Long-Term Efficacy of Mepolizumab at 3 Years in Patients with Severe Asthma: Comparison with Clinical Trials and Super Responders
Abstract
:1. Introduction
2. Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACT | Asthma Control Test |
| BMI | Body mass index |
| CRSwNP | Chronic Rhinosinusitis with nasal polyps |
| FeNO | Fractional exhaled nitric oxide |
| FEV1 | Forced expiratory volume 1 s |
| Ig | Immunoglobulin |
| IL | Interleukin |
| MEP | Mepolizumab |
| OCS | Oral Corticosteroids |
| MCID | Minimal clinically important difference |
| RCT | Randomized controlled trials |
| RL | Real Life |
| SANI | Severe Asthma Network Italy |
| SNOT-22 | Sinonasal Outcome Test |
| T2 | Type 2 |
Appendix A
References
- Chiu, C.-J.; Huang, M.-T. Asthma in the Precision Medicine Era: Biologics and Probiotics. Int. J. Mol. Sci. 2021, 22, 4528. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.F.; Wenzel, S.E.; Brozek, J.L.; Bush, A.; Castro, M.; Sterk, P.J.; Adcock, I.M.; Bateman, E.D.; Bel, E.H.; Bleecker, E.R.; et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur. Respir. J. 2013, 43, 343–373. [Google Scholar] [CrossRef] [PubMed]
- Kyriakopoulos, C.; Gogali, A.; Bartziokas, K.; Kostikas, K. Identification and treatment of T2-low asthma in the era of biologics. ERJ Open Res. 2021, 7. [Google Scholar] [CrossRef] [PubMed]
- Bonato, M.; Bazzan, E.; Snijders, D.; Turato, G.; Turrin, M.; Cosio, M.G.; Barbato, A.; Saetta, M.; Baraldo, S. Innate Lymphocytes -ILC2—Might Be the Drivers of T2-High Nonatopic Asthma in Children. Eur. Respir. J. 2021, 58, PA861. [Google Scholar] [CrossRef]
- Fahy, J.V. Type 2 Inflammation in Asthma-Present in Most, Absent in Many. Nat. Rev. Immunol. 2015, 15, 57–65. [Google Scholar] [CrossRef]
- Woodruff, P.G.; Modrek, B.; Choy, D.F.; Jia, G.; Abbas, A.R.; Ellwanger, A.; Arron, J.R.; Koth, L.L.; Fahy, J.V. T-helper Type 2–driven Inflammation Defines Major Subphenotypes of Asthma. Am. J. Respir. Crit. Care Med. 2009, 180, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Voorham, J.; Xu, X.; Price, D.B.; Golam, S.; Davis, J.; Ling, J.Z.J.; Kerkhof, M.; Ow, M.; Tran, T.N. Healthcare Resource Utilization and Costs Associated with Incremental Systemic Corticosteroid Exposure in Asthma. Allergy 2019, 74, 273–283. [Google Scholar] [CrossRef]
- Price, D.B.; Trudo, F.; Voorham, J.; Xu, X.; Kerkhof, M.; Jie, J.L.Z.; Tran, T.N. Adverse Outcomes from Initiation of Systemic Corticosteroids for Asthma: Long-Term Observational Study. J. Asthma Allergy 2018, 11, 193–204. [Google Scholar] [CrossRef]
- McGregor, M.C.; Krings, J.G.; Nair, P.; Castro, M. Role of Biologics in Asthma. Am. J. Respir. Crit. Care Med. 2019, 199, 433–445. [Google Scholar] [CrossRef]
- Agache, I.; Beltran, J.; Akdis, C.; Akdis, M.; Canelo-Aybar, C.; Canonica, G.W.; Casale, T.; Chivato, T.; Corren, J.; del Giacco, S.; et al. Efficacy and Safety of Treatment with Biologicals (Benralizumab, Dupilumab, Mepolizumab, Omalizumab and Reslizumab) for Severe Eosinophilic Asthma. A Systematic Review for the EAACI Guidelines—Recommendations on the Use of Biologicals in Severe Asthma. Allergy 2020, 75, 1023–1042. [Google Scholar] [CrossRef]
- Menzella, F.; Lusuardi, M.; Galeone, C.; Taddei, S.; Zucchi, L. Profile of Anti-IL-5 MAb Mepolizumab in the Treatment of Severe Refractory Asthma and Hypereosinophilic Diseases. J. Asthma Allergy 2015, 8, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Azim, A.; Pini, L.; Khakwani, Z.; Kumar, S.; Howarth, P. Severe Acute Respiratory Syndrome Coronavirus 2 Infection in Those on Mepolizumab Therapy. Ann. Allergy Asthma Immunol. 2021, 126, 438–440. [Google Scholar] [CrossRef] [PubMed]
- Greenfeder, S.; Umland, S.P.; Cuss, F.M.; Chapman, R.W.; Egan, R.W. Th2 Cytokines and Asthma—The Role of Interleukin-5 in Allergic Eosinophilic Disease. Respir. Res. 2001, 2, 71. [Google Scholar] [CrossRef] [PubMed]
- Potaczek, D.P.; Miethe, S.; Schindler, V.; Alhamdan, F.; Garn, H. Role of Airway Epithelial Cells in the Development of Different Asthma Phenotypes. Cell. Signal. 2020, 69, 109523. [Google Scholar] [CrossRef] [PubMed]
- Potaczek, D.P.; Harb, H.; Michel, S.; Alhamwe, B.A.; Renz, H.; Tost, J. Epigenetics and Allergy: From Basic Mechanisms to Clinical Applications. Epigenomics 2017, 9, 539–571. [Google Scholar] [CrossRef] [PubMed]
- Ortega, H.G.; Liu, M.C.; Pavord, I.D.; Brusselle, G.G.; FitzGerald, J.M.; Chetta, A.; Humbert, M.; Katz, L.E.; Keene, O.N.; Yancey, S.W.; et al. Mepolizumab Treatment in Patients with Severe Eosinophilic Asthma. N. Engl. J. Med. 2014, 371, 1198–1207. [Google Scholar] [CrossRef] [PubMed]
- Bel, E.H.; Wenzel, S.E.; Thompson, P.J.; Prazma, C.M.; Keene, O.N.; Yancey, S.W.; Ortega, H.G.; Pavord, I.D. Oral Glucocorticoid-Sparing Effect of Mepolizumab in Eosinophilic Asthma. N. Engl. J. Med. 2014, 371, 1189–1197. [Google Scholar] [CrossRef]
- Pavord, I.D.; Korn, S.; Howarth, P.; Bleecker, E.R.; Buhl, R.; Keene, O.N.; Ortega, H.; Chanez, P. Mepolizumab for Severe Eosinophilic Asthma (DREAM): A Multicentre, Double-Blind, Placebo-Controlled Trial. Lancet 2012, 380, 651–659. [Google Scholar] [CrossRef]
- Matucci, A.; Vivarelli, E.; Bormioli, S.; Francesca, N.; Chiccoli, F.; Valentina, M.; Francesca, G.; Oliviero, R.; Parronchi, P.; Vultaggio, A. Long-term retention rate of mepolizumab treatment in severe asthma: A 36-months real-life experience. J. Asthma 2022, 60, 158–166. [Google Scholar] [CrossRef]
- Bagnasco, D.; Massolo, A.; Bonavia, M.; Brussino, L.; Bucca, C.; Caminati, M.; Canonica, G.W.; Caruso, C.; D’Amato, M.; de Ferrari, L.; et al. The Importance of Being Not Significant: Blood Eosinophils and Clinical Responses Do Not Correlate in Severe Asthma Patients Treated with Mepolizumab in Real Life. Allergy 2019, 75, 1460–1463. [Google Scholar] [CrossRef]
- Bagnasco, D.; Menzella, F.; Caminati, M.; Caruso, C.; Guida, G.; Bonavia, M.; Riccio, A.; Milanese, M.; Manfredi, A.; Senna, G.; et al. Efficacy of Mepolizumab in Patients with Previous Omalizumab Treatment Failure: Real-Life Observation. Allergy Eur. J. Allergy Clin. Immunol. 2019, 74, 2539–2541. [Google Scholar] [CrossRef] [PubMed]
- Bagnasco, D.; Povero, M.; Pradelli, L.; Brussino, L.; Rolla, G.; Caminati, M.; Menzella, F.; Heffler, E.; Canonica, G.W.; Paggiaro, P.; et al. Economic Impact of Mepolizumab in Uncontrolled Severe Eosinophilic Asthma, in Real Life. World Allergy Organ. J. 2021, 14, 100509. [Google Scholar] [CrossRef] [PubMed]
- Pharmacoeconomic Review Report—Mepolizumab (Nucala)—NCBI Bookshelf. Available online: https://www.ncbi.nlm.nih.gov/books/NBK409872/ (accessed on 21 April 2022).
- Detoraki, A.; Tremante, E.; D’Amato, M.; Calabrese, C.; Casella, C.; Maniscalco, M.; Poto, R.; Brancaccio, R.; Boccia, M.; Martino, M.; et al. Mepolizumab Improves Sino-Nasal Symptoms and Asthma Control in Severe Eosinophilic Asthma Patients with Chronic Rhinosinusitis and Nasal Polyps: A 12-Month Real-Life Study. Ther. Adv. Respir. Dis. 2021, 15, 17534666211009398. [Google Scholar] [CrossRef]
- Kassem, F.; Cohen-Confino, R.; Meir-Shafrir, K.; Lachover-Roth, I.; Cohen-Engler, A.; Nageris, B.; Rosman, Y. Mepolizumab for Eosinophilic Chronic Sinusitis with Nasal Polyposis: Real-Life Experience. Rhinology 2021, 59, 110–112. [Google Scholar] [CrossRef]
- Blonde, L.; Khunti, K.; Harris, S.B.; Meizinger, C.; Skolnik, N.S. Interpretation and Impact of Real-World Clinical Data for the Practicing Clinician. Adv. Ther. 2018, 35, 1763–1774. [Google Scholar] [CrossRef]
- Heffler, E.; Blasi, F.; Latorre, M.; Menzella, F.; Paggiaro, P.; Pelaia, G.; Senna, G.; Canonica, G.W.; Barbuto, S.; Bradicich, M.; et al. The Severe Asthma Network in Italy: Findings and Perspectives. J. Allergy Clin. Immunol. Pract. 2019, 7, 1462–1468. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, J.E.; d’Ancona, G.; Elstad, M.; Green, L.; Fernandes, M.; Thomson, L.; Roxas, C.; Dhariwal, J.; Nanzer, A.M.; Kent, B.D.; et al. Real-World Effectiveness and the Characteristics of a “Super-Responder” to Mepolizumab in Severe Eosinophilic Asthma. Chest 2020, 158, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Bonini, M.; di Paolo, M.; Bagnasco, D.; Baiardini, I.; Braido, F.; Caminati, M.; Carpagnano, E.; Contoli, M.; Corsico, A.; del Giacco, S.; et al. Minimal Clinically Important Difference for Asthma Endpoints: An Expert Consensus Report. Eur. Respir. Rev. 2020, 29, 190137. [Google Scholar] [CrossRef]
- Haldar, P.; Brightling, C.E.; Hargadon, B.; Gupta, S.; Monteiro, W.; Sousa, A.; Marshall, R.P.; Bradding, P.; Green, R.H.; WardIaw, A.J.; et al. Mepolizumab and Exacerbations of Refractory Eosinophilic Asthma. N. Engl. J. Med. 2009, 360, 973–984. [Google Scholar] [CrossRef]
- Nair, P.; Pizzichini, M.M.M.; Kjarsgaard, M.; Inman, M.D.; Efthimiadis, A.; Pizzichini, E.; Hargreave, F.E.; O’Byrne, P.M. Mepolizumab for Prednisone-Dependent Asthma with Sputum Eosinophilia. N. Engl. J. Med. 2009, 360, 985–993. [Google Scholar] [CrossRef]
- Israel, E.; Canonica, G.W.; Brusselle, G.; Yang, S.; Howarth, P.H.; Martin, A.L.; Koufopoulou, M.; Smith, S.G.; Alfonso-Cristancho, R. Real-Life Effectiveness of Mepolizumab in Severe Asthma: A Systematic Literature Review. J. Asthma 2022, 59, 2201–2217. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.; Brusselle, G.G.; Bel, E.H.; FitzGerald, J.M.; Masoli, M.; Korn, S.; Kato, M.; Albers, F.C.; Bradford, E.S.; Gilson, M.J.; et al. Long-Term Safety and Clinical Benefit of Mepolizumab in Patients with the Most Severe Eosinophilic Asthma: The COSMEX Study. Clin. Ther. 2019, 41, 2041–2056.e5. [Google Scholar] [CrossRef]
- Bagnasco, D.; Milanese, M.; Rolla, G.; Lombardi, C.; Bucca, C.; Heffler, E.; Canonica, G.W.; Passalacqua, G. The North-Western Italian Experience with Anti IL-5 Therapy Amd Comparison with Regulatory Trials. World Allergy Organ. J. 2018, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- Lugogo, N.; Domingo, C.; Chanez, P.; Leigh, R.; Gilson, M.J.; Price, R.G.; Yancey, S.W.; Ortega, H.G. Long-Term Efficacy and Safety of Mepolizumab in Patients with Severe Eosinophilic Asthma: A Multi-Center, Open-Label, Phase IIIb Study. Clin. Ther. 2016, 38, 2058–2070.e1. [Google Scholar] [CrossRef] [PubMed]
- Bagnasco, D.; Caminati, M.; Menzella, F.; Milanese, M.; Rolla, G.; Lombardi, C.; Bucca, C.; Heffler, E.; Paoletti, G.; Testino, E.; et al. One Year of Mepolizumab. Efficacy and Safety in Real-Life in Italy. Pulm. Pharmacol. Ther. 2019, 58, 101836. [Google Scholar] [CrossRef]
- Pini, L.; Caruso, C.; Colantuono, S.; Bagnasco, D.; Maxwell, A.; Price, R.G.; Howarth, P.; Canonica, G.W. Prospective Italian Real-World Study of Mepolizumab in Severe Eosinophilic Asthma Validates Retrospective Outcome Reports. Clin. Transl. Allergy 2021, 11, e12067. [Google Scholar] [CrossRef] [PubMed]
- Pelaia, C.; Busceti, M.T.; Solinas, S.; Terracciano, R.; Pelaia, G. Real-Life Evaluation of the Clinical, Functional, and Hematological Effects of Mepolizumab in Patients with Severe Eosinophilic Asthma: Results of a Single-Centre Observational Study. Pulm. Pharmacol. Ther. 2018, 53, 1–5. [Google Scholar] [CrossRef]
- Pilette, C.; Canonica, G.W.; Chaudhuri, R.; Chupp, G.; Lee, F.E.-H.; Lee, J.K.; Almonacid, C.; Welte, T.; Alfonso-Cristancho, R.; Jakes, R.W.; et al. REALITI-A Study: Real-World Oral Corticosteroid-Sparing Effect of Mepolizumab in Severe Asthma. J. Allergy Clin. Immunol. Pract. 2022, 10, 2646–2656. [Google Scholar] [CrossRef]
- Canonica, G.W.; Malvezzi, L.; Blasi, F.; Paggiaro, P.; Mantero, M.; Senna, G.; Heffler, E.; Bonavia, M.; Caiaffa, P.; Calabrese, C.; et al. Chronic Rhinosinusitis with Nasal Polyps Impact in Severe Asthma Patients: Evidences from the Severe Asthma Network Italy (SANI) Registry. Respir. Med. 2020, 166, 105947. [Google Scholar] [CrossRef]
- Harrison, T.; Canonica, G.W.; Chupp, G.; Lee, J.; Schleich, F.; Welte, T.; Valero, A.; Gemzoe, K.; Maxwell, A.; Joksaite, S.; et al. Real-World Mepolizumab in the Prospective Severe Asthma REALITI-A Study: Initial Analysis. Eur. Respir. J. 2020, 56, 2000151. [Google Scholar] [CrossRef]
- Chowdhury, N.I.; Mace, J.C.; Bodner, T.E.; Alt, J.A.; Deconde, A.S.; Levy, J.M.; Smith, T.L. Investigating the Minimal Clinically Important Difference for SNOT-22 Symptom Domains in Surgically Managed Chronic Rhinosinusitis. Int. Forum Allergy Rhinol. 2017, 7, 1149–1155. [Google Scholar] [CrossRef]
- Hopkins, C.; Gillett, S.; Slack, R.; Lund, V.J.; Browne, J.P. Psychometric Validity of the 22-Item Sinonasal Outcome Test. Clin. Otolaryngol. 2009, 34, 447–454. [Google Scholar] [CrossRef]
- Canonica, G.W.; Colombo, G.L.; Bruno, G.M.; di Matteo, S.; Martinotti, C.; Blasi, F.; Bucca, C.; Crimi, N.; Paggiaro, P.; Pelaia, G.; et al. Shadow Cost of Oral Corticosteroids-Related Adverse Events: A Pharmacoeconomic Evaluation Applied to Real-Life Data from the Severe Asthma Network in Italy (SANI) Registry. World Allergy Organ. J. 2019, 12, 100007. [Google Scholar] [CrossRef]
- Varricchi, G.; Ferri, S.; Pepys, J.; Poto, R.; Spadaro, G.; Nappi, E.; Paoletti, G.; Virchow, J.C.; Heffler, E.; Canonica, W.G. Biologics and Airway Remodeling in Severe Asthma. Allergy 2022, 77, 3538–3552. [Google Scholar] [CrossRef]
- Moore, W.C.; Kornmann, O.; Humbert, M.; Poirier, C.; Bel, E.H.; Kaneko, N.; Smith, S.G.; Martin, N.; Gilson, M.J.; Price, R.G.; et al. Stopping versus Continuing Long-Term Mepolizumab Treatment in Severe Eosinophilic Asthma (COMET Study). Eur. Respir. J. 2022, 59, 2100396. [Google Scholar] [CrossRef] [PubMed]
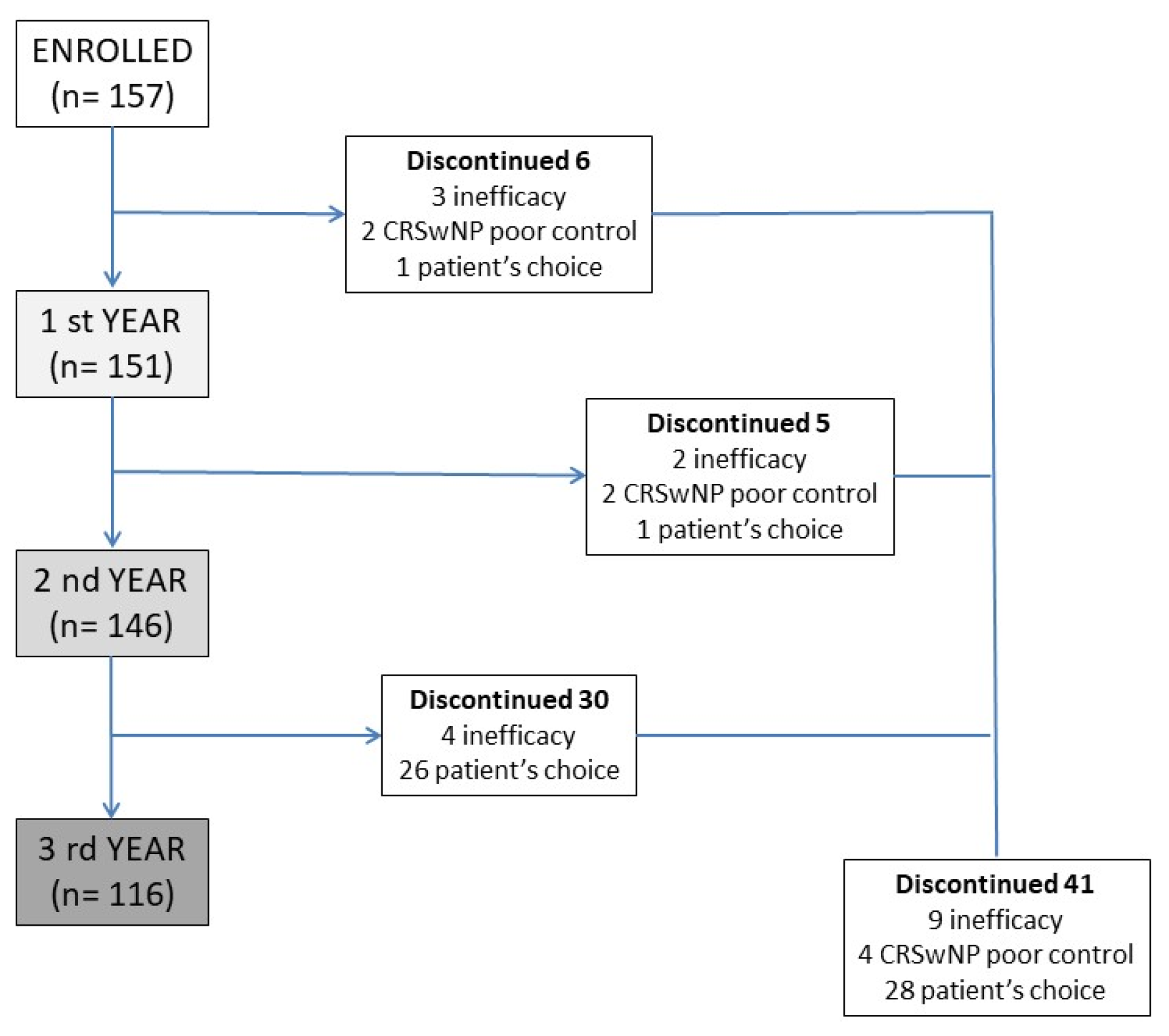
| Baseline (n = 157) | 1° Year (n = 151) | p-Value Baseline vs. 1° y | 2° Year (n = 146) | p-Value 1° vs. 2° y | 3° Year (n = 116) | p-Value 2° vs. 3° y | p-Value Baseline vs. 3° | |
|---|---|---|---|---|---|---|---|---|
| Male (%) | 80 (51) | 76 (50) | 0.864 | 77 (53) | 0.706 | 59 (51) | 0.833 | 0.937 |
| Age mean (range) | 59 (21–84) | 59 (22–85) | 0.945 | 59 (23–81) | 0.899 | 60 (24–82) | 0.910 | 0.904 |
| Age onset | 41 (15.7) | n.a. | - | n.a. | - | n.a. | - | - |
| BMI | 25.8 (8.8) | 26.1 (7.6) | 0.894 | 25.9 (6.4) | 0.903 | 26.0 (6.1) | 0.945 | 0.849 |
| CRSwNP (%) | 99 (63) | 95 (63) | 0.901 | 88 (60) | 0.811 | 70 (60) | >0.999 | 0.705 |
| Blood Eosinophils + | 718 (579) | 88 (43) | <0.0001 | 91 (23) | >0.999 | 90 (31) | >0.999 | <0.0001 |
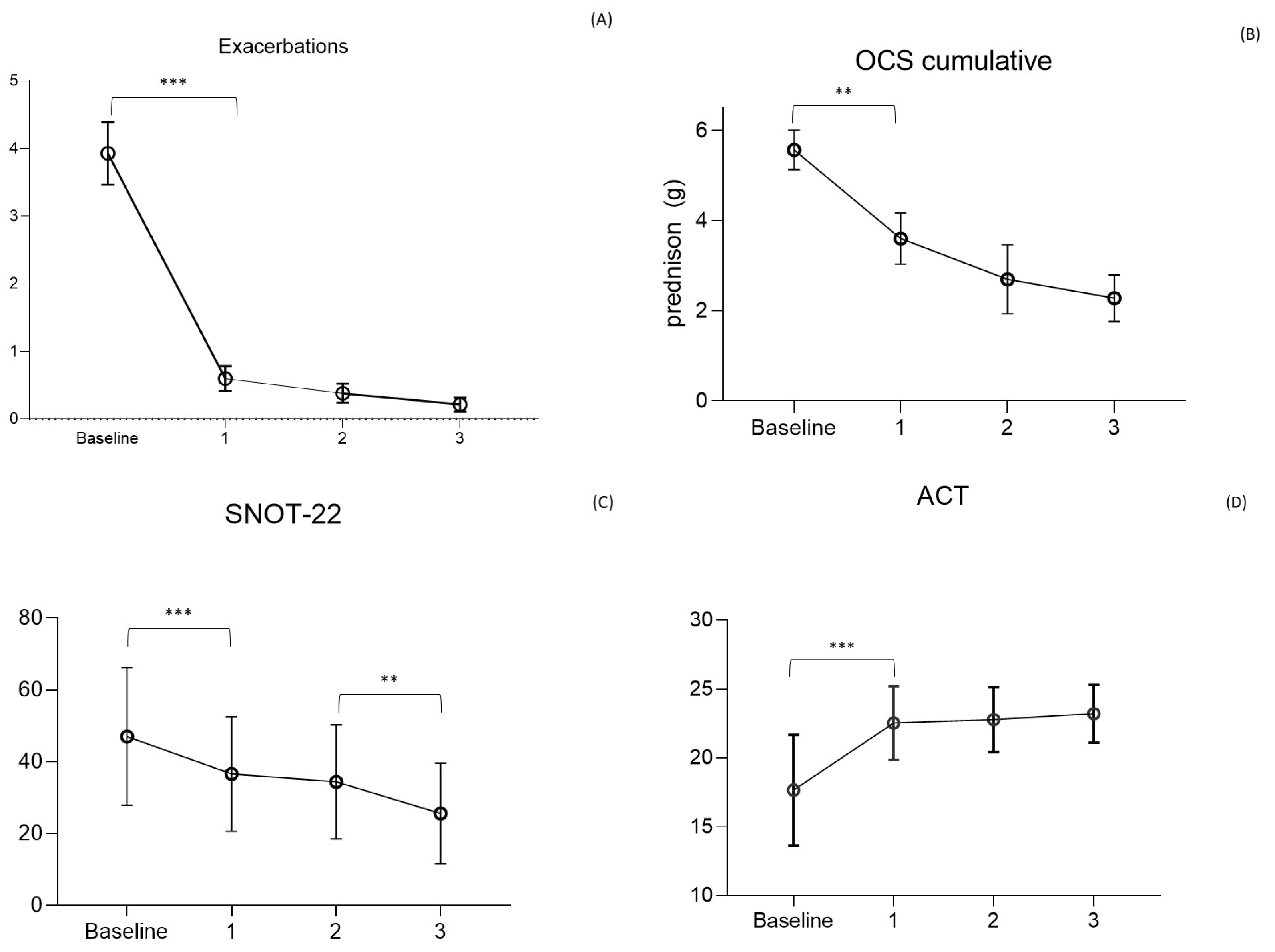
| OCS dependent (%) | 85 (54) | 31 (21) | <0.0001 | 16 (11) | 0.02 | 7 (6) | 0.19 | <0.001 |
| OCS daily dose ° | 15.0 (11) | 9.8 (10) | 0.022 | 7.6 (9) | 0.891 | 6.3 (4) | 0.933 | 0.046 |
| OCS cumulative yearly dose (g) | 5.8 (4.0) | 3.6 (3.11) | 0.039 | 2.7 (2.7) | 0.867 | 2.2 (1.3) | 0.822 | 0.049 |
| Exacerbations | 3.9 (2.8) | 0.6 (1.2) | <0.0001 | 0.4 (0.9) | 0.656 | 0.2 (0.5) | 0.842 | <0.0001 |
| Hospitalizated patients (%) | 35 (22) | 2 (1.3) | <0.0001 | 1 (0.7) | >0.999 | 1 (0.8) | >0.999 | <0.0001 |
| Hospitalization § | 1.4 (0.5) | 0.02 (0.18) | <0.0001 | 0.006 (0.08) | >0.999 | 0.03 (0.2) | >0.999 | <0.0001 |
| FEV1 % | 70 (33) | 83 (24) | 0.158 | 82 (22) | 0.981 | 84 (20) | 0.933 | 0.206 |
| FEV1 L | 2.21 (1.0) | 2.38 ¥ (1.0) | 0.044 | 2.33 (0.86) | >0.999 | 2.39 (0.9) | >0.999 | 0.078 |
| FeNO | 58 (42) | 34 (18) | <0.0001 | 38 (14) | 0.443 | 35 (18) | 0.718 | <0.0001 |
| ACT | 17 (4) | 23 (2) ¥ | <0.0001 | 23 (2) | 0.898 | 23 (2) | 0.691 | <0.0001 |
| SNOT-22 | 51 (15) | 37 (15) ¥ | 0.0002 | 34 (16) | 0.857 | 26 (14) | 0.05 | <0.0001 |
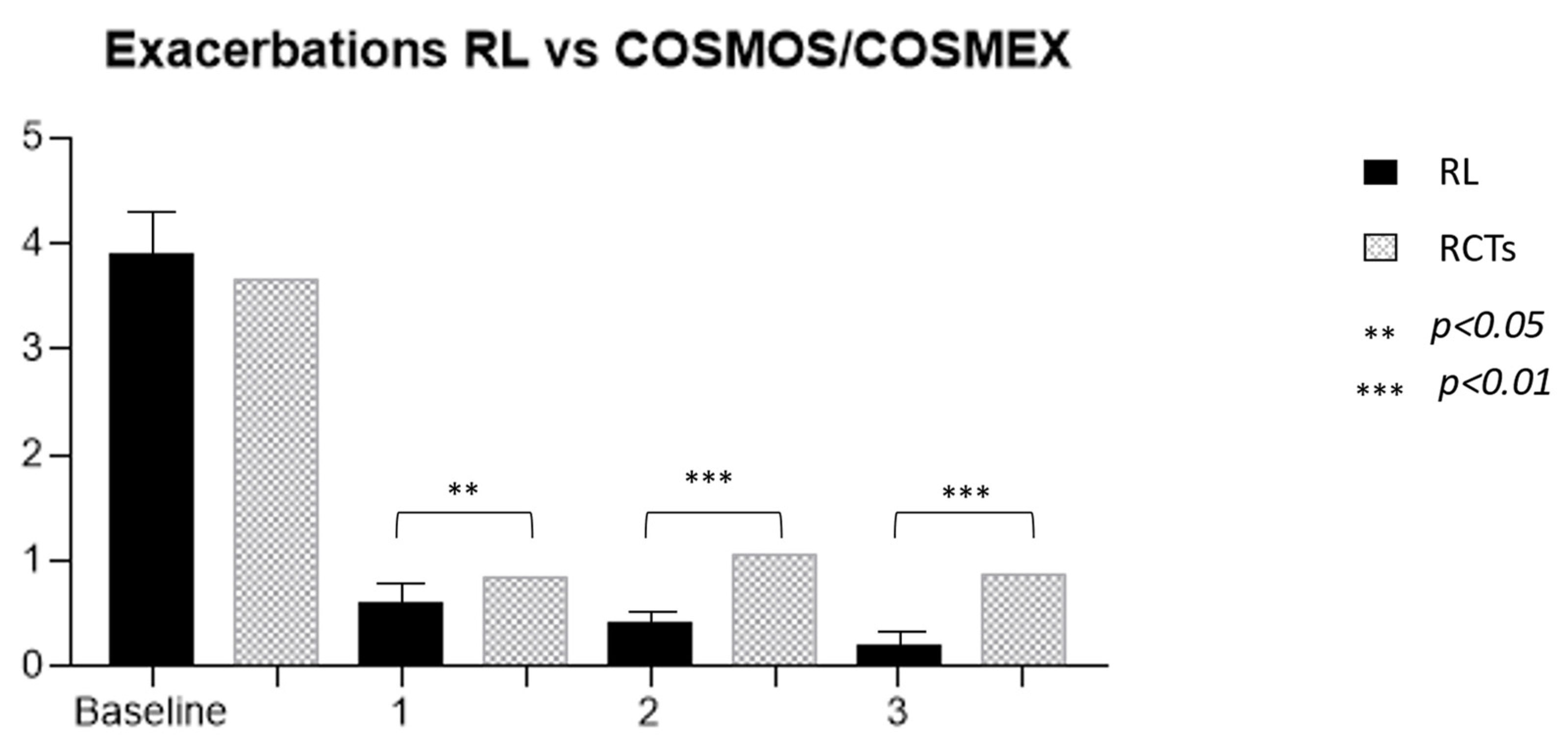
| RL vs. COSMEX/COSMOS | RL Cohort | Cosmos 651 | p-Value | Kavanagh 28 | p-Value (Cosmos vs. Kavanagh) | p-Value (RL vs. Kavanagh) |
|---|---|---|---|---|---|---|
| Exacerbations * | 3.9 (2.8) | 3.67 | 0.305 | 3.57 (2.2) | 0.811 | 0.142 |
| OCS dose ° | 15 (11) | 12.5 | <0.005 | 10 | 0.013 | <0.0001 |
| BMI | 25.8 (8.8) | 28.1 (6.1) | 0.001 | 28.2 (4.5) | 0.907 | 0.0008 |
| FEV1 (L) | 2.21 (1.0) | 1.99 (0.7) | 0.008 | 2.10 (0.65) | 0.378 | 0.171 |
| CRSwNP § | 99 (63%) | 24 (7%) | <0.0001 | 19 (67.9) | <0.001 | 0.188 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bagnasco, D.; Nicola, S.; Testino, E.; Brussino, L.; Pini, L.; Caminati, M.; Piccardo, F.; Canevari, R.F.; Melissari, L.; Ioppi, A.; et al. Long-Term Efficacy of Mepolizumab at 3 Years in Patients with Severe Asthma: Comparison with Clinical Trials and Super Responders. Biomedicines 2023, 11, 2424. https://doi.org/10.3390/biomedicines11092424
Bagnasco D, Nicola S, Testino E, Brussino L, Pini L, Caminati M, Piccardo F, Canevari RF, Melissari L, Ioppi A, et al. Long-Term Efficacy of Mepolizumab at 3 Years in Patients with Severe Asthma: Comparison with Clinical Trials and Super Responders. Biomedicines. 2023; 11(9):2424. https://doi.org/10.3390/biomedicines11092424
Chicago/Turabian StyleBagnasco, Diego, Stefania Nicola, Elisa Testino, Luisa Brussino, Laura Pini, Marco Caminati, Federica Piccardo, Rikki Frank Canevari, Laura Melissari, Alessandro Ioppi, and et al. 2023. "Long-Term Efficacy of Mepolizumab at 3 Years in Patients with Severe Asthma: Comparison with Clinical Trials and Super Responders" Biomedicines 11, no. 9: 2424. https://doi.org/10.3390/biomedicines11092424
APA StyleBagnasco, D., Nicola, S., Testino, E., Brussino, L., Pini, L., Caminati, M., Piccardo, F., Canevari, R. F., Melissari, L., Ioppi, A., Guastini, L., Lombardi, C., Milanese, M., Losa, F., Robbiano, M., De Ferrari, L., Riccio, A. M., Guida, G., Bonavia, M., ... on behalf of SANI. (2023). Long-Term Efficacy of Mepolizumab at 3 Years in Patients with Severe Asthma: Comparison with Clinical Trials and Super Responders. Biomedicines, 11(9), 2424. https://doi.org/10.3390/biomedicines11092424















