1. Introduction
The latest findings in medical research regarding hydrogen unequivocally highlight substantial prospects for harnessing hydrogen as a therapeutic intervention. Extensive observations in both clinical and experimental studies distinctly demonstrate that hydrogen holds significant promise as an innovative therapy to address unmet patient needs across various etiologies [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10]. Renowned for its antioxidant properties, hydrogen gas has emerged as a novel therapeutic agent with potential applications in various medical domains, including transplantation. In addition to its antioxidative attributes, hydrogen also exerts anti-inflammatory effects by modulating pro-inflammatory cytokines and signaling pathways. Moreover, hydrogen’s capacity to activate cytoprotective pathways enhances cellular resilience to stress.
Due to advancements in new medications and improved surgical techniques over the past few decades, the success rate of organ transplantation has risen significantly, establishing it as the primary treatment for end-stage organ failure. However, favorable outcomes of transplant recipients continue to be hindered by persistent challenges such as ischemia-reperfusion injury (IRI) and graft rejection. Despite extensive efforts to augment the pool of deceased organ donors in response to escalating demand, the number of available cadaveric donors has remained largely stagnant. This disparity has led to thousands of preventable deaths each year due to organ scarcity.
In an effort to mitigate patient fatalities on the waiting list, strategies have been implemented to broaden the organ donor pool, including the utilization of marginal donors. The relationship between hydrogen therapy and organ transplantation is intriguing, particularly the potential of hydrogen to enhance transplantation outcomes [
11].
Hydrogen plays a significant role in reducing graft rejection, improving graft survival, and modulating immune responses in transplantation, as presented in this comprehensive review. By analyzing both preclinical and clinical studies meticulously, we aim to provide valuable insight into hydrogen’s potential utility as a complementary therapy in transplantation (
Table 1).
Organ grafts may experience multiple injuries during warm ischemia, cold ischemia, and reperfusion injury. These injuries all contribute to primary graft dysfunction, which is a major cause of morbidity and mortality after organ transplantation. Hydrogen therapy entails the administration of hydrogen gas or hydrogen-enriched solutions to exert therapeutic effects. In the context of organ transplantation, hydrogen, with its antioxidant, anti-inflammatory, and cytoprotective properties, has been explored as a potential strategy to address major challenges associated with organ transplantation.
This review paper explores the potential applications and therapeutic implications of hydrogen in transplantation, shedding light on its role in mitigating IRI, improving graft survival, and modulating immune responses. This review clearly demonstrates that hydrogen possesses biological effects for mitigating graft injuries and that hydrogen may have a huge potential as a safe and potent therapeutic tool for its recipients. Through an in-depth analysis of preclinical and clinical studies, we aim to provide insights into the promising use of hydrogen as an adjunct therapy in transplantation.
2. Challenges in Organ Transplantation
Organ transplantation failures result from a range of immunological and non-immunological factors, presenting in either chronic or acute forms. These failures are influenced by complex networks of interconnected biological events. Organ grafts may undergo injuries at different stages, including during warm ischemia, cold ischemia, reperfusion, and the acute or chronic phases. IRI arises when there is a temporary disruption of the blood supply during organ removal and subsequent reimplantation. Reperfusion, while essential for restoring blood flow and oxygen to the graft, can paradoxically trigger oxidative stress, inflammation, and tissue damage. This can compromise the function and viability of the transplanted organ. IRI contributes to primary graft dysfunction, which is a significant cause of morbidity and mortality following organ transplantation [
35]. The immune responses responsible for graft rejection can imperil the success of transplants. Graft rejection is a complex process in which the recipient’s immune system detects the transplanted organ as foreign and mounts an immune response against it. This uncontrolled immune response can lead to a graft’s dysfunction and ultimate failure. Nevertheless, more research is necessary to gain a comprehensive understanding of hydrogen’s impact on immune responses in the context of transplantation. It is worth noting that long-term immunosuppressant medication may increase the risk of malignancy or infectious disease development.
Graft-versus-host disease (GVHD) primarily affects recipients of bone marrow or stem cell transplants, where the transplanted immune cells (graft) attack the recipient’s tissues and organs. This immune assault can result in inflammation and damage to various organs, including the skin, liver, gastrointestinal tract, and lungs. The immune response prompted by GVHD releases inflammatory molecules known as cytokines, which can provoke widespread inflammation which is detrimental to the function of the transplanted organ. The chronic inflammatory state induced by GVHD can lead to multi-organ dysfunction over time, impacting the overall health and function of transplanted organs. GVHD presents a substantial challenge in transplantation, prompting ongoing research to enhance prevention and treatment strategies [
36,
37].
Moreover, to address the current organ shortage for transplantation, the donor pool has been expanded to include marginal donors, such as elderly donors and non-heart-beating donors, and grafts subjected to prolonged cold storage [
38]. However, grafts from these donors exhibit more severe graft injury and a higher propensity for failure compared to non-marginal organs. Although there are various approaches for graft protection, including pre- and post-transplant management, an optimal prophylactic treatment for graft failure remains undetermined. Consequently, long-term graft survival and function are influenced by numerous factors, including ongoing alloimmune reactions, early IRI, recipient metabolic abnormalities (elevated cholesterol or lipids), other recipient conditions (viral infections, hypertension), and adverse reactions to chronic immunosuppressive therapy. Transplant medicine is a challenging and intricate field, and specific therapeutic strategies are crucial for improving both short-term and long-term graft and patient outcomes.
3. Hydrogen: Chemistry and Physiology
Hydrogen (molecular formula H2) is the lightest molecule, an odorless, tasteless, colorless, nonmetallic, and highly combustible diatomic gas. Hydrogen is chemically stable at room temperature, which is mainly determined by the strong covalent bond between hydrogen atoms. It is known to be quite flammable and reactive with specific catalysts or in the presence of heat.
Endogenously, humans do not produce hydrogen since enzymes with hydrogenase activity are not present in the human body. However, in the large intestine, anaerobic organisms generate hydrogen by breaking down carbohydrates, primarily from the undigested polysaccharide fraction of starches and plant cells, through hydrogenase activity. Every day, the human body continuously produces several liters of hydrogen under normal physiological conditions, mainly through the fermentation of non-digestible carbohydrates by microbiota in the large intestine [
39,
40]. In vivo hydrogen will be released to the outside in a manner similar to circulation and respiration. Hydrogen is excreted via the lungs or through the rectum in the form of flatulence. Since hydrogen has a large diffusion capacity, the proportion of hydrogen released from the skin cannot be ignored [
41]. It is noteworthy that the enterobacterial flora serves as a significant source of both hydrogen and hydrogen sulfide.
4. Clinical Use of Hydrogen and Toxicity
It is essential to identify safety concerns, potential side effects, and toxicity associated with the use of therapeutic gases [
11,
42,
43]. While research is ongoing, existing evidence suggests that hydrogen therapy is well-tolerated by most individuals, with minimal to no adverse effects reported. This favorable safety profile has piqued the interest in hydrogen therapy within medical research. However, it is crucial to emphasize that hydrogen therapy, like any medical treatment, should be administered under the guidance of qualified healthcare professionals. The safety and appropriateness of hydrogen therapy may vary depending on the specific medical condition, mode of administration, and individual patient factors. As is the case with any medical intervention, a comprehensive evaluation of potential risks and benefits should be conducted, and treatment should be tailored to each patient’s specific needs. Patients should consult their healthcare providers to determine whether hydrogen therapy is a suitable option for addressing their specific health concerns and to ensure its safe and effective use. When administered under appropriate medical supervision and following established protocols, hydrogen therapy in medicine is generally considered safe [
44].
There are two prominent challenges or enigmas concerning the impact of hydrogen. Firstly, the dose-dependent effect remains elusive. Whether in animal experiments or clinical observations, hydrogen is administered in small doses, yet it yields substantial effects. Secondly, both humans and animals produce a considerable quantity of hydrogen through their gut bacteria, but the following question remains: why does the increase in such a minute amount of hydrogen result in such pronounced effects? Furthermore, various issues, including the molecular mechanisms of hydrogen and the optimal methods for utilizing it in the treatment of different diseases—such as dosage, frequency, and so forth—still require further investigation [
45].
5. Protective Mechanism of Hydrogen
5.1. Scavenging Free Radicals
Although various mechanisms for the cellular and tissue protection provided by hydrogen exposure have been suggested, hydrogen’s role as a scavenger of reactive oxygen species has been advocated. Ohsawa et al. reported that, in vitro, hydrogen selectively reduces peroxynitrite and hydroxyl radicals, which are very strong oxidants which react indiscriminately with nucleic acids, proteins, and lipids, resulting in lipid peroxidation, DNA fragmentation, and protein inactivation. Biochemical experiments using electron resonance spectroscopy spin traps and fluorescent probes suggest that the effects of hydrogen against hydroxyl radicals are more potent than those against peroxynitrite [
46,
47,
48].
5.2. Protection of Mitochondrial Function
Hydrogen easily permeates biological membranes and diffuses into the nucleus, mitochondria, and cytosol, reaching target tissues. Treatment with hydrogen-rich saline significantly reduced the loss of mitochondrial membrane potential and preserved mitochondrial cytochrome c content [
49]. In an open-label trial, Ito et al. investigated the effects of drinking hydrogen-enriched water for 12 weeks in patients with mitochondrial metabolism diseases, including progressive muscular dystrophy and mitochondrial myopathies, and observed significant improvements in lactate-to-pyruvate ratios [
50]. Zhang et al. reported that hydrogen improved mitochondrial quality by upregulating heme oxygenase-1 (HO-1) expression through the nuclear factor erythroid 2-related factor 2 (Nrf2)/YY1 complex in vitro [
51].
5.3. Anti-Inflammation
Hydrogen can modulate the production of inflammatory cytokines like interleukin (IL)-1 beta (IL-1β), IL-6, and tumor necrosis factor-alpha (TNF-α). These cytokines are key players in the inflammatory response of the human body. Hydrogen attenuates this inflammatory response by inhibiting the nuclear factor-kappa B (NF-κB) and p38 MAPK pathways [
52]. Hydrogen can promote very early M1-to-M2 polarization without disturbing the functions of the M1 phenotype, suggesting that hydrogen could reduce inflammation by shifting early macrophage polarization in clinical settings [
53]. Hydrogen’s anti-inflammatory impact on the inflammatory response was demonstrated to occur through the phosphatidylinositol 3 kinase/protein kinase B signaling pathway [
54].
5.4. Induction of Antioxidant Enzyme
Another potential mechanism underlying hydrogen’s cellular protective function may be an increase in antioxidant enzymes such as superoxide dismutase, catalase, or HO-1 [
55]. Hydrogen-rich saline treatment considerably increased the antioxidant enzyme levels of serum superoxide dismutase and reduced glutathione [
56].
5.5. Induction of Surfactant-Related Genes
Tanaka et al. investigated the changes in genes after hydrogen inhalation using a gene array analysis and showed that clara cell protein 16 (CC16) was the most upregulated gene in response to preloading hydrogen in lung grafts. CC16 is one of the major proteins secreted by the respiratory epithelium and has antioxidant and anti-inflammatory properties. Preloading hydrogen via mechanical ventilation also significantly increased other surfactant-related mRNAs, including HSD11b1, SCGB3A2, and SP-A [
27].
5.6. Protection of Vascular Endothelial Cells
Nitric oxide (NO) plays a crucial role in maintaining the delicate equilibrium of factors that regulate vascular tone, blood flow, and coagulation, all of which are vital for the health of endothelial cells [
57]. Hydrogen can enhance endothelial NO synthase activity, leading to increased circulating NO levels, thus providing protection to vascular endothelial cells. The incubation in a hydrogen-rich medium significantly improves cell viability and shields human umbilical vein endothelial cells from cellular damage induced by hydrogen peroxide [
29]. Moreover, hydrogen effectively suppresses the release of cell adhesion molecules like intercellular cell adhesion molecule-1 and vascular cell adhesion molecule-1, as well as proinflammatory mediators, including high-mobility group box 1 protein, IL-1β, and TNF-α. Furthermore, hydrogen demonstrates the ability to elevate levels of the anti-inflammatory cytokine IL-10 [
58,
59].
5.7. Prevention of Apoptosis
Hydrogen’s anti-apoptotic functions have been proposed to occur via the inhibition of caspase-3 activation and the induction of anti-apoptotic gene B-cell lymphoma-2 (Bcl-2) [
32]. Terasaki et al. reported that the activation of the pro-apoptotic gene Bax was reduced via hydrogen treatment [
60]. The anti-apoptotic effects of hydrogen gas inhalation were partially mediated by the early activation of NF-κB during hydrogen treatment and correlated with decreased levels of Bax and elevated levels of the anti-apoptotic protein Bcl-2 [
61]. Zhang et al. demonstrated that the upregulation of Bcl-2, NF-κB, HO-1, and zinc finger protein A20 was seen in rats where only the donors received hydrogen [
23]. Meanwhile, in another study, the intraperitoneal administration of hydrogen-rich saline to a transplant recipient immediately after reperfusion protected them against acute kidney injury after liver transplantation, partly by reducing apoptosis, which is potentially involved in the modulation of p53-mediated autophagy [
21].
5.8. Inhibition of Infiltrating Cell Migration
Accelerated atherosclerosis caused by the immune response is a primary cause of graft loss after organ transplantation. Smooth muscle cell proliferation and migration play important roles in the progression of intimal hyperplasia. Sun et al. demonstrated that the incubation in a hydrogen-rich medium suppressed smooth muscle cell migration using an in vitro rat smooth muscle cell (A7r5) culture model [
29]. Matrix metalloproteinases (MMPs) are important mediators of intimal hyperplasia, and MMP-2 and MMP-9 promote the formation of neointima. In the same study, the oral intake of hydrogen-rich water effectively inhibited MMP-2 and MMP-9 in rat vein grafts [
29].
5.9. Inhibition of Fibrosis
Type III collagen plays a significant role in the interstitia of solid organs and in the formation of granulation tissue following ischemic tissue damage. Terasaki et al. conducted a study showing that both the inhalation of 3% hydrogen gas and the oral consumption of hydrogen-enriched water reduced oxidative stress and apoptosis, which are indicators of acute lung damage, in mice exposed to irradiation [
60]. This led to a decrease in the deposition of type III collagen and the development of lung fibrosis, which is a manifestation of late-stage damage. Therefore, hydrogen’s potential to mitigate fibrosis may prove effective in addressing chronic allograft injury.
5.10. Immunomodulation
Hydrogen has been found to influence various aspects of the immune response. By modulating cytokine profiles and immune cell interactions, hydrogen may promote an environment conducive to immune tolerance and decrease the likelihood of graft rejection. Using a mouse model, Itoh et al. demonstrated that drinking hydrogen-rich water could attenuate an immediate allergic reaction by inhibiting the phosphorylation of FcεRI-associated Lyn and its downstream signaling molecules, which subsequently reduced the generation of hydrogen peroxide and suppressed NADPH oxidase activity [
62]. Hydrogen’s immunomodulatory effects have been investigated in both adaptive and innate immune responses. Hydrogen regulates T cell differentiation, dendritic cell function, and cytokine profiles, potentially leading to immune tolerance and prolonged graft survival [
22]. In another study, T cell proliferation was significantly suppressed in the in vitro presence of hydrogen and accompanied by the lowered production of interferon-ɣ and IL-2 [
28].
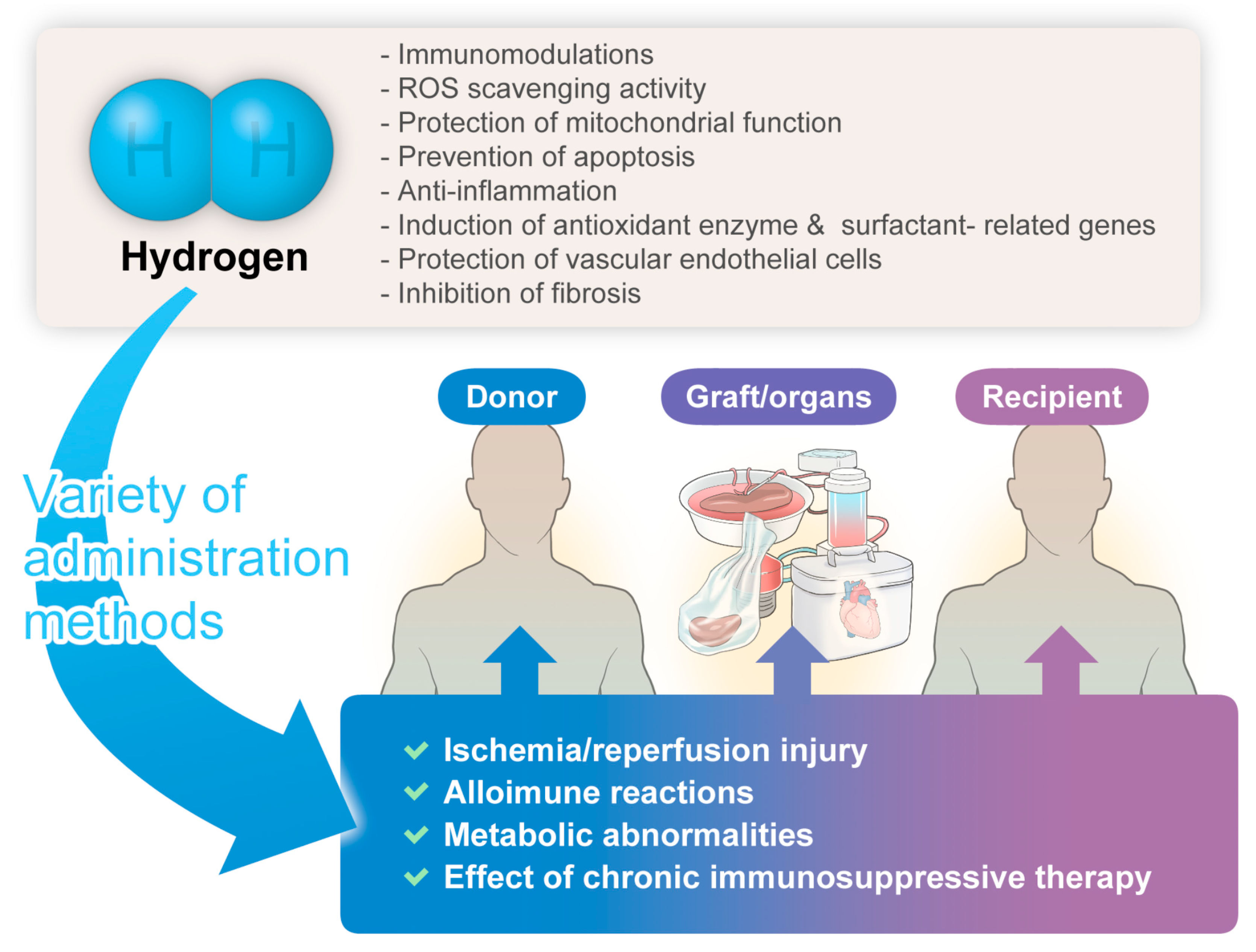
The hydrogen-related advantages described herein might hold significant implications for donors, grafts/organs, and recipients, manifesting anticipated organ-protective effects (
Figure 1).
6. Hydrogen Applications in Transplantation
6.1. Hydrogen Gas Inhalation
Since hydrogen is a gaseous molecule, a safe concentration of hydrogen inhalation would be a straightforward delivery method for employing hydrogen as a therapeutic tool. Buchholz et al. demonstrated that hydrogen inhalation by recipients at a 2% concentration significantly dampened transplant-induced muscularis inflammation, mitigated bowel dysfunction, and prevented bowel dysmotility in rat intestinal transplant models [
34]. In a lung transplantation brain death rat model, donor and recipient ventilation with 2% hydrogen hindered oxidative injuries by increasing the actions of superoxide dismutase and other antioxidants to protect lung function [
63]. Kawamura et al. also indicated the efficacy of donor treatment with 2% hydrogen for three hours on lung allograft function in rats [
31]. Zhang et al. demonstrated that one hour of donor treatment with 2% hydrogen inhalation significantly reduced liver injury after transplantation in a rat orthotopic liver transplant model [
23].
6.2. Lung Inflation during Cold Preservation
Among transplantable organs, the lung is unique because it is an organ that contains air, allowing for the incorporation of hydrogen into the air within the alveoli. Lung inflation with 3% hydrogen gas during the cold ischemia phase alleviated lung graft injury, determined by inhibiting apoptosis and inflammatory responses, and improved graft function [
16,
64]. Similarly, Duan et al. investigated the efficacy of lung inflation during cold ischemia with 3% hydrogen + 40% oxygen + 57% nitrogen in a rat model and demonstrated that hydrogen exposure during cold ischemia improved donor lung quality by mitigating mitochondrial structural anomalies, enhancing mitochondrial function, and reducing apoptosis, inflammation, and oxidative stress, which may be achieved through activation of the Nrf2/HO-1 pathway [
13].
6.3. Preservation Solution
The ongoing optimization of organ preservation solutions has always been an important component of donor protection for lung transplantation. Various ways of dissolving hydrogen gas in organ preservation solutions have been developed, including the use of electrolysis, a hydrogen gas cylinder, or a hydrogen-generating agent. The use of a hydrogen-rich preservation solution (more than 1.0 ppm) diminishes IRI in rat lungs during cold ischemia through anti-inflammatory and antioxidant effects [
18]. Abe et al. demonstrated that 24 to 48 h of organ preservation in hydrogen-rich University of Wisconsin solution attenuated renal cold IRI in a syngeneic rat kidney transplantation model and was associated with less interstitial macrophage infiltration, tubular apoptosis, and oxidative stress in the kidney grafts, better renal graft function, and longer graft survival compared to simple cold storage [
26].
Buchholz et al. indicated that luminal preservation, cold graft storage, and vascular flush in a hydrogen-bubbled preservation solution significantly preserved mucosal graft morphology and diminished graft malondialdehyde levels, showing significant reduction potential and weakened proinflammatory molecular responses within the re-perfused intestinal graft in rats [
30].
Similarly, a hydrogen gas-containing organ preservation solution impeded the development of acute injuries in a donor’s kidney after cardiac death in a preclinical miniature pig model with an optimal immunosuppressive protocol based on the human clinical setting. A marginal kidney processed using a hydrogen gas-containing preservation solution was engrafted for longer than 100 days [
14]. Kobayashi et al. studied a practical method of quickly dissolving hydrogen gas in organ preservation solutions using a canister containing a hydrogen-absorbing alloy [
19]. After 30 min of warm ischemic injury induced by circulatory arrest, the donor kidneys were harvested and perfused for five minutes with a hydrogen-containing cold ET-Kyoto (ETK) solution in a miniature pig kidney transplantation model. Preservation in a hydrogen-containing solution for either one or four hours resulted in better renal function with more blood flow [
19]. In another study, Kayawake et al. demonstrated that a hydrogen-rich preservation solution lessened IRI in a canine left lung transplantation model following 23 h of cold ischemia in an ETK solution. The graft function in this study, determined using blood gas analysis, significantly improved in the hydrogen-treated group, which was associated with lesser extents of lung edema and histopathological injury [
15].
6.4. Hydrogen Flush after Cold Storage
Protective effects can be achieved by subjecting hydrogen to a single flush ex vivo, even without placing it in a storage solution. In a previous study, hydrogen flush after 24 h of cold ischemia significantly lowered transaminases, high-mobility group box protein 1 release, and portal venous pressure compared to vehicle-treated controls. The portal venous route maintained the sinusoidal endothelia, and the arterial route attenuated biliary damage [
65].
6.5. Ex Vivo Perfusion
Ex vivo lung perfusion allows for the evaluation and recovery of an ex vivo donor lung by perfusion with normothermic perfusate. Haam et al. reported that lung graft ventilation after cardiac death with 2% hydrogen gas for four hours during ex vivo perfusion significantly mitigated inflammation-related lung injury and improved lung function in a porcine model [
66]. Noda et al. used a rat lung ex vivo perfusion model and ventilated the lungs with air supplemented with 2% hydrogen for four hours. Hydrogen administration decreased proinflammatory changes through the upregulation of HO-1, promoted mitochondrial biogenesis, and significantly reduced lactate production. In addition, the expression of hypoxia-inducible factor-1 in the hydrogen-treated lungs was significantly attenuated. Thus, the preconditioning of lung grafts with inhaled hydrogen diminished these proinflammatory changes, promoted mitochondrial biogenesis in the lungs throughout the procedure, and resulted in better post-transplant graft function [
67,
68]. Ishikawa et al. reported that 90 min of reperfusion with oxygenated buffer with hydrogen at 37° on an isolated perfused rat liver apparatus significantly reduced the apoptosis, energy depletion, liver enzyme leakage, redox status, impaired microcirculation, and necrosis associated with increased bile production. The phosphorylation of cytoplasmic MKK4 and JNK were suppressed by means of hydrogen treatment [
69].
6.6. Hydrogen Exposure Using a Hydrogen Bath
Noda et al. invented a unique cold storage device with a hydrogen-rich water bath, which enabled water saturation with hydrogen and maintained saturated hydrogen levels and a consistent temperature throughout the procedure. The grafts stored with the hydrogen-rich water bath also had a higher adenosine triphosphate content and less mitochondrial damage, which were associated with efficiently ameliorated myocardial injury [
29]. The use of a hydrogen-rich water bath in which hydrogen was dissolved into a solution for liver graft tissues enabled superior morphologic and functional protection against IRI in a rat liver transplant model [
20].
6.7. Venous/Intraperitoneal Injection
Luo et al. demonstrated that the intravenous administration of hydrogen-saturated saline to the recipient enhances the migration and proliferation capacity of bone marrow mesenchymal stem cells (BMSCs) to repair spinal cord injury by reducing the inflammatory response and oxidative stress in the injured area, suggesting that hydrogen and BMSC co-delivery is an effective method of improving BMSC transplantation in the treatment of spinal cord injury [
12]. Intravenous hydrogen-rich saline administered via the tail vein at the beginning of reperfusion significantly diminished the severity of pancreatic IRI in rats, possibly by decreasing inflammation and oxidative stress [
24].
6.8. Intraluminal Administration
The small intestine is a unique organ because it has both luminal and vascular routes through which preservation solutions can be administered. In addition to the vessel walls, the epithelial cell layers forming the mucosa and covering the inner part of the lumen are also highly susceptible to IRI and, thus, a potential therapeutic target [
70]. Yamamoto et al. demonstrated that the intraluminal administration of hydrogen-rich saline regulated the loss of the transmembrane protein ZO-1 in the graft intestine and modulated IRI to the transplanted intestine in rats [
17].
6.9. Oral Intake of a Hydrogen-Rich Solution
Oral consumption of hydrogen-rich solutions could be implemented easily into everyday clinical practice [
71]. Solubilized hydrogen may be valuable since it is a safe, easily administered, and portable method of delivering hydrogen to the human body. Cardinal et al. showed that the oral intake of water containing dissolved hydrogen resulted in a sustained increase in the hydrogen levels in the serum and kidney, a better kidney allograft function over a 60-day follow-up period, and a reduction in the markers of inflammation and tissue oxidation. Noda et al. showed that drinking hydrogen-rich water prolongs the survival of cardiac allografts and decreases intimal hyperplasia in aortic allografts [
66]. Furthermore, in another study, T cell proliferation was significantly restrained in the presence of hydrogen in vitro, accompanied by a lower production of interferon-ɣ and IL-2. In yet another study, hydrogen treatment was also associated with higher graft ATP levels and higher activity of the mitochondrial respiratory chain enzymes [
25].
7. Clinical Studies
While numerous preclinical studies have showcased hydrogen’s potential in transplantation, human clinical trials remain relatively scarce. Initial clinical data indicate its safety and potential efficacy, but more extensive, controlled trials are required to firmly establish hydrogen’s therapeutic advantages. It is important to recognize that, despite the promising prospects of hydrogen in transplantation, research in this field is still in its early stages. Key considerations, such as the most effective administration method, dosage, and timing of hydrogen therapy, are pivotal aspects warranting further investigation [
72,
73] and must be carefully evaluated to maximize hydrogen’s therapeutic benefits while mitigating potential drawbacks. Moreover, comprehending how hydrogen interacts with existing immunosuppressive regimens is crucial for optimizing its utilization. Of note, it is important to be aware of the negative results of hydrogen therapy. Hosgood et al. showed that the administration of hydrogen gas did not increase renal function or decrease oxidative damage or inflammation during the reperfusion of kidneys with ischemia damage [
74].
8. Future Direction
While data support the use of hydrogen in many aspects of our current transplant practices, transplant surgeons cannot assume that treatments successful in lab animals or even critically ill transplant patients will yield the same results in critically ill trauma patients. Further studies, including well-designed clinical trials and investigations exploring detailed mechanisms, are required to fully unlock hydrogen’s potential and integrate it into transplantation protocols [
75,
76].
The ability to administer medical gases through inhalation makes this treatment highly attractive for translation into a human clinical setting. Some gases like supplemental oxygen are already being administered in intensive care units. In most cases, medical gas treatment via inhalation can seamlessly complement existing therapeutic strategies by incorporating the gas into conventional delivery mechanism. Hydrogen’s limited reactivity with other gases at therapeutic concentrations allows it to be administered alongside other therapeutic gases, including inhaled anesthesia agents, as part of a combined gas therapy. Recent research has demonstrated that combined therapy with hydrogen and carbon monoxide (CO) enhances therapeutic efficacy over single-gas treatment in preventing cold IRI after heart transplantation, operating through anti-inflammatory and antioxidant mechanisms [
33]. Meng et al. showed that lung inflation with CO or hydrogen during cold ischemia protected against IRI through anti-apoptotic, antioxidant, and anti-inflammatory mechanisms in a rat lung transplantation model, with enhanced benefits observed when CO and hydrogen were used in combination [
77]. Shinbo et al. demonstrated that the inhalation of a combined gas (80 ppm of NO and 2% hydrogen) effectively mitigated myocardial infarction and improved left ventricular function in a mouse left anterior descending coronary artery ligation model, outperforming NO inhalation alone. This suggests that the inhibitory effect of NO on inflammation may be enhanced by adding hydrogen to the inhaled NO gas, thereby eliminating highly reactive byproducts like peroxynitrite [
78].
9. Conclusions
As is clear in this review, hydrogen gas therapy may have well-defined benefits in transplant medicine. Hydrogen holds the potential to address critical challenges in organ transplantation, primarily IRI and graft rejection, through its antioxidative, anti-inflammatory, and immunomodulatory properties. However, more comprehensive research, including well-designed clinical trials, is needed to determine the safety, efficacy, and long-term impact of hydrogen therapy on transplant outcomes. At this moment, there are no available clinical studies investigating the efficacies of hydrogen in transplantation. Appropriately designed randomized controlled trials with patient-important outcomes, such as the improvement of the functioning of target organs, decreased intensive care unit and hospital stays, and decreased cost of therapy, are sorely needed to establish the role of hydrogen therapy in patients with disease and discover its advantages over preexisting standard therapies for transplant patients.
Author Contributions
Conceptualization, T.O., H.N. and A.N.; methodology, T.O. and A.N.; investigation, T.O., H.N. and A.N.; data curation, T.O., H.N. and A.N.; writing—original draft preparation, T.O., H.N. and A.N.; writing—review and editing, T.N., T.H. (Takahiro Hirayama), T.H. (Takashi Hongo), K.A., T.A., M.H. and T.Y.; supervision, H.N. and A.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical consent and approval were waived for this study because it is not a research study.
Informed Consent Statement
Not applicable.
Data Availability Statement
No research data were collected.
Acknowledgments
We thank Christine Burr for editing the manuscript.
Conflicts of Interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Cardinal, J.S.; Zhan, J.; Wang, Y.; Sugimoto, R.; Tsung, A.; McCurry, K.R.; Billiar, T.R.; Nakao, A. Oral hydrogen water prevents chronic allograft nephropathy in rats. Kidney Int. 2010, 77, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Snijder, P.M.; van den Berg, E.; Whiteman, M.; Bakker, S.J.; Leuvenink, H.G.; van Goor, H. Emerging role of gasotransmitters in renal transplantation. Am. J. Transplant. 2013, 13, 3067–3075. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Song, Y.; Yi, Y.; Jiang, X.; Ma, S.; Ma, C.; Li, J.; Zhanghuang, Z.; Liu, M.; Zhao, P.; et al. Therapeutic Potential of Molecular Hydrogen in Metabolic Diseases from Bench to Bedside. Pharmaceuticals 2023, 16, 541. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Cui, H.; Xu, W. Hydrogen inhibits the proliferation and migration of gastric cancer cells by modulating lncRNA MALAT1/miR-124-3p/EZH2 axis. Cancer Cell Int. 2021, 21, 70. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.B.; Kong, X.F.; Mu, F.; Lu, T.Y.; Lu, Y.Y.; Xu, K.C. Hydrogen therapy can be used to control tumor progression and alleviate the adverse events of medications in patients with advanced non-small cell lung cancer. Med. Gas Res. 2020, 10, 75–80. [Google Scholar]
- Niu, Y.; Nie, Q.; Dong, L.; Zhang, J.; Liu, S.F.; Song, W.; Wang, X.; Wu, G.; Song, D. Hydrogen Attenuates Allergic Inflammation by Reversing Energy Metabolic Pathway Switch. Sci. Rep. 2020, 10, 1962. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.S.; Kawamura, T.; Toyoda, Y.; Nakao, A. Recent advances in hydrogen research as a therapeutic medical gas. Free Radic. Res. 2010, 44, 971–982. [Google Scholar] [CrossRef]
- Yang, F.; Lei, Y.; Liu, R.; Luo, X.; Li, J.; Zeng, F.; Lu, S.; Huang, X.; Lan, Y. Hydrogen: Potential Applications in Solid Organ Transplantation. Oxid. Med. Cell Longev. 2021, 2021, 6659310. [Google Scholar] [CrossRef]
- Quan, L.; Zheng, B.; Zhou, H. Protective effects of molecular hydrogen on lung injury from lung transplantation. Exp. Biol. Med. 2021, 246, 1410–1418. [Google Scholar] [CrossRef]
- Hasegawa, T.; Ito, M.; Hasegawa, S.; Teranishi, M.; Takeda, K.; Negishi, S.; Nishiwaki, H.; Takeda, J.I.; LeBaron, T.W.; Ohno, K. Molecular Hydrogen Enhances Proliferation of Cancer Cells That Exhibit Potent Mitochondrial Unfolded Protein Response. Int. J. Mol. Sci. 2022, 23, 2888. [Google Scholar] [CrossRef]
- Iida, A.; Nosaka, N.; Yumoto, T.; Knaup, E.; Naito, H.; Nishiyama, C.; Yamakawa, Y.; Tsukahara, K.; Terado, M.; Sato, K.; et al. The Clinical Application of Hydrogen as a Medical Treatment. Acta Med. Okayama 2016, 70, 331–337. [Google Scholar]
- Luo, S.; Wu, J.; Qiu, Y.; Xiao, B.; Xi, Y.; Yang, C.; Narita, Y.; Yoshii, D.; Zhong, L.; Komohara, Y.; et al. Hydrogen Promotes the Effectiveness of Bone Mesenchymal Stem Cell Transplantation in Rats with Spinal Cord Injury. Stem Cells Int. 2023, 2023, 8227382. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Quan, L.; Zheng, B.; Li, Z.; Zhang, G.; Zhang, M.; Zhou, H. Inflation using hydrogen improves donor lung quality by regulating mitochondrial function during cold ischemia phase. BMC Pulm. Med. 2023, 23, 213. [Google Scholar] [CrossRef] [PubMed]
- Nishi, K.; Iwai, S.; Tajima, K.; Okano, S.; Sano, M.; Kobayashi, E. Prevention of Chronic Rejection of Marginal Kidney Graft by Using a Hydrogen Gas-Containing Preservation Solution and Adequate Immunosuppression in a Miniature Pig Model. Front. Immunol. 2020, 11, 626295. [Google Scholar] [CrossRef] [PubMed]
- Kayawake, H.; Chen-Yoshikawa, T.F.; Saito, M.; Yamagishi, H.; Yoshizawa, A.; Hirano, S.I.; Kurokawa, R.; Date, H. Protective Effects of a Hydrogen-Rich Preservation Solution in a Canine Lung Transplantation Model. Ann. Thorac. Surg. 2021, 111, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Kang, J.; Xing, E.; Zheng, B.; Wang, X.; Zhou, H. Lung Inflation With Hydrogen During the Cold Ischemia Phase Alleviates Lung Ischemia-Reperfusion Injury by Inhibiting Pyroptosis in Rats. Front. Physiol. 2021, 12, 699344. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Aokage, T.; Igawa, T.; Hirayama, T.; Seya, M.; Ishikawa-Aoyama, M.; Nojima, T.; Nakao, A.; Naito, H. Luminal preloading with hydrogen-rich saline ameliorates ischemia-reperfusion injury following intestinal transplantation in rats. Pediatr. Transplant. 2020, 24, e13848. [Google Scholar] [CrossRef]
- Saito, M.; Chen-Yoshikawa, T.F.; Takahashi, M.; Kayawake, H.; Yokoyama, Y.; Kurokawa, R.; Hirano, S.I.; Date, H. Protective effects of a hydrogen-rich solution during cold ischemia in rat lung transplantation. J. Thorac. Cardiovasc. Surg. 2020, 159, 2110–2118. [Google Scholar] [CrossRef]
- Kobayashi, E.; Sano, M. Organ preservation solution containing dissolved hydrogen gas from a hydrogen-absorbing alloy canister improves function of transplanted ischemic kidneys in miniature pigs. PLoS ONE 2019, 14, e0222863. [Google Scholar] [CrossRef]
- Uto, K.; Sakamoto, S.; Que, W.; Shimata, K.; Hashimoto, S.; Sakisaka, M.; Narita, Y.; Yoshii, D.; Zhong, L.; Komohara, Y.; et al. Hydrogen-rich solution attenuates cold ischemia-reperfusion injury in rat liver transplantation. BMC Gastroenterol. 2019, 19, 25. [Google Scholar] [CrossRef]
- Du, H.; Sheng, M.; Wu, L.; Zhang, Y.; Shi, D.; Weng, Y.; Xu, R.; Yu, W. Hydrogen-Rich Saline Attenuates Acute Kidney Injury After Liver Transplantation via Activating p53-Mediated Autophagy. Transplantation 2016, 100, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Chen, X.; Qian, L.; Shen, J.; Cai, J. Administration of hydrogen-rich saline in mice with allogeneic hematopoietic stem-cell transplantation. Med. Sci. Monit. 2015, 21, 749–754. [Google Scholar] [PubMed]
- Zhang, C.B.; Tang, Y.C.; Xu, X.J.; Guo, S.X.; Wang, H.Z. Hydrogen gas inhalation protects against liver ischemia/reperfusion injury by activating the NF-kappaB signaling pathway. Exp. Ther. Med. 2015, 9, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.L.; Cheng, L.; Ren, J.D.; Fang, C.; Xiang, K.; Xu, H.T.; Tang, L.J.; Wang, T.; Tian, F.Z. Hydrogen-rich saline protects against ischemia/reperfusion injury in grafts after pancreas transplantations by reducing oxidative stress in rats. Mediat. Inflamm. 2015, 2015, 281985. [Google Scholar] [CrossRef] [PubMed]
- Noda, K.; Tanaka, Y.; Shigemura, N.; Kawamura, T.; Wang, Y.; Masutani, K.; Sun, X.; Toyoda, Y.; Bermudez, C.A.; Nakao, A. Hydrogen-supplemented drinking water protects cardiac allografts from inflammation-associated deterioration. Transpl. Int. 2012, 25, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Li, X.K.; Yazawa, K.; Hatayama, N.; Xie, L.; Sato, B.; Kakuta, Y.; Tsutahara, K.; Okumi, M.; Tsuda, H.; et al. Hydrogen-rich University of Wisconsin solution attenuates renal cold ischemia-reperfusion injury. Transplantation 2012, 94, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Shigemura, N.; Kawamura, T.; Noda, K.; Isse, K.; Stolz, D.B.; Billiar, T.R.; Toyoda, Y.; Bermudez, C.A.; Lyons-Weiler, J.; et al. Profiling molecular changes induced by hydrogen treatment of lung allografts prior to procurement. Biochem. Biophys. Res. Commun. 2012, 425, 873–879. [Google Scholar] [CrossRef]
- Noda, K.; Shigemura, N.; Tanaka, Y.; Kawamura, T.; Hyun Lim, S.; Kokubo, K.; Billiar, T.R.; Bermudez, C.A.; Kobayashi, H.; Nakao, A. A novel method of preserving cardiac grafts using a hydrogen-rich water bath. J. Heart Lung Transplant. 2013, 32, 241–250. [Google Scholar] [CrossRef]
- Sun, Q.; Kawamura, T.; Masutani, K.; Peng, X.; Sun, Q.; Stolz, D.B.; Pribis, J.P.; Billiar, T.R.; Sun, X.; Bermudez, C.A.; et al. Oral intake of hydrogen-rich water inhibits intimal hyperplasia in arterialized vein grafts in rats. Cardiovasc. Res. 2012, 94, 144–153. [Google Scholar] [CrossRef]
- Buchholz, B.M.; Masutani, K.; Kawamura, T.; Peng, X.; Toyoda, Y.; Billiar, T.R.; Bauer, A.J.; Nakao, A. Hydrogen-enriched preservation protects the isogeneic intestinal graft and amends recipient gastric function during transplantation. Transplantation 2011, 92, 985–992. [Google Scholar] [CrossRef]
- Kawamura, T.; Huang, C.S.; Peng, X.; Masutani, K.; Shigemura, N.; Billiar, T.R.; Okumura, M.; Toyoda, Y.; Nakao, A. The effect of donor treatment with hydrogen on lung allograft function in rats. Surgery 2011, 150, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, T.; Huang, C.S.; Tochigi, N.; Lee, S.; Shigemura, N.; Billiar, T.R.; Okumura, M.; Nakao, A.; Toyoda, Y. Inhaled hydrogen gas therapy for prevention of lung transplant-induced ischemia/reperfusion injury in rats. Transplantation 2010, 90, 1344–1351. [Google Scholar] [CrossRef] [PubMed]
- Nakao, A.; Kaczorowski, D.J.; Wang, Y.; Cardinal, J.S.; Buchholz, B.M.; Sugimoto, R.; Tobita, K.; Lee, S.; Toyoda, Y.; Billiar, T.R.; et al. Amelioration of rat cardiac cold ischemia/reperfusion injury with inhaled hydrogen or carbon monoxide, or both. J. Heart Lung Transplant. 2010, 29, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, B.M.; Kaczorowski, D.J.; Sugimoto, R.; Yang, R.; Wang, Y.; Billiar, T.R.; McCurry, K.R.; Bauer, A.J.; Nakao, A. Hydrogen inhalation ameliorates oxidative stress in transplantation induced intestinal graft injury. Am. J. Transplant. 2008, 8, 2015–2024. [Google Scholar] [CrossRef] [PubMed]
- Naito, H.; Nojima, T.; Fujisaki, N.; Tsukahara, K.; Yamamoto, H.; Yamada, T.; Aokage, T.; Yumoto, T.; Osako, T.; Nakao, A. Therapeutic strategies for ischemia reperfusion injury in emergency medicine. Acute Med. Surg. 2020, 7, e501. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, J.L.; Levine, J.E.; Reddy, P.; Holler, E. Graft-versus-host disease. Lancet 2009, 373, 1550–1561. [Google Scholar] [CrossRef]
- Jamil, M.O.; Mineishi, S. State-of-the-art acute and chronic GVHD treatment. Int. J. Hematol. 2015, 101, 452–466. [Google Scholar] [CrossRef]
- Staubli, S.M.; Ceresa, C.D.L.; Pollok, J.M. The Current Role and Future Applications of Machine Perfusion in Liver Transplantation. Bioengineering 2023, 10, 593. [Google Scholar] [CrossRef]
- Strocchi, A.; Levitt, M.D. Maintaining intestinal H2 balance: Credit the colonic bacteria. Gastroenterology 1992, 102, 1424–1426. [Google Scholar] [CrossRef]
- Carbonero, F.; Benefiel, A.C.; Gaskins, H.R. Contributions of the microbial hydrogen economy to colonic homeostasis. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 504–518. [Google Scholar] [CrossRef]
- Levitt, M.D. Volume and composition of human intestinal gas determined by means of an intestinal washout technic. N. Engl. J. Med. 1971, 284, 1394–1398. [Google Scholar] [CrossRef] [PubMed]
- Nakao, A.; Yamada, T.; Kohama, K.; Yoshie, N.; Fujisaki, N.; Kotani, J. Application of carbon monoxide for treatment of acute kidney injury. Acute Med. Surg. 2014, 1, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Ageta, K.; Hirayama, T.; Aokage, T.; Seya, M.; Meng, Y.; Nojima, T.; Yamamoto, H.; Obara, T.; Nakao, A.; Yumoto, T.; et al. Hydrogen inhalation attenuates lung contusion after blunt chest trauma in mice. Surgery 2023, 174, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Zhao, J.N.; Bao, N.R. Hydrogen applications: Advances in the field of medical therapy. Med. Gas Res. 2023, 13, 99–107. [Google Scholar] [PubMed]
- Ishibashi, T.; Sato, B.; Rikitake, M.; Seo, T.; Kurokawa, R.; Hara, Y.; Nagao, T. Consumption of water containing a high concentration of molecular hydrogen reduces oxidative stress and disease activity in patients with rheumatoid arthritis: An open-label pilot study. Med. Gas Res. 2012, 2, 27. [Google Scholar] [CrossRef] [PubMed]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.S.; Kawamura, T.; Lee, S.; Tochigi, N.; Shigemura, N.; Buchholz, B.M.; Kloke, J.D.; Billiar, T.R.; Toyoda, Y.; Nakao, A. Hydrogen inhalation ameliorates ventilator-induced lung injury. Crit. Care. 2010, 14, R234. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Ito, M.; Fujita, Y.; Ito, M.; Ichihara, M.; Masuda, A.; Suzuki, Y.; Maesawa, S.; Kajita, Y.; Hirayama, M.; et al. Molecular hydrogen is protective against 6-hydroxydopamine-induced nigrostriatal degeneration in a rat model of Parkinson’s disease. Neurosci. Lett. 2009, 453, 81–85. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, H.; Ji, M.; Jia, M.; Chen, H.; Yang, J.; Duan, M. Hydrogen-rich saline attenuates neuronal ischemia--reperfusion injury by protecting mitochondrial function in rats. J. Surg. Res. 2014, 192, 564–572. [Google Scholar] [CrossRef]
- Ito, M.; Ibi, T.; Sahashi, K.; Ichihara, M.; Ito, M.; Ohno, K. Open-label trial and randomized, double-blind, placebo-controlled, crossover trial of hydrogen-enriched water for mitochondrial and inflammatory myopathies. Med. Gas Res. 2011, 1, 24. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Wu, H.; Li, L.; Yang, X.; Lai, K.; Bao, J.; Xie, K.; Yu, Y. Hydrogen regulates mitochondrial quality to protect glial cells and alleviates sepsis-associated encephalopathy by Nrf2/YY1 complex promoting HO-1 expression. Int. Immunopharmacol. 2023, 118, 110009. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Li, Z.; Meng, C.; Kang, J.; Zhang, M.; Ma, L.; Zhou, H. The Anti-inflammatory Effect of Hydrogen on Lung Transplantation Model of Pulmonary Microvascular Endothelial Cells During Cold Storage Period. Transplantation 2018, 102, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Cai, Z.; Zhang, X.; Liu, M.; Xie, F.; Liu, Z.; Lu, S.; Ma, X. Hydrogen Attenuates Inflammation by Inducing Early M2 Macrophage Polarization in Skin Wound Healing. Pharmaceuticals 2023, 16, 885. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Liu, X.; Diao, Y.; Sun, Y.; Gao, G.; Zhang, T.; Chen, K.; Pei, L. Hydrogen-rich solution against myocardial injury and aquaporin expression via the PI3K/Akt signaling pathway during cardiopulmonary bypass in rats. Mol. Med. Rep. 2018, 18, 1925–1938. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; An, Z.; Liao, J.; Ran, N.; Zhu, Y.; Ren, S.; Meng, X.; Cui, N.; Yu, Y.; Fan, H. The Role and Mechanism of Hydrogen-Rich Water in the Cucumis sativus Response to Chilling Stress. Int. J. Mol. Sci. 2023, 24, 6702. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Feng, X.; Chen, J.; Wu, Y.; Shen, L. Hydrogen-rich saline alleviates early brain injury through inhibition of necroptosis and neuroinflammation via the ROS/HO-1 signaling pathway after traumatic brain injury. Exp. Ther. Med. 2022, 23, 126. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Ma, Z.; Guo, Z.; Zhao, F.; Wang, Y.; Cai, L.; Yang, J. Type 1 Diabetes Mellitus is an Independent Risk Factor for Pulmonary Fibrosis. Cell Biochem. Biophys. 2014, 70, 1385–1391. [Google Scholar] [CrossRef]
- Chen, H.; Xie, K.; Han, H.; Li, Y.; Liu, L.; Yang, T.; Yu, Y. Molecular hydrogen protects mice against polymicrobial sepsis by ameliorating endothelial dysfunction via an Nrf2/HO-1 signaling pathway. Int. Immunopharmacol. 2015, 28, 643–654. [Google Scholar] [CrossRef]
- Aoyama-Ishikawa, M.; Seishu, A.; Kawakami, S.; Maeshige, N.; Miyoshi, M.; Ueda, T.; Usami, M.; Nakao, A.; Kotani, J. Intravenous immunoglobulin-induced neutrophil apoptosis in the lung during murine endotoxemia. Surg. Infect. 2014, 15, 36–42. [Google Scholar] [CrossRef]
- Terasaki, Y.; Ohsawa, I.; Terasaki, M.; Takahashi, M.; Kunugi, S.; Dedong, K.; Urushiyama, H.; Amenomori, S.; Kaneko-Togashi, M.; Kuwahara, N.; et al. Hydrogen therapy attenuates irradiation-induced lung damage by reducing oxidative stress. Am. J. Physiol. Lung Cell Mol. Physiol. 2011, 301, L415–L426. [Google Scholar] [CrossRef]
- Huang, C.S.; Kawamura, T.; Peng, X.; Tochigi, N.; Shigemura, N.; Billiar, T.R.; Nakao, A.; Toyoda, Y. Hydrogen inhalation reduced epithelial apoptosis in ventilator-induced lung injury via a mechanism involving nuclear factor-kappa B activation. Biochem. Biophys. Res. Commun. 2011, 408, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Hamada, N.; Terazawa, R.; Ito, M.; Ohno, K.; Ichihara, M.; Nozawa, Y.; Ito, M. Molecular hydrogen inhibits lipopolysaccharide/interferon gamma-induced nitric oxide production through modulation of signal transduction in macrophages. Biochem. Biophys. Res. Commun. 2011, 411, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Fu, Z.; Wei, Y.; Liu, J.; Cui, X.; Yang, W.; Ding, W.; Pan, P.; Li, W. Hydrogen inhalation decreases lung graft injury in brain-dead donor rats. J. Heart Lung Transplant. 2013, 32, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Fang, X.; Meng, C.; Xing, J.; Liu, J.; Yang, W.; Li, W.; Zhou, H. Lung inflation with hydrogen during the cold ischemia phase decreases lung graft injury in rats. Exp. Biol. Med. 2015, 240, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, I.; Hata, K.; Okamura, Y.; Nigmet, Y.; Hirao, H.; Kubota, T.; Inamoto, O.; Kusakabe, J.; Goto, T.; Tajima, T.; et al. Hydrogen Flush After Cold Storage as a New End-Ischemic Ex Vivo Treatment for Liver Grafts Against Ischemia/Reperfusion Injury. Liver Transpl. 2018, 24, 1589–1602. [Google Scholar] [CrossRef] [PubMed]
- Haam, S.; Lee, S.; Paik, H.C.; Park, M.S.; Song, J.H.; Lim, B.J.; Nakao, A. The effects of hydrogen gas inhalation during ex vivo lung perfusion on donor lungs obtained after cardiac death. Eur. J. Cardiothorac. Surg. 2015, 48, 542–547. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Noda, K.; Shigemura, N.; Tanaka, Y.; Bhama, J.; D’Cunha, J.; Kobayashi, H.; Luketich, J.D.; Bermudez, C.A. Hydrogen Preconditioning During Ex Vivo Lung Perfusion Improves the Quality of Lung Grafts in Rats. Transplantation 2014, 98, 499–506. [Google Scholar] [CrossRef]
- Dark, J. Hydrogen in lung reconditioning—More than just inflation. Transplantation 2014, 98, 497–498. [Google Scholar] [CrossRef]
- Ishikawa, T.; Shimada, S.; Fukai, M.; Kimura, T.; Umemoto, K.; Shibata, K.; Fujiyoshi, M.; Fujiyoshi, S.; Hayasaka, T.; Kawamura, N.; et al. Post-reperfusion hydrogen gas treatment ameliorates ischemia reperfusion injury in rat livers from donors after cardiac death: A preliminary study. Surg. Today. 2018, 48, 1081–1088. [Google Scholar] [CrossRef]
- Obara, T.; Yamamoto, H.; Aokage, T.; Igawa, T.; Nojima, T.; Hirayama, T.; Seya, M.; Ishikawa-Aoyama, M.; Nakao, A.; Motterlini, R.; et al. Luminal Administration of a Water-soluble Carbon Monoxide-releasing Molecule (CORM-3) Mitigates Ischemia/Reperfusion Injury in Rats Following Intestinal Transplantation. Transplantation 2021, 106, 1365–1375. [Google Scholar] [CrossRef]
- Simon, A.R. Hydrogen-supplemented drinking water, just soda or an elixir of life? Transpl. Int. 2012, 25, 1211–1212. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Jiang, X.; Yi, Y.; Liu, Z.J.; Ma, C.; He, J.; Xun, Z.M.; Wang, M.; Liu, M.Y.; Mawulikplimi Adzavon, Y.; et al. Different effects of hydrogen-rich water intake and hydrogen gas inhalation on gut microbiome and plasma metabolites of rats in health status. Sci. Rep. 2022, 12, 7231. [Google Scholar] [CrossRef] [PubMed]
- Xun, Z.M.; Zhao, Q.H.; Zhang, Y.; Ju, F.D.; He, J.; Yao, T.T.; Zhang, X.K.; Yi, Y.; Ma, S.N.; Zhao, P.X.; et al. Effects of long-term hydrogen intervention on the physiological function of rats. Sci. Rep. 2020, 10, 18509. [Google Scholar] [CrossRef]
- Hosgood, S.A.; Moore, T.; Qurashi, M.; Adams, T.; Nicholson, M.L. Hydrogen Gas Does Not Ameliorate Renal Ischemia Reperfusion Injury in a Preclinical Model. Artif. Organs. 2018, 42, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Yoritaka, A.; Ohtsuka, C.; Maeda, T.; Hirayama, M.; Abe, T.; Watanabe, H.; Saiki, H.; Oyama, G.; Fukae, J.; Shimo, Y.; et al. Randomized, double-blind, multicenter trial of hydrogen water for Parkinson’s disease. Mov. Disord. 2018, 33, 1505–1507. [Google Scholar] [CrossRef]
- Nishiwaki, H.; Ito, M.; Negishi, S.; Sobue, S.; Ichihara, M.; Ohno, K. Molecular hydrogen upregulates heat shock response and collagen biosynthesis, and downregulates cell cycles: Meta-analyses of gene expression profiles. Free Radic. Res. 2018, 52, 434–445. [Google Scholar] [CrossRef]
- Meng, C.; Ma, L.; Niu, L.; Cui, X.; Liu, J.; Kang, J.; Liu, R.; Xing, J.; Jiang, C.; Zhou, H. Protection of donor lung inflation in the setting of cold ischemia against ischemia-reperfusion injury with carbon monoxide, hydrogen, or both in rats. Life Sci. 2016, 151, 199–206. [Google Scholar] [CrossRef]
- Shinbo, T.; Kokubo, K.; Sato, Y.; Hagiri, S.; Hataishi, R.; Hirose, M.; Kobayashi, H. Breathing nitric oxide plus hydrogen gas reduces ischemia-reperfusion injury and nitrotyrosine production in murine heart. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H542–H550. [Google Scholar] [CrossRef][Green Version]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).