Levodopa Rescues Retinal Function in the Transgenic A53T Alpha-Synuclein Model of Parkinson’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice and Levodopa Treatment
2.2. Optical Coherence Tomography
2.3. Electroretinography
2.4. Immunohistochemistry
2.5. Data and Statistical Analysis
3. Results
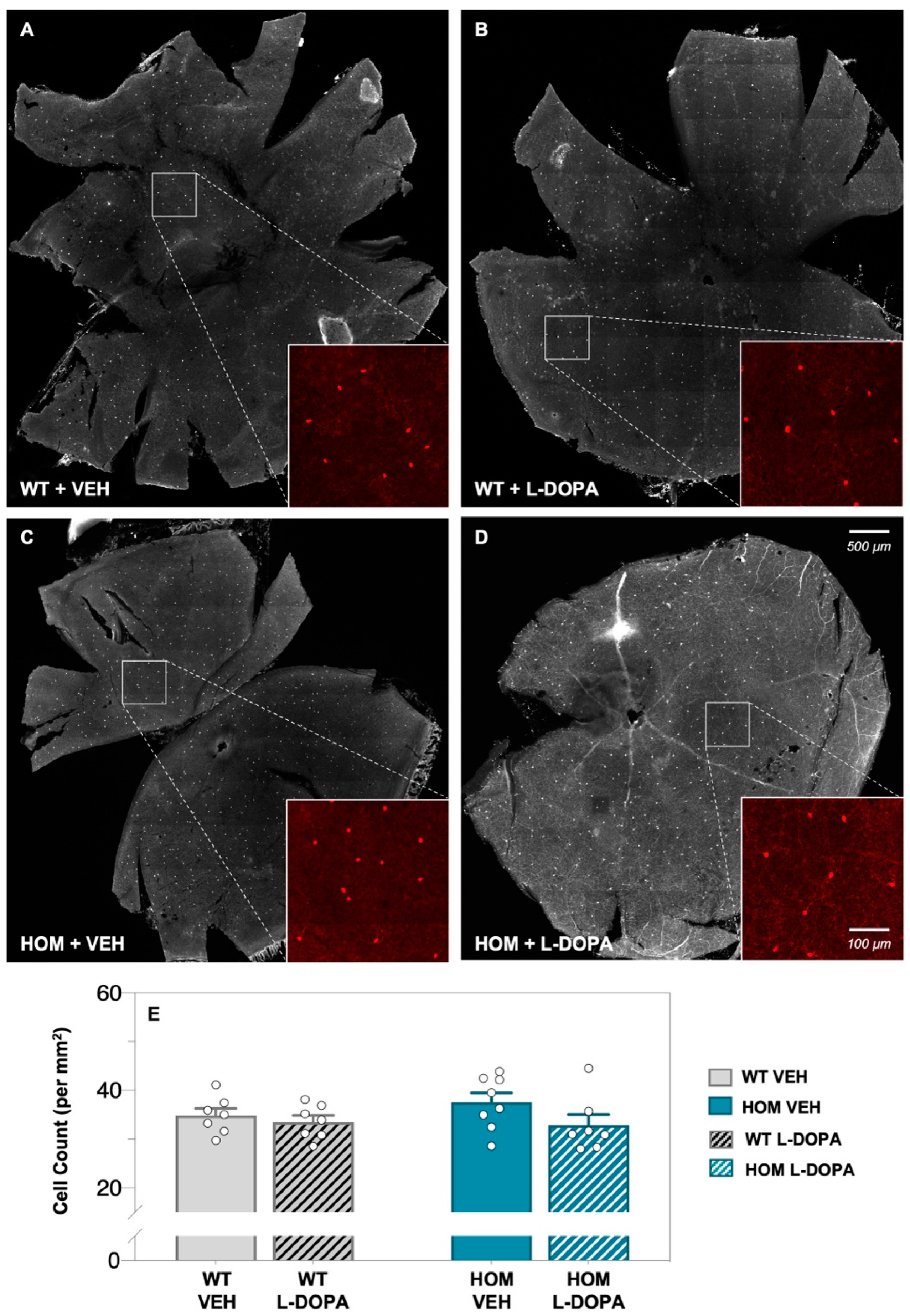
3.1. Acute Levodopa Treatment on Retinal Amacrine Cell Expression in A53T Mice
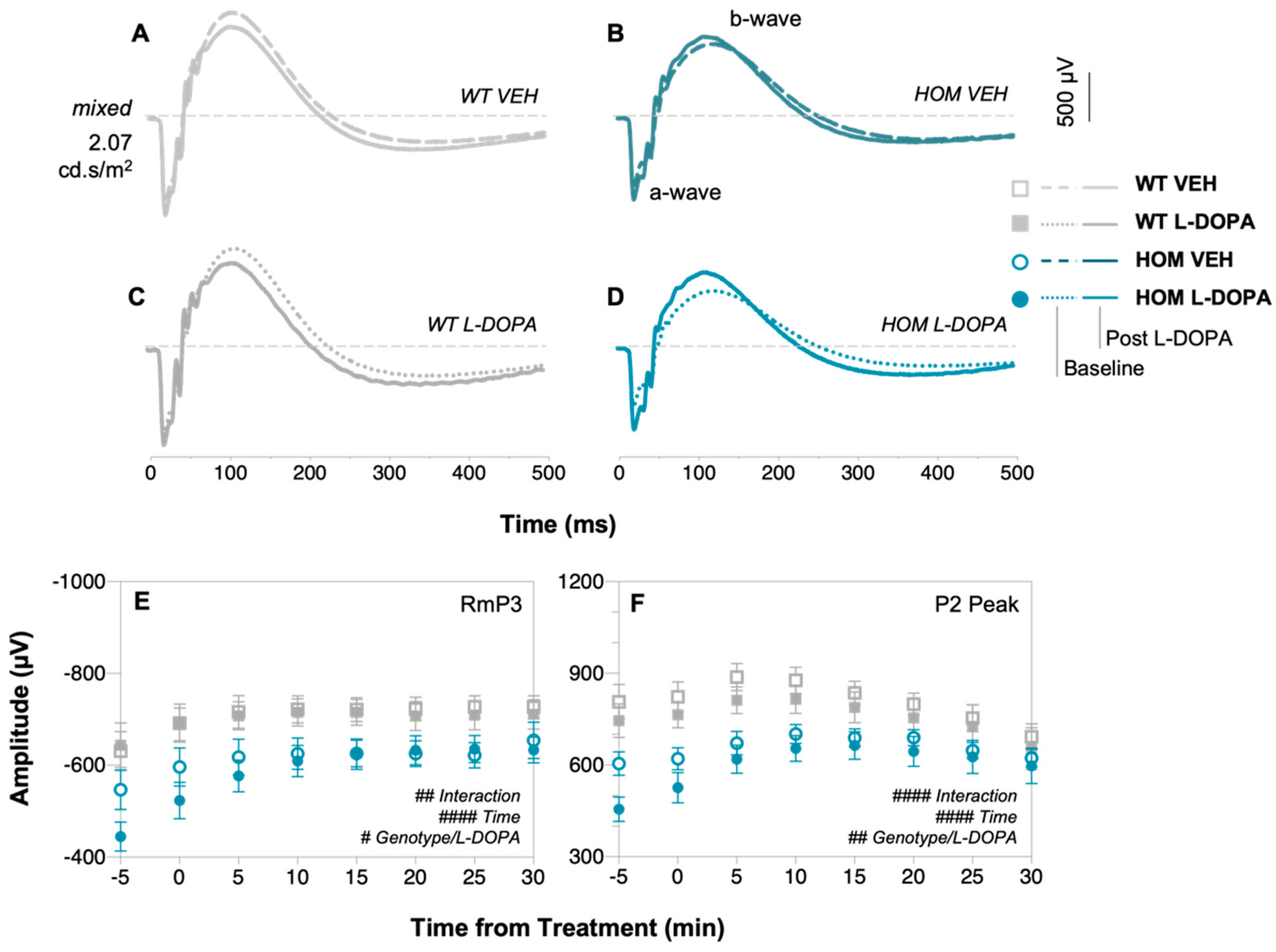
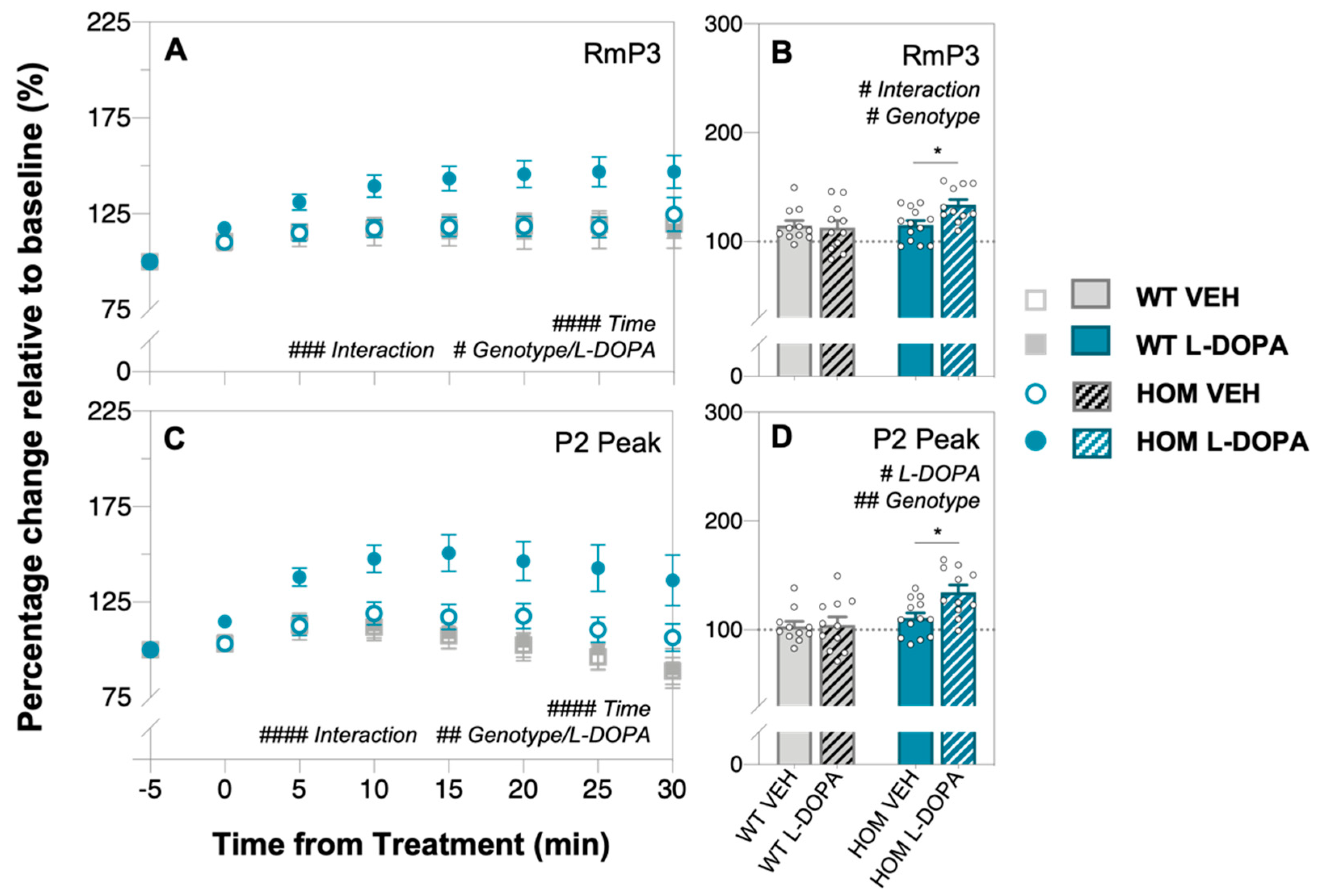
3.2. Acute Levodopa Treatment on In Vivo Retinal Function in A53T Mice
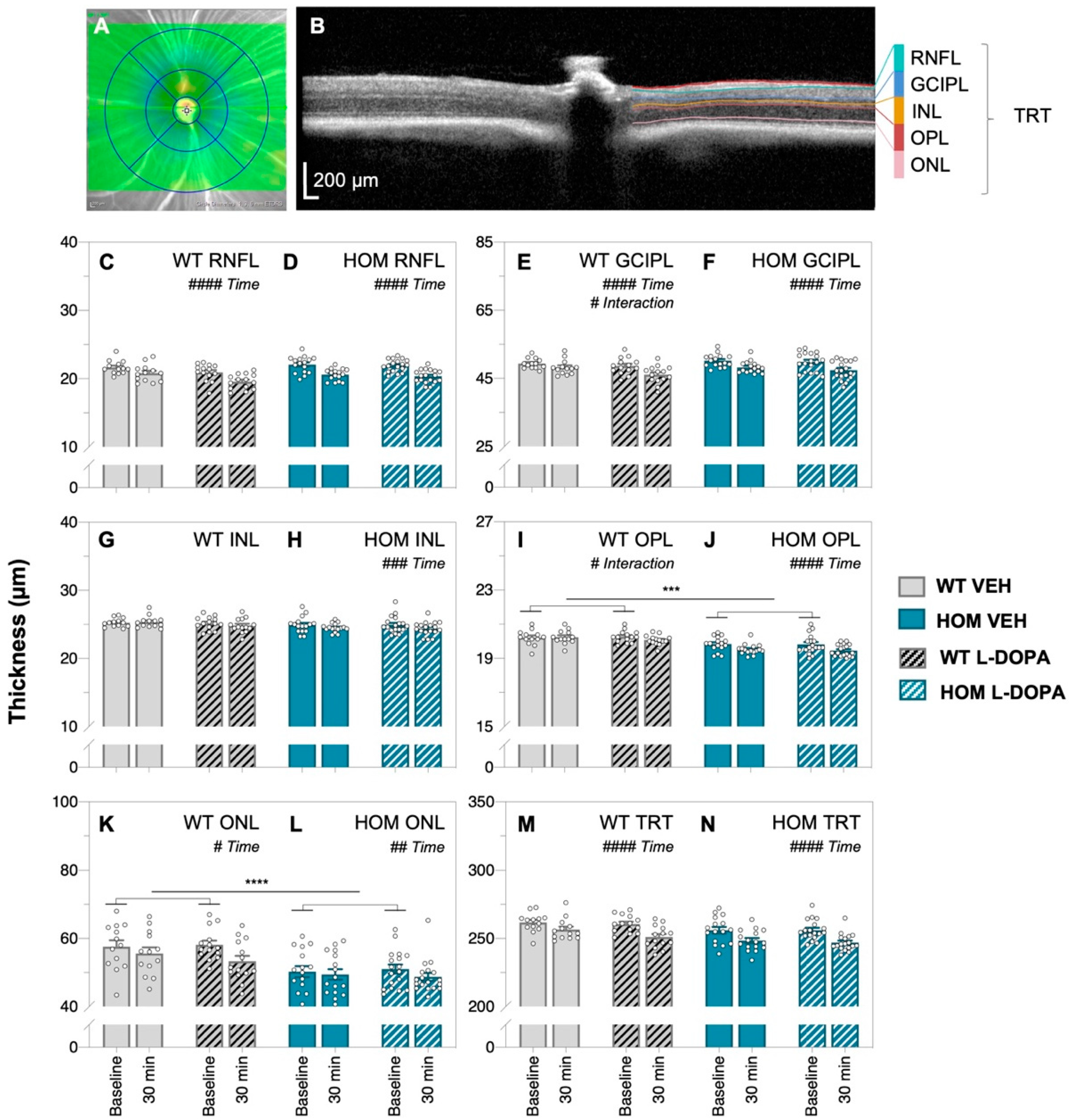
3.3. Acute Levodopa Treatment on In Vivo Retinal Structure in A53T Mice
4. Discussion
4.1. Amacrine Cells Are Preserved in 6-Month-Old A53T Mice
4.2. Acute L-DOPA Treatment Ameliorates Retinal Dysfunction in A53T Mice
4.3. Retinal Thinning Occurs in A53T Mice after Acute L-DOPA Treatment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elbaz, A.; Carcaillon, L.; Kab, S.; Moisan, F. Epidemiology of Parkinson’s disease. Rev. Neurol. 2016, 172, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Tolosa, E.; Garrido, A.; Scholz, S.W.; Poewe, W. Challenges in the diagnosis of Parkinson’s disease. Lancet Neurol. 2021, 20, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Marino, B.L.B.; Souza, L.R.; Sousa, K.P.A.; Ferreira, J.V.; Padilha, E.C.; Silva, C.H.T.P.; Taft, C.A.; Hage-Melim, L.I.S. Parkinson’s disease: A Review from the Pathophysiology to Diagnosis, New Perspectives for Pharmacological Treatment. Mini Rev. Med. Chem. 2019, 20, 754–767. [Google Scholar] [CrossRef] [PubMed]
- Pringsheim, T.; Day, G.S.; Smith, D.B.; Rae-Grant, A.; Licking, N.; Armstrong, M.J.; de Bie, R.M.A.; Roze, E.; Miyasaki, J.M.; Hauser, R.A.; et al. Dopaminergic Therapy for Motor Symptoms in Early Parkinson Disease Practice Guideline Summary: A Report of the AAN Guideline Subcommittee. Neurology 2021, 97, 942–957. [Google Scholar] [CrossRef] [PubMed]
- Lane, E.L. L-DOPA for Parkinson’s disease-a bittersweet pill. Eur. J. Neurosci. 2019, 49, 384–398. [Google Scholar] [CrossRef] [PubMed]
- Ovallath, S.; Sulthana, B. Levodopa: History and Therapeutic Applications. Ann. Indian Acad. Neurol. 2017, 20, 185–189. [Google Scholar] [CrossRef]
- Rajput, A.H. Levodopa prolongs life expectancy and is non-toxic to substantia nigra. Park. Relat. Disord. 2001, 8, 95–100. [Google Scholar] [CrossRef]
- Rajput, A.H.; Uitti, R.J.; Rajput, A.H.; Offord, K.P. Timely levodopa (LD) administration prolongs survival in Parkinson’s disease. Park. Relat. Disord. 1997, 3, 159–165. [Google Scholar] [CrossRef]
- Shan, L.; Diaz, O.; Zhang, Y.; Ladenheim, B.; Cadet, J.-L.; Chiang, Y.-H.; Olson, L.; Hoffer, B.J.; Bäckman, C.M. L-Dopa induced dyskinesias in Parkinsonian mice: Disease severity or L-Dopa history. Brain Res. 2015, 1618, 261–269. [Google Scholar] [CrossRef]
- Miguelez, C.; Benazzouz, A.; Ugedo, L.; De Deurwaerdère, P. Impairment of Serotonergic Transmission by the Antiparkinsonian Drug L-DOPA: Mechanisms and Clinical Implications. Front. Cell Neurosci. 2017, 11, 274. [Google Scholar] [CrossRef]
- Martínez-Fernández, R.; Kibleur, A.; Chabardès, S.; Fraix, V.; Castrioto, A.; Lhommée, E.; Moro, E.; Lescoules, L.; Pelissier, P.; David, O.; et al. Different effects of levodopa and subthalamic stimulation on emotional conflict in Parkinson’s disease. Hum. Brain Mapp. 2018, 39, 5014–5027. [Google Scholar] [CrossRef] [PubMed]
- Beckers, M.; Bloem, B.R.; Verbeek, M.M. Mechanisms of peripheral levodopa resistance in Parkinson’s disease. NPJ Park. Dis. 2022, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Kempster, P.A.; Frankel, J.P.; Bovingdon, M.; Webster, R.; Lees, A.J.; Stern, G.M. Levodopa peripheral pharmacokinetics and duration of motor response in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1989, 52, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Nutt, J.G. Pharmacokinetics and pharmacodynamics of levodopa. Mov. Disord. 2008, 23 (Suppl. S3), S580–S584. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA 2020, 323, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, A.A.; Chakravarthy, S.; Phillips, J.R.; Gupta, A.; Keri, S.; Polner, B.; Frank, M.J.; Jahanshahi, M. Motor symptoms in Parkinson’s disease: A unified framework. Neurosci. Biobehav. Rev. 2016, 68, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. 2008, 23, 2129–2170. [Google Scholar] [CrossRef]
- Lashuel, H.A.; Overk, C.R.; Oueslati, A.; Masliah, E. The many faces of α-synuclein: From structure and toxicity to therapeutic target. Nat. Rev. Neurosci. 2013, 14, 38–48. [Google Scholar] [CrossRef]
- Garcia-Ruiz, P.J.; Chaudhuri, K.R.; Martinez-Martin, P. Non-motor symptoms of Parkinson’s disease A review…from the past. J. Neurol. Sci. 2014, 338, 30–33. [Google Scholar] [CrossRef]
- Schapira, A.H.V.; Chaudhuri, K.R.; Jenner, P. Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 2017, 18, 435–450. [Google Scholar] [CrossRef]
- Pfeiffer, R.F. Non-motor symptoms in Parkinson’s disease. Park. Relat. Disord. 2016, 22 (Suppl. S1), S119–S122. [Google Scholar] [CrossRef] [PubMed]
- Doty, R.L. Olfactory dysfunction in Parkinson disease. Nat. Rev. Neurol. 2012, 8, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Fanciulli, A.; Wenning, G.K. Autonomic failure: A neglected presentation of Parkinson’s disease. Lancet Neurol. 2021, 20, 781–782. [Google Scholar] [CrossRef] [PubMed]
- Weil, R.S.; Schrag, A.E.; Warren, J.D.; Crutch, S.J.; Lees, A.J.; Morris, H.R. Visual dysfunction in Parkinson’s disease. Brain 2016, 139, 2827–2843. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Kågedal, K.; Halliday, G.M. Alpha-synuclein biology in Lewy body diseases. Alzheimers Res. Ther. 2014, 6, 73. [Google Scholar] [CrossRef] [PubMed]
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. Alpha-synuclein in Lewy bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef] [PubMed]
- Post, M.R.; Lieberman, O.J.; Mosharov, E.V. Can Interactions Between α-Synuclein, Dopamine and Calcium Explain Selective Neurodegeneration in Parkinson’s Disease? Front. Neurosci. 2018, 12, 161. [Google Scholar] [CrossRef]
- Mor, D.E.; Ischiropoulos, H. The Convergence of Dopamine and α-Synuclein: Implications for Parkinson’s Disease. J. Exp. Neurosci. 2018, 12, 1179069518761360. [Google Scholar] [CrossRef]
- Conway, K.A.; Rochet, J.C.; Bieganski, R.M.; Lansbury, P.T. Kinetic stabilization of the alpha-synuclein protofibril by a dopamine-alpha-synuclein adduct. Science 2001, 294, 1346–1349. [Google Scholar] [CrossRef]
- Bucolo, C.; Leggio, G.M.; Drago, F.; Salomone, S. Dopamine outside the brain: The eye, cardiovascular system and endocrine pancreas. Pharmacol. Ther. 2019, 203, 107392. [Google Scholar] [CrossRef]
- Frederick, J.M.; Rayborn, M.E.; Laties, A.M.; Lam, D.M.; Hollyfield, J.G. Dopaminergic neurons in the human retina. J. Comp. Neurol. 1982, 210, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Chen, V.; Saez-Atienzar, S. A tango for two: Dopamine and α-synuclein synergy may explain nigrostriatal degeneration in Parkinson’s disease. Mov. Disord. 2018, 33, 249. [Google Scholar] [CrossRef] [PubMed]
- Mor, D.E.; Tsika, E.; Mazzulli, J.R.; Gould, N.S.; Kim, H.; Daniels, M.J.; Doshi, S.; Gupta, P.; Grossman, J.L.; Tan, V.X.; et al. Dopamine induces soluble α-synuclein oligomers and nigrostriatal degeneration. Nat. Neurosci. 2017, 20, 1560–1568. [Google Scholar] [CrossRef] [PubMed]
- Sinn, R.; Wittbrodt, J. An eye on eye development. Mech. Dev. 2013, 130, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Juárez Olguín, H.; Calderón Guzmán, D.; Hernández García, E.; Barragán Mejía, G. The Role of Dopamine and Its Dysfunction as a Consequence of Oxidative Stress. Oxidative Med. Cell Longev. 2016, 2016, 9730467. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H. Neurotransmitters in the Retina. In Webvision: The Organization of the Retina and Visual System; Kolb, H., Fernandez, E., Nelson, R., Eds.; University of Utah Health Sciences Center: Salt Lake City, UT, USA, 1995. Available online: http://www.ncbi.nlm.nih.gov/books/NBK11546/ (accessed on 20 September 2021).
- Popova, E. Role of Dopamine in Retinal Function. In Webvision: The Organization of the Retina and Visual System; Kolb, H., Fernandez, E., Nelson, R., Eds.; University of Utah Health Sciences Center: Salt Lake City, UT, USA, 1995. Available online: http://www.ncbi.nlm.nih.gov/books/NBK561740/ (accessed on 21 September 2021).
- Witkovsky, P. Dopamine and retinal function. Doc. Ophthalmol. 2004, 108, 17–40. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Pardue, M.T.; Iuvone, P.M.; Qu, J. Dopamine signaling and myopia development: What are the key challenges. Prog. Retin. Eye Res. 2017, 61, 60–71. [Google Scholar] [CrossRef]
- Korshunov, K.S.; Blakemore, L.J.; Trombley, P.Q. Dopamine: A Modulator of Circadian Rhythms in the Central Nervous System. Front. Cell Neurosci. 2017, 11, 91. [Google Scholar] [CrossRef]
- Linden, R.; Martins, R.A.P.; Silveira, M.S. Control of programmed cell death by neurotransmitters and neuropeptides in the developing mammalian retina. Prog. Retin. Eye Res. 2005, 24, 457–491. [Google Scholar] [CrossRef]
- Kolb, H. Amacrine cells of the mammalian retina: Neurocircuitry and functional roles. Eye 1997, 11 Pt 6, 904–923. [Google Scholar] [CrossRef]
- Price, M.J.; Feldman, R.G.; Adelberg, D.; Kayne, H. Abnormalities in color vision and contrast sensitivity in Parkinson’s disease. Neurology 1992, 42, 887–890. [Google Scholar] [CrossRef] [PubMed]
- Peppe, A.; Stanzione, P.; Pierelli, F.; De Angelis, D.; Pierantozzi, M.; Bernardi, G. Visual alterations in de novo Parkinson’s disease: Pattern electroretinogram latencies are more delayed and more reversible by levodopa than are visual evoked potentials. Neurology 1995, 45, 1144–1148. [Google Scholar] [CrossRef] [PubMed]
- Gottlob, I.; Weghaupt, H.; Vass, C. Effect of levodopa on the human luminance electroretinogram. Investig. Ophthalmol. Vis. Sci. 1990, 31, 1252–1258. [Google Scholar]
- Jaffe, M.J.; Bruno, G.; Campbell, G.; Lavine, R.A.; Karson, C.N.; Weinberger, D.R. Ganzfeld electroretinographic findings in parkinsonism: Untreated patients and the effect of levodopa intravenous infusion. J. Neurol. Neurosurg. Psychiatry 1987, 50, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Hutton, J.T.; Morris, J.L.; Elias, J.W. Levodopa improves spatial contrast sensitivity in Parkinson’s disease. Arch. Neurol. 1993, 50, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Büttner, T.; Kuhn, W.; Patzold, T.; Przuntek, H. L-Dopa improves colour vision in Parkinson’s disease. J. Neural Transm. Park. Dis. Dement. Sect. 1994, 7, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.T.O.; Hui, F.; Charng, J.; Velaedan, S.; van Koeverden, A.K.; Lim, J.K.H.; He, Z.; Wong, V.H.Y.; Vingrys, A.J.; Bui, B.V.; et al. Retinal biomarkers provide “insight” into cortical pharmacology and disease. Pharmacol. Ther. 2017, 175, 151–177. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Tugcu, B.; Coskun, C.; Ekinci, C.; Nacaroglu, S.A. Effects of levodopa on retina in Parkinson disease. Eur. J. Ophthalmol. 2014, 24, 114–119. [Google Scholar] [CrossRef]
- Lee, M.K.; Stirling, W.; Xu, Y.; Xu, X.; Qui, D.; Mandir, A.S.; Dawson, T.M.; Copeland, N.G.; Jenkins, N.A.; Price, D.L. Human α-synuclein-harboring familial Parkinson’s disease-linked Ala-53 → Thr mutation causes neurodegenerative disease with α-synuclein aggregation in transgenic mice. Proc. Natl. Acad. Sci. USA 2002, 99, 8968–8973. [Google Scholar] [CrossRef]
- Oaks, A.W.; Frankfurt, M.; Finkelstein, D.I.; Sidhu, A. Age-dependent effects of A53T alpha-synuclein on behavior and dopaminergic function. PLoS ONE 2013, 8, e60378. [Google Scholar] [CrossRef]
- Paumier, K.L.; Sukoff Rizzo, S.J.; Berger, Z.; Chen, Y.; Gonzales, C.; Kaftan, E.; Li, L.; Lotarski, S.; Monaghan, M.; Shen, W.; et al. Behavioral characterization of A53T mice reveals early and late stage deficits related to Parkinson’s disease. PLoS ONE 2013, 8, e70274. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Song, N.; Jia, F.; Tang, T.; Bao, W.; Zuo, C.; Xie, J.; Jiang, H. Genomic DNA levels of mutant alpha-synuclein correlate with non-motor symptoms in an A53T Parkinson’s disease mouse model. Neurochem. Int. 2018, 114, 71–79. [Google Scholar] [CrossRef]
- Billings, J.L.; Hare, D.J.; Nurjono, M.; Volitakis, I.; Cherny, R.A.; Bush, A.I.; Adlard, P.A.; Finkelstein, D.I. Effects of Neonatal Iron Feeding and Chronic Clioquinol Administration on the Parkinsonian Human A53T Transgenic Mouse. ACS Chem. Neurosci. 2016, 7, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, D.I.; Hare, D.J.; Billings, J.L.; Sedjahtera, A.; Nurjono, M.; Arthofer, E.; George, S.; Culvenor, J.G.; Bush, A.I.; Adlard, P.A. Clioquinol Improves Cognitive, Motor Function, and Microanatomy of the Alpha-Synuclein hA53T Transgenic Mice. ACS Chem. Neurosci. 2016, 7, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, D.I.; Billings, J.L.; Adlard, P.A.; Ayton, S.; Sedjahtera, A.; Masters, C.L.; Wilkins, S.; Shackleford, D.M.; Charman, S.A.; Bal, W.; et al. The novel compound PBT434 prevents iron mediated neurodegeneration and alpha-synuclein toxicity in multiple models of Parkinson’s disease. Acta Neuropathol. Commun. 2017, 5, 53. [Google Scholar] [CrossRef] [PubMed]
- Billings, J.L.; Gordon, S.L.; Rawling, T.; Doble, P.A.; Bush, A.I.; Adlard, P.A.; Finkelstein, D.I.; Hare, D.J. l-3,4-dihydroxyphenylalanine (l-DOPA) modulates brain iron, dopaminergic neurodegeneration and motor dysfunction in iron overload and mutant alpha-synuclein mouse models of Parkinson’s disease. J. Neurochem. 2019, 150, 88–106. [Google Scholar] [CrossRef] [PubMed]
- Tran, K.K.N.; Wong, V.H.Y.; Hoang, A.; Finkelstein, D.I.; Bui, B.V.; Nguyen, C.T.O. Retinal alpha-synuclein accumulation correlates with retinal dysfunction and structural thinning in the A53T mouse model of Parkinson’s disease. Front. Neurosci. 2023, 17, 1146979. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. J. Physiol. 2020, 598, 3793–3801. [Google Scholar] [CrossRef]
- Parish, C.L.; Stanic, D.; Drago, J.; Borrelli, E.; Finkelstein, D.I.; Horne, M.K. Effects of long-term treatment with dopamine receptor agonists and antagonists on terminal arbor size. Eur. J. Neurosci. 2002, 16, 787–794. [Google Scholar] [CrossRef]
- Blades, F.; Wong, V.H.Y.; Nguyen, C.T.O.; Bui, B.V.; Kilpatrick, T.J.; Binder, M.D. Tyro3 Contributes to Retinal Ganglion Cell Function, Survival and Dendritic Density in the Mouse Retina. Front. Neurosci. 2020, 14, 840. [Google Scholar] [CrossRef]
- Tran, K.K.N.; Wong, V.H.Y.; Lim, J.K.H.; Shahandeh, A.; Hoang, A.; Finkelstein, D.I.; Bui, B.V.; Nguyen, C.T.O. Characterization of retinal function and structure in the MPTP murine model of Parkinson’s disease. Sci. Rep. 2022, 12, 7610. [Google Scholar] [CrossRef]
- Behn, D.; Doke, A.; Racine, J.; Casanova, C.; Chemtob, S.; Lachapelle, P. Dark adaptation is faster in pigmented than albino rats. Doc. Ophthalmol. 2003, 106, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Lamb, T.D.; Pugh, E.N. A quantitative account of the activation steps involved in phototransduction in amphibian photoreceptors. J. Physiol. 1992, 449, 719–758. [Google Scholar] [CrossRef] [PubMed]
- Hood, D.C.; Birch, D.G. A computational model of the amplitude and implicit time of the b-wave of the human ERG. Vis. Neurosci. 1992, 8, 107–126. [Google Scholar] [CrossRef]
- Wang, H.H.; Cuenca, N.; Kolb, H. Development of morphological types and distribution patterns of amacrine cells immunoreactive to tyrosine hydroxylase in the cat retina. Vis. Neurosci. 1990, 4, 159–175. [Google Scholar] [CrossRef]
- Costa, G.; Sisalli, M.J.; Simola, N.; Della Notte, S.; Casu, M.A.; Serra, M.; Pinna, A.; Feliciello, A.; Annunziato, L.; Scorziello, A.; et al. Gender Differences in Neurodegeneration, Neuroinflammation and Na+-Ca2+ Exchangers in the Female A53T Transgenic Mouse Model of Parkinson’s Disease. Front. Aging Neurosci. 2020, 12, 118. [Google Scholar] [CrossRef] [PubMed]
- Marrocco, E.; Indrieri, A.; Esposito, F.; Tarallo, V.; Carboncino, A.; Alvino, F.G.; De Falco, S.; Franco, B.; De Risi, M.; De Leonibus, E. α-synuclein overexpression in the retina leads to vision impairment and degeneration of dopaminergic amacrine cells. Sci. Rep. 2020, 10, 9619. [Google Scholar] [CrossRef] [PubMed]
- Mello, L.G.M.; Paraguay, I.B.B.; Andrade, T.d.S.; Rocha, A.A.d.N.; Barbosa, E.R.; Oyamada, M.K.; Monteiro, M.L.R. Electroretinography reveals retinal dysfunction in Parkinson’s disease despite normal high-resolution optical coherence tomography findings. Park. Relat. Disord. 2022, 101, 90–95. [Google Scholar] [CrossRef]
- Ellis, C.; Allen, T.; Marsden, C.; Ikeda, H. Electroretinographic Abnormalities in Idiopathic Parkinsons-Disease and the Effect of Levodopa Administration. Clin. Vis. Sci. 1987, 1, 347–355. [Google Scholar]
- Netser, R.; Demmin, D.L.; Dobkin, R.; Goldstein, A.; Roché, M.; Netser Zernik, A.; Silverstein, S.M. Flash Electroretinography Parameters and Parkinson’s Disease. J. Park. Dis. 2021, 11, 251–259. [Google Scholar] [CrossRef]
- Ortuño-Lizarán, I.; Sánchez-Sáez, X.; Lax, P.; Serrano, G.E.; Beach, T.G.; Adler, C.H.; Cuenca, N. Dopaminergic Retinal Cell Loss and Visual Dysfunction in Parkinson Disease. Ann. Neurol. 2020, 88, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Legros, J.; Versaux-Botteri, C.; Vernier, P. Dopamine receptor localization in the mammalian retina. Mol. Neurobiol. 1999, 19, 181–204. [Google Scholar] [CrossRef] [PubMed]
- Shimozawa, A.; Fujita, Y.; Kondo, H.; Takimoto, Y.; Terada, M.; Sanagi, M.; Hisanaga, S.; Hasegawa, M. Effect of L-DOPA/Benserazide on Propagation of Pathological α-Synuclein. Front. Neurosci. 2019, 13, 595. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, P.; Müller, A.-K.; Südmeyer, M.; Ferrea, S.; Ringelstein, M.; Cohn, E.; Aktas, O.; Dietlein, T.; Lappas, A.; Foerster, A.; et al. Optical coherence tomography in parkinsonian syndromes. PLoS ONE 2012, 7, e34891. [Google Scholar] [CrossRef]
- Müller, A.-K.; Blasberg, C.; Südmeyer, M.; Aktas, O.; Albrecht, P. Photoreceptor layer thinning in parkinsonian syndromes. Mov. Disord. 2014, 29, 1222–1223. [Google Scholar] [CrossRef] [PubMed]
- Roth, N.M.; Saidha, S.; Zimmermann, H.; Brandt, A.U.; Isensee, J.; Benkhellouf-Rutkowska, A.; Dornauer, M.; Kühn, A.A.; Müller, T.; Calabresi, P.A.; et al. Photoreceptor layer thinning in idiopathic Parkinson’s disease. Mov. Disord. 2014, 29, 1163–1170. [Google Scholar] [CrossRef]
- Schneider, M.; Müller, H.-P.; Lauda, F.; Tumani, H.; Ludolph, A.C.; Kassubek, J.; Pinkhardt, E.H. Retinal single-layer analysis in Parkinsonian syndromes: An optical coherence tomography study. J. Neural. Transm. 2014, 121, 41–47. [Google Scholar] [CrossRef]
- Unlu, M.; Gulmez Sevim, D.; Gultekin, M.; Karaca, C. Correlations among multifocal electroretinography and optical coherence tomography findings in patients with Parkinson’s disease. Neurol. Sci. 2018, 39, 533–541. [Google Scholar] [CrossRef]
- Tremoleda, J.L.; Kerton, A.; Gsell, W. Anaesthesia and physiological monitoring during in vivo imaging of laboratory rodents: Considerations on experimental outcomes and animal welfare. EJNMMI Res. 2012, 2, 44. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, K.K.N.; Wong, V.H.Y.; Vessey, K.A.; Finkelstein, D.I.; Bui, B.V.; Nguyen, C.T.O. Levodopa Rescues Retinal Function in the Transgenic A53T Alpha-Synuclein Model of Parkinson’s Disease. Biomedicines 2024, 12, 130. https://doi.org/10.3390/biomedicines12010130
Tran KKN, Wong VHY, Vessey KA, Finkelstein DI, Bui BV, Nguyen CTO. Levodopa Rescues Retinal Function in the Transgenic A53T Alpha-Synuclein Model of Parkinson’s Disease. Biomedicines. 2024; 12(1):130. https://doi.org/10.3390/biomedicines12010130
Chicago/Turabian StyleTran, Katie K. N., Vickie H. Y. Wong, Kirstan A. Vessey, David I. Finkelstein, Bang V. Bui, and Christine T. O. Nguyen. 2024. "Levodopa Rescues Retinal Function in the Transgenic A53T Alpha-Synuclein Model of Parkinson’s Disease" Biomedicines 12, no. 1: 130. https://doi.org/10.3390/biomedicines12010130
APA StyleTran, K. K. N., Wong, V. H. Y., Vessey, K. A., Finkelstein, D. I., Bui, B. V., & Nguyen, C. T. O. (2024). Levodopa Rescues Retinal Function in the Transgenic A53T Alpha-Synuclein Model of Parkinson’s Disease. Biomedicines, 12(1), 130. https://doi.org/10.3390/biomedicines12010130







