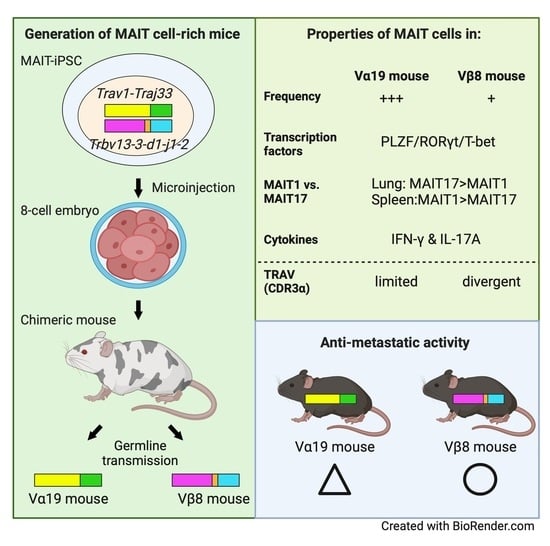
Mice Generated with Induced Pluripotent Stem Cells Derived from Mucosal-Associated Invariant T Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Cell Lines
2.3. Generation of Chimeric Mice from MAIT-iPSCs
2.4. Generation of Vα19 and Vβ8 Mice from the Chimeric Mouse
2.5. Mouse Genotyping
2.6. Cell Isolation from Tissues
2.7. Flow Cytometry
2.8. MAIT Cell Activation
2.9. TCR Repertoire Analysis
2.10. Tumor Resistance
2.11. Quantification and Statistical Analysis
2.12. Relevant Materials Used in this Study
3. Results
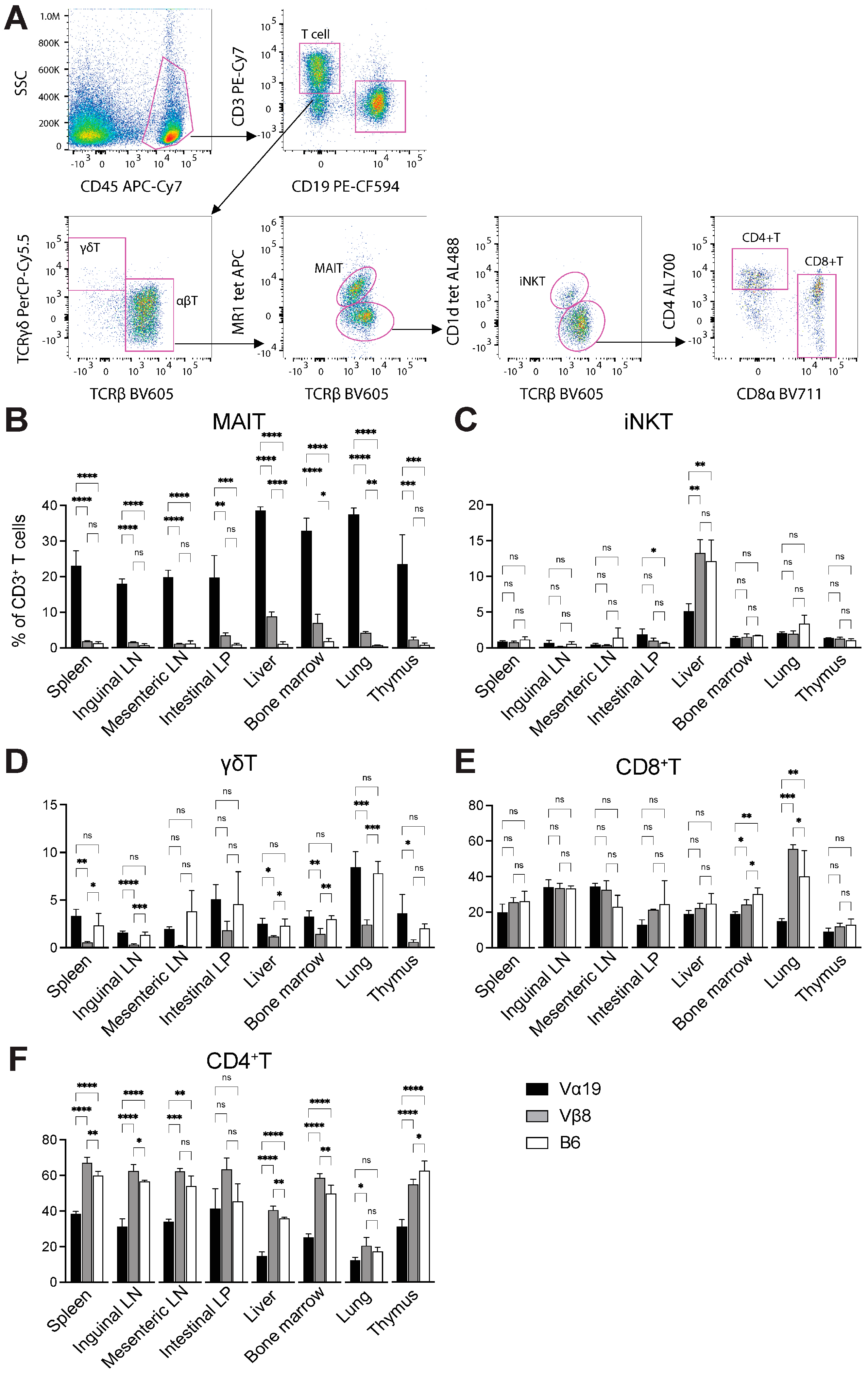
3.1. Mice Harboring Rearranged TCR Loci Specific for MAIT Cells
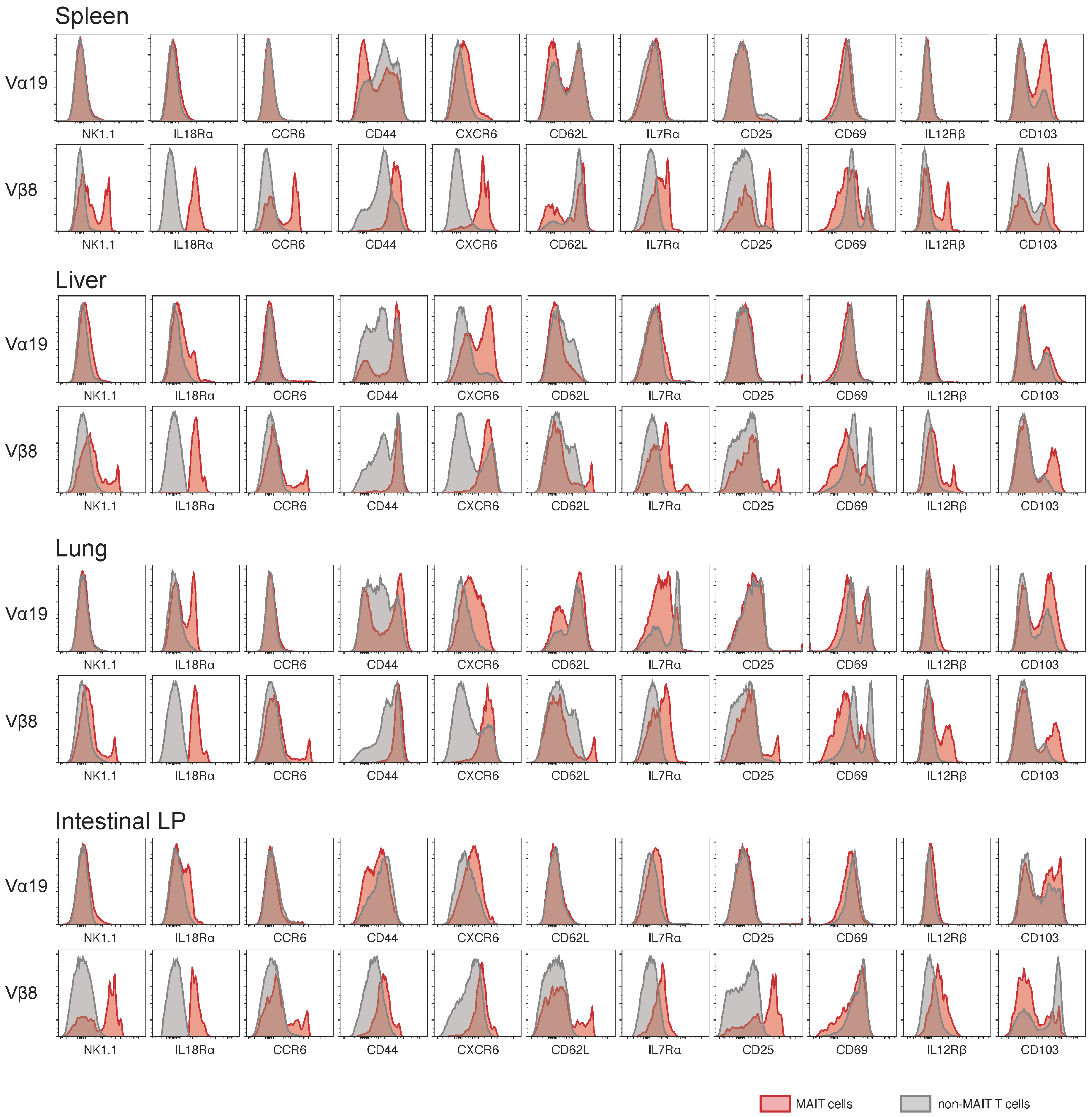
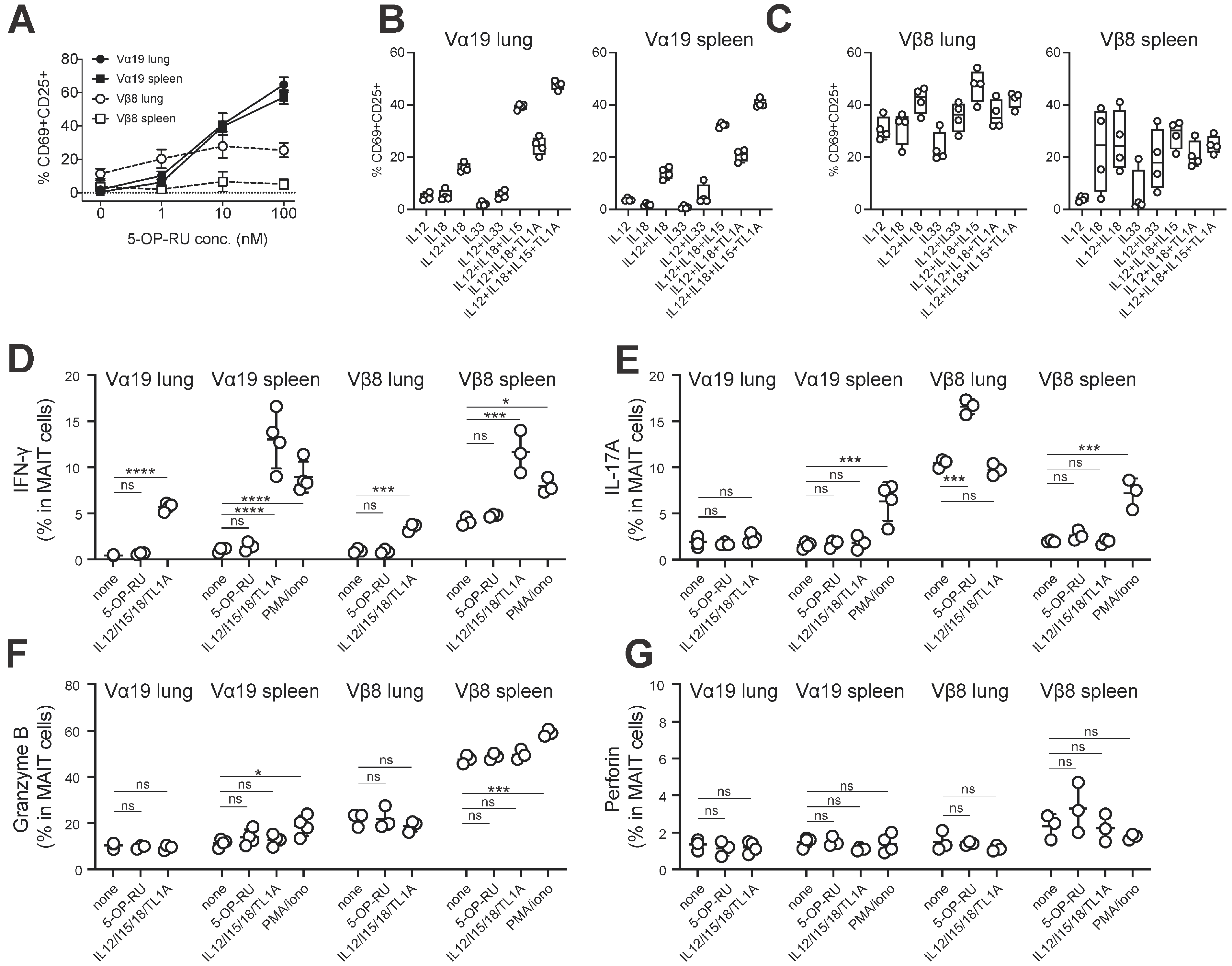
3.2. Expression of Molecules Relevant to MAIT Cells
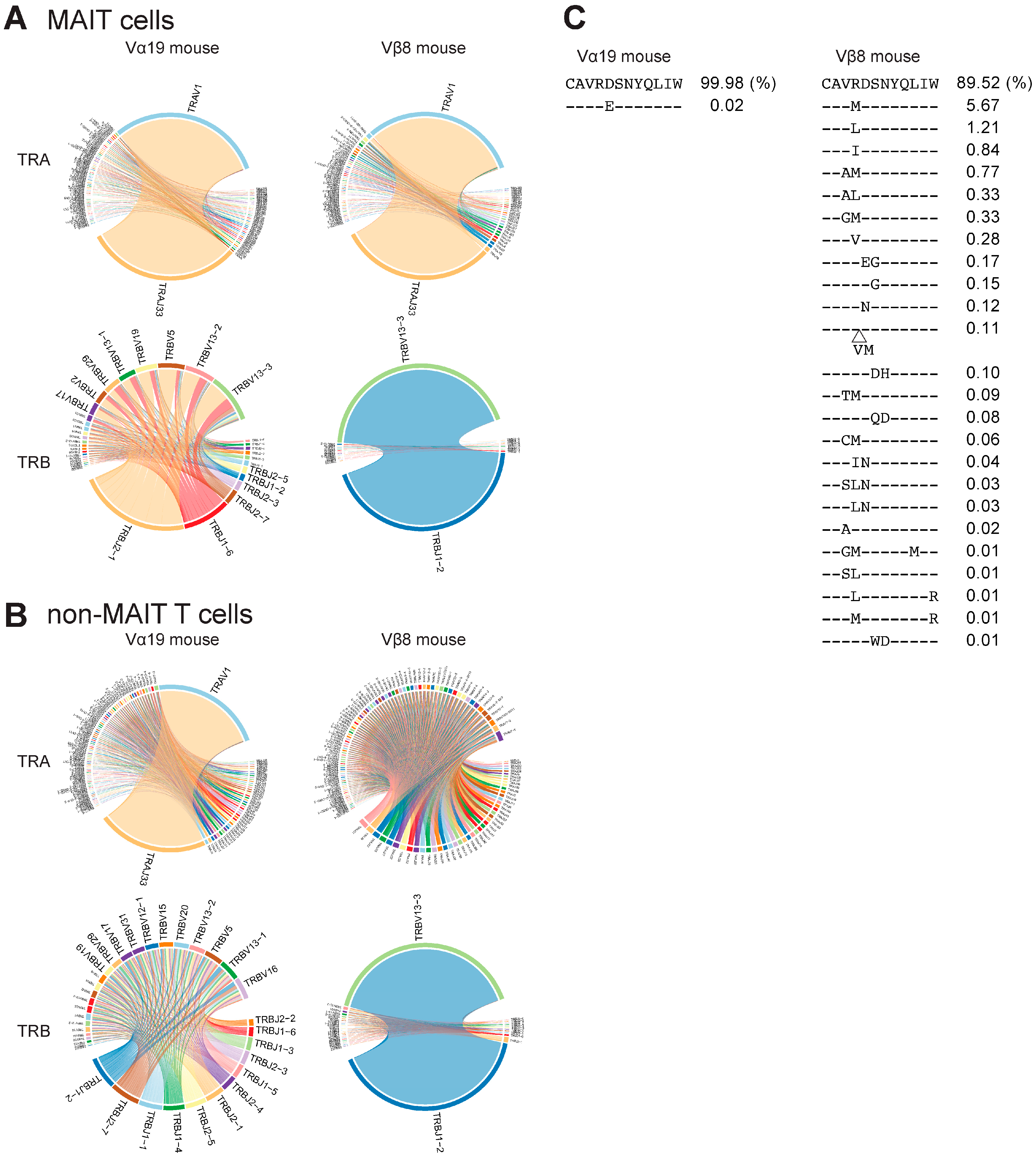
3.3. TCR Repertoire of Vα19 and Vβ8 Mice
3.4. MAIT Cell Activation and Production of IFN-γ and IL-17A
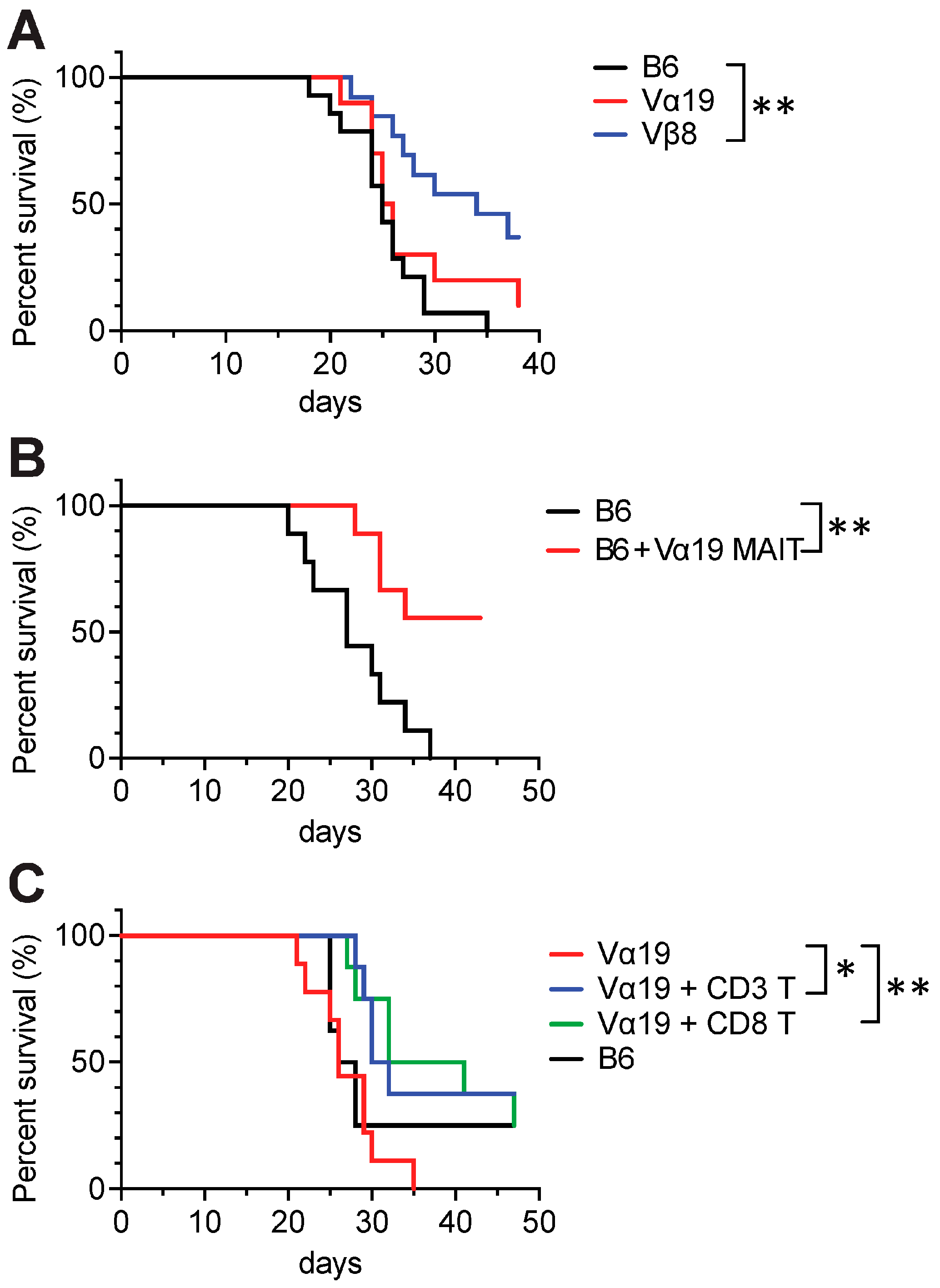
3.5. Tumor Resistance in Vβ8 and Vα19 Mice
4. Discussion
Limitations of the Study
5. Resource Availability
5.1. Lead Contact
5.2. Materials Availability
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Key Resources Table
| Reagent or Resource | Source | Identifier |
| Antibodies | ||
| CD3 (145-2C11), APC | BioLegend (Tokyo, Japan) | Cat# 100312, RRID:AB_312677 |
| CD3 (145-2C11), PE/Cy7 | BioLegend (Tokyo, Japan) | Cat# 100320, RRID:AB_312685 |
| CD4 (RM4-4), PerCP/Cy5.5 | BioLegend (Tokyo, Japan) | Cat# 116012; RRID:AB_2563023 |
| CD4 (RM4-5), Alexa Fluor 700 | BD Biosciences (Tokyo, Japan) | Cat# 561025, RRID:AB_2034006 |
| CD8α (53-6.7), APC/Cy7 | BioLegend (Tokyo, Japan) | Cat# 100714; RRID:AB_312753 |
| CD8α (53-6.7), BV711 | BioLegend (Tokyo, Japan) | Cat# 100747, RRID:AB_11219594 |
| CD11b (M1/70), BV605 | BioLegend (Tokyo, Japan) | Cat# 563015, RRID:AB_2737951 |
| CD19 (1D3), PE/CF594 | BD Biosciences (Tokyo, Japan) | Cat# 562329, RRID:AB_11154580 |
| CD25 (PC61), APC | BD Biosciences (Tokyo, Japan) | Cat# 557192; RRID:AB_398623 |
| CD25 (PC61), APC | BioLegend (Tokyo, Japan) | Cat# 102012; RRID:AB_312861 |
| CD25 (PC61), PE | BioLegend (Tokyo, Japan) | Cat# 102007; RRID:AB_312856 |
| CD44 (IM7), FITC | BioLegend (Tokyo, Japan) | Cat#103006; RRID:AB_312957 |
| CD44 (IM7), BV510 | BD Biosciences (Tokyo, Japan) | Cat# 563114, RRID:AB_2738011 |
| CD45 (30-F11), APC/Cy7 | BioLegend (Tokyo, Japan) | Cat# 103116, RRID:AB_312981 |
| CD45R/B220 (RA3-6B2), PE | BioLegend (Tokyo, Japan) | Cat# 103207; RRID:AB_312992 |
| CD62L (MEL-14), PE | BioLegend(Tokyo, Japan) | Cat# 104407, RRID:AB_313094 |
| CD69 (H1.2F3), APC/Cy7 | BioLegend (Tokyo, Japan) | Cat# 104525; RRID:AB_10683447 |
| CD69 (H1.2F3), PE/Cy7 | BioLegend (Tokyo, Japan) | Cat# 104511; RRID:AB_493565 |
| CD69 (H1.2F3), BV421 | BD Biosciences (Tokyo, Japan) | Cat# 562920, RRID:AB_2737893 |
| CD103 (2E7), PE | BioLegend (Tokyo, Japan) | Cat# 121405, RRID:AB_535948 |
| CD127 (IL-7Rα) (A7R34), PerCP/Cy5.5 | BioLegend (Tokyo, Japan) | Cat# 135021; RRID:AB_1937274 |
| CD186 (CXCR6) (SAO51D1), PE | BioLegend (Tokyo, Japan) | Cat# 151103; RRID:AB_2566545 |
| CD196 (CCR6) (29-2L17), BV605 | BioLegend (Tokyo, Japan) | Cat# 129819, RRID:AB_2562513 |
| CD212 (IL12Rb) (114), PE | BD Biosciences (Tokyo, Japan) | Cat# 551974, RRID:AB_394310 |
| CD218a (IL-18Rα) (P3TUNYA), eFluor450 | Thermo Fisher Scientifics (Tokyo, Japan) | Cat# 48-5183-80; RRID:AB_2574068 |
| CD314 (NKG2D) (CX5), PE | BioLegend (Tokyo, Japan) | Cat# 130207, RRID:AB_1227713 |
| F4/80 (BM8), PE | BioLegend (Tokyo, Japan) | Cat# 123109; RRID:AB_893498 |
| Gr1 (Ly6G/Ly6C) (RB6-8C5), FITC | BD Biosciences (Tokyo, Japan) | Cat# 553126, RRID:AB_394642 |
| I-A/I-E (M5/114.15.2), BV421 | BD Biosciences (Tokyo, Japan) | Cat# 562564, RRID:AB_2716857 |
| Ly6C (HK1.4), PerCP/Cy5.5 | Thermo Fisher Scientifics (Tokyo, Japan) | Cat# 45-5932-82, RRID:AB_2723343 |
| Ly6G, PE/Cy7 | BD Biosciences (Tokyo, Japan) | Cat# 560601, RRID:AB_1727562 |
| NK1.1 (PK136), BV510 | BD Biosciences (Tokyo, Japan) | Cat# 563096; RRID:AB_2738002 |
| NK1.1 (PK136), BV605 | BD Biosciences (Tokyo, Japan) | Cat# 563220, RRID:AB_2738077 |
| TCRβ (H57-597), BV605 | BD Biosciences (Tokyo, Japan) | Cat# 562840; RRID:AB_2687544 |
| TCRβ (H57-597), BV605 | BioLegend (Tokyo, Japan) | Cat# 109241; RRID:AB_2629563 |
| TCRβ (H57-597), PE/Cy7 | BioLegend (Tokyo, Japan) | Cat# 109222; RRID:AB_893625 |
| TCRγ/δ (GL3), PerCP/Cy5.5 | BioLegend (Tokyo, Japan) | Cat# 118117, RRID:AB_10612572 |
| Eomes (Dan11mag), Alexa488 | Thermo Fisher Scientifics (Tokyo, Japan) | Cat# 53-4875-82, RRID:AB_10854265 |
| T-bet (4B10), PerCP/Cy5.5 | BioLegend (Tokyo, Japan) | Cat# 644805, RRID:AB_1595593 |
| PLZF (9E12), PE/Cy7 | BioLegend (Tokyo, Japan) | Cat# 145805, RRID:AB_2566164 |
| RORγt (Q31-378), BV421 | BD Biosciences (Tokyo, Japan) | Cat# 562894, RRID:AB_2687545 |
| IFN-γ (XMG1.2), FITC | BioLegend (Tokyo, Japan) | Cat# 505806, RRID:AB_315400 |
| IL-17A (TC11-18H10), BV421 | BD Biosciences (Tokyo, Japan) | Cat# 563354, RRID:AB_2687547 |
| Perforin (S16009A), APC/Fire750 | BioLegend (Tokyo, Japan) | Cat# 154317, RRID:AB_2924481 |
| Granzyme B (QA16A02), PerCP/Cy5.5 | BioLegend (Tokyo, Japan) | Cat# 372211, RRID:AB_2728378 |
| rat IgG2a, κ (RTK2758), Alexa488 | BioLegend(Tokyo, Japan) | Cat# 400525, RRID:AB_2864283 |
| mouse IgG1, κ (MOPC-21), PerCP/Cy5.5 | BioLegend (Tokyo, Japan) | 400150, RRID:AB_893664 |
| hamster IgG (HTK888), PE/Cy7 | BioLegend (Tokyo, Japan) | Cat# 400921, RRID:AB_2905473 |
| mouse IgG2a, κ (G155-178), BV421 | BD Biosciences (Tokyo, Japan) | Cat# 562439, RRID:AB_11151914 |
| rat IgG1, κ (RTK2071), FITC | BioLegend (Tokyo, Japan) | Cat# 400405, RRID:AB_326511 |
| rat IgG1, κ (R3-34), BV421 | BD Biosciences (Tokyo, Japan) | Cat# 562868, RRID:AB_2734711 |
| rat IgG2a, κ (RTK2758), APC/Fire750 | BioLegend (Tokyo, Japan) | Cat# 400567, RRID:AB_2923254 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| DMEM | Nacalai tesque (Kyoto, Japan) | Car# 08458-16 |
| RPMI1640 | Nacalai tesque (Kyoto, Japan) | Cat# 30264-56 |
| HBSS | FUJIFILM Wako (Osaka, Japan) | Cat# 084-08345 |
| HEPES | FUJIFILM Wako (Osaka, Japan) | Cat# 047861 |
| penicillin/streptomycin | Lonza (Tokyo, Japan) | Cat# 09-757F |
| 2-mercaptoethanol | FUJIFILM Wako (Osaka, Japan) | Cat# 133-06864 |
| Collagenase Yakult | Yakult Pharmaceutical(Tokyo, Japan) | Cat# YK102 |
| Collagenase type II | Worthington (NJ, USA) | Cat# LS004176 |
| Dispase II | FUJIFILM Wako (Osaka, Japan) | Cat# 383-02281 |
| Percoll | Cytiva (Tokyo, Japan) | Cat# 17089101 |
| 5-Amino-4-D-ribitylaminouracil Dihydrochloride | Toronto Research Chemicals (ON, Canada) | Cat# A629245 |
| Methylglyoxal solution | Sigma-Aldrich (Tokyo, Japan) | Cat# M0252 |
| Mouse MR1 5-OP-RU tetramer, APC-labeled | NIH Tetramer Core Facility (GA, USA) | N/A |
| Mouse MR1 5-OP-RU tetramer, BV421-labeled | NIH Tetramer Core Facility (GA, USA) | N/A |
| Mouse CD1d PBS-57 tetramer, Alexa Fluor 488 | NIH Tetramer Core Facility (GA, USA) | N/A |
| Critical Commercial Assays | ||
| OneTaq master mix | New England Biolabs (Tokyo, Japan) | Cat# M0482L |
| Anti-APC MicroBeads | Miltenyi Biotec (Tokyo, Japan) | Cat# 130-090-855; RRID:AB_244367 |
| RNeasy Mini kit | Qiagen (Tokyo, Japan) | Cat# 74104 |
| SMARTer Mouse TCR a/b profiling kit | Takara Bio (Shiga, Japan) | Cat# 634402 |
| MojoSort mouse CD3 T cell isolation kit | BioLegend (Tokyo, Japan) | Cat# 480031 |
| Cell Stimulation Cocktail | Thermo Fisher Scientifics (Tokyo, Japan) | Cat# 00-4970-93 |
| Protein Transport Inhibitor Cocktail | Thermo Fisher Scientifics (Tokyo, Japan) | Cat# 00-4980-93 |
| Transcription factor buffer set | BD Biosciences (Tokyo, Japan) | Cat# 562574 |
| Cytofix/Cytoperm Fixation/Permeabilization kit | BD Biosciences (Tokyo, Japan) | Cat# 554714 |
| Experimental Models: Cell Lines | ||
| Murine MAIT-iPSC clone L7-1 | [17] | N/A |
| Murine MAIT-iPSC clone L11-1 | [17] | N/A |
| Murine MAIT-iPSC clone L19-1 | [17] | N/A |
| Lewis lung carcinoma (LLC) | Riken Bioresource Center (Ibaraki, Japan) | Cat# RCB0558; RRID: CVCL_4358 |
| Experimental Models: Organisms/Strains | ||
| C57BL/6NJcl | CLEA Japan (Tokyo, Japan) | N/A |
| Vα19 | This study | N/A |
| Vβ8 | This study | N/A |
| Vα19/Vβ8 | This study | N/A |
| Oligonucleotides | ||
| A1 5′-TCAACTGCACATACAGCACCTC-3′ | This study | N/A |
| A2 5′-CATGCATTATTCAGCCAGTGCCTTCT-3′ | This study | N/A |
| B1 5′-GTACTGGTATCGGCAGGAC-3′ | This study | N/A |
| B2 5′-GAGCCGAAGGTGTAGTCGG-3′ | This study | N/A |
| Software and Algorithms | ||
| FlowJo software v9 and v10 | BD Bioscience (Tokyo, Japan) | N/A |
| Prism 9 for macOS | GraphPad Software (Boston, MA, USA) | N/A |
| MiXCR-3.0.5 | [22] | N/A |
| VDJtools-1.2.1 | [23] | N/A |
References
- Franciszkiewicz, K.; Salou, M.; Legoux, F.; Zhou, Q.; Cui, Y.; Bessoles, S.; Lantz, O. MHC class I-related molecule, MR1, and mucosal-associated invariant T cells. Immunol. Rev. 2016, 272, 120–138. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, D.I.; Koay, H.; McCluskey, J.; Gherardin, N.A. The biology and functional importance of MAIT cells. Nat. Immunol. 2019, 20, 1110–1128. [Google Scholar] [CrossRef] [PubMed]
- Treiner, E.; Duban, L.; Bahram, S.; Radosavljevic, M.; Wanner, V.; Tilloy, F.; Affaticati, P.; Gilfillan, S.; Lantz, O. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature 2003, 422, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, A.M.; Sant’Angelo, D.B. The BTB-ZF family of transcription factors: Key regulators of lineage commitment and effector function development in the immune system. J. Immunol. 2011, 187, 2841–2847. [Google Scholar] [CrossRef] [PubMed]
- Koay, H.; Gherardin, N.A.; Enders, A.; Loh, L.; Mackay, L.K.; Almeida, C.F.; Russ, B.E.; Nold-Petry, C.A.; Nold, M.F.; Bedoui, S.; et al. A three-stage intrathymic development pathway for the mucosal-associated invariant T cell lineage. Nat. Immunol. 2016, 17, 1300–1311. [Google Scholar] [CrossRef] [PubMed]
- Eberl, G. RORγt, a multitask nuclear receptor at mucosal surfaces. Mucosal Immunol. 2017, 10, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Dzopalić, T.; Božić-Nedeljković, B.; Jurišić, V. Function of innate lymphoid cells in the immune-related disorders. Hum. Cell Off. J. Hum. Cell Res. Soc. 2019, 32, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Mazzurana, L.; Rao, A.; Van Acker, A.; Mjösberg, J. The roles for innate lymphoid cells in the human immune system. Semin. Immunopathol. 2018, 40, 407–419. [Google Scholar] [CrossRef]
- Corbett, A.J.; Eckle, S.B.G.; Birkinshaw, R.W.; Liu, L.; Patel, O.; Mahony, J.; Chen, Z.; Reantragoon, R.; Meehan, B.; Cao, H.; et al. T-cell activation by transitory neo-antigens derived from distinct microbial pathways. Nature 2014, 509, 361–365. [Google Scholar] [CrossRef]
- Kjer-Nielsen, L.; Patel, O.; Corbett, A.J.; Le Nours, J.; Meehan, B.; Liu, L.; Bhati, M.; Chen, Z.; Kostenko, L.; Reantragoon, R.; et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature 2012, 491, 717–723. [Google Scholar] [CrossRef]
- Tilloy, F.; Treiner, E.; Park, S.H.; Garcia, C.; Lemonnier, F.; de la Salle, H.; Bendelac, A.; Bonneville, M.; Lantz, O. An invariant T cell receptor α chain defines a novel TAP-independent major histocompatibility complex class ib-restricted α/β T cell subpopulation in mammals. J. Exp. Med. 1999, 189, 1907–1921. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, D.I.; Uldrich, A.P.; McCluskey, J.; Rossjohn, J.; Moody, D.B. The burgeoning family of unconventional T cells. Nat. Immunol. 2015, 16, 1114–1123. [Google Scholar] [CrossRef] [PubMed]
- Toubal, A.; Nel, I.; Lotersztajn, S.; Lehuen, A. Mucosal-associated invariant T cells and disease. Nat. Rev. Immunol. 2019, 19, 643–657. [Google Scholar] [CrossRef] [PubMed]
- Dusseaux, M.; Martin, E.; Serriari, N.; Péguillet, I.; Premel, V.; Louis, D.; Milder, M.; Le Bourhis, L.; Soudais, C.; Treiner, E.; et al. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17–secreting T cells. Blood 2011, 117, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Franciszkiewicz, K.; Mburu, Y.K.; Mondot, S.; Le Bourhis, L.; Premel, V.; Martin, E.; Kachaner, A.; Duban, L.; Ingersoll, M.A.; et al. Mucosal-associated invariant T cell-rich congenie mouse strain allows functional evaluation. J. Clin. Investig. 2015, 125, 4171. [Google Scholar] [CrossRef] [PubMed]
- Rahimpour, A.; Koay, H.F.; Enders, A.; Clanchy, R.; Eckle, S.B.G.; Meehan, B.; Chen, Z.; Whittle, B.; Liu, L.; Fairlie, D.P.; et al. Identification of phenotypically and functionally heterogeneous mouse mucosal-associated invariant T cells using MR1 tetramers. J. Exp. Med. 2015, 212, 1095–1108. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, C.; Murakami, Y.; Ishii, E.; Fujita, H.; Wakao, H. Reprogramming and redifferentiation of mucosal-associated invariant T cells reveal tumor inhibitory activity. eLife 2022, 11, e70848. [Google Scholar] [CrossRef]
- Inoue, K.; Wakao, H.; Ogonuki, N.; Miki, H.; Seino, K.; Nambu-Wakao, R.; Noda, S.; Miyoshi, H.; Koseki, H.; Taniguchi, M.; et al. Generation of cloned mice by direct nuclear transfer from natural killer T cells. Curr. Biol. 2005, 15, 1114–1118. [Google Scholar] [CrossRef]
- Wakao, H.; Wakao, R.; Sakata, S.; Iwabuchi, K.; Oda, A.; Fujita, H. In vitro induction of natural killer T cells from embryonic stem cells prepared using somatic cell nuclear transfer. FASEB J. 2008, 22, 2223–2231. [Google Scholar] [CrossRef]
- Wakao, H.; Kawamoto, H.; Sakata, S.; Inoue, K.; Ogura, A.; Wakao, R.; Oda, A.; Fujita, H. A novel mouse model for invariant NKT cell study. J. Immunol. 2007, 179, 3888–3895. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Bolotin, D.A.; Poslavsky, S.; Mitrophanov, I.; Shugay, M.; Mamedov, I.Z.; Putintseva, E.V.; Chudakov, D.M. MiXCR: Software for comprehensive adaptive immunity profiling. Nat. Methods 2015, 12, 380–381. [Google Scholar] [CrossRef] [PubMed]
- Shugay, M.; Bagaev, D.V.; Turchaninova, M.A.; Bolotin, D.A.; Britanova, O.V.; Putintseva, E.V.; Pogorelyy, M.V.; Nazarov, V.I.; Zvyagin, I.V.; Kirgizova, V.I.; et al. VDJtools: Unifying post-analysis of T cell receptor repertoires. PLoS Comput. Biol. 2015, 11, e1004503. [Google Scholar] [CrossRef] [PubMed]
- Legoux, F.; Salou, M.; Lantz, O. MAIT cell development and functions: The microbial connection. Immunity 2020, 53, 710–723. [Google Scholar] [CrossRef] [PubMed]
- Koay, H.; Su, S.; Amann-Zalcenstein, D.; Daley, S.R.; Comerford, I.; Miosge, L.; Baldwin, T.; Hickey, P.F.; Berzins, S.P.; Mak, J.Y.W.; et al. A divergent transcriptional landscape underpins the development and functional branching of MAIT cells. Sci. Immunol. 2019, 4, eaay6039. [Google Scholar] [CrossRef] [PubMed]
- Reantragoon, R.; Corbett, A.J.; Sakala, I.G.; Gherardin, N.A.; Furness, J.B.; Chen, Z.; Eckle, S.B.G.; Uldrich, A.P.; Birkinshaw, R.W.; Patel, O.; et al. Antigen-loaded MR1 tetramers define T cell receptor heterogeneity in mucosal-associated invariant T cells. J. Exp. Med. 2013, 210, 2305–2320. [Google Scholar] [CrossRef] [PubMed]
- Jesteadt, E.; Zhang, I.; Yu, H.; Meierovics, A.; Chua Yankelevich, W.; Cowley, S. Interleukin-18 is critical for mucosa-associated invariant T cell gamma interferon responses to francisella species in vitro but not in vivo. Infect. Immun. 2018, 86, 117. [Google Scholar] [CrossRef]
- Leng, T.; Akther, H.D.; Hackstein, C.; Powell, K.; King, T.; Friedrich, M.; McCuaig, S.; Neyazi, M.; Arancibia-Cárcamo, C.V.; Hagel, J.; et al. TCR and inflammatory signals tune human MAIT cells to exert specific tissue repair and effector functions. Cell Rep. 2019, 28, 3077–3091.e5. [Google Scholar] [CrossRef]
- Hinks, T.S.C.; Zhang, X. MAIT cell activation and functions. Front. Immunol. 2020, 11, 1014. [Google Scholar] [CrossRef]
- Ussher, J.E.; Bilton, M.; Attwod, E.; Shadwell, J.; Richardson, R.; Lara, C.; Mettke, E.; Kurioka, A.; Hansen, T.H.; Klenerman, P.; et al. CD161++CD8+ T cells, including the MAIT cell subset, are specifically activated by IL-12+IL-18 in a TCR-independent manner. Eur. J. Immunol. 2014, 44, 195–203. [Google Scholar] [CrossRef]
- Croxford, J.L.; Miyake, S.; Huang, Y.; Shimamura, M.; Yamamura, T. Invariant V(alpha)19i T cells regulate autoimmune inflammation. Nat. Immunol. 2006, 7, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Kawachi, I.; Maldonado, J.; Strader, C.; Gilfillan, S. MR1-restricted V alpha 19i mucosal-associated invariant T cells are innate T cells in the gut lamina propria that provide a rapid and diverse cytokine response. J. Immunol. 2006, 176, 1618–1627. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.; Treiner, E.; Duban, L.; Guerri, L.; Laude, H.; Toly, C.; Premel, V.; Devys, A.; Moura, I.C.; Tilloy, F.; et al. Stepwise development of MAIT cells in mouse and human. PLoS Biol. 2009, 7, e1000054. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, N.; Kanie, O.; Huang, Y.; Fujii, R.; Watanabe, H.; Shimamura, M. Synthetic alpha-mannosyl ceramide as a potent stimulant for an NKT cell repertoire bearing the invariant Valpha19-Jalpha26 TCR alpha chain. Chem. Biol. 2005, 12, 677–683. [Google Scholar] [CrossRef]
- Dey, R.J.; Dey, B.; Harriff, M.; Canfield, E.T.; Lewinsohn, D.M.; Bishai, W.R. Augmentation of the riboflavin-biosynthetic pathway enhances mucosa-associated invariant T (MAIT) cell activation and diminishes mycobacterium tuberculosis virulence. mBio 2022, 13, e0386521. [Google Scholar] [CrossRef]
- Xu, C.; Li, S.; Fulford, T.S.; Christo, S.N.; Mackay, L.K.; Gray, D.H.; Uldrich, A.P.; Pellicci, D.G.; Godfrey, D.I.; Koay, H.-F. Expansion of MAIT cells in the combined absence of NKT and γδ-T cells. Mucosal Immunol. 2023, 16, 446–461. [Google Scholar] [CrossRef]
- Vuletić, A.; Jovanić, I.; Jurišić, V.; Milovanović, Z.; Nikolić, S.; Spurnić, I.; Konjević, G. IL-2 and IL-15 induced NKG2D, CD158a and CD158b expression on T, NKT- like and NK cell lymphocyte subsets from regional lymph nodes of melanoma patients. Pathol. Oncol. Res. 2020, 26, 223–231. [Google Scholar] [CrossRef]

| iPS Clones | TCRα | TCRβ | Νο. of Chimeric Male (Animal ID) (% of Donor Coat Color) | |||||
|---|---|---|---|---|---|---|---|---|
| Vα | Jα | Vβ | D | Jβ | (60–90%) | (30–60%) | (<30%) | |
| L7 | Trav1 (Vα19) | Traj33 (Jα33) | Trbv13-3*01 (Vβ8) | Trbd1*01 | Trbj1-2*01 | 2 (#25, 26) | 2 (#27, 28) | 1 (#13) |
| L11 | Trav1 (Vα19) | Traj33 (Jα33) | Trbv19*01, *03 (Vβ6) | Trbd2*01 | Trbj2-3*01 | 2 (#29, 30) | 2 (#31, 32) | - |
| L19 | Trav1 (Vα19) | Traj33 (Jα33) | Trbv19*01, *03 (Vβ6) | Trbd2*01 | Trbj2-3*01 | - | - | 2 (#33, 34) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugimoto, C.; Fujita, H.; Wakao, H. Mice Generated with Induced Pluripotent Stem Cells Derived from Mucosal-Associated Invariant T Cells. Biomedicines 2024, 12, 137. https://doi.org/10.3390/biomedicines12010137
Sugimoto C, Fujita H, Wakao H. Mice Generated with Induced Pluripotent Stem Cells Derived from Mucosal-Associated Invariant T Cells. Biomedicines. 2024; 12(1):137. https://doi.org/10.3390/biomedicines12010137
Chicago/Turabian StyleSugimoto, Chie, Hiroyoshi Fujita, and Hiroshi Wakao. 2024. "Mice Generated with Induced Pluripotent Stem Cells Derived from Mucosal-Associated Invariant T Cells" Biomedicines 12, no. 1: 137. https://doi.org/10.3390/biomedicines12010137
APA StyleSugimoto, C., Fujita, H., & Wakao, H. (2024). Mice Generated with Induced Pluripotent Stem Cells Derived from Mucosal-Associated Invariant T Cells. Biomedicines, 12(1), 137. https://doi.org/10.3390/biomedicines12010137