Anti-Aging Drugs and the Related Signal Pathways
Abstract
1. Introduction

2. Rapamycin and the Aging-Related mTOR Signal Pathway (Figure 2)

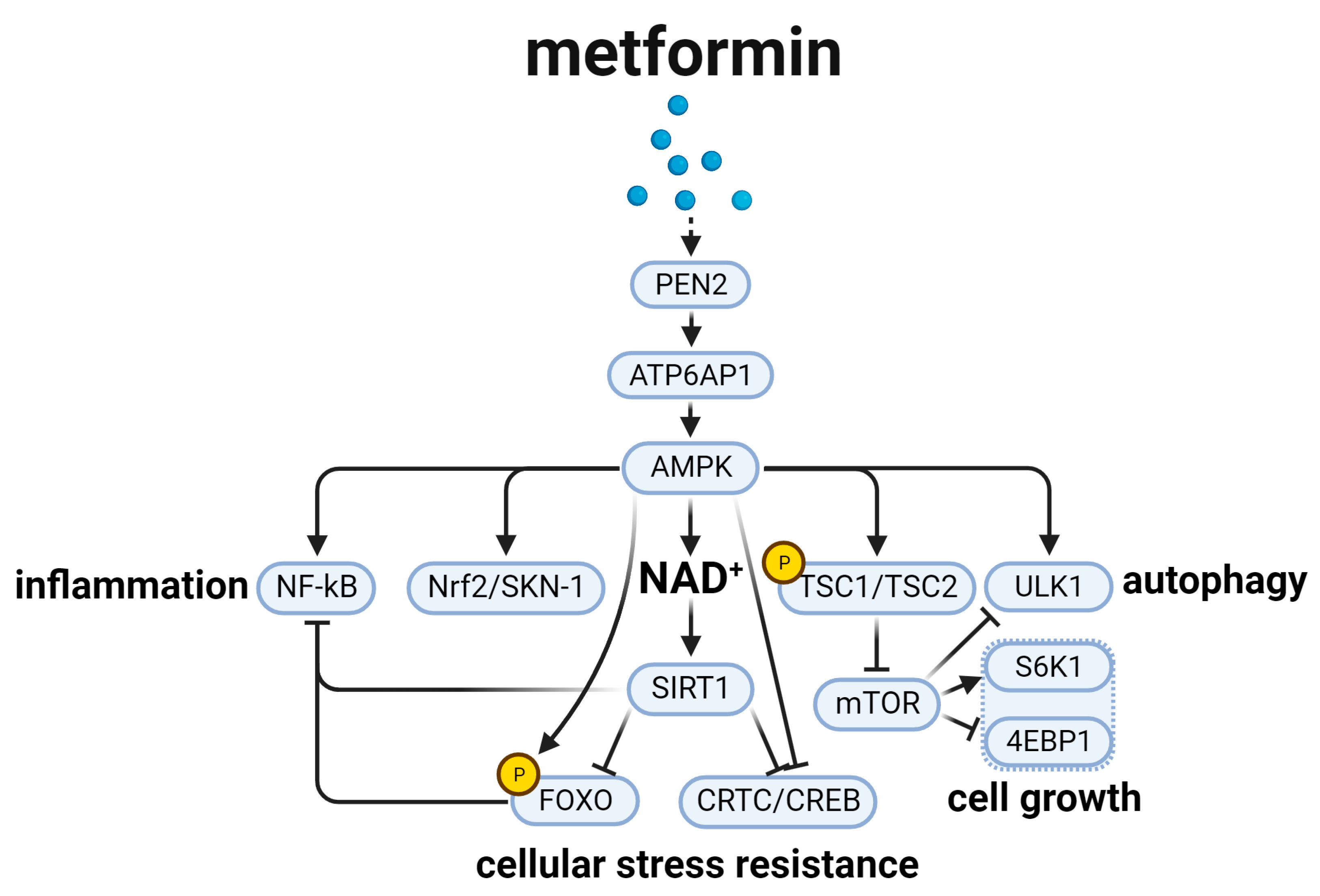
3. Metformin and the Aging-Related AMPK Signal Pathway (Figure 3)
4. Acarbose and Aging-Related Nutrient Signal Pathways (Figure 4)

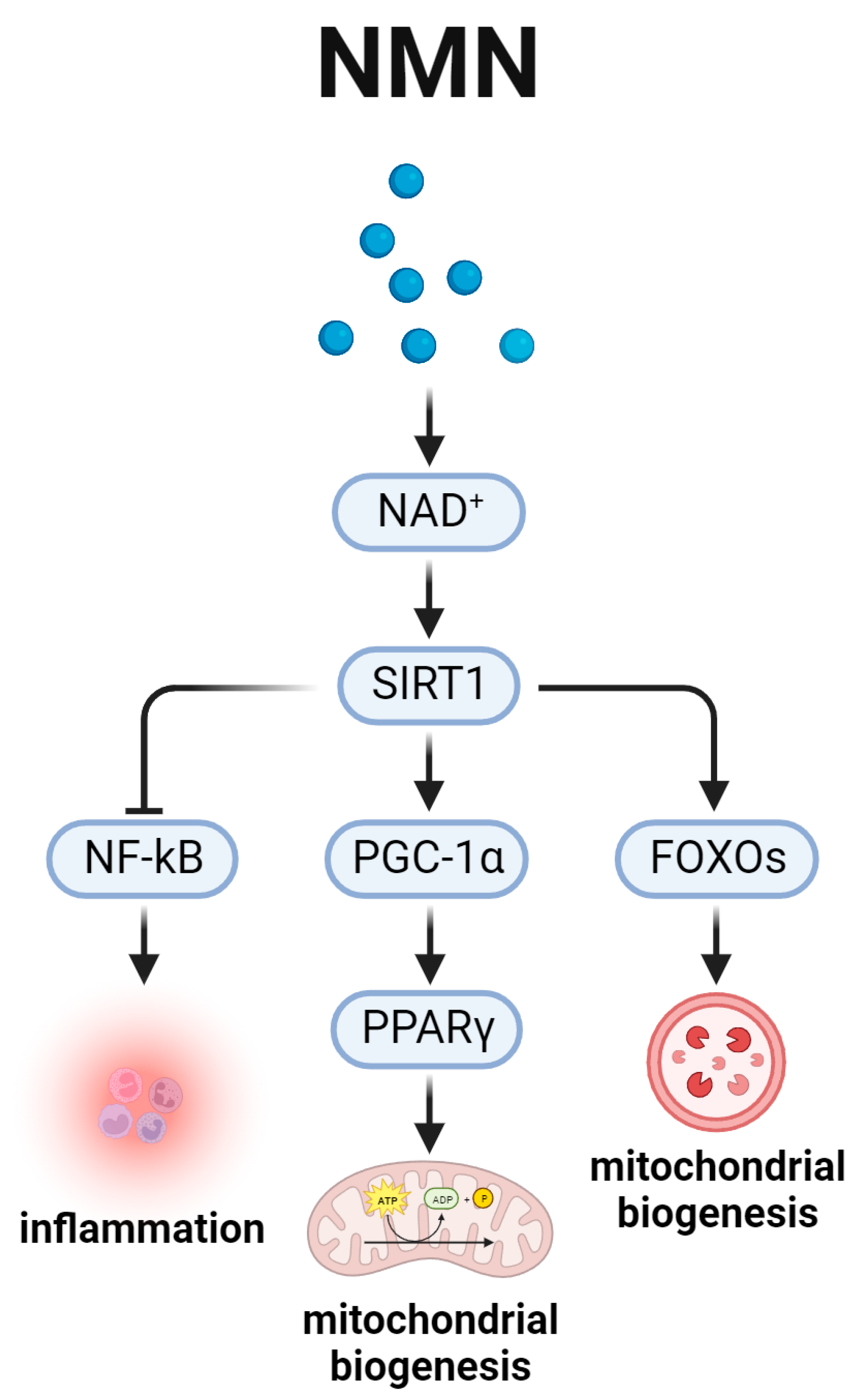
5. Nicotinamide Adenine Dinucleotide and the Aging-Related SIRT1 Signal Pathway (Figure 5)
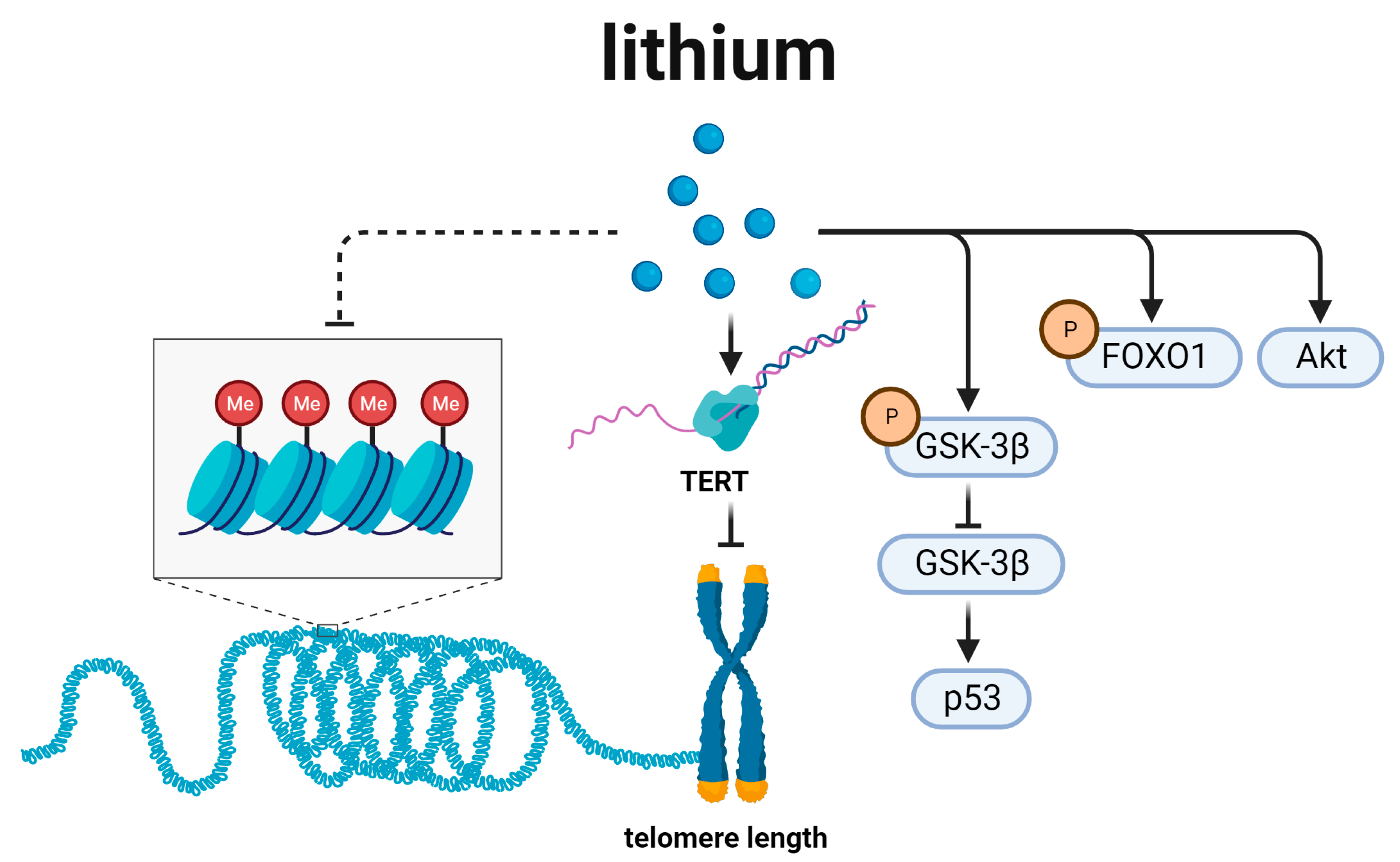
6. Lithium and Telomere Length Regulation (Figure 6)
7. NSAIDs and the Aging-Related Energy Metabolism (Figure 7)

8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cho, S.J.; Stout-Delgado, H.W. Aging and Lung Disease. Annu. Rev. Physiol. 2020, 82, 433–459. [Google Scholar] [CrossRef] [PubMed]
- Escoubas, C.C.; Silva-García, C.G.; Mair, W.B. Deregulation of CRTCs in Aging and Age-Related Disease Risk. Trends Genet. 2017, 33, 303–321. [Google Scholar] [CrossRef] [PubMed]
- Moskalev, A.; Zhikrivetskaya, S.; Shaposhnikov, M.; Dobrovolskaya, E.; Gurinovich, R.; Kuryan, O.; Pashuk, A.; Jellen, L.C.; Aliper, A.; Peregudov, A.; et al. Aging Chart: A community resource for rapid exploratory pathway analysis of age-related processes. Nucleic Acids Res. 2016, 44, D894–D899. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Song, W.; Li, J.; Jing, Y.; Liang, C.; Zhang, L.; Zhang, X.; Zhang, W.; Liu, B.; An, Y.; et al. The landscape of aging. Sci. China Life Sci. 2022, 65, 2354–2454. [Google Scholar] [CrossRef] [PubMed]
- Han, L.K.M.; Aghajani, M.; Clark, S.L.; Chan, R.F.; Hattab, M.W.; Shabalin, A.A.; Zhao, M.; Kumar, G.; Xie, L.Y.; Jansen, R.; et al. Epigenetic Aging in Major Depressive Disorder. Am. J. Psychiatry 2018, 175, 774–782. [Google Scholar] [CrossRef] [PubMed]
- de Magalhães, J.P.; Wuttke, D.; Wood, S.H.; Plank, M.; Vora, C. Genome-environment interactions that modulate aging: Powerful targets for drug discovery. Pharmacol. Rev. 2012, 64, 88–101. [Google Scholar] [CrossRef]
- Tang, D.; Tao, S.; Chen, Z.; Koliesnik, I.O.; Calmes, P.G.; Hoerr, V.; Han, B.; Gebert, N.; Zörnig, M.; Löffler, B.; et al. Dietary restriction improves repopulation but impairs lymphoid differentiation capacity of hematopoietic stem cells in early aging. J. Exp. Med. 2016, 213, 535–553. [Google Scholar] [CrossRef]
- Soultoukis, G.A.; Partridge, L. Dietary Protein, Metabolism, and Aging. Annu. Rev. Biochem. 2016, 85, 5–34. [Google Scholar] [CrossRef]
- Piper, M.D.; Partridge, L.; Raubenheimer, D.; Simpson, S.J. Dietary restriction and aging: A unifying perspective. Cell Metab. 2011, 14, 154–160. [Google Scholar] [CrossRef]
- Hood, D.A.; Memme, J.M.; Oliveira, A.N.; Triolo, M. Maintenance of Skeletal Muscle Mitochondria in Health, Exercise, and Aging. Annu. Rev. Physiol. 2019, 81, 19–41. [Google Scholar] [CrossRef]
- Huang, Q.; Ning, Y.; Liu, D.; Zhang, Y.; Li, D.; Zhang, Y.; Yin, Z.; Fu, B.; Cai, G.; Sun, X.; et al. A Young Blood Environment Decreases Aging of Senile Mice Kidneys. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2018, 73, 421–428. [Google Scholar] [CrossRef]
- Castellano, J.M.; Mosher, K.I.; Abbey, R.J.; McBride, A.A.; James, M.L.; Berdnik, D.; Shen, J.C.; Zou, B.; Xie, X.S.; Tingle, M.; et al. Human umbilical cord plasma proteins revitalize hippocampal function in aged mice. Nature 2017, 544, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Firsanov, D.; Zhang, Z.; Cheng, Y.; Luo, L.; Tombline, G.; Tan, R.; Simon, M.; Henderson, S.; Steffan, J.; et al. SIRT6 Is Responsible for More Efficient DNA Double-Strand Break Repair in Long-Lived Species. Cell 2019, 177, 622–638.e622. [Google Scholar] [CrossRef] [PubMed]
- Partridge, L.; Fuentealba, M.; Kennedy, B.K. The quest to slow ageing through drug discovery. Nat. Rev. Drug Discov. 2020, 19, 513–532. [Google Scholar] [CrossRef] [PubMed]
- Lindenfeld, J.; Miller, G.G.; Shakar, S.F.; Zolty, R.; Lowes, B.D.; Wolfel, E.E.; Mestroni, L.; Kobashigawa, J. Drug therapy in the heart transplant recipient: Part II: Immunosuppressive drugs. Circulation 2004, 110, 3858–3865. [Google Scholar] [CrossRef] [PubMed]
- Powers, R.W., III; Kaeberlein, M.; Caldwell, S.D.; Kennedy, B.K.; Fields, S. Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes Dev. 2006, 20, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Houtkooper, R.H.; Mouchiroud, L.; Ryu, D.; Moullan, N.; Katsyuba, E.; Knott, G.; Williams, R.W.; Auwerx, J. Mitonuclear protein imbalance as a conserved longevity mechanism. Nature 2013, 497, 451–457. [Google Scholar] [CrossRef]
- Bjedov, I.; Toivonen, J.M.; Kerr, F.; Slack, C.; Jacobson, J.; Foley, A.; Partridge, L. Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metab. 2010, 11, 35–46. [Google Scholar] [CrossRef]
- Harrison, D.E.; Strong, R.; Sharp, Z.D.; Nelson, J.F.; Astle, C.M.; Flurkey, K.; Nadon, N.L.; Wilkinson, J.E.; Frenkel, K.; Carter, C.S.; et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 2009, 460, 392–395. [Google Scholar] [CrossRef]
- Neff, F.; Flores-Dominguez, D.; Ryan, D.P.; Horsch, M.; Schröder, S.; Adler, T.; Afonso, L.C.; Aguilar-Pimentel, J.A.; Becker, L.; Garrett, L.; et al. Rapamycin extends murine lifespan but has limited effects on aging. J. Clin. Investig. 2013, 123, 3272–3291. [Google Scholar] [CrossRef]
- Chung, C.L.; Lawrence, I.; Hoffman, M.; Elgindi, D.; Nadhan, K.; Potnis, M.; Jin, A.; Sershon, C.; Binnebose, R.; Lorenzini, A.; et al. Topical rapamycin reduces markers of senescence and aging in human skin: An exploratory, prospective, randomized trial. GeroScience 2019, 41, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.C.; Rabinovitch, P.S.; Kaeberlein, M. mTOR is a key modulator of ageing and age-related disease. Nature 2013, 493, 338–345. [Google Scholar] [CrossRef]
- Liu, G.Y.; Sabatini, D.M. mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 2020, 21, 183–203. [Google Scholar] [CrossRef] [PubMed]
- Ferrara-Romeo, I.; Martinez, P.; Saraswati, S.; Whittemore, K.; Graña-Castro, O.; Thelma Poluha, L.; Serrano, R.; Hernandez-Encinas, E.; Blanco-Aparicio, C.; Maria Flores, J.; et al. The mTOR pathway is necessary for survival of mice with short telomeres. Nat. Commun. 2020, 11, 1168. [Google Scholar] [CrossRef] [PubMed]
- Laberge, R.M.; Sun, Y.; Orjalo, A.V.; Patil, C.K.; Freund, A.; Zhou, L.; Curran, S.C.; Davalos, A.R.; Wilson-Edell, K.A.; Liu, S.; et al. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat. Cell Biol. 2015, 17, 1049–1061. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, J.T.; Rodgers, J.T.; Arlow, D.H.; Vazquez, F.; Mootha, V.K.; Puigserver, P. mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature 2007, 450, 736–740. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Gravel, S.P.; Chénard, V.; Sikström, K.; Zheng, L.; Alain, T.; Gandin, V.; Avizonis, D.; Arguello, M.; Zakaria, C.; et al. mTORC1 controls mitochondrial activity and biogenesis through 4E-BP-dependent translational regulation. Cell Metab. 2013, 18, 698–711. [Google Scholar] [CrossRef]
- Sarkar, S.; Ravikumar, B.; Floto, R.A.; Rubinsztein, D.C. Rapamycin and mTOR-independent autophagy inducers ameliorate toxicity of polyglutamine-expanded huntingtin and related proteinopathies. Cell Death Differ. 2009, 16, 46–56. [Google Scholar] [CrossRef]
- Mafi, S.; Ahmadi, E.; Meehan, E.; Chiari, C.; Mansoori, B.; Sadeghi, H.; Milani, S.; Jafarinia, M.; Taeb, S.; Mafakheri, B.B.; et al. The mTOR Signaling Pathway Interacts with the ER Stress Response and the Unfolded Protein Response in Cancer. Cancer Res. 2023, 83, 2450–2460. [Google Scholar] [CrossRef]
- Hansen, M.; Taubert, S.; Crawford, D.; Libina, N.; Lee, S.J.; Kenyon, C. Lifespan extension by conditions that inhibit translation in Caenorhabditis elegans. Aging Cell 2007, 6, 95–110. [Google Scholar] [CrossRef]
- Selman, C.; Tullet, J.M.; Wieser, D.; Irvine, E.; Lingard, S.J.; Choudhury, A.I.; Claret, M.; Al-Qassab, H.; Carmignac, D.; Ramadani, F.; et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science 2009, 326, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Robida-Stubbs, S.; Glover-Cutter, K.; Lamming, D.W.; Mizunuma, M.; Narasimhan, S.D.; Neumann-Haefelin, E.; Sabatini, D.M.; Blackwell, T.K. TOR signaling and rapamycin influence longevity by regulating SKN-1/Nrf and DAF-16/FoxO. Cell Metab. 2012, 15, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Thoreen, C.C.; Chantranupong, L.; Keys, H.R.; Wang, T.; Gray, N.S.; Sabatini, D.M. A unifying model for mTORC1-mediated regulation of mRNA translation. Nature 2012, 485, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Bonawitz, N.D.; Chatenay-Lapointe, M.; Pan, Y.; Shadel, G.S. Reduced TOR signaling extends chronological life span via increased respiration and upregulation of mitochondrial gene expression. Cell Metab. 2007, 5, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Shadel, G.S. Extension of chronological life span by reduced TOR signaling requires down-regulation of Sch9p and involves increased mitochondrial OXPHOS complex density. Aging 2009, 1, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.F.; Latorre-Muro, P.; Puigserver, P. Mechanisms of mitochondrial respiratory adaptation. Nat. Rev. Mol. Cell Biol. 2022, 23, 817–835. [Google Scholar] [CrossRef]
- Ohsumi, Y. Historical landmarks of autophagy research. Cell Res. 2014, 24, 9–23. [Google Scholar] [CrossRef]
- Mihaylova, M.M.; Shaw, R.J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 2011, 13, 1016–1023. [Google Scholar] [CrossRef]
- Rosenbluth, J.M.; Pietenpol, J.A. mTOR regulates autophagy-associated genes downstream of p73. Autophagy 2009, 5, 114–116. [Google Scholar] [CrossRef]
- Qin, L.; Wang, Z.; Tao, L.; Wang, Y. ER stress negatively regulates AKT/TSC/mTOR pathway to enhance autophagy. Autophagy 2010, 6, 239–247. [Google Scholar] [CrossRef]
- Chen, D.; Thomas, E.L.; Kapahi, P. HIF-1 modulates dietary restriction-mediated lifespan extension via IRE-1 in Caenorhabditis elegans. PLoS Genet. 2009, 5, e1000486. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.S.; Yu, W.S.; Lim, L.W. Exploring ER stress response in cellular aging and neuroinflammation in Alzheimer’s disease. Ageing Res. Rev. 2021, 70, 101417. [Google Scholar] [CrossRef] [PubMed]
- Trelinska, J.; Dachowska, I.; Kotulska, K.; Fendler, W.; Jozwiak, S.; Mlynarski, W. Complications of mammalian target of rapamycin inhibitor anticancer treatment among patients with tuberous sclerosis complex are common and occasionally life-threatening. Anti Cancer Drugs 2015, 26, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Lamming, D.W.; Ye, L.; Sabatini, D.M.; Baur, J.A. Rapalogs and mTOR inhibitors as anti-aging therapeutics. J. Clin. Investig. 2013, 123, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, B.K.; Lamming, D.W. The Mechanistic Target of Rapamycin: The Grand ConducTOR of Metabolism and Aging. Cell Metab. 2016, 23, 990–1003. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, B.; Qazi, Y.; Wellen, J.R. Strategies for the management of adverse events associated with mTOR inhibitors. Transplant. Rev. 2014, 28, 126–133. [Google Scholar] [CrossRef]
- Hurvitz, S.A.; Andre, F.; Jiang, Z.; Shao, Z.; Mano, M.S.; Neciosup, S.P.; Tseng, L.M.; Zhang, Q.; Shen, K.; Liu, D.; et al. Combination of everolimus with trastuzumab plus paclitaxel as first-line treatment for patients with HER2-positive advanced breast cancer (BOLERO-1): A phase 3, randomised, double-blind, multicentre trial. Lancet Oncol. 2015, 16, 816–829. [Google Scholar] [CrossRef]
- Yao, J.C.; Fazio, N.; Singh, S.; Buzzoni, R.; Carnaghi, C.; Wolin, E.; Tomasek, J.; Raderer, M.; Lahner, H.; Voi, M.; et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): A randomised, placebo-controlled, phase 3 study. Lancet 2016, 387, 968–977. [Google Scholar] [CrossRef]
- Krentz, A.J.; Bailey, C.J. Oral antidiabetic agents: Current role in type 2 diabetes mellitus. Drugs 2005, 65, 385–411. [Google Scholar] [CrossRef]
- Massi-Benedetti, M.; Orsini-Federici, M. Treatment of type 2 diabetes with combined therapy: What are the pros and cons? Diabetes Care 2008, 31 (Suppl. S2), S131–S135. [Google Scholar] [CrossRef]
- Cabreiro, F.; Au, C.; Leung, K.Y.; Vergara-Irigaray, N.; Cochemé, H.M.; Noori, T.; Weinkove, D.; Schuster, E.; Greene, N.D.; Gems, D. Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell 2013, 153, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Foretz, M.; Guigas, B.; Bertrand, L.; Pollak, M.; Viollet, B. Metformin: From mechanisms of action to therapies. Cell Metab. 2014, 20, 953–966. [Google Scholar] [CrossRef] [PubMed]
- Rosell-Díaz, M.; Fernández-Real, J.M. Metformin, cognitive function, and changes in the gut microbiome. Endocr. Rev. 2023, bnad029. [Google Scholar] [CrossRef] [PubMed]
- Maruthur, N.M.; Tseng, E.; Hutfless, S.; Wilson, L.M.; Suarez-Cuervo, C.; Berger, Z.; Chu, Y.; Iyoha, E.; Segal, J.B.; Bolen, S. Diabetes Medications as Monotherapy or Metformin-Based Combination Therapy for Type 2 Diabetes: A Systematic Review and Meta-analysis. Ann. Intern. Med. 2016, 164, 740–751. [Google Scholar] [CrossRef] [PubMed]
- Şeylan, C.; Tarhan, Ç. Metformin extends the chronological lifespan of fission yeast by altering energy metabolism and stress resistance capacity. FEMS Yeast Res. 2023, 23, foad018. [Google Scholar] [CrossRef] [PubMed]
- Admasu, T.D.; Chaithanya, B.K.; Barardo, D.; Ng, L.F.; Lam, V.Y.M.; Xiao, L.; Cazenave-Gassiot, A.; Wenk, M.R.; Tolwinski, N.S.; Gruber, J. Drug Synergy Slows Aging and Improves Healthspan through IGF and SREBP Lipid Signaling. Dev. Cell 2018, 47, 67–79.e65. [Google Scholar] [CrossRef] [PubMed]
- Martin-Montalvo, A.; Mercken, E.M.; Mitchell, S.J.; Palacios, H.H.; Mote, P.L.; Scheibye-Knudsen, M.; Gomes, A.P.; Ward, T.M.; Minor, R.K.; Blouin, M.J.; et al. Metformin improves healthspan and lifespan in mice. Nat. Commun. 2013, 4, 2192. [Google Scholar] [CrossRef]
- Bannister, C.A.; Holden, S.E.; Jenkins-Jones, S.; Morgan, C.L.; Halcox, J.P.; Schernthaner, G.; Mukherjee, J.; Currie, C.J. Can people with type 2 diabetes live longer than those without? A comparison of mortality in people initiated with metformin or sulphonylurea monotherapy and matched, non-diabetic controls. Diabetes Obes. Metab. 2014, 16, 1165–1173. [Google Scholar] [CrossRef]
- Ma, T.; Tian, X.; Zhang, B.; Li, M.; Wang, Y.; Yang, C.; Wu, J.; Wei, X.; Qu, Q.; Yu, Y.; et al. Low-dose metformin targets the lysosomal AMPK pathway through PEN2. Nature 2022, 603, 159–165. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K. AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res. Rev. 2012, 11, 230–241. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Zandkarimi, F.; Zhang, Y.; Meena, J.K.; Kim, J.; Zhuang, L.; Tyagi, S.; Ma, L.; Westbrook, T.F.; Steinberg, G.R.; et al. Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat. Cell Biol. 2020, 22, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Greer, E.L.; Dowlatshahi, D.; Banko, M.R.; Villen, J.; Hoang, K.; Blanchard, D.; Gygi, S.P.; Brunet, A. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr. Biol. CB 2007, 17, 1646–1656. [Google Scholar] [CrossRef] [PubMed]
- Greer, E.L.; Oskoui, P.R.; Banko, M.R.; Maniar, J.M.; Gygi, M.P.; Gygi, S.P.; Brunet, A. The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J. Biol. Chem. 2007, 282, 30107–30119. [Google Scholar] [CrossRef] [PubMed]
- Cantó, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009, 458, 1056–1060. [Google Scholar] [CrossRef] [PubMed]
- Brunet, A.; Sweeney, L.B.; Sturgill, J.F.; Chua, K.F.; Greer, P.L.; Lin, Y.; Tran, H.; Ross, S.E.; Mostoslavsky, R.; Cohen, H.Y.; et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 2004, 303, 2011–2015. [Google Scholar] [CrossRef]
- Tullet, J.M.; Araiz, C.; Sanders, M.J.; Au, C.; Benedetto, A.; Papatheodorou, I.; Clark, E.; Schmeisser, K.; Jones, D.; Schuster, E.F.; et al. DAF-16/FoxO directly regulates an atypical AMP-activated protein kinase gamma isoform to mediate the effects of insulin/IGF-1 signaling on aging in Caenorhabditis elegans. PLoS Genet. 2014, 10, e1004109. [Google Scholar] [CrossRef]
- Linehan, W.M.; Srinivasan, R.; Schmidt, L.S. The genetic basis of kidney cancer: A metabolic disease. Nat. Rev. Urol. 2010, 7, 277–285. [Google Scholar] [CrossRef]
- Saito, T.; Uchiumi, T.; Yagi, M.; Amamoto, R.; Setoyama, D.; Matsushima, Y.; Kang, D. Cardiomyocyte-specific loss of mitochondrial p32/C1qbp causes cardiomyopathy and activates stress responses. Cardiovasc. Res. 2017, 113, 1173–1185. [Google Scholar] [CrossRef]
- Egan, D.F.; Shackelford, D.B.; Mihaylova, M.M.; Gelino, S.; Kohnz, R.A.; Mair, W.; Vasquez, D.S.; Joshi, A.; Gwinn, D.M.; Taylor, R.; et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 2011, 331, 456–461. [Google Scholar] [CrossRef]
- Lin, M.; Hua, R.; Ma, J.; Zhou, Y.; Li, P.; Xu, X.; Yu, Z.; Quan, S. Bisphenol A promotes autophagy in ovarian granulosa cells by inducing AMPK/mTOR/ULK1 signalling pathway. Environ. Int. 2021, 147, 106298. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.M.; Lombardo, P.S.; Malik, N.; Brun, S.N.; Hellberg, K.; Van Nostrand, J.L.; Garcia, D.; Baumgart, J.; Diffenderfer, K.; Asara, J.M.; et al. AMPK/ULK1-mediated phosphorylation of Parkin ACT domain mediates an early step in mitophagy. Science Adv. 2021, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Andris, F.; Leo, O. AMPK in lymphocyte metabolism and function. Int. Rev. Immunol. 2015, 34, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Quaile, M.P.; Melich, D.H.; Jordan, H.L.; Nold, J.B.; Chism, J.P.; Polli, J.W.; Smith, G.A.; Rhodes, M.C. Toxicity and toxicokinetics of metformin in rats. Toxicol. Appl. Pharmacol. 2010, 243, 340–347. [Google Scholar] [CrossRef]
- Feng, J.; Wang, X.; Ye, X.; Ares, I.; Lopez-Torres, B.; Martínez, M.; Martínez-Larrañaga, M.R.; Wang, X.; Anadón, A.; Martínez, M.A. Mitochondria as an important target of metformin: The mechanism of action, toxic and side effects, and new therapeutic applications. Pharmacol. Res. 2022, 177, 106114. [Google Scholar] [CrossRef]
- Smith, D.L., Jr.; Orlandella, R.M.; Allison, D.B.; Norian, L.A. Diabetes medications as potential calorie restriction mimetics-a focus on the alpha-glucosidase inhibitor acarbose. GeroScience 2021, 43, 1123–1133. [Google Scholar] [CrossRef]
- Gibbs, V.K.; Brewer, R.A.; Miyasaki, N.D.; Patki, A.; Smith, D.L., Jr. Sex-dependent Differences in Liver and Gut Metabolomic Profiles With Acarbose and Calorie Restriction in C57BL/6 Mice. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2018, 73, 157–165. [Google Scholar] [CrossRef]
- Harrison, D.E.; Strong, R.; Alavez, S.; Astle, C.M.; DiGiovanni, J.; Fernandez, E.; Flurkey, K.; Garratt, M.; Gelfond, J.A.L.; Javors, M.A.; et al. Acarbose improves health and lifespan in aging HET3 mice. Aging Cell 2019, 18, e12898. [Google Scholar] [CrossRef]
- Yu, A.Q.; Le, J.; Huang, W.T.; Li, B.; Liang, H.X.; Wang, Q.; Liu, Y.T.; Young, C.A.; Zhang, M.Y.; Qin, S.L. The Effects of Acarbose on Non-Diabetic Overweight and Obese Patients: A Meta-Analysis. Adv. Ther. 2021, 38, 1275–1289. [Google Scholar] [CrossRef]
- Strong, R.; Miller, R.A.; Antebi, A.; Astle, C.M.; Bogue, M.; Denzel, M.S.; Fernandez, E.; Flurkey, K.; Hamilton, K.L.; Lamming, D.W.; et al. Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an α-glucosidase inhibitor or a Nrf2-inducer. Aging Cell 2016, 15, 872–884. [Google Scholar] [CrossRef]
- Harrison, D.E.; Strong, R.; Allison, D.B.; Ames, B.N.; Astle, C.M.; Atamna, H.; Fernandez, E.; Flurkey, K.; Javors, M.A.; Nadon, N.L.; et al. Acarbose, 17-α-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell 2014, 13, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.J.; Miller, R.A.; Ericsson, A.C.; Harrison, D.C.; Strong, R.; Schmidt, T.M. Changes in the gut microbiome and fermentation products concurrent with enhanced longevity in acarbose-treated mice. BMC Microbiol. 2019, 19, 130. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, X.; Miller, R.A. ATF4 activity: A common feature shared by many kinds of slow-aging mice. Aging Cell 2014, 13, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.W.; Chen, G.H.; Wang, F.; Tong, J.J.; Tao, F. Long-term acarbose administration alleviating the impairment of spatial learning and memory in the SAMP8 mice was associated with alleviated reduction of insulin system and acetylated H4K8. Brain Res. 2015, 1603, 22–31. [Google Scholar] [CrossRef]
- Tong, J.J.; Chen, G.H.; Wang, F.; Li, X.W.; Cao, L.; Sui, X.; Tao, F.; Yan, W.W.; Wei, Z.J. Chronic acarbose treatment alleviates age-related behavioral and biochemical changes in SAMP8 mice. Behav. Brain Res. 2015, 284, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Dodds, S.G.; Parihar, M.; Javors, M.; Nie, J.; Musi, N.; Dave Sharp, Z.; Hasty, P. Acarbose improved survival for Apc(+/Min) mice. Aging Cell 2020, 19, e13088. [Google Scholar] [CrossRef]
- Diaz-Cuadros, M.; Miettinen, T.P.; Skinner, O.S.; Sheedy, D.; Díaz-García, C.M.; Gapon, S.; Hubaud, A.; Yellen, G.; Manalis, S.R.; Oldham, W.M.; et al. Metabolic regulation of species-specific developmental rates. Nature 2023, 613, 550–557. [Google Scholar] [CrossRef]
- Müller, V.; Chowdhury, N.P.; Basen, M. Electron Bifurcation: A Long-Hidden Energy-Coupling Mechanism. Annu. Rev. Microbiol. 2018, 72, 331–353. [Google Scholar] [CrossRef]
- Yoshino, J.; Baur, J.A.; Imai, S.I. NAD(+) Intermediates: The Biology and Therapeutic Potential of NMN and NR. Cell Metab. 2018, 27, 513–528. [Google Scholar] [CrossRef]
- Chini, C.C.S.; Peclat, T.R.; Warner, G.M.; Kashyap, S.; Espindola-Netto, J.M.; de Oliveira, G.C.; Gomez, L.S.; Hogan, K.A.; Tarragó, M.G.; Puranik, A.S.; et al. CD38 ecto-enzyme in immune cells is induced during aging and regulates NAD(+) and NMN levels. Nat. Metab. 2020, 2, 1284–1304. [Google Scholar] [CrossRef]
- Mills, K.F.; Yoshida, S.; Stein, L.R.; Grozio, A.; Kubota, S.; Sasaki, Y.; Redpath, P.; Migaud, M.E.; Apte, R.S.; Uchida, K.; et al. Long-Term Administration of Nicotinamide Mononucleotide Mitigates Age-Associated Physiological Decline in Mice. Cell Metab. 2016, 24, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Hong, Q.; Bai, X.Y.; Fu, B.; Xie, Y.; Zhang, X.; Li, J.; Shi, S.; Lv, Y.; Sun, X.; et al. High-affinity Na(+)-dependent dicarboxylate cotransporter promotes cellular senescence by inhibiting SIRT1. Mech. Ageing Dev. 2010, 131, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Minor, R.K.; Baur, J.A.; Gomes, A.P.; Ward, T.M.; Csiszar, A.; Mercken, E.M.; Abdelmohsen, K.; Shin, Y.K.; Canto, C.; Scheibye-Knudsen, M.; et al. SRT1720 improves survival and healthspan of obese mice. Sci. Rep. 2011, 1, 70. [Google Scholar] [CrossRef] [PubMed]
- Verdin, E. NAD⁺ in aging, metabolism, and neurodegeneration. Science 2015, 350, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G.; Zipkin, R.E.; Chung, P.; Kisielewski, A.; Zhang, L.L.; et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003, 425, 191–196. [Google Scholar] [CrossRef]
- Sun, H.J.; Xiong, S.P.; Cao, X.; Cao, L.; Zhu, M.Y.; Wu, Z.Y.; Bian, J.S. Polysulfide-mediated sulfhydration of SIRT1 prevents diabetic nephropathy by suppressing phosphorylation and acetylation of p65 NF-κB and STAT3. Redox Biol. 2021, 38, 101813. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, X.; Hou, W.; Chen, E.; Ye, C.; Chen, M.; Lu, Q.; Yu, X.; Li, W. Reversing the imbalance in bone homeostasis via sustained release of SIRT-1 agonist to promote bone healing under osteoporotic condition. Bioact. Mater. 2023, 19, 429–443. [Google Scholar] [CrossRef]
- Xu, W.; Yan, J.; Ocak, U.; Lenahan, C.; Shao, A.; Tang, J.; Zhang, J.; Zhang, J.H. Melanocortin 1 receptor attenuates early brain injury following subarachnoid hemorrhage by controlling mitochondrial metabolism via AMPK/SIRT1/PGC-1α pathway in rats. Theranostics 2021, 11, 522–539. [Google Scholar] [CrossRef]
- Salminen, A.; Huuskonen, J.; Ojala, J.; Kauppinen, A.; Kaarniranta, K.; Suuronen, T. Activation of innate immunity system during aging: NF-kB signaling is the molecular culprit of inflamm-aging. Ageing Res. Rev. 2008, 7, 83–105. [Google Scholar] [CrossRef]
- Zhang, T.; Hu, J.; Wang, X.; Zhao, X.; Li, Z.; Niu, J.; Steer, C.J.; Zheng, G.; Song, G. MicroRNA-378 promotes hepatic inflammation and fibrosis via modulation of the NF-κB-TNFα pathway. J. Hepatol. 2019, 70, 87–96. [Google Scholar] [CrossRef]
- Hariharan, N.; Maejima, Y.; Nakae, J.; Paik, J.; Depinho, R.A.; Sadoshima, J. Deacetylation of FoxO by Sirt1 Plays an Essential Role in Mediating Starvation-Induced Autophagy in Cardiac Myocytes. Circ. Res. 2010, 107, 1470–1482. [Google Scholar] [CrossRef] [PubMed]
- Najt, C.P.; Khan, S.A.; Heden, T.D.; Witthuhn, B.A.; Perez, M.; Heier, J.L.; Mead, L.E.; Franklin, M.P.; Karanja, K.K.; Graham, M.J.; et al. Lipid Droplet-Derived Monounsaturated Fatty Acids Traffic via PLIN5 to Allosterically Activate SIRT1. Mol. Cell 2020, 77, 810–824.e818. [Google Scholar] [CrossRef] [PubMed]
- Gujar, A.D.; Le, S.; Mao, D.D.; Dadey, D.Y.; Turski, A.; Sasaki, Y.; Aum, D.; Luo, J.; Dahiya, S.; Yuan, L.; et al. An NAD+-dependent transcriptional program governs self-renewal and radiation resistance in glioblastoma. Proc. Natl. Acad. Sci. USA 2016, 113, e8247–e8256. [Google Scholar] [CrossRef]
- Guscott, R.; Taylor, L. Lithium prophylaxis in recurrent affective illness. Efficacy, effectiveness and efficiency. Br. J. Psychiatry J. Ment. Sci. 1994, 164, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Zarse, K.; Terao, T.; Tian, J.; Iwata, N.; Ishii, N.; Ristow, M. Low-dose lithium uptake promotes longevity in humans and metazoans. Eur. J. Nutr. 2011, 50, 387–389. [Google Scholar] [CrossRef] [PubMed]
- Baldessarini, R.J.; Tondo, L. Testing for Antisuicidal Effects of Lithium Treatment. JAMA Psychiatry 2022, 79, 9–10. [Google Scholar] [CrossRef] [PubMed]
- Katz, I.R.; Rogers, M.P.; Lew, R.; Thwin, S.S.; Doros, G.; Ahearn, E.; Ostacher, M.J.; DeLisi, L.E.; Smith, E.G.; Ringer, R.J.; et al. Lithium Treatment in the Prevention of Repeat Suicide-Related Outcomes in Veterans With Major Depression or Bipolar Disorder: A Randomized Clinical Trial. JAMA Psychiatry 2022, 79, 24–32. [Google Scholar] [CrossRef]
- McIntyre, R.S.; Berk, M.; Brietzke, E.; Goldstein, B.I.; López-Jaramillo, C.; Kessing, L.V.; Malhi, G.S.; Nierenberg, A.A.; Rosenblat, J.D.; Majeed, A.; et al. Bipolar disorders. Lancet 2020, 396, 1841–1856. [Google Scholar] [CrossRef]
- McKnight, R.F.; Adida, M.; Budge, K.; Stockton, S.; Goodwin, G.M.; Geddes, J.R. Lithium toxicity profile: A systematic review and meta-analysis. Lancet 2012, 379, 721–728. [Google Scholar] [CrossRef]
- Pisanu, C.; Congiu, D.; Manchia, M.; Caria, P.; Cocco, C.; Dettori, T.; Frau, D.V.; Manca, E.; Meloni, A.; Nieddu, M.; et al. Differences in telomere length between patients with bipolar disorder and controls are influenced by lithium treatment. Pharmacogenomics 2020, 21, 533–540. [Google Scholar] [CrossRef]
- Manrique, T.; Morón, I.; Ballesteros, M.A.; Guerrero, R.M.; Fenton, A.A.; Gallo, M. Hippocampus, aging, and segregating memories. Hippocampus 2009, 19, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, M.; Biernacka, J.M.; Lavebratt, C.; Druliner, B.; Ryu, E.; Geske, J.; Colby, C.; Boardman, L.; Frye, M.; Schalling, M. Expression of telomerase reverse transcriptase positively correlates with duration of lithium treatment in bipolar disorder. Psychiatry Res. 2020, 286, 112865. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Chen, B.; Liu, Z.; Gong, A.Y.; Gunning, W.T.; Ge, Y.; Malhotra, D.; Gohara, A.F.; Dworkin, L.D.; Gong, R. Age-related GSK3β overexpression drives podocyte senescence and glomerular aging. J. Clin. Investig. 2022, 132, 4. [Google Scholar] [CrossRef] [PubMed]
- Polter, A.; Yang, S.; Zmijewska, A.A.; van Groen, T.; Paik, J.H.; Depinho, R.A.; Peng, S.L.; Jope, R.S.; Li, X. Forkhead box, class O transcription factors in brain: Regulation and behavioral manifestation. Biol. Psychiatry 2009, 65, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Chalecka-Franaszek, E.; Chuang, D.M. Lithium activates the serine/threonine kinase Akt-1 and suppresses glutamate-induced inhibition of Akt-1 activity in neurons. Proc. Natl. Acad. Sci. USA 1999, 96, 8745–8750. [Google Scholar] [CrossRef] [PubMed]
- Coutts, F.; Palmos, A.B.; Duarte, R.R.R.; de Jong, S.; Lewis, C.M.; Dima, D.; Powell, T.R. The polygenic nature of telomere length and the anti-ageing properties of lithium. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2019, 44, 757–765. [Google Scholar] [CrossRef] [PubMed]
- McColl, G.; Killilea, D.W.; Hubbard, A.E.; Vantipalli, M.C.; Melov, S.; Lithgow, G.J. Pharmacogenetic analysis of lithium-induced delayed aging in Caenorhabditis elegans. J. Biol. Chem. 2008, 283, 350–357. [Google Scholar] [CrossRef]
- Zmijewski, J.W.; Jope, R.S. Nuclear accumulation of glycogen synthase kinase-3 during replicative senescence of human fibroblasts. Aging Cell 2004, 3, 309–317. [Google Scholar] [CrossRef]
- Pons, V.; Lévesque, P.; Plante, M.M.; Rivest, S. Conditional genetic deletion of CSF1 receptor in microglia ameliorates the physiopathology of Alzheimer’s disease. Alzheimer Res. Ther. 2021, 13, 8. [Google Scholar] [CrossRef]
- Tajes, M.; Yeste-Velasco, M.; Zhu, X.; Chou, S.P.; Smith, M.A.; Pallàs, M.; Camins, A.; Casadesús, G. Activation of Akt by lithium: Pro-survival pathways in aging. Mech. Ageing Dev. 2009, 130, 253–261. [Google Scholar] [CrossRef]
- Tajes, M.; Gutierrez-Cuesta, J.; Folch, J.; Ferrer, I.; Caballero, B.; Smith, M.A.; Casadesus, G.; Camins, A.; Pallás, M. Lithium treatment decreases activities of tau kinases in a murine model of senescence. J. Neuropathol. Exp. Neurol. 2008, 67, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Gaziano, J.M.; Brotons, C.; Coppolecchia, R.; Cricelli, C.; Darius, H.; Gorelick, P.B.; Howard, G.; Pearson, T.A.; Rothwell, P.M.; Ruilope, L.M.; et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): A randomised, double-blind, placebo-controlled trial. Lancet 2018, 392, 1036–1046. [Google Scholar] [CrossRef]
- McNeil, J.J.; Wolfe, R.; Woods, R.L.; Tonkin, A.M.; Donnan, G.A.; Nelson, M.R.; Reid, C.M.; Lockery, J.E.; Kirpach, B.; Storey, E.; et al. Effect of Aspirin on Cardiovascular Events and Bleeding in the Healthy Elderly. N. Engl. J. Med. 2018, 379, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Agüero-Torres, H.; Viitanen, M.; Fratiglioni, L.; Louhija, J. The effect of low-dose daily aspirin intake on survival in the Finnish centenarians cohort. J. Am. Geriatr. Soc. 2001, 49, 1578–1580. [Google Scholar] [CrossRef]
- Atchison, C.R.; Balakumaran, A.; West, A.B.; Hoffmann, W.E.; Treinen-Moslen, M. Aging enhances susceptibility of diclofenac-treated rats to gastric ulceration, while attenuating enteropathy. Dig. Dis. Sci. 2000, 45, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.B.; Wu, G.S.; Ke, L.Y.; Zhou, X.G.; Wang, Y.H.; Luo, H.R. Aspirin Derivative 5-(Bis(3-methylbut-2-enyl)amino)-2-hydroxybenzoic Acid Improves Thermotolerance via Stress Response Proteins in Caenorhabditis elegans. Molecules 2018, 23, 1359. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Qadeer, A.; Xie, Y.; Jin, Y.; Li, Q.; Xiao, Y.; She, K.; Zheng, X.; Li, J.; Ji, S.; et al. Dietary Supplementation of Aspirin Promotes Drosophila Defense against Viral Infection. Molecules 2023, 28, 5300. [Google Scholar] [CrossRef]
- Lin, M.; He, J.; Zhang, X.; Sun, X.; Dong, W.; Zhang, R.; Xu, Y.; Lv, L. Targeting fibrinogen-like protein 1 enhances immunotherapy in hepatocellular carcinoma. J. Clin. Investig. 2023, 133, 9. [Google Scholar] [CrossRef]
- Ching, T.T.; Chiang, W.C.; Chen, C.S.; Hsu, A.L. Celecoxib extends C. elegans lifespan via inhibition of insulin-like signaling but not cyclooxygenase-2 activity. Aging Cell 2011, 10, 506–519. [Google Scholar] [CrossRef]
- Ayyadevara, S.; Bharill, P.; Dandapat, A.; Hu, C.; Khaidakov, M.; Mitra, S.; Shmookler Reis, R.J.; Mehta, J.L. Aspirin inhibits oxidant stress, reduces age-associated functional declines, and extends lifespan of Caenorhabditis elegans. Antioxid. Redox Signal. 2013, 18, 481–490. [Google Scholar] [CrossRef]
- Keaney, M.; Matthijssens, F.; Sharpe, M.; Vanfleteren, J.; Gems, D. Superoxide dismutase mimetics elevate superoxide dismutase activity in vivo but do not retard aging in the nematode Caenorhabditis elegans. Free Radic. Biol. Med. 2004, 37, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yu, X.; Meng, F.; Zhao, Z.; Guan, S.; Wang, L. Ferulic Acid Supplementation Increases Lifespan and Stress Resistance via Insulin/IGF-1 Signaling Pathway in C. elegans. Int. J. Mol. Sci. 2021, 22, 4279. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Xia, W.; Zheng, B.; Li, T.; Liu, R.H. DAF-16 is involved in colonic metabolites of ferulic acid-promoted longevity and stress resistance of Caenorhabditis elegans. J. Sci. Food Agric. 2022, 102, 7017–7029. [Google Scholar] [CrossRef]
- Huang, X.B.; Mu, X.H.; Wan, Q.L.; He, X.M.; Wu, G.S.; Luo, H.R. Aspirin increases metabolism through germline signalling to extend the lifespan of Caenorhabditis elegans. PLoS ONE 2017, 12, e0184027. [Google Scholar] [CrossRef]
- Rieu, I.; Magne, H.; Savary-Auzeloux, I.; Averous, J.; Bos, C.; Peyron, M.A.; Combaret, L.; Dardevet, D. Reduction of low grade inflammation restores blunting of postprandial muscle anabolism and limits sarcopenia in old rats. J. Physiol. 2009, 587, 5483–5492. [Google Scholar] [CrossRef]
- Bendlin, B.B.; Newman, L.M.; Ries, M.L.; Puglielli, L.; Carlsson, C.M.; Sager, M.A.; Rowley, H.A.; Gallagher, C.L.; Willette, A.A.; Alexander, A.L.; et al. NSAIDs may protect against age-related brain atrophy. Front. Aging Neurosci. 2010, 2, 35. [Google Scholar] [CrossRef]
- Grosser, T.; Ricciotti, E.; FitzGerald, G.A. The Cardiovascular Pharmacology of Nonsteroidal Anti-Inflammatory Drugs. Trends Pharmacol. Sci. 2017, 38, 733–748. [Google Scholar] [CrossRef]
| mTOR | AMPK | Nutrient Signal Pathway | SIRT1 | Telomere Length and GSK-3 | Energy Metabolism | |||
|---|---|---|---|---|---|---|---|---|
| Akt |  | |||||||
| ATF4 |  | |||||||
| BDNF |  | |||||||
| DAF-12/16 |  | |||||||
| DAF-16/FoxO |  | |||||||
| ATP6AP1 |  | |||||||
| NF-kB |  |  | ||||||
| Nrf2/SKN-1 |  |  | ||||||
| H4K8ac |  | |||||||
| HIF1α |  | |||||||
| IRE-1 |  | |||||||
| SIRT1 |  |  | ||||||
| CRTC/CREB | ||||||||
| p53 |  |  | ||||||
| p73 |  | |||||||
| PDK1 |  | |||||||
| PEN2 |  | |||||||
| TSC1/TSC2 |  | |||||||
| mTOR |  |  | ||||||
| ULK1 |  |  | ||||||
| S6K1 |  |  | ||||||
| 4EBP1 |  |  | ||||||
| FOXOs |  |  |  |  | rapamycin | |||
| Syt1/Stx1 |  |  | metformin | |||||
| TERT |  |  | acarbose | |||||
| PGC-1α |  |  |  | NMN | ||||
| PPARγ |  |  | lithium | |||||
| GSK-3β |  |  | NSAIDS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, N.; Yang, R.; Jiang, S.; Niu, Z.; Zhou, W.; Liu, C.; Gao, L.; Sun, Q. Anti-Aging Drugs and the Related Signal Pathways. Biomedicines 2024, 12, 127. https://doi.org/10.3390/biomedicines12010127
Du N, Yang R, Jiang S, Niu Z, Zhou W, Liu C, Gao L, Sun Q. Anti-Aging Drugs and the Related Signal Pathways. Biomedicines. 2024; 12(1):127. https://doi.org/10.3390/biomedicines12010127
Chicago/Turabian StyleDu, Nannan, Ruigang Yang, Shengrong Jiang, Zubiao Niu, Wenzhao Zhou, Chenyu Liu, Lihua Gao, and Qiang Sun. 2024. "Anti-Aging Drugs and the Related Signal Pathways" Biomedicines 12, no. 1: 127. https://doi.org/10.3390/biomedicines12010127
APA StyleDu, N., Yang, R., Jiang, S., Niu, Z., Zhou, W., Liu, C., Gao, L., & Sun, Q. (2024). Anti-Aging Drugs and the Related Signal Pathways. Biomedicines, 12(1), 127. https://doi.org/10.3390/biomedicines12010127







