2-Aminoethyl Dihydrogen Phosphate (2-AEH2P) Associated with Cell Metabolism-Modulating Drugs Presents a Synergistic and Pro-Apoptotic Effect in an In Vitro Model of the Ascitic Ehrlich Tumor
Abstract
1. Introduction
2. Methods
2.1. Acquisition of Ehrlich Ascitic Carcinoma (EAT) Cells
2.2. Cell Culture
2.3. Determination of Cytotoxic Activity Using the MTT Method
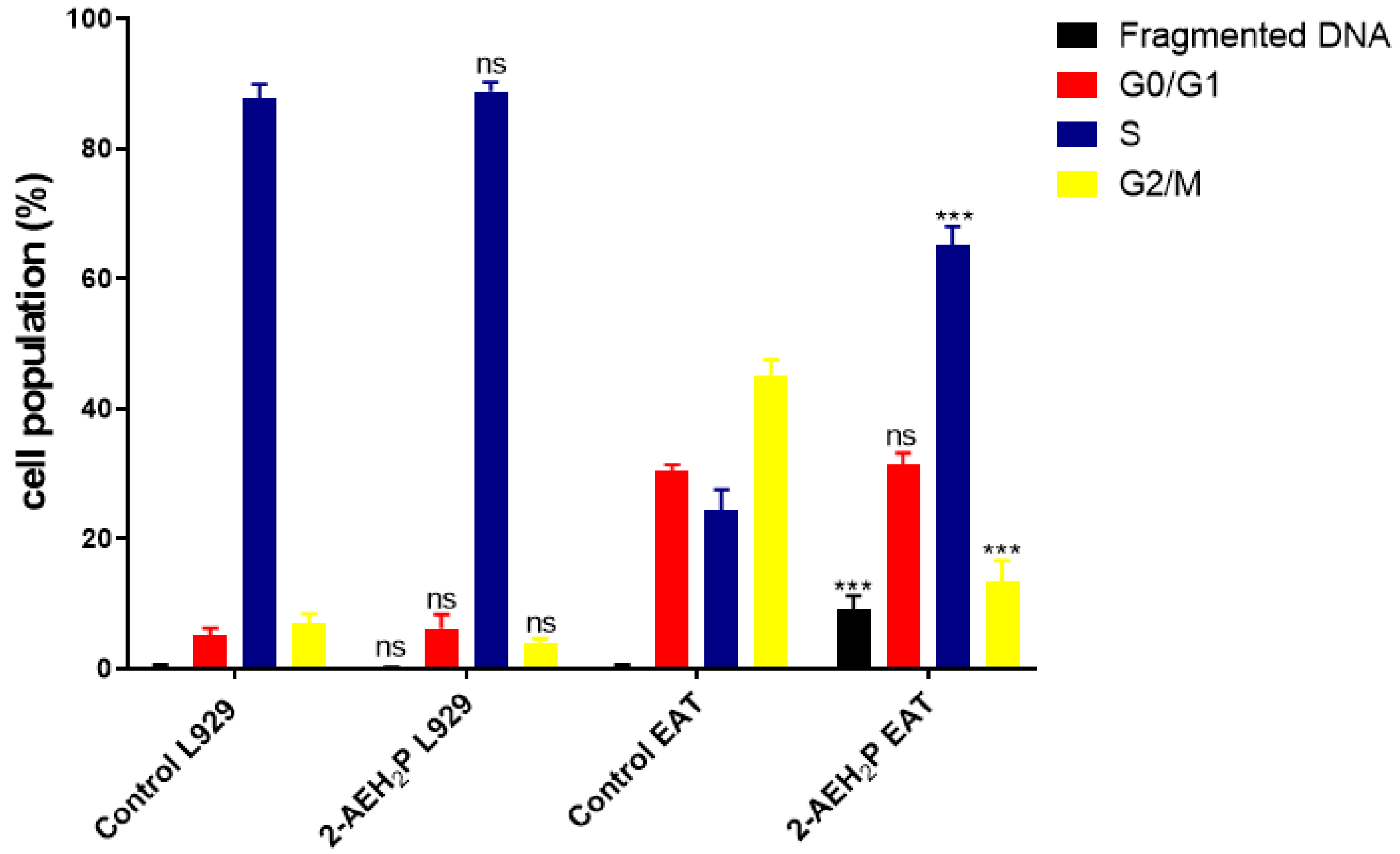
2.4. Analysis of Cell Cycle Phases and Fragmented DNA by Flow Cytometry
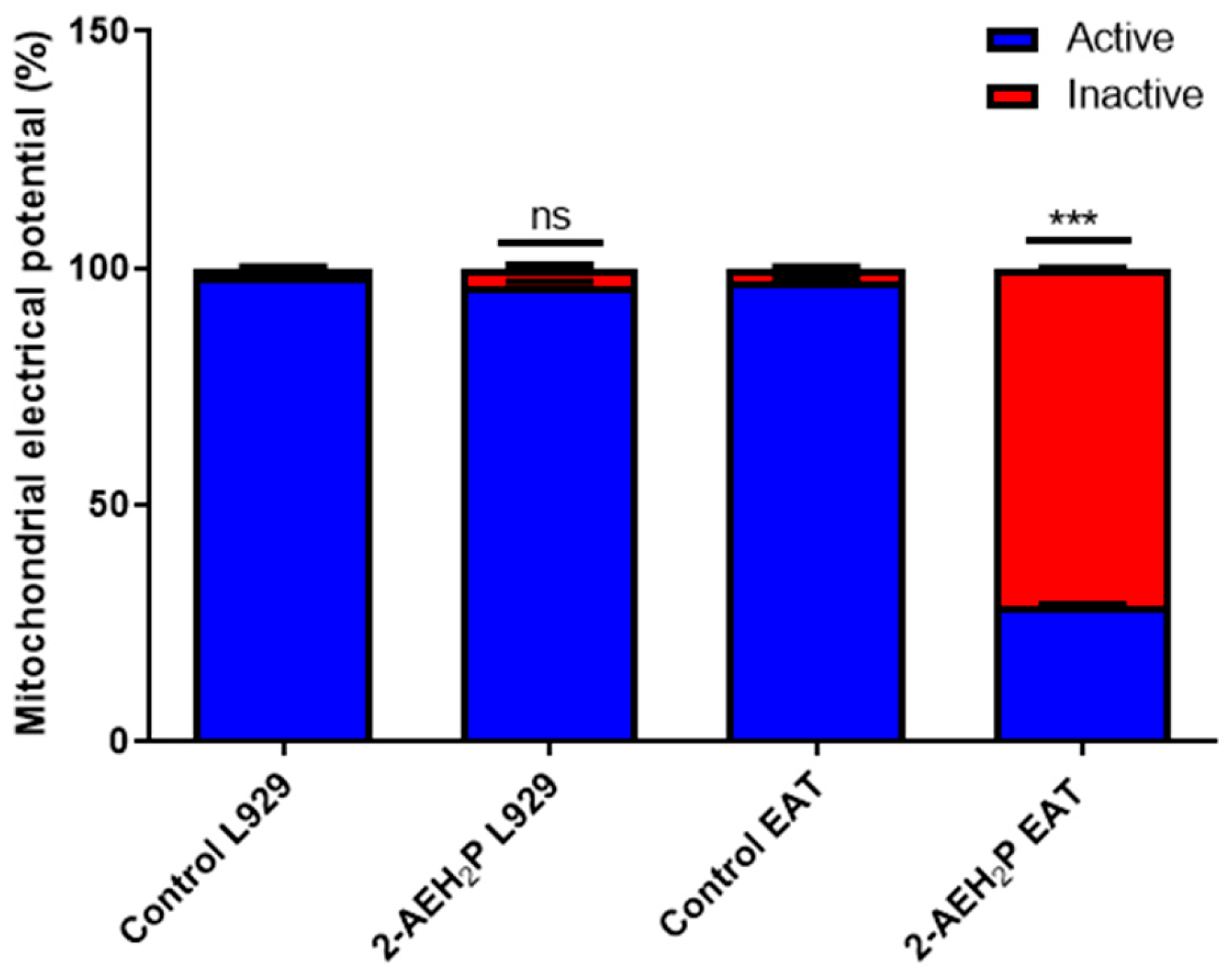
2.5. Analysis of Mitochondrial Membrane Potential
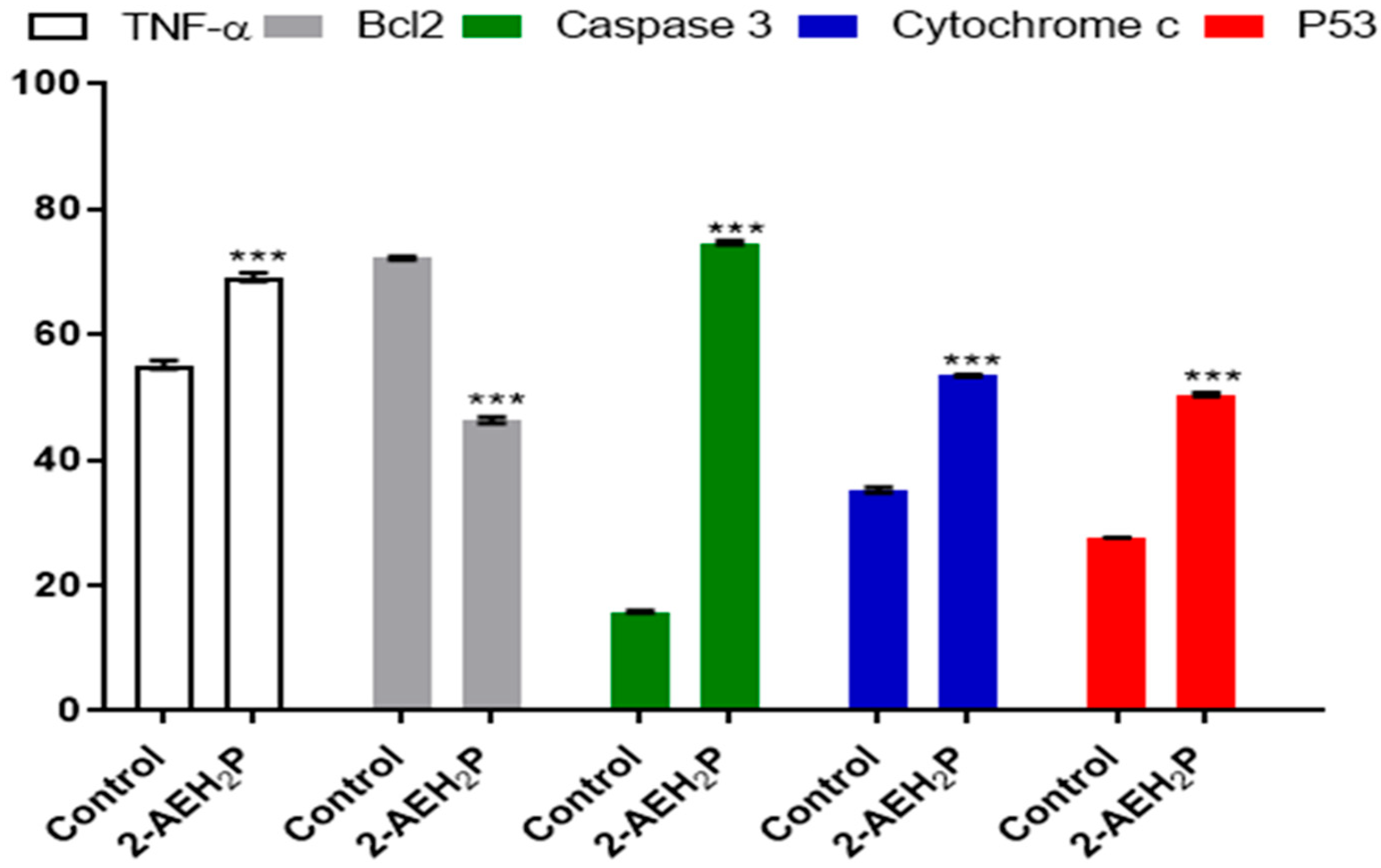
2.6. Evaluation of Cell Marker Expression by Flow Cytometry
2.7. Analysis of Pharmacological Effect and Drug Combination
2.8. Statistical Analysis
3. Results
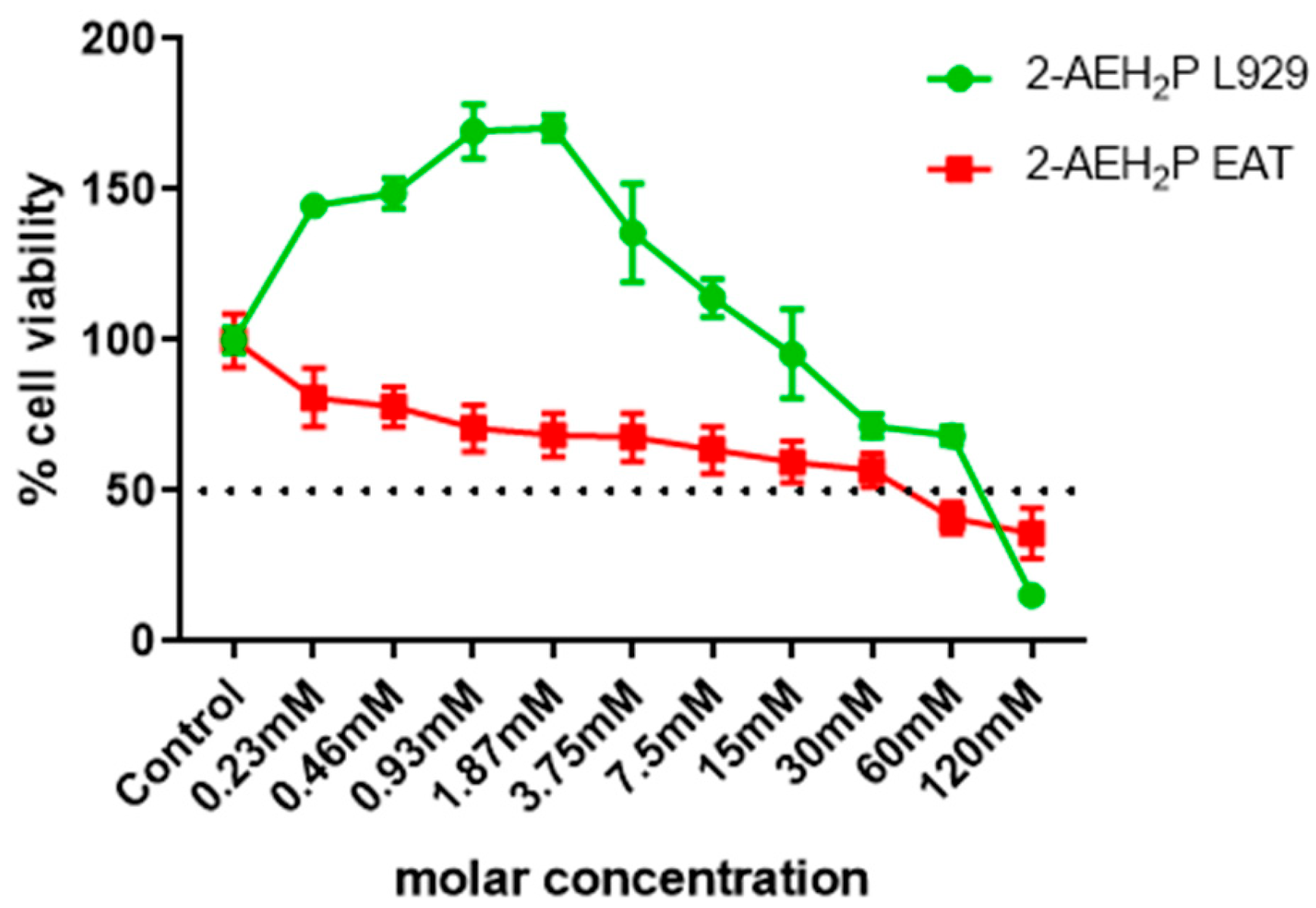
3.1. Analysis of the Compound 2-AEH2P in Murine Fibroblast Cells (L929) and Ehrlich Ascitic Tumor (EAT) Cells
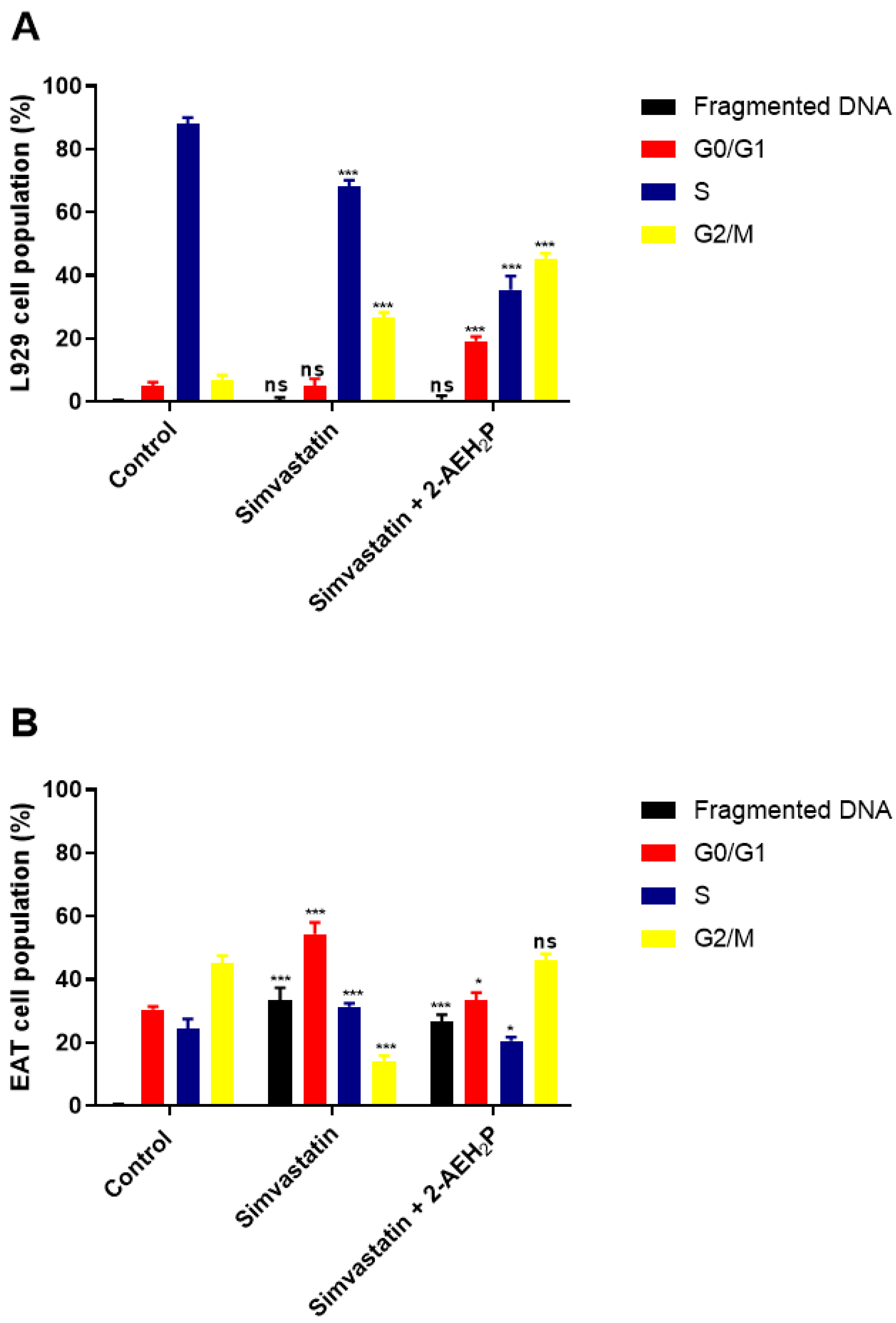
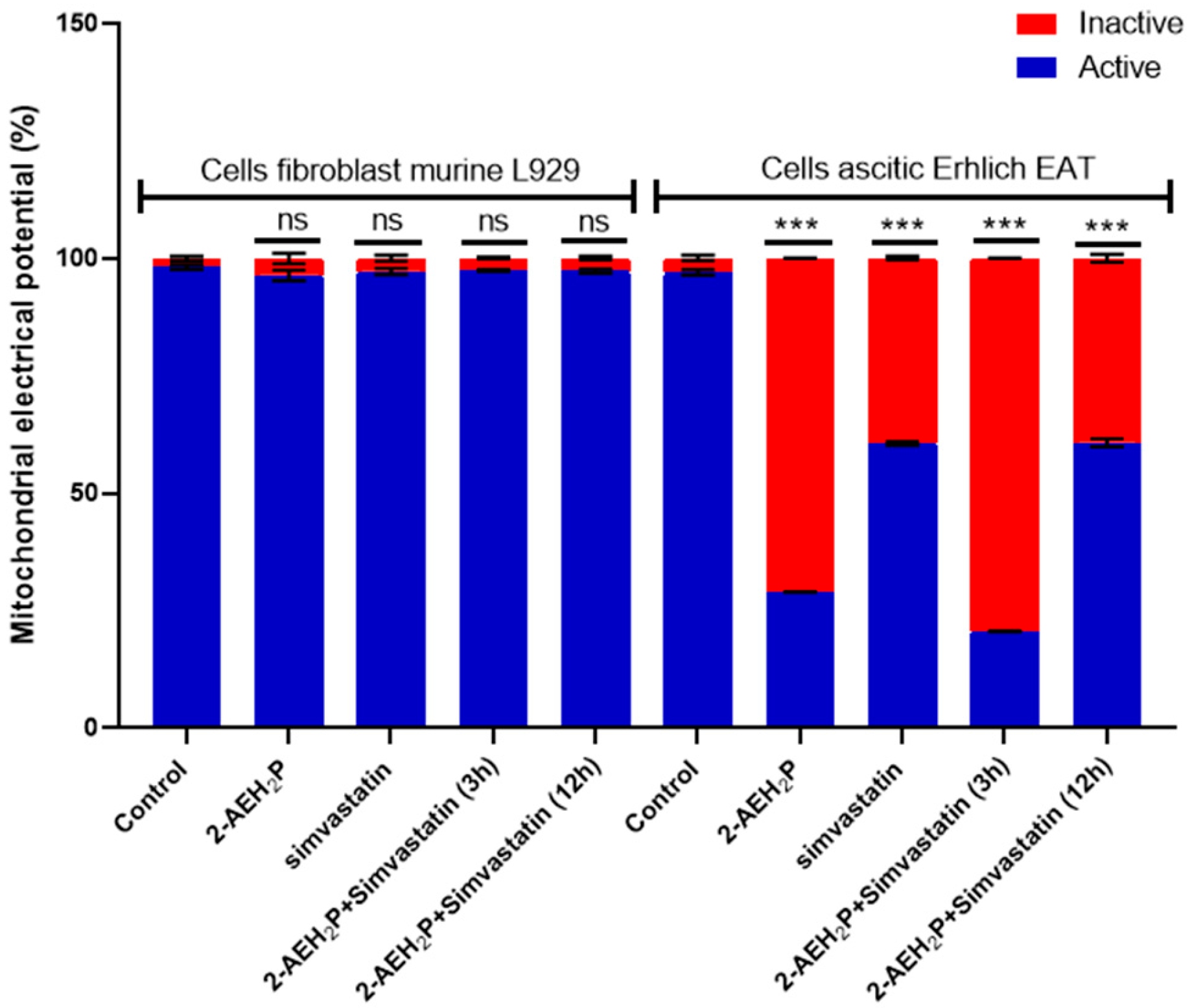
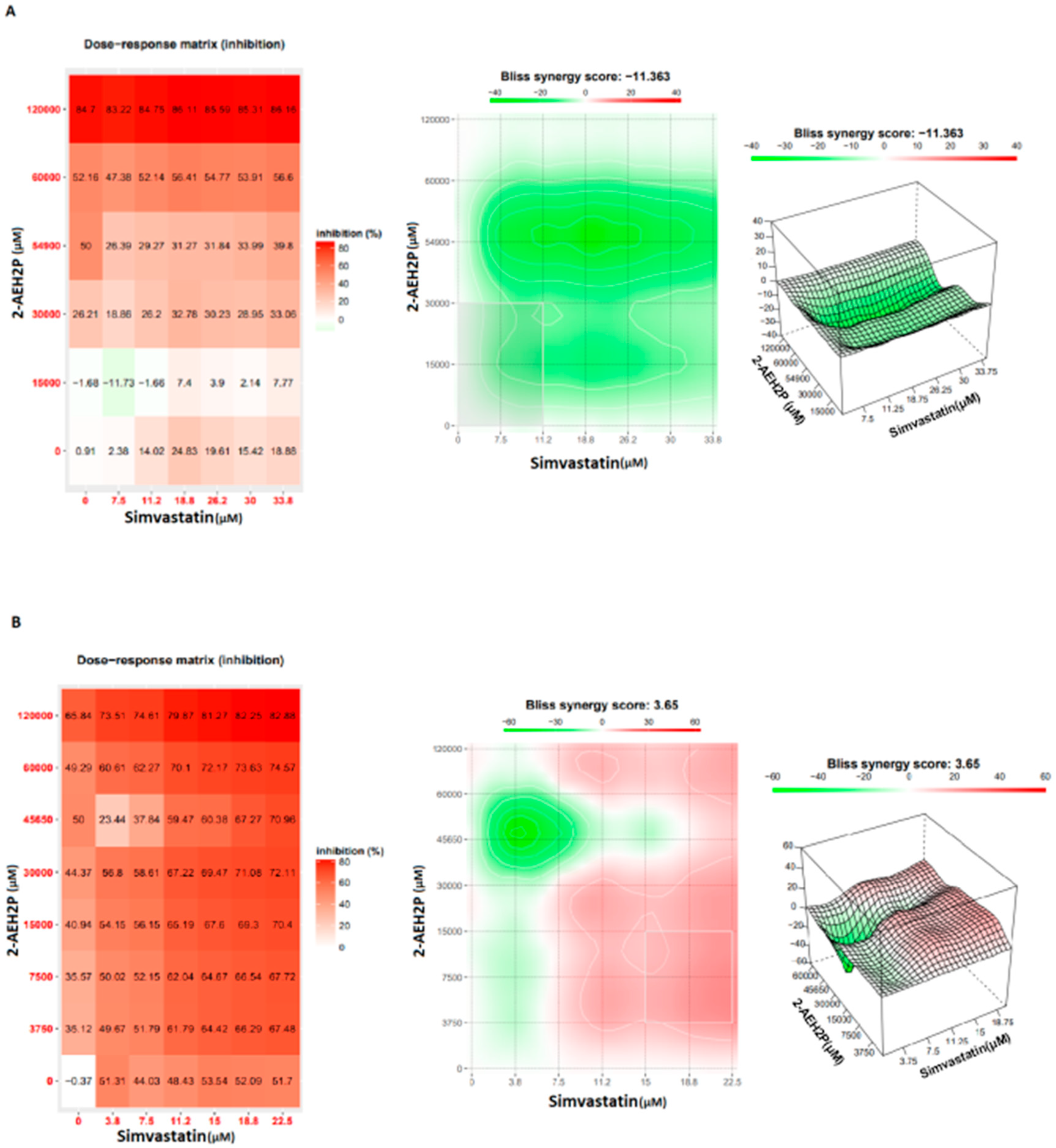
3.2. Analysis of the Compound 2-AEH2P Associated with Simvastatin in Murine Fibroblast Cells (L929) and Ehrlich Ascitic Tumor (EAT) Cells
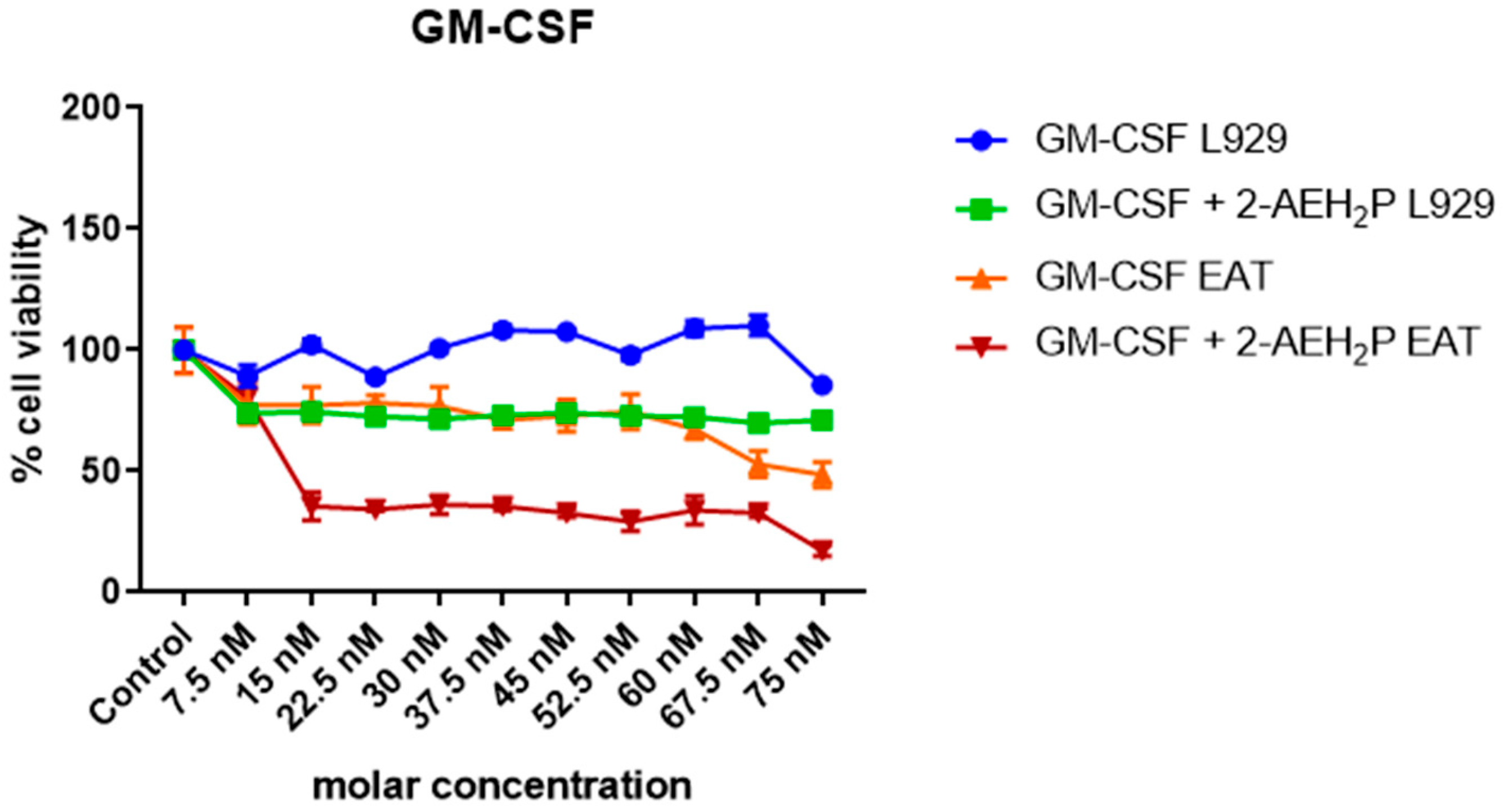
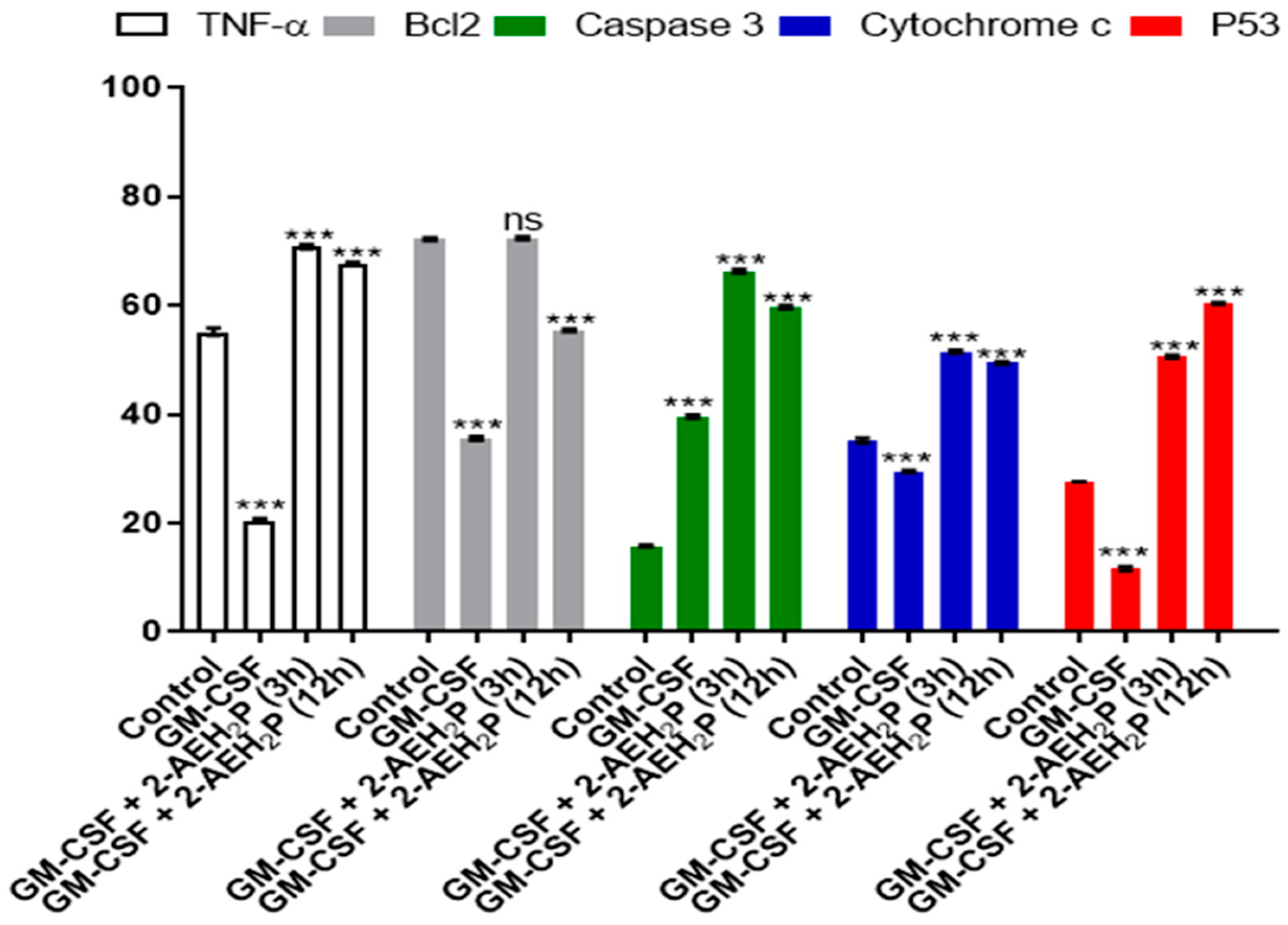
3.3. Analysis of the Compound 2-AEH2P associated with GM-CSF in Normal Murine Fibroblast Cells (L929) and Ehrlich Ascitic Tumor (EAT) Cells
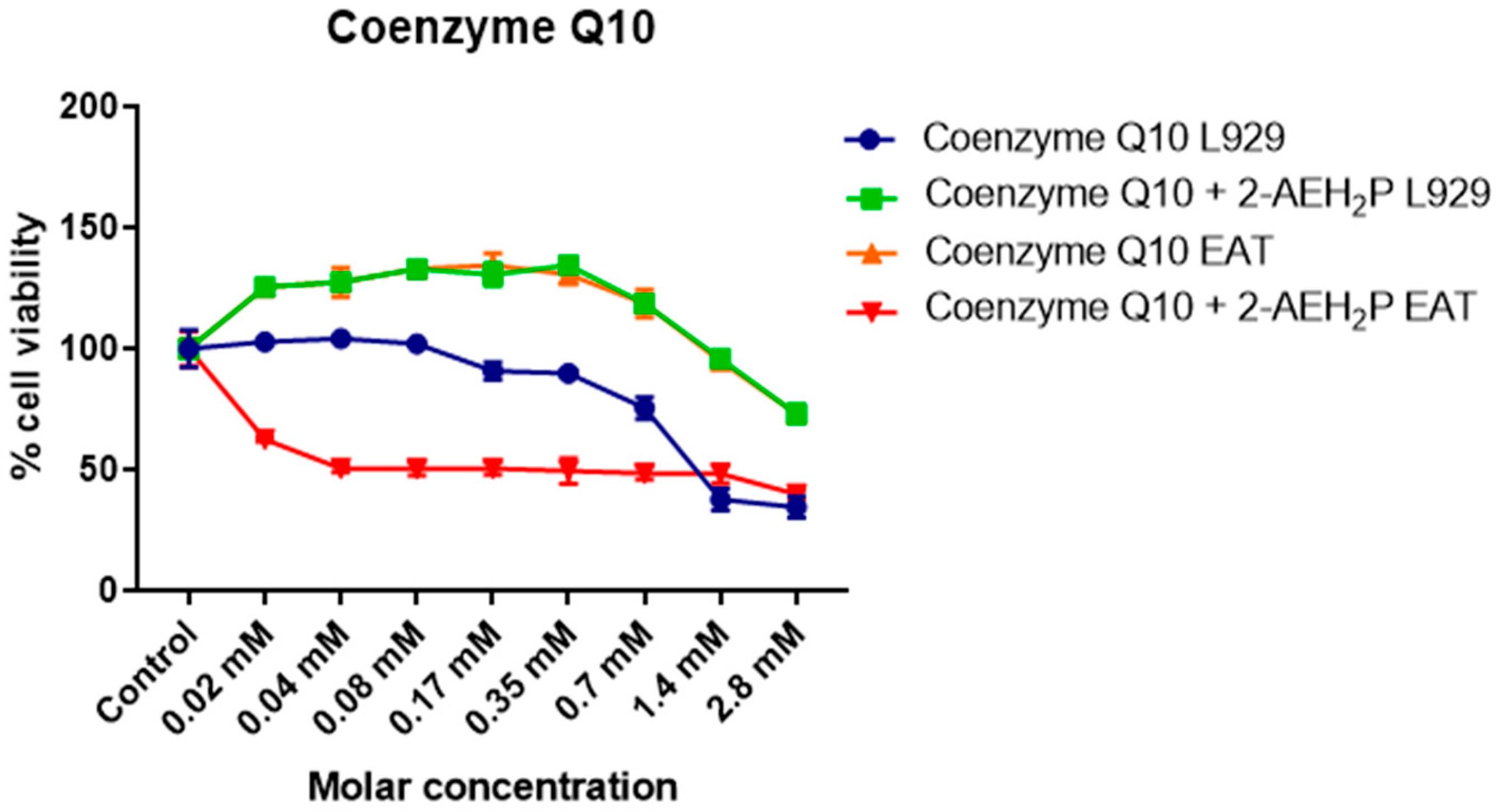
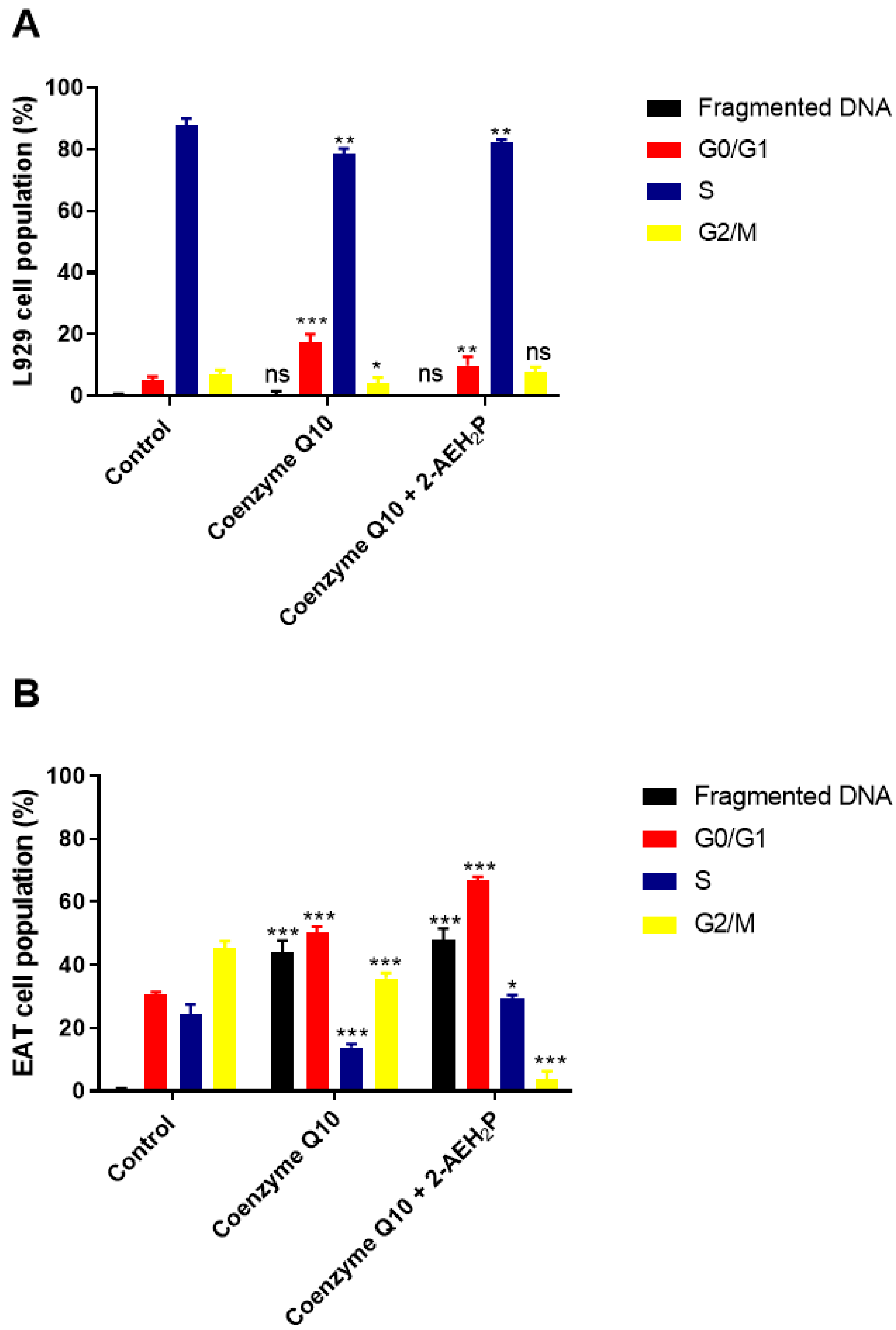
3.4. Analysis of the Compound 2-AEH2P Associated with Coenzyme Q10 in Normal Murine Fibroblast Cells (L929) and Ehrlich Ascitic Tumor (EAT) Cells
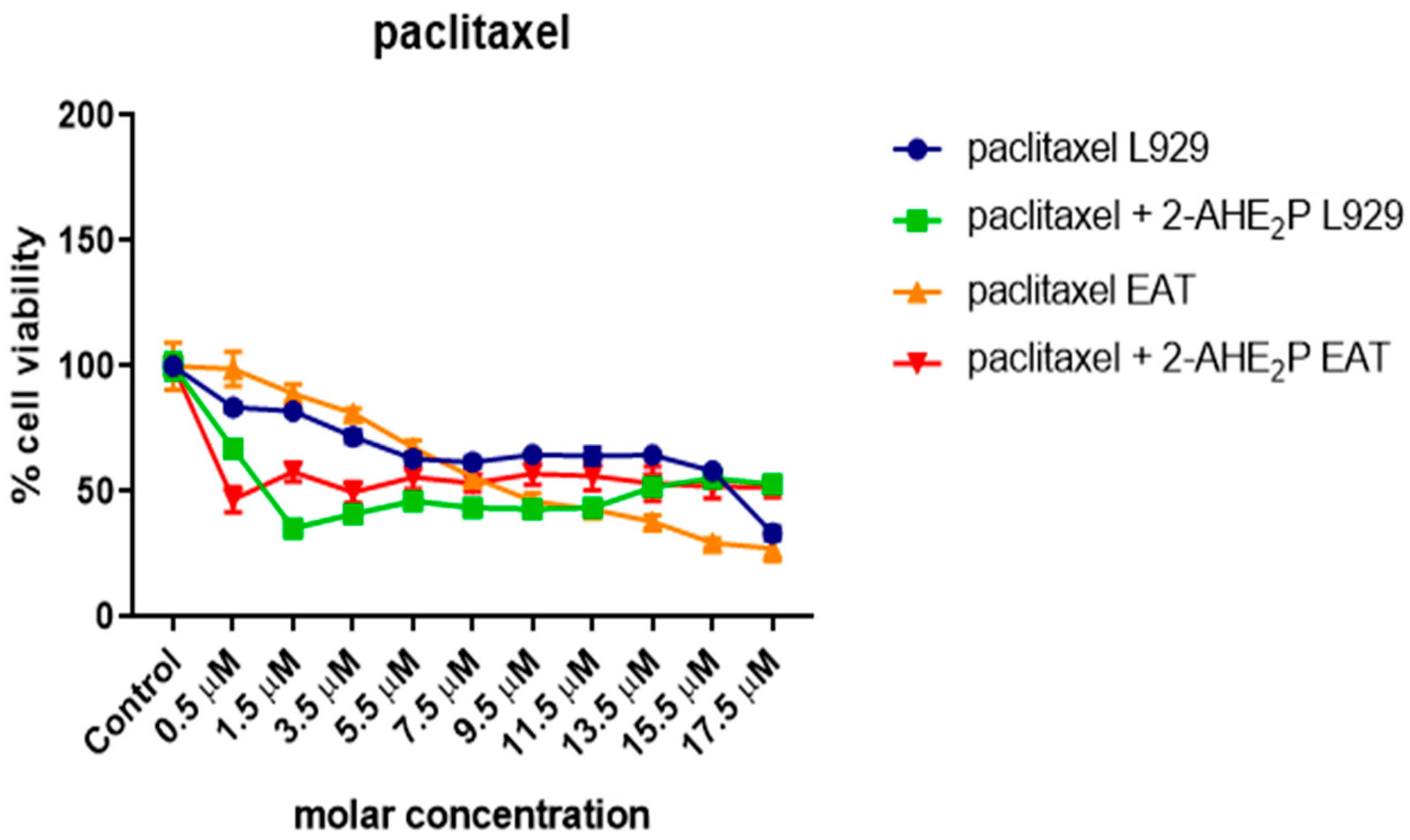
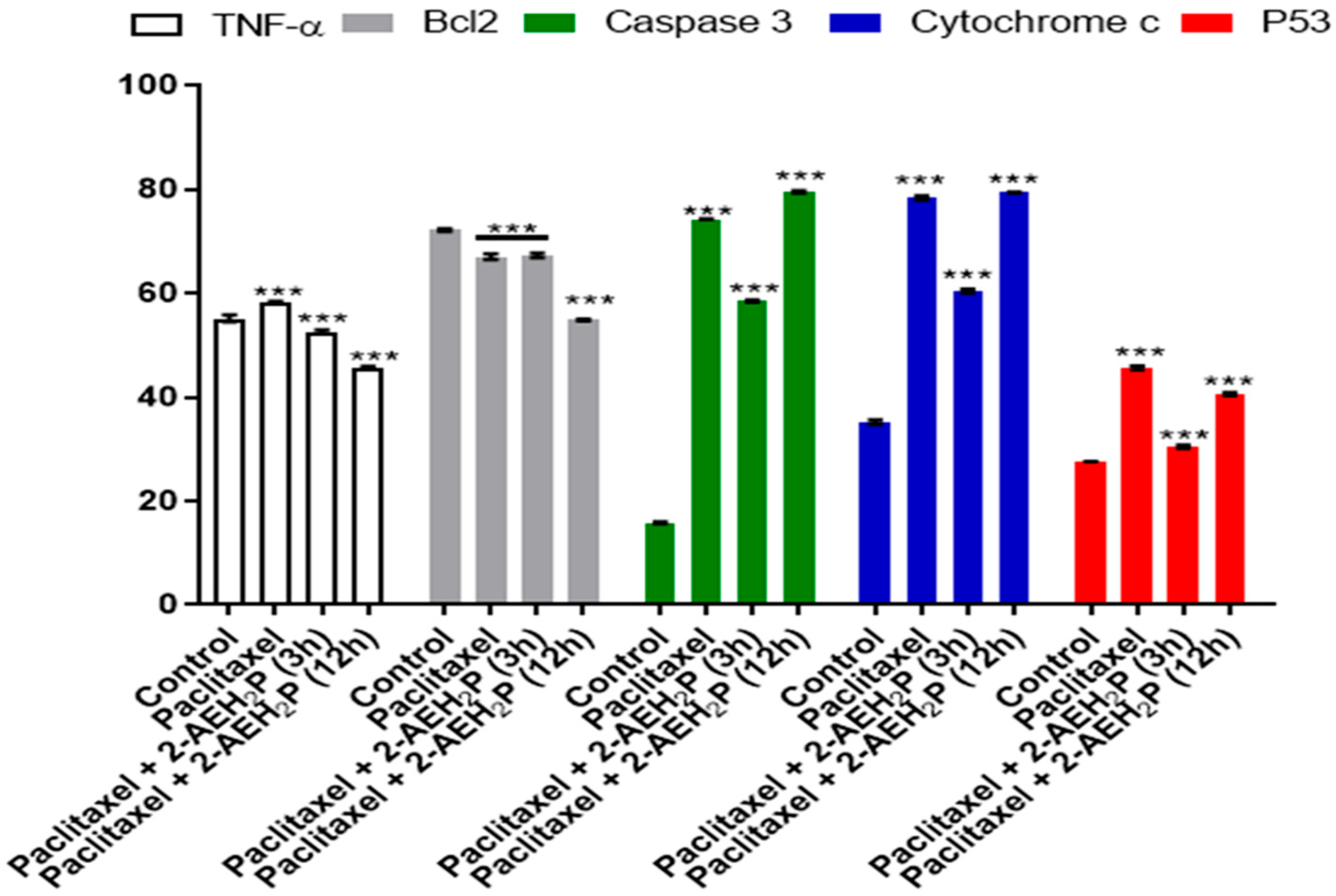
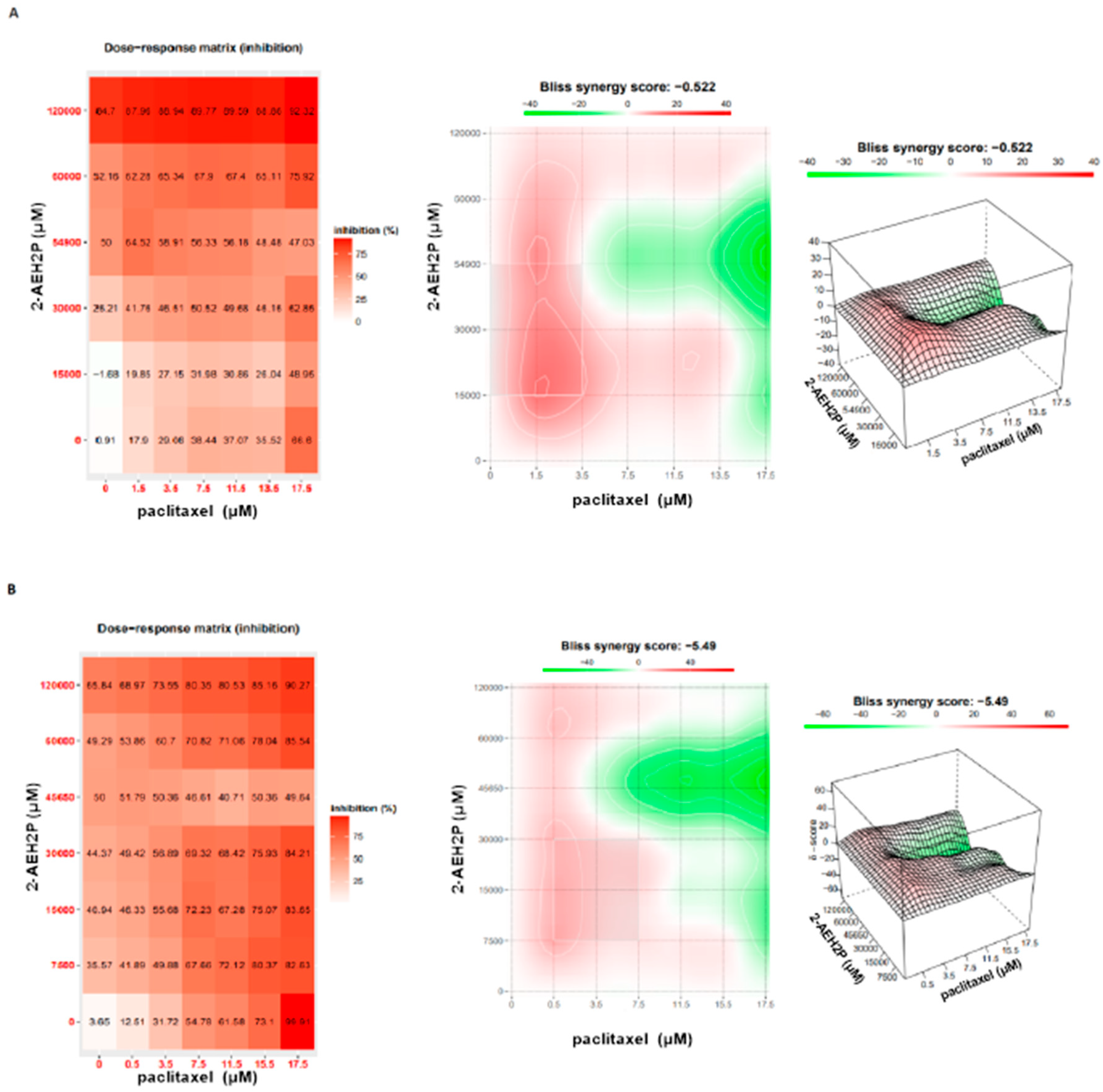
3.5. Analysis of the Compound 2-AEH2P Associated with the Chemotherapeutic Paclitaxel in Murine Fibroblast Cells (L929) and Ehrlich Ascitic Tumor (EAT) Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Xiao, Y.; Yu, D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol. Ther. 2021, 221, 107753. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Miao, K.; Sun, H.; Deng, C.X. Tumor heterogeneity reshapes the tumor microenvironment to influence drug resistance. Int. J. Biol. Sci. 2022, 18, 3019. [Google Scholar] [CrossRef] [PubMed]
- Saatci, O.; Huynh-Dam, K.-T.; Sahin, O. Endocrine resistance in breast cancer: From molecular mechanisms to therapeutic strategies. J. Mol. Med. 2021, 99, 1691–1710. [Google Scholar] [CrossRef] [PubMed]
- Medina, M.A.; Oza, G.; Sharma, A.; Arriaga, L.G.; Hernández Hernández, J.M.; Rotello, V.M.; Ramirez, J.T. Triple-negative breast cancer: A review of conventional and advanced therapeutic strategies. Int. J. Environ. Res. Public Health 2020, 17, 2078. [Google Scholar] [CrossRef] [PubMed]
- Elhanani, O.; Ben-Uri, R.; Keren, L. Spatial profiling technologies illuminate the tumor microenvironment. Cancer Cell 2023, 41, 404–420. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.-Z.; Jin, W.-L. The updated landscape of tumor microenvironment and drug repurposing. Signal Transduct. Target. Ther. 2020, 5, 166. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.R.; Wang, J.; Wang, Z.J.; Xi, M.J.; Xia, B.H.; Deng, K.; Yang, J.L. Lipid metabolic reprogramming in tumor microenvironment: From mechanisms to therapeutics. J. Hematol. Oncol. 2023, 16, 103. [Google Scholar] [CrossRef] [PubMed]
- Koundouros, N.; Poulogiannis, G. Reprogramming of fatty acid metabolism in cancer. Br. J. Cancer 2020, 122, 4–22. [Google Scholar] [CrossRef]
- Kim, H.Y.; Bae, S.J.; Choi, J.W.; Han, S.; Bae, S.H.; Cheong, J.H.; Jang, H. Cholesterol synthesis is important for breast cancer cell tumor sphere formation and invasion. Biomedicines 2022, 10, 1908. [Google Scholar] [CrossRef]
- Halimi, H.; Farjadian, S. Cholesterol: An important actor on the cancer immune scene. Front. Immunol. 2022, 13, 1057546. [Google Scholar] [CrossRef]
- Greife, A.; Tukova, J.; Steinhoff, C.; Scott, S.D.; Schulz, W.A.; Hatina, J. Establishment and characterization of a bladder cancer cell line with enhanced doxorubicin resistance by mevalonate pathway activation. Tumor Biol. 2015, 36, 3293–3300. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cai, L.; Li, H.; Chen, H.; Yang, T.; Tan, Y.; Guo, Z.; Wang, X. Overcoming Cancer Resistance to Platinum Drugs by Inhibiting Cholesterol Metabolism. Angew. Chem. Int. Ed. 2023, 62, e202309043. [Google Scholar] [CrossRef] [PubMed]
- Göbel, A.; Breining, D.; Rauner, M.; Hofbauer, L.C.; Rachner, T.D. Induction of 3-hydroxy-3-methylglutaryl-CoA reductase mediates statin resistance in breast cancer cells. Cell Death Dis. 2019, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Mihalik, N.E.; Steinberger, K.J.; Stevens, A.M.; Bobko, A.A.; Hoblitzell, E.H.; Tseytlin, O.; Akhter, H.; Dziadowicz, S.A.; Wang, L.; O’Connell, R.C.; et al. Dose-Specific Intratumoral GM-CSF Modulates Breast Tumor Oxygenation and Antitumor Immunity. J. Immunol. 2023, 211, 1589–1604. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, M.G.; Benavides, L.C.; Holmes, J.P.; Gates, J.D.; Mittendorf, E.A.; Ponniah, S.; Peoples, G.E. Results of the first phase 1 clinical trial of the HER-2/neu peptide (GP2) vaccine in disease-free breast cancer patients: United States Military Cancer Institute Clinical Trials Group Study I-04. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2010, 116, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Clifton, G.T.; Mittendorf, E.A.; Peoples, G.E. Adjuvant HER2/neu peptide cancer vaccines in breast cancer. Immunotherapy 2015, 7, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Abbaspour, M.; Akbari, V. Cancer vaccines as a targeted immunotherapy approach for breast cancer: An update of clinical evidence. Expert Rev. Vaccines 2022, 21, 337–353. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y. HER2/neu-based vaccination with li-Key hybrid, GM-CSF immunoadjuvant and trastuzumab as a potent triple-negative breast cancer treatment. J. Cancer Res. Clin. Oncol. 2023, 149, 6711–6718. [Google Scholar] [CrossRef]
- Zanetti, B.F.; Ferreira, C.P.; Vasconcelos, J.R.; Han, S.W. Adjuvant properties of IFN-γ and GM-CSF in the scFv6. C4 DNA vaccine against CEA-expressing tumors. Gene Ther. 2023, 30, 41–50. [Google Scholar] [CrossRef]
- Yang, H.L.; Lin, P.Y.; Vadivalagan, C.; Lin, Y.A.; Lin, K.Y.; Hseu, Y.C. Coenzyme Q0 defeats NLRP3-mediated inflammation, EMT/metastasis, and Warburg effects by inhibiting HIF-1α expression in human triple-negative breast cancer cells. Arch. Toxicol. 2023, 97, 1047–1068. [Google Scholar] [CrossRef]
- Sood, B.; Keenaghan, M. Coenzyme Q10; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Ambrosone, C.B.; Zirpoli, G.R.; Hutson, A.D.; McCann, W.E.; McCann, S.E.; Barlow, W.E.; Kelly, K.M.; Cannioto, R.; Sucheston-Campbell, L.E.; Hershman, D.L.; et al. Dietary supplement use during chemotherapy and survival outcomes of patients with breast cancer enrolled in a cooperative group clinical trial (SWOG S0221). J. Clin. Oncol. 2020, 38, 804. [Google Scholar] [CrossRef] [PubMed]
- PDQ® Integrative, Alternative, and Complementary Therapies Editorial Board. PDQ Coenzyme Q10; National Cancer Institute: Bethesda, MD, USA, 2022; Updated <06/072022>. Available online: https://www.cancer.gov/about-cancer/treatment/cam/hp/coenzyme-q10-pdq (accessed on 21 November 2023).
- Ferreira, A.K.; Meneguelo, R.; Pereira, A.; RFilho, O.M.; Chierice, G.O.; Maria, D.A. Synthetic phosphoethanolamine induces cell cycle arrest and apoptosis in human breast cancer MCF-7 cells through the mitochondrial pathway. Biomed. Pharmacother. 2013, 67, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Laveli da Silva, M.G.; Garcia de Sousa Cabral, L.; Alves, M.G.; Conceição, T.D.; Hesse, H.; Laiso, R.A.; Rabelo, D.D.; Maria, D.A. 2-aminoethyl Dihydrogen Phosphate as a Modulator of Proliferative and Apoptotic Effects in Breast Cancer Cell Lines. J. Pharm. Pharmacol. 2021, 9, 83–97. [Google Scholar]
- De Lima Luna, A.C.; de Assis Santos Filho, J.R.; Hesse, H.; Neto, S.C.; Chierice, G.O.; Maria, D.A. Modulation of pro-apoptotic effects and mitochondrial potential on B16F10 cells by DODAC/PHO-S liposomes. BMC Res. Notes 2018, 11, 126. [Google Scholar]
- Conceição, T.D.; Cabral, L.G.; Laveli-Silva, M.G.; Pacheco, J.C.; Alves, M.G.; Rabelo, D.C.; Laiso, R.A.; Maria, D.A. New potential antiproliferative monophosphoester 2-aminoethyl dihydrogen phosphate in K-562 and K-562 MDR+ leukemia cells. Biomed. Pharmacother. 2021, 142, 112054. [Google Scholar] [CrossRef] [PubMed]
- Luna, A.C.; Saraiva, G.K.; Chierice, G.O.; Hesse, H.; Maria, D.A. Antiproliferative and proapoptotic effects of DODAC/synthetic phosphoethanolamine on hepatocellular carcinoma cells. BMC Pharmacol. Toxicol. 2018, 19, 44. [Google Scholar] [CrossRef] [PubMed]
- Dan, V.M.; Raveendran, R.S.; Baby, S. Resistance to intervention: Paclitaxel in breast cancer. Mini Rev. Med. Chem. 2021, 21, 1237–1268. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, S.H.; Earnshaw, W.C. Induction of apoptosis by cancer chemotherapy. Exp. Cell Res. 2000, 256, 42–49. [Google Scholar] [CrossRef]
- Jordan, M.A. Mechanism of action of antitumor drugs that interact with microtubules and tubulin. Curr. Med. Chem. Anti-Cancer Agents 2002, 2, 1–17. [Google Scholar] [CrossRef]
- Kellokumpu-Lehtinen, P.; Tuunanen, T.; Asola, R.; Elomaa, L.; Heikkinen, M.; Kokko, R.; Järvenpää, R.; Lehtinen, I.; Maiche, A.; Kaleva-Kerola, J.; et al. Weekly paclitaxel—An effective treatment for advanced breast cancer. Anticancer Res. 2013, 33, 2623–2627. [Google Scholar]
- Van Vuuren, R.J.; Visagie, M.H.; Theron, A.E.; Joubert, A.M. Antimitotic drugs in the treatment of cancer. Cancer Chemother. Pharmacol. 2015, 76, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Avila, A.; Gollahon, L. Paclitaxel induces apoptosis in breast cancer cells through different calcium—Regulating mechanisms depending on external calcium conditions. Int. J. Mol. Sci. 2014, 15, 2672–2694. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.A.; Al-Zahrani, M.H.; Khan, S.H.; Khan, M.H.; Al-Subhi, H.A.; Kuerban, A.; Aslam, M.; Al-Abbasi, F.A.; Anwar, F. Tubulin proteins in cancer resistance: A review. Curr. Drug Metab. 2020, 21, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Berrieman, H.K.; Lind, M.J.; Cawkwell, L. Do β-tubulin mutations have a role in resistance to chemotherapy? Lancet Oncol. 2004, 5, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Feitosa, I.B.; Mori, B.; Teles, C.B.; da Costa, A.G. What are the immune responses during the growth of Ehrlich’s tumor in ascitic and solid form? Life Sci. 2021, 264, 118578. [Google Scholar] [CrossRef] [PubMed]
- Aydiner, A.; Ridvanogullari, M.; Anil, D.; Topuz, E.; Nurten, R.; Disci, R. Combined effects of epirubicin and tamoxifen on the cell-cycle phases in estrogen-receptor-negative Ehrlich ascites tumor cells. J. Cancer Res. Clin. Oncol. 1997, 123, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Gabai, V.L.; Zamulaeva, I.V.; Mosin, A.F.; Makarova, Y.M.; Mosina, V.A.; Budagova, K.R.; Malutina, Y.V.; Kabakov, A.E. Resistance of Ehrlich tumor cells to apoptosis can be due to accumulation of heat shock proteins. FEBS Lett. 1995, 375, 21–26. [Google Scholar] [CrossRef]
- Abdin, A.A.; Soliman, N.A.; Saied, E.M. Effect of propranolol on IL-10, visfatin, Hsp70, iNOS, TLR2, and survivin in amelioration of tumor progression and survival in Solid Ehrlich Carcinoma-bearing mice. Pharmacol. Rep. 2014, 66, 1114–1121. [Google Scholar] [CrossRef]
- Beniey, M. Peritoneal metastases from breast cancer: A scoping review. Cureus 2019, 11, e5367. [Google Scholar] [CrossRef]
- Palermo-Neto, J.; De Oliveira Massoco, C.; De Souza, W.R. Effects of physical and psychological stressors on behavior, macrophage activity, and Ehrlich tumor growth. Brain Behav. Immun. 2003, 17, 43–54. [Google Scholar] [CrossRef]
- Albalawi, A.E.; Althobaiti, N.A.; Alhasani, R.H.; Alnomasy, S.F. Anti-tumor effects and cellular mechanisms of Pistacia atlantica methanolic extract against Ehrlich solid tumor in mice. Asian Pac. J. Trop. Biomed. 2022, 12, 69–77. [Google Scholar]
- Gowda, N.G.; Shiragannavar, V.D.; Prabhuswamimath, S.C.; Tuladhar, S.; Chidambaram, S.B.; Santhekadur, P.K. Ehrlich Ascites carcinoma mice model for studying liver inflammation and fibrosis. Adv. Cancer Biol. Metastasis 2022, 4, 2667–3940. [Google Scholar]
- Zeng, L.; Li, W.; Chen, C.S. Breast cancer animal models and applications. Zool. Res. 2020, 41, 477–494. [Google Scholar] [CrossRef] [PubMed]
- Alberti, G.; Vergilio, G.; Paladino, L.; Barone, R.; Cappello, F.; Conway de Macario, E.; Macario, A.J.; Bucchieri, F.; Rappa, F. The chaperone system in breast cancer: Roles and therapeutic prospects of the molecular chaperones Hsp27, Hsp60, Hsp70, and Hsp90. Int. J. Mol. Sci. 2022, 23, 7792. [Google Scholar] [CrossRef]
- Loewenthal, H.; Jahn, G. Übertragungsversuche mit carcinomatöser Mäuse-Ascitesflüssigkeit und ihr Verhalten gegen physikalische und chemische Einwirkungen. Z. Krebsforsch. 1932, 37, 439–447. [Google Scholar] [CrossRef]
- Ahmed, H.; Chatterjee, B.P.; Debnath, A.K. Interaction and in vivo growth inhibition of Ehrlich ascites tumor cells by jacalin. J. Biosci. 1988, 13, 419–424. [Google Scholar] [CrossRef]
- De Sousa Cabral, L.G.; Hesse, H.; Freire, K.A.; de Oliveira, C.S.; Pedron, C.N.; Alves, M.G.; Carlstron, J.P.; Poyet, J.L.; Oliveira, V.X., Jr.; Maria, D.A. The BR2 peptide associated with 2-aminoethyl dihydrogen phosphate is a formulation with antiproliferative potential for a triple-negative breast cancer model. Biomed. Pharmacother. 2022, 153, 113398. [Google Scholar] [CrossRef] [PubMed]
- Bock, F.J.; Tait, S.W.G. Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell Biol. 2020, 21, 85–100. [Google Scholar] [CrossRef]
- Sainero-Alcolado, L.; Liaño-Pons, J.; Ruiz-Pérez, M.V.; Arsenian-Henriksson, M. Targeting mitochondrial metabolism for precision medicine in cancer. Cell Death Differ. 2022, 29, 1304–1317. [Google Scholar] [CrossRef]
- Yan, C.; Zheng, L.; Jiang, S.; Yang, H.; Guo, J.; Jiang, L.Y.; Li, T.; Zhang, H.; Bai, Y.; Lou, Y.; et al. Exhaustion-associated cholesterol deficiency dampens the cytotoxic arm of antitumor immunity. Cancer Cell 2023, 41, 1276–1293.E11. [Google Scholar] [CrossRef]
- Jiang, W.; Hu, J.W.; He, X.R.; Jin, W.L.; He, X.Y. Statins: A repurposed drug to fight cancer. J. Exp. Clin. Cancer Res. 2021, 40, 241. [Google Scholar] [CrossRef] [PubMed]
- Kopecka, J.; Trouillas, P.; Gašparović, A.Č.; Gazzano, E.; Assaraf, Y.G.; Riganti, C. Phospholipids and cholesterol: Inducers of cancer multidrug resistance and therapeutic targets. Drug Resist. Updates 2020, 49, 100670. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.C. Mitocôndrias e câncer. Nat. Rev. Câncer 2012, 10, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Chamoto, K.; Chowdhury, P.S.; Honjo, T. Tumors attenuating the mitochondrial activity in T cells escape from PD-1 blockade therapy. eLife 2020, 9, e52330. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, P.S.; Chamoto, K.; Kumar, A.; Honjo, T. PPAR-induced fatty acid oxidation in T cells increases the number of tumor-reactive CD8+ T cells and facilitates anti-PD-1 therapy. Cancer Immunol. Res. 2018, 6, 1375–1387. [Google Scholar] [CrossRef] [PubMed]
- Najjar, Y.G.; Menk, A.V.; Sander, C.; Rao, U.; Karunamurthy, A.; Bhatia, R.; Zhai, S.; Kirkwood, J.M.; Delgoffe, G.M. Tumor cell oxidative metabolism as a barrier to PD-1 blockade immunotherapy in melanoma. JCI Insight 2019, 4, e124989. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-F.; Tseng, L.-M.; Lee, H.-C. Role of mitochondrial alterations in human cancer progression and cancer immunity. J. Biomed. Sci. 2023, 30, 61. [Google Scholar] [CrossRef]
- Mantle, D.; Lopez-Lluch, G.; Hargreaves, I.P. Coenzyme Q10 metabolism: A review of unresolved issues. Int. J. Mol. Sci. 2023, 24, 2585. [Google Scholar] [CrossRef]
- Rusciani, L.; Proietti, I.; Paradisi, A.; Rusciani, A.; Guerriero, G.; Mammone, A.; De Gaetano, A.; Lippa, S. Recombinant interferon α-2b and coenzyme Q10 as a postsurgical adjuvant therapy for melanoma: A 3-year trial with recombinant interferon-α and 5-year follow-up. Melanoma Res. 2007, 17, 177–183. [Google Scholar] [CrossRef]
- Testai, L.; Martelli, A.; Flori, L.; Cicero, A.F.; Colletti, A. Coenzyme Q10: Clinical applications beyond cardiovascular diseases. Nutrients 2021, 13, 1697. [Google Scholar] [CrossRef]
- Neto, A.P.; Garcia de Sousa Cabral, L.; Oliveira, T.A.; Pacheco, J.C.; Alves, M.G.; Hernández, M.R.; Laiso, R.A.; Maria, D.A. The Antiproliferative and Pro-apoptotic Role of 2-aminoethyl dihydrogen Phosphate in Triple-negative Breast Tumor Cells. J. Pharm. Pharmacol. 2021, 9, 160–173. [Google Scholar]
- Vringer, E.; Tait, S.W.G. Mitochondria and cell death-associated inflammation. Cell Death Differ. 2023, 30, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Laiso, R.A.; Chammas, S.M.; de Sousa Cabral, L.G.; Maria, D.A. Clinical oncology translational study phase-1 with antitumor phosphorylethanolamine (2-AEH2P) in dogs with neoplasms. J. Pharm. Pharmacol. 2021, 9, 179–196. [Google Scholar]










| Treatment | Drug Concentration |
|---|---|
| 2-AEH2P | 45.6 mM |
| Simvastatin | 9.6 μM |
| GM-CSF | 144 nM |
| Coenzyme Q10 | 5.5 μM |
| Paclitaxel | 8.7 μM |
| Simvastatin + 2-AEH2P | 10.1 μM + 22.8 mM |
| GM-CSF + 2-AEH2P | 16 nM + 22.8 mM |
| Coenzyme Q10 + 2-AEH2P | 5.8 μM + 22.8 mM |
| Paclitaxel + 2-AEH2P | 6.2 μM + 22.8 mM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, M.G.; Cabral, L.G.S.; Totti, P.G.F.; Azarias, F.R.; Pomini, K.T.; Rici, R.E.G.; Laiso, R.A.N.; Maria, D.A. 2-Aminoethyl Dihydrogen Phosphate (2-AEH2P) Associated with Cell Metabolism-Modulating Drugs Presents a Synergistic and Pro-Apoptotic Effect in an In Vitro Model of the Ascitic Ehrlich Tumor. Biomedicines 2024, 12, 109. https://doi.org/10.3390/biomedicines12010109
Alves MG, Cabral LGS, Totti PGF, Azarias FR, Pomini KT, Rici REG, Laiso RAN, Maria DA. 2-Aminoethyl Dihydrogen Phosphate (2-AEH2P) Associated with Cell Metabolism-Modulating Drugs Presents a Synergistic and Pro-Apoptotic Effect in an In Vitro Model of the Ascitic Ehrlich Tumor. Biomedicines. 2024; 12(1):109. https://doi.org/10.3390/biomedicines12010109
Chicago/Turabian StyleAlves, Monique G., Laertty G. S. Cabral, Paulo G. F. Totti, Felipe R. Azarias, Karine T. Pomini, Rose E. G. Rici, Rosa A. N. Laiso, and Durvanei A. Maria. 2024. "2-Aminoethyl Dihydrogen Phosphate (2-AEH2P) Associated with Cell Metabolism-Modulating Drugs Presents a Synergistic and Pro-Apoptotic Effect in an In Vitro Model of the Ascitic Ehrlich Tumor" Biomedicines 12, no. 1: 109. https://doi.org/10.3390/biomedicines12010109
APA StyleAlves, M. G., Cabral, L. G. S., Totti, P. G. F., Azarias, F. R., Pomini, K. T., Rici, R. E. G., Laiso, R. A. N., & Maria, D. A. (2024). 2-Aminoethyl Dihydrogen Phosphate (2-AEH2P) Associated with Cell Metabolism-Modulating Drugs Presents a Synergistic and Pro-Apoptotic Effect in an In Vitro Model of the Ascitic Ehrlich Tumor. Biomedicines, 12(1), 109. https://doi.org/10.3390/biomedicines12010109








