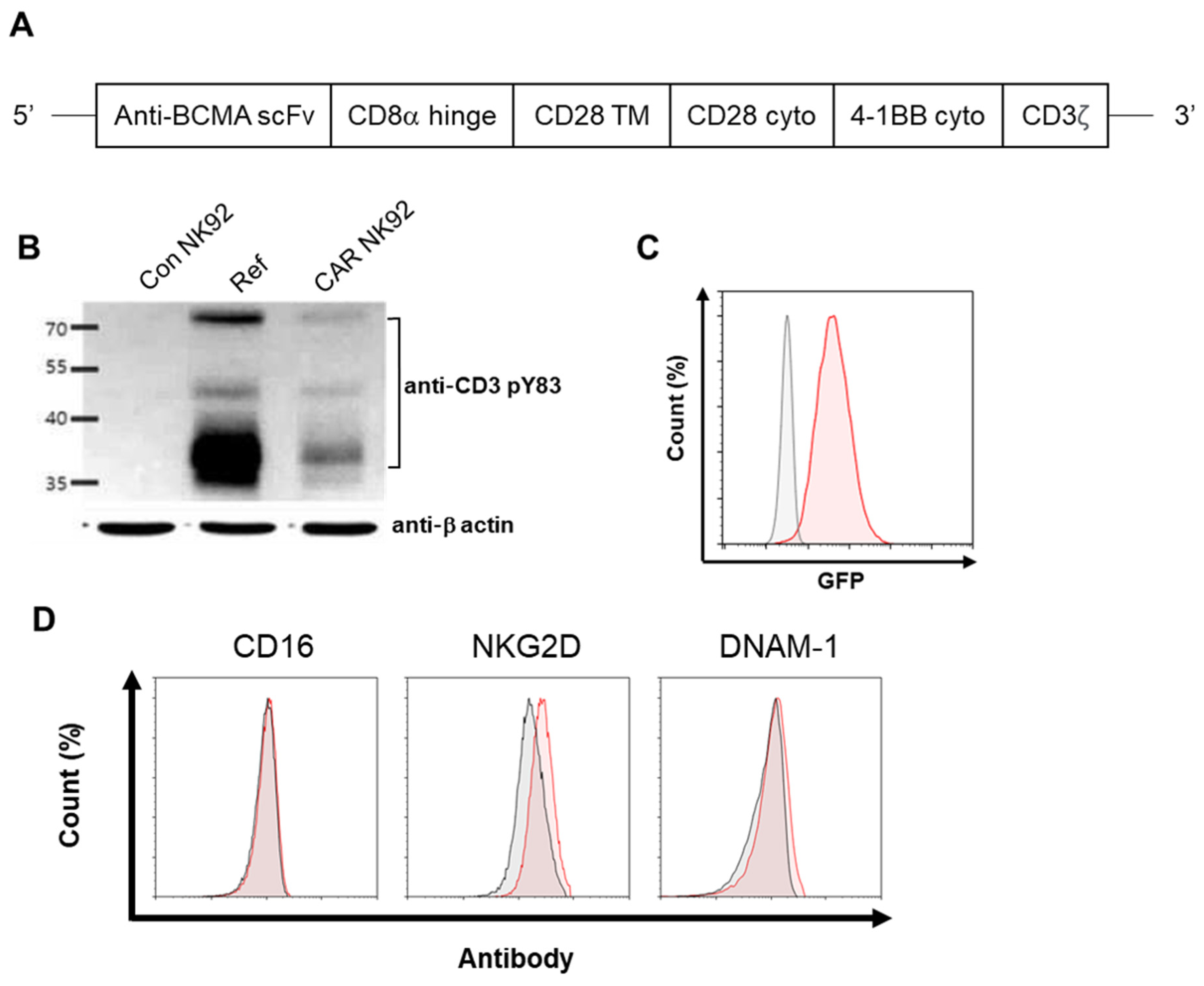
An anti-BCMA scFv was derived from DNA sequences encoding a specific monoclonal antibody against BCMA. The anti-BCMA sequence was provided by ABLbio lnc., after signing a material transfer agreement (MTA). The entire anti-BCMA scFv-CD28-4 1BB-CD3ζ fragment was then ligated into a lentiviral vector designated as pCDH-EF1-turboGFP to generate a pCDH-BCMA CAR-turboGFP construct. The lentiviral particles were produced by a commercial vendor (Lugen SCI, Inc., Bucheon, Republic of Korea).
2.2. Lentiviral Transduction of NK92 Cells
For lentivirus infection, NK92 cells were adjusted to 1 × 105 cells/mL using α-MEM with IL-2 and 2-mercaptoethanol. They were then loaded into 12-well plates followed by the addition of lentivirus at an MOI of 10 in the presence of Lenti-TD-MAX (Lugen SCI, Inc., Bucheon, Republic of Korea). Cells were then incubated for 24 h. Following this step, the infection protocol was repeated. Starting from day 1 after the second infection, transduced NK92 cells were purified using a FACS Aria II cell sorter (BD Biosciences, San Jose, CA, USA) based on expression of a GFP marker on the cell surface encoded by the vector.
2.3. Cell Culture
NK92 cells, part of a human NK-cell line, were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA) and seeded into a T75 flask (1 × 105 cells/mL) using Minimum Essential Medium (MEM) α (Welgene, Gyeongsan, Republic of Korea) supplemented with 12.5% fetal bovine serum (FBS) (Gibco, Waltham, MA, USA), 12.5% horse serum (Gibco), 1% penicillin–streptomycin (Gibco), 1 × 2-mercaptoethanol (Gibco), and 100U/mL Proleukin IL-2 (Bayer HealthCare Pharmaceuticals, Emeryville, CA, USA). The human B-lymphocyte cell lines H929, MM.1R, U266, and RPMI8226 and lymphoblast cell line K562 were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). H929, MM.1R, RPMI8226, and K562 cells were cultured using RPMI 1640 (Welgene, Republic of Korea) supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin. U266 cells were cultured using RPMI 1640 supplemented with 15% fetal bovine serum and 1% penicillin–streptomycin. All cells were incubated at 37 °C in a humidified atmosphere with 5% CO2.
2.4. Flow Cytometry Analysis of Cell Phenotype
Anti-NKG2D-APC (#130-117-718) and anti-DNAM-1-APC (#130-124-241) were purchased from Miltenyi Biotec (Auburn, CA, USA) and anti-CD16-Pacific Blue (#562874) was purchased from BD Bioscience (San Jose, CA, USA). Cells were stained with antibodies for 20 min at 4 °C. After 20 min of staining, unincorporated dye was removed by washing with 1% FBS containing PBS. Samples were then centrifuged at 1000 rpm for 3 min and the pellets were resuspended in 500 μL of 1% FBS containing PBS. Measurements were performed on a Novocyte flow cytometer (Novocyte, Agilent Technologies, Santa Clara, CA, USA).
2.5. Western Blot
Cells were harvested using a Passive Lysis Buffer (#E1941, Promega, Madison, WI, USA). Protein concentrations of cell lysates were determined using a Pierce BCA protein assay kit (#23225, Thermo Fisher Scientific, Rockford, IL, USA). Samples were resolved with 12% sodium dodecyl sulfate–PAGE gels and transferred to polyvinylidene difluoride (PVDF) membranes (Bio-Rad, Hercules, CA, USA). Membranes were blocked with a 5% nonfat milk solution in TBST buffer (20 mM Tris–HCl, pH 7.4, 500 mM NaCl, 0.1% Tween 20) for 2 h at room temperature. Blots were then incubated with CD3ζ antibodies (diluted 1:1000) (phosphor Y83, Abcam, Boston, MA, USA) in TBST overnight at room temperature. Equal loading was confirmed with an anti-β-actin (Sigma-Aldrich Co. LLC., St Louis, MO, USA). After blots were washed three times in TBST, they were incubated with an anti-rabbit secondary antibody in TBST for 1 h at room temperature. Finally, immunoblots were detected by SuperSignal® West Pico Chemiluminescent Substrate (#34580, Thermo Fisher Scientific, Waltham, MA, USA) and exposure to an image analysis system (Amersham Imager 600, GE Healthcare, Uppsala, Sweden).
2.6. Cytotoxicity
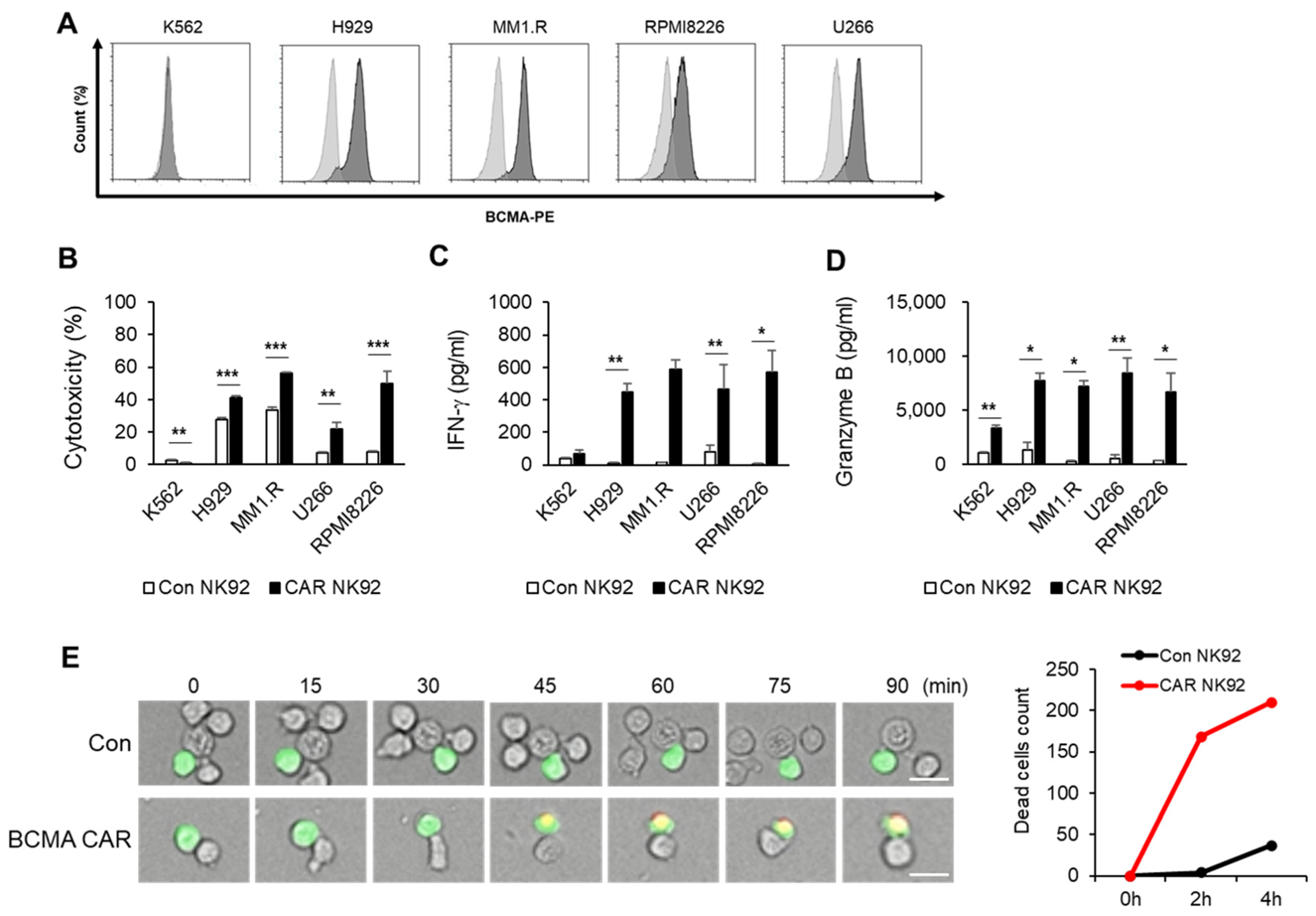
Target cells (K562, H929, MM.1R, U266, RPMI8226) were stained with a 50 nM CellTrace™ CFSE dye (#21888, Sigma-Aldrich, St Louis, MO, USA) at 37 °C for 15 min. Target cells were plated at a density of 2 × 104 cells/well in a 96-well U-bottom plate and co-cultured with effector cells (Con NK92, CAR NK92) at an E/T ratio of 1:1 for 4 h at 37 °C. After 4 h of reaction, plates were centrifuged and washed using PBS containing 1% FBS. Target cell cytotoxicity against effector cells were assessed using 7-amino-actinomycin (7-AAD) (#559925, BD Biosciences, San Jose, CA, USA). 7-AAD-positive cells were analyzed using flow cytometry (Novocyte, Agilent Technologies, Santa Clara, CA, USA). Cytotoxicity was calculated using the following equation: Co-culture 7-AAD positive (%) − Target cell 7-AAD positive (%) − effector cell 7-AAD positive (%).
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
Target cells (K562, H929, MM.1R, U266, RPMI8226) were plated into a 96-well U-bottom plate at a density of 2 × 104 cells/well and co-cultured with effector cells (Coc NK92, CAR NK92) at a ratio of 1:1 for 4 h at 37 °C. After 4 h of co-culturing, plates were centrifuged at 1000× g for 5 min and supernatants were harvested for ELISA. The concentration of IFN-γ in each well was determined using a human IFN-γ ELISA kit (#421203, Biolegend, San Diego, CA, USA). The concentration of granzyme B was determined using a human granzyme B ELISA kit (#DY2906, R&D system, Minneapolis, MN, USA). All groups were analyzed in duplicate.
2.8. Live Cell Imaging (LCI)
Target cells were stained with 5 µM CellTracker™ Green CMFDA dye (#C7025, Thermo Fisher Scientific) for 30 min at 37 °C, washed three times, and maintained with complete medium. Target cells were seeded into a 96-well plate at a density of 2 × 104 cells/well. They were then added to effector cells immediately before the start of imaging at a 1:1 E:T ratio (2 × 104 cells/well). LCI was performed at 37 °C with 5% CO2 in a total volume of 300 µL complete medium without IL-2, containing propidium iodide (Thermo Fisher Scientific) for dead cells. Time-lapse imaging was performed using an ImageXpress Nano Automated Imaging System (Molecular devices, San Jose, CA, USA). Images were acquired every 5 min for 4 h in a single z plane for transmitted light and fluorescent channels.
2.9. CD138-Positive Cell Isolation from Bone Marrow of MM Patients
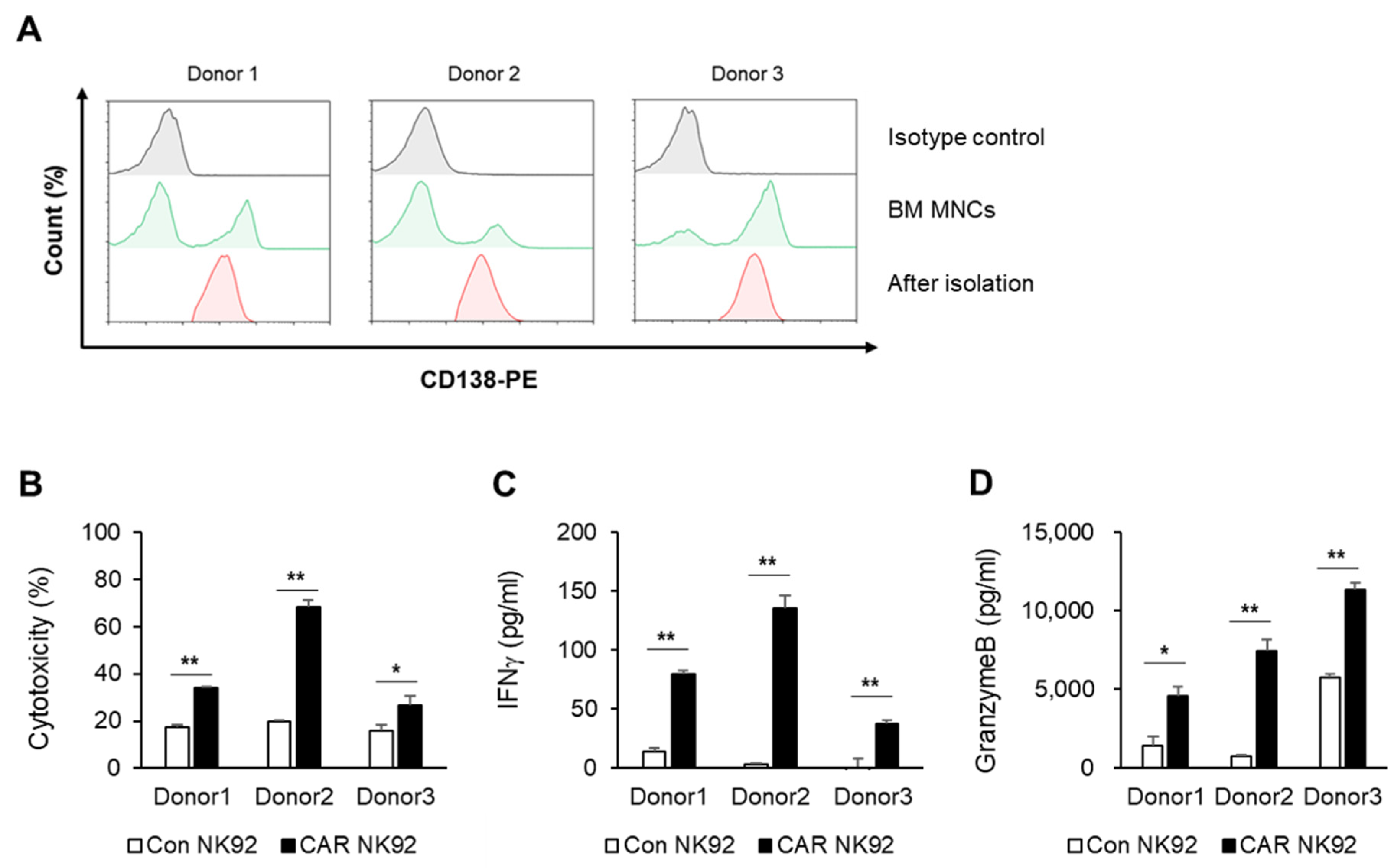
Bone marrow specimens were obtained from multiple myeloma patients at the Department of Hematology–Oncology, Chungbuk National University Hospital. This study was approved by the Medical Ethics Committee of the Chungbuk National University Hospital (IRB No. 2020-01-002-001). Human mononuclear cells were isolated from bone marrow by density gradient centrifugation (25 min, 500× g) using Ficoll–Paque medium (d = 1.078, # 17-1440-03, Cytiva, Marlborough, MA, USA) and washed twice with phosphate-buffered saline (Gibco, Waltham, MA, USA). CD138-positive cells from peripheral blood were isolated using CD138 microbeads (#130-105-961, Miltenyi Biotec, Auburn, CA, USA) according to the manufacturer’s instructions. Briefly, PBMC at a concentration of 1 × 108 cells/mL were incubated with CD138 microbeads at a titer of 1:5. After 30 min of incubation at 4 °C, cells were washed once with PBS containing 5 mm EDTA and 0.5% BSA (PBS/EDTA/BSA). After resuspending cells in 2 mL PBS/EDTA/BSA, cells were separated using two sequential MS columns (Miltenyi Biotec).
2.10. In Vivo Efficacy Studies
All protocols for animal studies were reviewed and approved by the Osong Medical Innovation Foundation Laboratory Animal Center, KBIO (Permit NO. KBIO-IACUC-2019-112). Six-week-old female NOD mice were purchased from KOATECH Co., Ltd. (Pyeongtaek, Republic of Korea). The housing conditions of the mice included a constant temperature of 23 °C with ventilation of 10–20 times per hour. Every day, 12 h of illumination and 12 h of darkness were maintained. Food and water were sterilized by high-pressure steam. Each mouse was inoculated in the right flank subcutaneously with 3 × 106 NCI-H929luc+ cells (NCI-H929 cells expressing luciferase) on day 0. After tumor cell injection, when tumor size reached 100 mm3, mice were randomly assigned into three groups, (1) an untreated group, (2) a Con NK92 group, and (3) a CAR NK92 group. At 24 h after tumor cell injection, mice were intravenously injected with 1.5 × 107 Con NK92 cells or CAR NK92 cells in 300 µL PBS or the same volume of PBS without cells. The NK92 cell treatment was repeated every three days until the experiment was terminated. Before NK92 injection, NK92 and CAR NK92 cells were irradiated with 10 Gy (X-RAD IR160, PRECISION, Madison, CT, USA). During the treatment, the tumor size and body weight of mice were monitored every three days. Tumors were assessed with a caliper at their greatest length and width to estimate tumor volume. Tumor volume was calculated using the following formula: tumor volume = (length × width)2/2. On day 18, the experiment was terminated. After mice were sacrificed, tumors were separated and their weights were measured.
2.11. Statistical Analysis
All results were confirmed with at least three independent experiments. Data from one representative experiment are shown. All data were analyzed using GraphPad Prism 5.0 software (GraphPad Inc., San Diego, CA, USA). Quantitative data are shown as means ± standard deviation. Significance of statistical analysis was determined with two-tailed, unpaired Student’s t-tests. p-values less than 0.05 were considered significant.
4. Discussion
Multiple myeloma (MM) is the second most common hematological malignancy [
1]. In a therapeutic environment of MM therapy, B-cell mature antigen (BCMA), one of the most specific and highly expressed antigens in bone marrow cells, occupies promising targets [
21]. In CAR T-cell immunotherapy, the CAR strategy targeting BCMA is known to be innovative by combining the target specificity of mAB and the cell toxicity of T cells [
22]. Most recent in March 2021, FDA approved the use of Abecma
®, a BCMA, for treating MM. However, many factors such as cost, cytokine release syndrome, and individuality of patients can contribute to failure of CAR T-cell therapy.
NK cells are a group of cytotoxic lymphocytes of the innate immune system that can mount a rapid response to non-self cells [
15]. CAR NK cells can overcome the disadvantages of CAR T cells mentioned above. Additionally, NK92 NK cells can proliferate indefinitely. Thus, they can be developed as stable, easy-to-use “off-the-shelf” cells [
12]. In the present study, we constructed a third-generation CAR against BCMA and developed a CAR-modified NK92 cell line. Although NK92 cells require an irradiation step before clinical use due to the immortality of the cell line and resulting safety concerns, they have advantages of being easily scalable and highly available as an “off-the-shelf” product, which can significantly reduce both the treatment cycle and treatment cost. The goal of this study was to generate BCMA CAR NK92 cells to cause MM cell death and determine whether they could be used as a potential therapy. To demonstrate the function of BCMA CAR NK cells, we tested CAR constructs using multiple myeloma cell lines. As shown in
Figure 2, targeting BCMA can selectively cause death of myeloma cells that overexpress the BCMA antigen. However, cell death was not observed for cells that did not express BCMA, such as K562 cells. IFN-γ is known to directly enhance NK cell-mediated induction of cancer cell death due to apoptosis and cytolysis. Our results showed that levels of IFN-γ and granzyme B were higher in BCMA CAR NK cells than in control NK92 cells. This phenomenon was confirmed not only in MM cell lines but also in primary MM cells. Experiments using primary MM cells isolated from bone marrow samples of MM patients showed the same results as those using cell lines. BCMA CAR NK cells exhibited significantly increased cytotoxicity to MM cells isolated from BM samples of patients with MM and released more cytokines than control NK cells (
Figure 3).
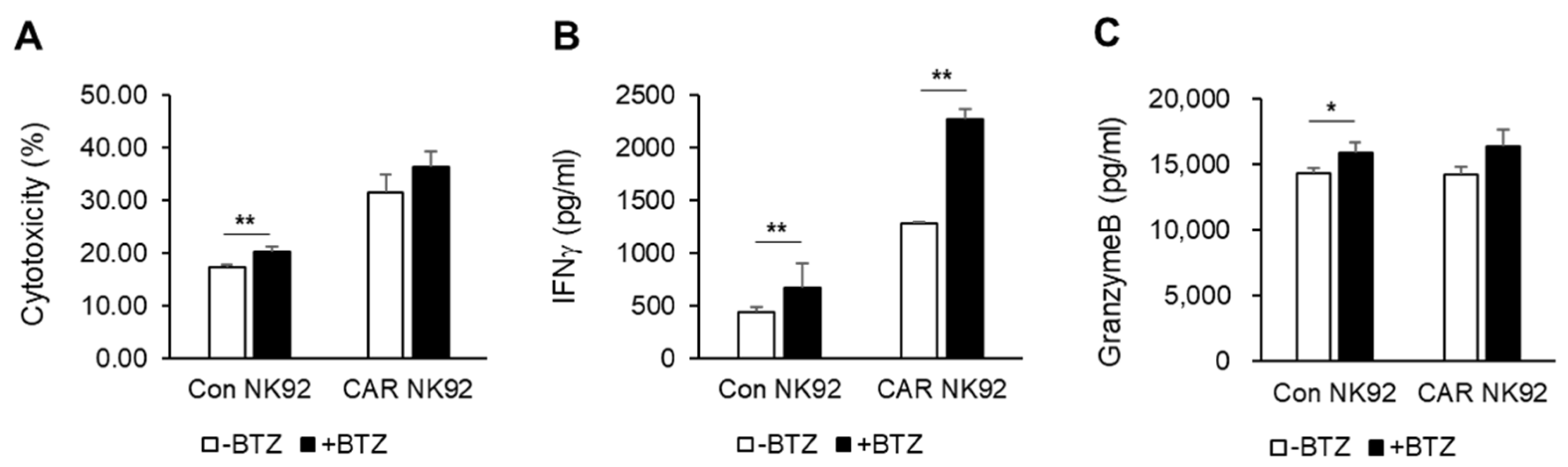
To enhance the efficiency of CAR NK cells against MM, combination therapy with already used drugs can be considered. We used bortezomib, the most commonly used proteasome inhibitor in patients with MM. Bortezomib activates immune responses through upregulating the expression of NK-cell-activating receptors NKG2D, DNAM-1, and TRAIL [
11]. In vitro studies have shown that bortezomib and BCMA CAR NK92 cells exhibited synergistic effects by releasing cytokines such as IFN-γ. However, the mechanism involved was not revealed in this study.
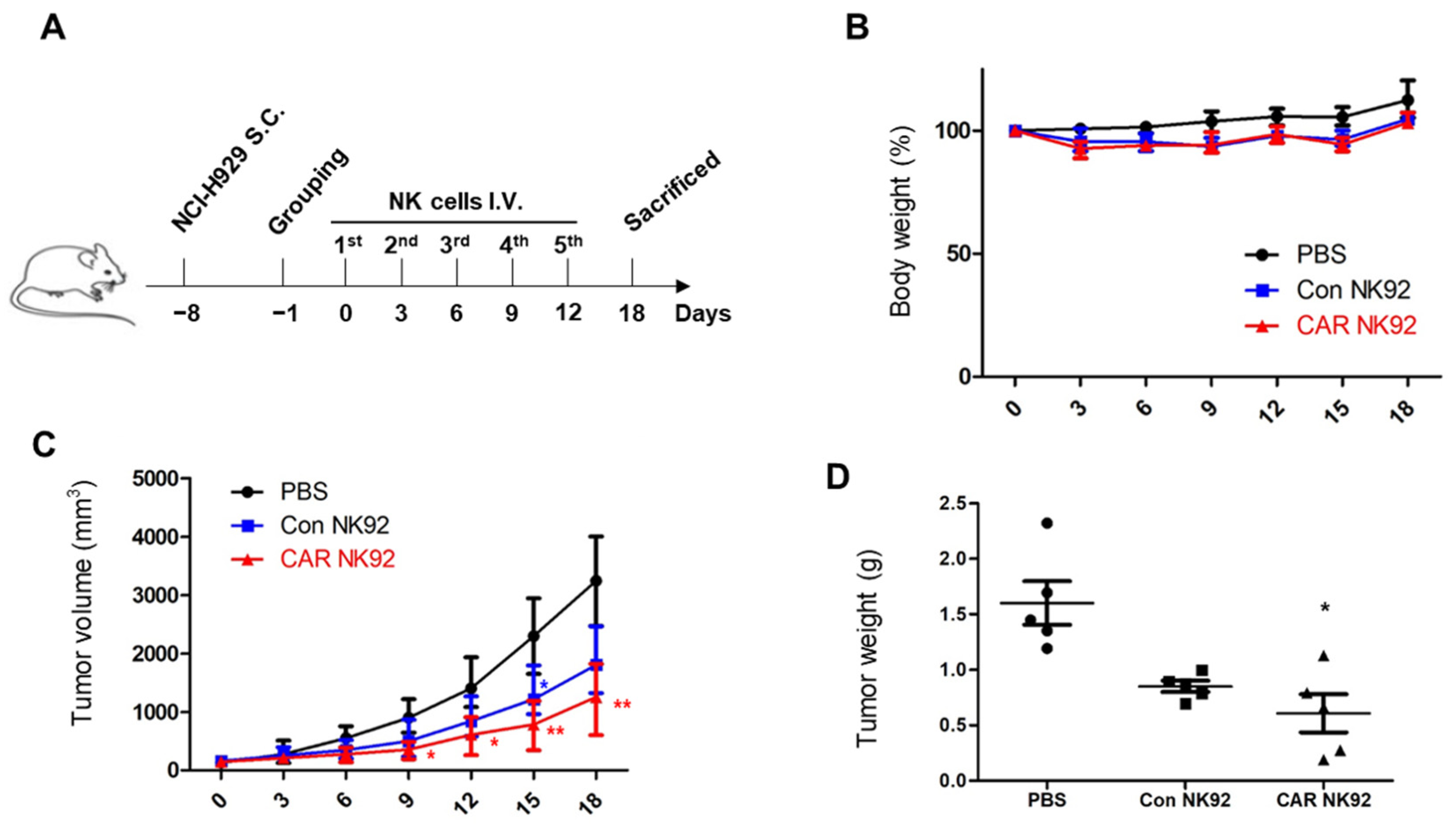
BCMA CAR NK92 cells led tumor regression in an NCI-H929-derived MM subcutaneous tumor model without causing serious side effects. A subcutaneous tumor model was established with NCI-H929, a BCMA-positive MM cell line. Surely, BCMA CAR NK92 cells induced obvious tumor remission in a MM subcutaneous tumor model, indicating their potentially therapy (
Figure 5). These results demonstrate the therapeutic efficacy of BCMA CAR NK92 cells in immunotherapy of MM. These findings indicate the contribution of BCMA CAR NK92 cells to antitumor immune responses in clinical trials.
Our current study has a few limitations. In vivo studies show reduced tumor regression only in the NCI-H929 subcutaneous model. Therefore, a metastatic model or another tumor model should be considered in the future. The NK92 cell line used in this study is easy to culture but requires irradiation before clinical use. Most current clinical trials with NK cells administer autologous or allogeneic ex vivo expanded NK cells. Thus, clinical trials of CAR NK immunotherapy using primary NK cells must be prepared.
In conclusion, BCMA-specific CAR NK92 cells are effective against MM cell lines and primary MM cells from patients. This study indicates that BCMA-specific CAR NK92 cells have great potential to kill MM cell by releasing cytokines, providing an experimental platform for new clinical therapies of BCMA-specific CAR-modified NK cells for treating MM. They could also be expanded to research autologous or allogeneic primary NK cells based on NK cell lines.