Abstract
In our life scenarios, we are involuntarily exposed to many heavy metals that are well-distributed in water, food, and air and have adverse health effects on animals and humans. Cadmium (Cd) is one of the most toxic 10 chemicals reported by The World Health Organization (WHO), affecting organ structure and function. In our present study, we use one of the green microalga Chlorella vulgaris (ChV, 500 mg/kg body weight) to investigate the beneficial effects against CdCl2-induced hepato-renal toxicity (Cd, 2 mg/kg body weight for 10 days) on adult male Sprague-Dawley rats. In brief, 40 adult male rats were divided into four groups (n = 10); Control, ChV, Cd, and Cd + ChV. Cadmium alters liver and kidney architecture and disturbs the cellular signaling cascade, resulting in loss of body weight, alteration of the hematological picture, and increased ALT, AST, ALP, and urea in the blood serum. Moreover, cadmium puts hepatic and renal cells under oxidative stress due to the up-regulation of lipid peroxidation resulting in a significant increase in the IgG level as an innate immunity protection and induction of the pro-inflammatory cytokines (IL-1β and TNF-α) that causes hepatic hemorrhage, irregular hepatocytes in the liver and focal glomeruli swelling and proximal tubular degeneration in the kidney. ChV additive to CdCl2, could organize the protein translation process via NF-kB/Nrf2 pathways to prevent oxidative damage by maintaining cellular redox homeostasis and improving the survival of and tolerance of cells against oxidative damage caused by cadmium. The present study shed light on the anti-inflammatory and antioxidative properties of Chlorella vulgaris that suppress the toxicity influence of CdCl2.
1. Introduction
The diverse health influence of exposure to environmental heavy metals became a global issue due to their toxic effects on living organisms, either by metabolic interference or mutagenesis [1]. Cadmium (Cd) has been reported to be among the highly toxic metals entering the water supply and contaminating food or air from different industrial and agricultural sources [2]. Long-term exposure to Cd has been found to cause variable physiological, histological, and biochemical alterations to different body organs of humans and laboratory animals with slow elimination from the body. Hence, they accumulate mainly in the liver, kidney, and brain [3,4,5,6]. Moreover, acute Cd exposure caused hepatotoxicity and death in different living species [7,8,9,10].
Furthermore, various in vitro and in vivo research revealed that Cd preferentially accumulated in the hepatocytes resulted in enhancement of lipid peroxidation and alteration of mitochondrial function [11,12]. Alongside, Cd resulted in genotoxicity and apoptotic effects in the human hepatocytes [13]. Sufficient evidence has emerged to reveal the mechanisms of hepatotoxicity induced by Cd, including ischemia initiated by endothelial damages and activation of Kupffer cells that induce serial cascades of events, including the proinflammatory and cytotoxic mediator, and activation of Nrf2 pathway that induce oxidative stress via suppression of antioxidant related genes [14,15,16].
Additionally, many studies revealed that Cd could induce renal injury via synthesizing the Cd-metallothionein (Cd-Mt) complex inside the hepatic tissues which circulated into blood, reaching the proximal renal tubules [17,18,19]. The free Cd-ions might lead to free radicals accumulation, oxidative stress, and lipid peroxidation [20,21]. Generally, oxidative stress might adversely affect the structure and physiological function of cells and impair the translation and transcription of RNA besides the structures and functions of cell membranes [22]. Furthermore, it has been investigated that metabolic alteration, hormonal disorders, and secretion of pro-inflammatory biomarkers have also been observed in association with Cd-induced oxidative damage [23,24]. Additionally, the reactive oxygen species (ROS) control the activity of heat shock protein70 (HSP70), nuclear factor erythroid 2-related factor 2 (Nrf2) and nuclear factor kappa B (NF-kB), and heme oxygenase-1 (HO-1), which could regulate the antioxidant responses of the cells in oxidative stress conditions. Nrf2 and NF-kB significantly enhance the antioxidant defense of stressed cells and help to eliminate and detoxify exogenous chemicals and their toxic metabolites [25].
Chlorella vulgaris (C. vulgaris) is a single-cell, easily cultivated, highly productive green microalga most often used in food supplements owing to its high content of valuable nutrients [26,27]. Accordingly, the Food and Drug Administration (FDA) documented C. vulgaris as a safe alga for the dietary supplement [28]. It is a superfood, containing 18% amino acids, 20% vitamins, 60% protein, and essential elements such as calcium, iron, magnesium, phosphorous, and potassium [29]. Furthermore, microalgae have valuable antioxidants and therapeutic properties as they contain carotenoids, chlorophyll, phycobiliproteins, lutein, and astaxanthin [30]. Chlorella sp. supplementation has many beneficial physiological properties as they are antihypertensive [31], antioxidative [32], hypocholesterolemic [33], have antitumor activities [34], and hypolipidemic and hypoglycemic roles in human and animal research [35].
Furthermore, C. vulgaris has been reported to protect against hepatic injuries induced by carbon tetrachloride in mice and rats and is highly resistant to toxic heavy metals, including cadmium [36,37]. Moreover, the detox efficacy of C. vulgaris was also reported against CCl4, which causes renal damage, including glomerulosclerosis, atrophy, and loss of cellular entity in the renal cortex [38]. The hepatorenal protective effects of C. vulgaris against cadmium toxicity in rats were reported [3,4]. Perhaps the supposed mechanisms of detoxification in the presence of C. vulgaris are illustrated through the inductions of metal binding metallothionein MT-like protein in the cell, which is involved in heavy metal detoxification, and once the toxic elements fixed to the C. vulgaris fibers, which could not be reabsorbed, they are eliminated from the body in the stool [39,40]. Recently, C. vulgaris has been reported to safely remove pesticides, heavy metals, and herbicides from the bodies by increasing their excretion in urine and feces [41]. However, scientific information concerning the exact mode of action and molecular mechanisms of C. vulgaris on the toxicological effects of cadmium is relatively little. Therefore, this work aimed to investigate the benefits of C. vulgaris supplementation in cadmium detoxification by evaluating function markers of the liver and kidney, immunological markers, biomarkers of oxidative stress, stress hormones, stress-responsive genes mRNA expression, and histopathological changes in the liver and kidney of rat.
2. Materials and Methods
2.1. Animal Husbandry
Adult male rats (Sprague–Dawley; n = 40), with 180–190 gm average body weight (BW), were used in our study. Rats were housed under hygienic conditions in stainless-steel cages with wood shavings as bedding and acclimatized before use for two weeks on a basal diet, with free access to water, with 12 h light-darkness cycle.
2.2. Tested Compounds
CdCl2 (analytical grade with 99% purity) was brought by El-Faraana Company for trading in Giza, Cairo, Egypt, as a white powder. Chlorella vulgaris was kindly supplied by The National Research Center, Giza, Cairo, Egypt. The rest of the chemicals were obtained from Sigma Chemical Co. (Sigma-Aldrich, Inc., PO Box 14508, St. Louis, MI, USA) and were of analytical grade.
2.3. Experimental Design
After two weeks of acclimatization, the 40 rats were divided randomly into 4 equal groups, each containing 10 rats; group 1 (control group). Group 2 is the ChV group (received Chlorella vulgaris at a dose of 500 mg/kg body weight/day orally for 10 days). Group 3 (treated with 2 mg/kg CdCl2 via subcutaneous injection daily for 10 days) and group 4 (Cd + ChV) co-treated with both 2 mg/kg CdCl2 and 500 mg/kg Chlorella vulgaris daily for 10 days with the same indicated routes.
2.4. Blood Collecting and Tissue Preservation
At the experimental end (after 10 days), rats were weighed and then euthanized by cervical dislocation under anesthesia using intramuscular injection with 1 mL/kg of ketamine xylazine mixture (2:1). For each experimental group, samples of blood (5 samples per group) were harvested from the median canthus in an anticoagulant-free tube for separation of serum to be used for biochemical analysis. Another set of blood (5 samples per group) was collected in tubes containing EDTA for hematological analysis. For tissue collection, dissected liver and kidney specimens were weighed to obtain the relative weight (organ weight ×100/body weight) and then divided into 3 parts; the 1st part was centrifuged at 4 °C for 15 min at 3000 rpm to obtain a homogenate, then the supernatants were harvested and kept at −20 °C to be used in biochemical analysis. The 2nd part was kept at −80 °C for quantitative real-time qRT-PCR, and the 3rd specimens were fixed in 10% neutral buffered formalin for the histopathological procedure.
2.5. Hematological Analysis
Collected blood samples were used to measure hematologic parameters by blood cell analyzer. Total blood picture, white blood cell count, and differential leukocyte count were indicated using an automatic cell counter (Hospitex Hemascreen 18, Hospitex International CO, Via Baldanzese, Calenzano, Italy) [42].
2.6. Biochemical Analysis
After serum collection (5 samples/group) was used to determine the total protein [43], albumin [44], total cholesterol [45], high-density lipoprotein (HDL) low-density lipoprotein (LDL) [46], triglycerides [47] creatinine, urea, glucose were estimated spectrophotometrically using commercial diagnostic kits purchased from Biodiagnostic Company, Giza, Egypt. Alkaline phosphatase (ALP), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) were estimated spectrophotometrically using commercial diagnostic kits purchased from Biodiagnostic Company (Giza, Egypt) as described in [48,49], respectively, according to the manufacturer’s instructions. The serum levels of immunoglobulin (IgG and IgM) were measured as described in [50] using commercial ELISA kits for rats (CusabioBiotech Co., Ltd., Wuhan, China) according to the manufacturer’s protocols.
2.7. Inflammatory Markers Evaluation in the Serum
Inflammation parameters, including Interleukin-1 beta (IL-1β), tumor necrosis factor-α (TNF-α), and nitric oxide (NO) were estimated [51] using commercial rat ELISA kits (Catalog no: MBS825017, MBS843321, MBS010567, respectively, from MyBioSource, San Diego, CA, USA) following the manufacturer’s directives.
2.8. Oxidative Stress Markers Detection in the Serum
Oxidative stress is indicated by determining reactive oxygen species (ROS) and total antioxidant capacity (TAC) [52] by the ELISA kit (Cat No. MBS164653, MyBioSource, San Diego, CA, USA for ROS and TA 2513 kit, Biodiagnostic Co., Egypt for TAC). Additionally, total antioxidant capacity was detected using commercial kits. While malondialdehyde; MDA (lipid peroxidation marker) [53] was estimated using commercial kits (Cat No. ab118970, Abcam Co., Cambridge, UK) according to the manufacturer’s instructions.
2.9. Determination of Stress-Related Hormones
Cortisone, adrenaline, and noradrenaline were evaluated in the serum by rat ELISA kits (Cat. no: MBS024061, MBS031232, and MBS269993, respectively, from MyBioSource, San Diego, CA, USA) according to the instructions of the manufacturer [54].
2.10. Separation of mRNA and Quantitative Real-Time PCR (qRT-PCR)
RNA was extracted from the liver tissues, and its concentration and integrity were checked by agarose (1%) and spectrophotometry. QuantiTect RT kit (Qiagen, Germany) synthesized first-strand cDNA. The primers of the targeted genes (HSP70, Nrf2, NF-kB, HO-1, and the internal housekeeping gene β-actin) [55,56,57] are shown in Supplementary Table S1. QuantiTect SYBR Green PCR kit (Qiagen, Germany) and a Rotor-Gene Q apparatus Real-time were used for performing the PCR. The thermocycler condition was 95 °C for 15 min for the initial activation, followed by 40 cycles of denaturation at 94 °C for 15 s, annealing at 60 °C for 10 s, and elongation at 72 °C for 15 s. The relative expressions of the targeted genes were analyzed using the 2−ΔΔCt equation [58].
2.11. Histopathological Investigation
Dissected kidney and liver samples were fixed for 48 h in 10% neutral-buffered formalin. Then samples were washed carefully under running tap water for one night, followed by dehydration in ascending ethanol series (70–100%). After that, tissues were cleared in xylene and embedded in paraffin wax. Paraffin blocks were cut at 3–5 µm thickness, stained with H&E (hematoxylin and eosin) stain, and examined by Olympus BX51 Light Microscope, Tokyo, Japan [59].
2.12. Statistical Analysis
Data were analyzed using One-way ANOVA followed by Duncan’s Multiple Range test to compare means value between groups. Data were expressed as mean ± standard error (SE), and a p < 0.05 was considered significant.
3. Results
3.1. Chlorella vulgaris Improved the Hematological Parameters Altered by Cadmium
Table 1 shows the hematological parameters after Cd and C. vulgaris treatment. The RBC count was not significantly altered in the different experimental groups; however, PCV and Hb declined significantly after Cd administration. Interestingly, co-exposure to C. vulgaris in the presence of Cd could elevate the Hb and PCV levels and restore Hb to the normal value. Furthermore, the results of the leukogram revealed that the total leukocyte count was significantly decreased with a concomitant reduction in the lymphocyte count compared with the control group after Cd treatment. Contrarily, co-administration of C. vulgaris + Cd restored the lymphocytic count to the control value and significantly elevated the WBC count, though it was still below normal level. While no significant differences were found in the neutrophils and eosinophils counts among the different experimental groups.

Table 1.
Effects of separate and concurrent exposure to CdCl2 and ChV on hematological parameters.
3.2. C. vulgaris Restores the Body and Organs Weight Affected by Cd Treatment
After Cadmium administration, the body weight of treated rats showed a significant reduction compared with the control and C. vulgaris groups. Adding Chlorella vulgaris could restore the final weight of exposed rats in the Cd + C. vulgaris group to the normal level. However, the different treatments did not significantly alter the relative weight of the kidneys, liver, and spleen (Table 2).

Table 2.
Effect of separate and concurrent exposure to CdCl2 and Chlorella on body weight and other organs.
3.3. C. vulgaris Modulates the Serum Biochemical Parameters Altered by Cd Treatment
Rats injected with cadmium exhibited a remarkable decline in serum levels of albumin and total protein (p < 0.001) relative to the control. Conversely, C. vulgaris + Cd elevated the total protein and albumin values without returning them to the normal level (Table 3). ALT, AST, ALP, and urea levels were upregulated significantly (p < 0.001) in the serum of the cadmium-treated group relative to the control. Interestingly, C. vulgaris addition to Cd significantly decreased their levels, unlike Cd alone. Moreover, data also showed a significant elevation in the creatinine level in Cd-treated rats relative to the control; however, its level in the C. vulgaris + Cd group was comparable to the control.

Table 3.
Effects of separation and concurrent exposure to CdCl2 and Chlorella on serum biochemical parameters.
Concerning the lipid profile, rats exposed to Cd showed significant elevations in the LDL-cholesterol and total cholesterol levels, accompanied by a significant reduction in the HDL-cholesterol level in serum compared to the control. Contrarily, the co-exposure to C. vulgaris + Cd significantly lowered the LDL-cholesterol and total cholesterol levels and elevated the HDL-cholesterol compared to the Cd group. However, the different treatments could not restore the serum triglycerides to the normal value (Table 3).
3.4. Cd Administration Induces the Whole Body’s Oxidative Stress
The level of oxidative stress and TAC were evaluated in the serum of treated groups. Herein, the MDA total and ROS were increased significantly by Cd (p ˂ 0.01) compared to the control. Co-administration of C. vulgaris + Cd modulated the level of MDA to normal value. Moreover, C. vulgaris + Cd group exhibited a significant reduction in ROS level; however, it did not normalize to the control level. Collectively, the findings indicated that the lowest level of TAC was observed in the Cd-treated group, followed by C. vulgaris + Cd; while the highest values were observed in the C. vulgaris group compared to the control (Table 4).

Table 4.
Effects of separate and concurrent exposure to CdCl2 and Chlorella on oxidative stress, immunity, and inflammatory markers in serum.
3.5. Cd Administration Elevates the Innate Immunity Response
Data in Table 4 indicated a significant increase in the level of IgG in the Cd group relative to the control; however, its level in the C. vulgaris + Cd group was comparable to the control. IgM levels did not significantly differ among the different groups.
3.6. Cd Administration Stimulates the Pro-Inflammatory Mediators
Regarding the inflammatory response, Cd significantly increased the serum levels of TNF-α and IL-1β, which were significantly suppressed (p ˂ 0.01) by the co-administration of C. vulgaris but did not reach to normal value (Table 4).
3.7. C. vulgaris Additive Modulates the Adrenal Hormones Induced by Cd Administration
Cd induced significant elevations in the levels of stress-related hormones, including cortisone, noradrenaline, and adrenaline, compared to the control (Figure 1). However, their levels exhibited no remarkable changes in the C. vulgaris group compared to the control one. Interestingly, these hormones modulated again in the C. vulgaris + Cd co-treated group to the control value.
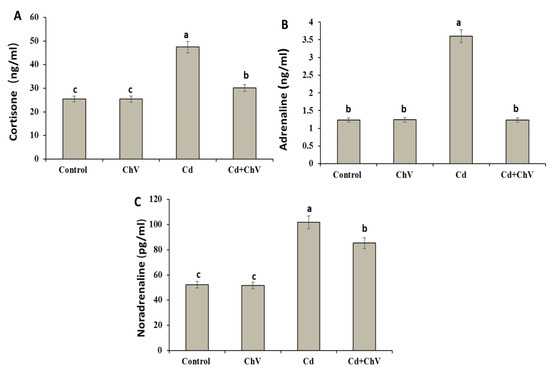
Figure 1.
Serum levels of cortisone (A), adrenaline (B), and noradrenaline (C) in rats exposed to Cd and C. vulgaris separately or in combination. Data were analyzed using One-way ANOVA followed by Duncan’s Multiple Range test to compare mean value between groups (control, ChV: chlorella vulgaris-treated group, Cd: cadmium-treated group, Cd + ChV: cadmium + C. vulgaris treated group). Data were expressed as mean ± SE. Values not sharing a common superscript letter (a, b, c where a: the highest value, c: the lowest value) differ significantly at p < 0.05.
3.8. Chlorella vulgaris Restores the Cellular Redox Homeostasis by Modulating Stress Key Mediators at the Genetic Level
Cellular key mediators control the expressions of various genes shared in cellular antioxidant, antitoxic, and anti-inflammatory responses. Some of these mediators include the NF-KB, 70 kDa heat shock protein (Hsp70), Nrf2, and HO-1 [60,61]. Herein, mRNA expressions of hepatic HSP70 and NF-kB involved in the cellular mediation against Cd toxicity exhibited significant up-regulations compared to the control and C. vulgaris groups. Co-administration of C. vulgaris + Cd significantly down-regulated the relative expressions of both genes compared with the Cd group (Figure 2A,B).
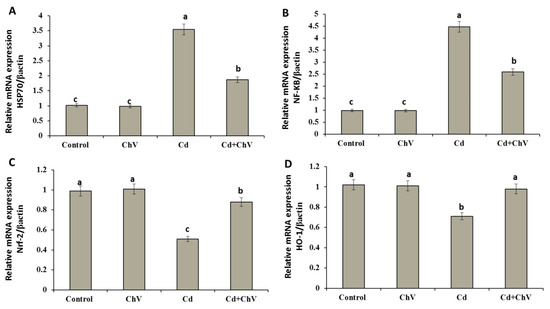
Figure 2.
Relative expression of HSP70 (A), NF-kB (B), Nrf-2 (C), or HO-1 (D) mRNA in hepatic tissue of rats exposed to Cd and C. vulgaris separately or in combination. Data were analyzed using One-way ANOVA followed by Duncan’s Multiple Range test to compare mean value between groups (control, ChV: chlorella vulgaris-treated group, Cd: cadmium-treated group, Cd + ChV: cadmium + C. vulgaris treated group). Data were expressed as mean ± SE. Values not sharing a common superscript letter (a, b, c where a: the highest value, c: the lowest value) differ significantly at p < 0.05.
Conversely, treatment with Cd showed significant decreases in the relative expression of HO-1 and Nrf-2 mRNAs compared to the control and C. vulgaris groups. Contrarily, the co-administration of C. vulgaris + Cd significantly improved the Nrf-2 mRNAs expression than Cd alone. Similarly, HO-1 expression was up-regulated significantly. Chlorella v. restored Nrf-2 and Ho-1 expressions to the control value (Figure 2C,D).
3.9. C. vulgaris Relief the Toxicity of CADMIUM in the Hepatic and Renal Tissues at the Histological Levels
At the histological level, the liver of the control as well as C. vulgaris groups showed normal hepatic architecture with central veins and hepatocyte cords in the portal area (Figure 3A,B). Contrarily, Cadmium causes alternation in the hepatic tissue architecture exhibited by hepatocyte degeneration, inflammatory cellular infiltration, and blood congestion in the portal area (Figure 3C). Interestingly, C. vulgaris inhibited the cadmium-induced inflammatory pathway and reduced the hepatic tissue damage (Figure 3D) not only in the liver but also the renal corpuscles of the kidney as the control and C. vulgaris groups exhibited normal renal cortex with Bowman’s capsule containing glomerulus and fine-arranged proximal and distal convoluted tubules with central arranged nuclei in the lining epithelia (Figure 4A,B). In the cadmium-treated group, histopathological alternation in the renal cortex was observed in segmented glomeruli, dilated tubules, edema exudate, and congested blood vessels (Figure 4C). Interestingly, adding C. vulgaris to the cadmium in the co-exposed group ameliorated the renal tissue with fine-arranged proximal and distal convoluted tubules (Figure 4D).
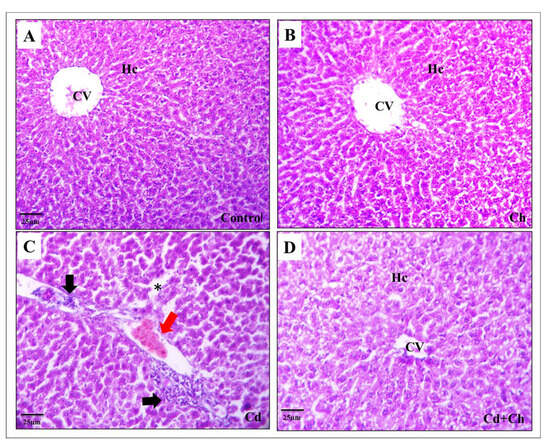
Figure 3.
Cadmium alters hepatic tissue architecture: (A) Photomicrograph of a hepatic tissue section of the control group stained with H&E showing normal histology with a lobular area of central vein (CV) and hepatocytes (Hc). (B) C. vulgaris-fed group showing no mild difference from the control group. (C) Cadmium treated group showing congestion of the central vein and portal area with inflammatory cellular infiltration (black arrow) and blood congestion (red arrow). Notice the dilated sinusoidal spaces with hepatocyte damage (black asterisk). (D) Treated group of cadmium and C. vulgaris showing the relief effect of chlorella against the toxic effect of cadmium on the central vein and hepatic cords.
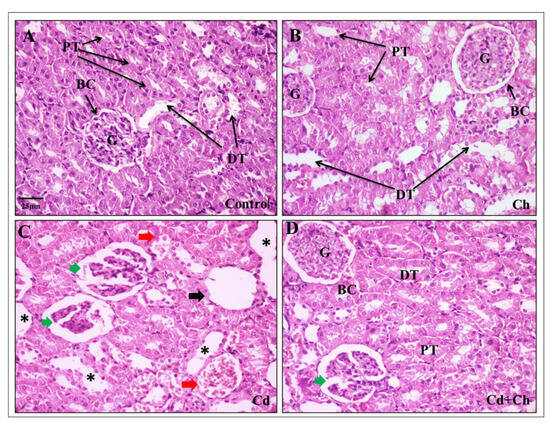
Figure 4.
Effects of cadmium and chlorella on the kidney: (A) Photomicrograph of control kidney showing normal histology of Bowman capsule (BC), glomerulus (G), proximal convoluted tubule (PT), and distal convoluted tubule (DT). (B) Photomicrograph of chlorella-fed group showing normal renal corpuscles. (C) Cadmium treated group showing severe degenerative alternations in the renal tubules (black asterisk), corpuscle degeneration (black arrow), glomeruli segmentation with Bowman space widening (green arrow), and blood congestion (red arrow). (D) Cd + C. vulgaris co-administrated group showing the protective change of C. vulgaris on the glomeruli and tubules epithelial cells except for mild corpuscle segmentation (green arrow).
4. Discussion
Cadmium is a highly toxic metal that is widely distributed in the surrounding environment with a destructive impact on many organ systems [62,63,64,65]. In vitro studies revealed that ROS production underlined cadmium’s toxicity mechanisms because cadmium induces the production of nitric oxide, superoxide anion, and hydrogen peroxidase, which is implicated in many deleterious health effects [66,67,68].
Furthermore, hematological profiles are considered good indicators to evaluate the physiological response of the animal to internal or external stressors [69]. In our present study, the administration of CdCl2 significantly decreased Hb, PVC, WBC count, and lymphocytes (which are immune cells fundamental in cellular and humoral immunity. In the blood, they represent 20 to 45% of WBC), and this agreed with earlier studies which reported that CdCl2 could alter the hematological indices [70,71]. Additionally, Cd induced a remarkable reduction in the total body weight of rats. However, the relative weight of the liver and kidneys were not significantly altered by different treatments, Similar findings were observed by other authors [71,72]. C. vulgaris in our present study could improve the altered hematological parameters and normalize the Cd-reducing effect on body weight.
C. vulgaris is rich in pigments (chlorophyll), amino acids, vitamins (A, B complex, C, and E), and minerals (iron, calcium, manganese, phosphorus) [73,74]. Accordingly, its content of antioxidant vitamins could protect cells against the unwanted actions of free radicals [75]. Vitamin E could protect cellular membranes against lipid peroxidation by scavenging lipid peroxyl radicals, counteract oxidative damage, and keep the ascorbic acid and GSH contents in damaged cells after exposure to xenobiotics, including Cd [76,77]. Moreover, C. vulgaris has been used as a useful food additive for fish diets as it could improve immunity, enhance digestibility, and organize growth performance owing to the considerable content of crude proteins, minerals, polysaccharides, lipids, and other bioactive constituents, which are important for various physiological functions [78]. Alongside, Kang et al. concluded that C. vulgaris addition to the diet of broilers improved their body weights [79]. This could explain the improving effect of C. vulgaris on the hematological indices of rats.
The liver is the organ of drug transformations and is considered the main hub for protein synthesis; therefore, the cellular damage induced by CdCl2 could be monitored by measuring the function of hepatic enzymes in the blood serum, including; AST, ALT and ALP besides the total protein and albumin content [80]. Hence, in case of liver damage due to inflammations, necrosis, or bile duct obstruction, liver enzymes are released in the serum or plasma carrying all the physiological information about the health status [81]. Herein, CdCl2 induced liver enzyme synthesis while decreasing the albumin and total protein content compared to the control indicating liver dysfunctions [17,82]. Our results agree with Renugadevi and Prabu, who observed that the level of these enzymes was increased significantly in Cd-intoxicated rats relative to the control [20]. Collectively, the up-regulation of hepatic markers in the blood serum suggested extensive liver injuries in the presence of Cd due to the increased lipid peroxidation that caused membrane damage and increased the membrane’s permeability and the leakage of hepatic enzymes into the circulation [83]. This is in line with the present findings, where the administration of CdCl2 significantly increased ROS production and lipid peroxidation while decreasing the TAC in the exposed rat.
Normally, there is a balance between ROS and cellular antioxidants. Hence, oxidative stress occurs if this balance is disturbed by the overproduction of ROS or/and depletion of antioxidants. Subsequently, increasing ROS production can disturb the cell’s physiology and induce DNA, protein, and lipid malformations [84,85]. Moreover, the present findings revealed significant alterations in the lipid profile of Cd-exposed rats relative to the control. Herein, the altered hepatic lipogenesis could be explained by the deleterious effects of Cd on liver functions and structure, which agreed with Wu et al. [86].
The administration of Cd in this study resulted in hepatocyte degeneration, inflammatory cellular infiltration, and blood congestion in the portal area. This is similar to the chronic and acute effects of Cd reported in previous works [82]. Furthermore, hepatic necrosis has been reported in rats after parenteral administration of soluble salt of Cd [87]. In concurrent administration of C. vulgaris and Cd, the levels of liver enzyme activity were significantly reduced compared to the Cd group alone. This finding indicates the palliative effects of C. vulgaris in ameliorating the hepatotoxic effect of Cd.
Regarding kidney functions, the changes in creatinine and urea levels in the present work showed the adverse effects of CdCl2 on renal function, which agreed with Abdel-Moneim and Ghafeer [17]. It has been documented that Cd induced nephrotoxicity via the cross-talk between the liver and kidney via the hepatic Cd-metallothionein complex (Cd-Mt) [19]. For more clarification, the insufficient synthesis of Mt, the unbound Cd ions resulting in hepatic injuries, and the bound ions excreting through the kidney lead to improper function of the renal and lipid peroxidation [20,88,89]. This is parallel to our results concerning the oxidative damage and histopathological alternation in renal tissues, including renal edema [90], proximal tubular apoptosis, necrosis, and degenerations [91] of glomerular capillaries in favor of Bowman’s space [92].
In our present study, Cd induced stressful hormone synthesis suggesting the ability of Cd to activate the hypothalamus-pituitary axis (HPA) and consequently, the release of glucocorticoids from the adrenals served as a significant monitor in estimating the immediate physiological responses to various stressors [93]. On the other hand, administration of C. vulgaris with Cd could return the level of these hormones to normal values suggesting its importance as a naturally occurring anti-stress agent [94].
Nuclear factor kB (NF-kB) and heat shock protein 70 (HSP70) are important proteins that help the stability of DNA and organize the transcription processes that protect the cell during stress [95]. In this work, Cd significantly increases the expressions of the NF-kB and HSP70 in the liver, suggesting its significant role in improving the survivability and tolerance of cells against Cd-induced oxidative damage. Contrarily, C. vulgaris additions down-regulate the relative expressions of these genes, which may be ascribed to the antioxidant property of C. vulgaris [96]. Additionally, NF-κB has a strategic position at the crossroads between oxidative stress and inflammation; it was suggested that ROS might act as a key secondary mediator responsible for the NF-κB activation in response to multiple stimuli [25].
Moreover, the transcription factor, Nrf2, can assist in preventing oxidative damage to cells by maintaining cellular redox homeostasis and promoting the activities of detoxification and biotransformation enzymes [97,98]. Additionally, Nrf2 is essential for producing HO-1, which protects and neutralizes free radicals in cells under stress [99]. Herein, Cd administration downregulated the expressions of Nrf2 and HO-1, indicating the hepatotoxic and oxidative damaging effects of CdCl2. However, co-administration of C. vulgaris with Cd substantially increased the expressions of HO-1 and Nrf-2, indicating that C. vulgaris was successful in triggering the Nrf2/HO-1-dependent pathway against cellular damage caused by CdCl2 intoxication.
Regarding the inflammation pathway, the current work studied the activation effect of CdCl2 on inflammatory markers, including TNF-α and IL-1β. It may be linked to the enhanced ROS production that occurs after Cd administration and helps recruit more inflammatory cells and fibroblasts to the injury site and stimulates the production of certain cytokines such as TNF-α [100]. Sequentially, TNF-α triggers the inductions of other cytokines such as interferon-γ and IL-1β [101]. Like TNF-α and IL-1β, NO has been regarded as a hepatic injury mediator [102]. Elevating the NO content could lead to lipid peroxidation and more destructive effects on the tissues [103]. Additionally, some research has linked the HSP70 to the induction of TNF-α, IL-1β, and IL-6, which are crucial in the initial stages of liver regeneration [104]. Alongside, HSP 70 has also been demonstrated to be involved in the in vitro induction of NO in bone marrow macrophages [105], and this is consistent with the elevated expressions of HSP 70 in Cd-exposed rats in our study.
In our study, C. vulgaris exhibited an excellent hepatoprotective effect, maintained the integrity of membranes of hepatocytes, prevented the leakage of enzymes into the circulation, and repaired the hepatic tissue damage after Cd administration. These results agree with [106,107]. Furthermore, Cheng et al. recorded that the underlined mechanisms of C. vulgaris protections might be associated with its immunomodulation activity that could stimulate macrophages’ phagocytic activities and enhance natural killer (NK) cells [108]. C. vulgaris microalga has anti-inflammatory and antioxidant properties that protect against membrane fragility [31,32]. These beneficial antioxidants include chlorophyll, carotenoids, astaxanthin, lutein, and phycobili-proteins [109]. The antioxidant properties of C. vulgaris and its phenolics provide cellular protective effects due to their redox potentials that could suppress oxygen and decomposing peroxides [110,111,112]. Herein, the protective effect of C. vulgaris co-administered with Cd has been elucidated
However, pretreatment of rats with C. vulgaris alga, alongside its health benefits, can prevent damage and protect against oxidative harmful effects induced by paracetamol through their free radical scavenging and powerful antioxidant effects, and they can be used as prophylactic agents against paracetamol-induced toxicity [113]. Therefore, there is a possible need for a future investigation on the preventive effect of C. vulgaris when administered before exposure to Cd and the curative and restoring effects when administered following damage induction by Cd.
5. Conclusions
This study showed that cadmium chloride altered the physiological response, including hematological and serum biochemical parameters (liver and kidney function markers), immunological and inflammatory biomarkers, and induced oxidative stress and histopathological alterations in the liver and kidney of adult male rats. On the other hand, C. vulgaris succeeded in preventing the disruption of organ functions by protecting them from oxidative stress and inflammation and enhancing immunity. These effects of C. vulgaris could be the mechanisms of their hepato-renal protection. Moreover, the beneficial role of C. vulgaris in this study could be suggestive of their use as an immunomodulatory and antioxidant supplement and could be a base for further research on its importance in detoxifying the body from environmental pollutants.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomedicines11092414/s1, Table S1: Primer sequences (5′-3′) used for the quantitative real-time qPCR.
Author Contributions
Conceptualization, E.A.A.M., E.E.-H. and A.M.A.A.-E., methodology, E.A.A.M., E.E.-H. and A.M.A.A.-E.; software, M.R.F. and M.A.; validation, M.R.F. and M.A.; formal analysis M.R.F.; investigation, E.A.A.M., E.E.-H. and A.M.A.A.-E. resources, S.M.A.-Z. and S.A.M.; data curation M.R.F., M.A., M.M.A., G.C. and A.M.A.A.-E.; writing—original draft preparation, M.R.F., M.A., E.A.A.M., E.E.-H., S.M.A.-Z., S.A.M., M.M.A., G.C. and A.M.A.A.-E.; writing—review and editing, M.R.F., M.A., E.A.A.M., E.E.-H., S.M.A.-Z., S.A.M., M.M.A., G.C. and A.M.A.A.-E.; project administration, M.R.F., M.A., G.C. and M.M.A. funding acquisition, M.R.F., M.A., G.C. and M.M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Researchers Supporting Project (RSPD2023R731), King Saud University (Riyadh, Saudi Arabia).
Institutional Review Board Statement
All the experimental steps were approved by the Institutional Animal Care and Use Committee, Zagazig University, Egypt (ZU-IACUC/2F/388/2022).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pandey, G.; Madhuri, S. Heavy metals causing toxicity in animals and fishes. Res. J. Anim. Vet. Fish. Sci. 2014, 2, 17–23. [Google Scholar]
- Järup, L.; Åkesson, A. Current status of cadmium as an environmental health problem. Toxicol. Appl. Pharmacol. 2009, 238, 201–208. [Google Scholar] [CrossRef]
- Gaurav, D.; Preet, S.; Dua, K. Protective effect of Spirulina platensis on cadmium induced renal toxicity in wistar rats. Arch. Appl. Sci. Res. 2010, 2, 390–397. [Google Scholar]
- Bernard, A. Renal dysfunction induced by cadmium: Biomarkers of critical effects. Biometals 2004, 17, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Gao, X.; Luo, X.; Li, L.; Ma, M.; Zhu, Y.; Zhao, L.; Li, R. The effects of long-term exposure to low doses of cadmium on the health of the next generation of mice. Chem.-Biol. Interact. 2019, 312, 108792. [Google Scholar] [CrossRef] [PubMed]
- Lane, E.; Canty, M.J.; More, S.J. Cadmium exposure and consequence for the health and productivity of farmed ruminants. Res. Vet. Sci. 2015, 101, 132–139. [Google Scholar] [CrossRef]
- Souza, V.; Bucio, L.; Gutierrez-Ruiz, M. Cadmium uptake by a human hepatic cell line (WRL-68 cells). Toxicology 1997, 120, 215–220. [Google Scholar] [CrossRef]
- Koyu, A.; Gokcimen, A.; Ozguner, F.; Bayram, D.S.; Kocak, A. Evaluation of the effects of cadmium on rat liver. Mol. Cell. Biochem. 2006, 284, 81–85. [Google Scholar] [CrossRef]
- Kang, M.-Y.; Cho, S.-H.; Lim, Y.-H.; Seo, J.-C.; Hong, Y.-C. Effects of environmental cadmium exposure on liver function in adults. Occup. Environ. Med. 2013, 70, 268–273. [Google Scholar] [CrossRef]
- Elazab, S.T.; Hsu, W.H. Antagonism of cadmium-induced liver injury in ducks by α-bisabolol. Front. Vet. Sci. 2022, 9, 1024549. [Google Scholar] [CrossRef]
- Al-Nasser, I.A.; Al-Nasser, I. Cadmium hepatotoxicity and alterations of the mitochondrial function. J. Toxicol. Clin. Toxicol. 2000, 38, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Berzina, N.; Markovs, J.; Isajevs, S.; Apsite, M.; Smirnova, G. Cadmium-Induced Enteropathy in Domestic Cocks: A Biochemical and Histological Study after Subchronic Exposure. Basic Clin. Pharmacol. Toxicol. 2007, 101, 29–34. [Google Scholar] [CrossRef]
- Skipper, A.; Sims, J.N.; Yedjou, C.G.; Tchounwou, P.B. Cadmium chloride induces DNA damage and apoptosis of human liver carcinoma cells via oxidative stress. Int. J. Environ. Res. Public Health 2016, 13, 88. [Google Scholar] [CrossRef]
- Rikans, L.E.; Yamano, T. Mechanisms of cadmium-mediated acute hepatotoxicity. J. Biochem. Mol. Toxicol. 2000, 14, 110–117. [Google Scholar] [CrossRef]
- Shaikh, Z.A.; Vu, T.T.; Zaman, K. Oxidative stress as a mechanism of chronic cadmium-induced hepatotoxicity and renal toxicity and protection by antioxidants. Toxicol. Appl. Pharmacol. 1999, 154, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Ma, H.; Liu, G.; Fan, S.; Guo, Z. Mechanism of cadmium exposure induced hepatotoxicity in the mud crab (Scylla paramamosain): Activation of oxidative stress and Nrf2 signaling pathway. Antioxidants 2022, 11, 978. [Google Scholar] [CrossRef]
- Abdel-Moneim, W.M.; Ghafeer, H.H. The potential protective effect of natural honey against cadmium-induced hepatotoxicity and nephrotoxicity. Mansoura J. Forensic Med. Clin. Toxicol. 2007, 15, 75–98. [Google Scholar] [CrossRef]
- Trzcinka-Ochocka, M.; Jakubowski, M.; Razniewska, G.; Halatek, T.; Gazewski, A. The effects of environmental cadmium exposure on kidney function: The possible influence of age. Environ. Res. 2004, 95, 143–150. [Google Scholar] [CrossRef]
- Dudley, R.E.; Gammal, L.M.; Klaassen, C.D. Cadmium-induced hepatic and renal injury in chronically exposed rats: Likely role of hepatic cadmium-metallothionein in nephrotoxicity. Toxicol. Appl. Pharmacol. 1985, 77, 414–426. [Google Scholar] [CrossRef]
- Renugadevi, J.; Prabu, S.M. Naringenin protects against cadmium-induced oxidative renal dysfunction in rats. Toxicology 2009, 256, 128–134. [Google Scholar] [CrossRef]
- Branca, J.J.V.; Pacini, A.; Gulisano, M.; Taddei, N.; Fiorillo, C.; Becatti, M. Cadmium-InducedCytotoxicity: Effects on MitochondrialElectron Transport Chain. Front. Cell Dev. Biol. 2020, 8, 604377. [Google Scholar] [CrossRef] [PubMed]
- Iwagami, Y. Changes in the ultrastructure of human cells related to certain biological responses under hyperthermic culture conditions. Human Cell 1996, 9, 353–366. [Google Scholar] [PubMed]
- Sabir, S.; Akash, M.S.H.; Fiayyaz, F.; Saleem, U.; Mehmood, M.H.; Rehman, K. Role of cadmium and arsenic as endocrine disruptors in the metabolism of carbohydrates: Inserting the association into perspectives. Biomed. Pharmacother. 2019, 114, 108802. [Google Scholar] [CrossRef] [PubMed]
- Das, S.C.; Al-Naemi, H.A. Cadmium toxicity: Oxidative stress, inflammation and tissue injury. Occup. Dis. Environ. Med. 2019, 7, 144–163. [Google Scholar] [CrossRef]
- Farag, M.R.; Elhady, W.M.; Ahmed, S.Y.A.; Taha, H.S.A.; Alagawany, M. Astragalus polysaccharides alleviate tilmicosin- induced toxicity in rats byinhibiting oxidative damage and modulating the expressions of HSP70, NF-kB and Nrf2/HO-1 pathway. Res. Vet. Sci. 2019, 124, 137–148. [Google Scholar] [CrossRef]
- Jeon, J.-Y.; Kim, K.-E.; Im, H.-J.; Oh, S.-T.; Lim, S.-U.; Kwon, H.-S.; Moon, B.-H.; Kim, J.-M.; An, B.-K.; Kang, C.-W. The production of lutein-enriched eggs with dietary Chlorella. Food Sci. Anim. Resour. 2012, 32, 13–17. [Google Scholar] [CrossRef]
- Buono, S.; Langellotti, A.L.; Martello, A.; Rinna, F.; Fogliano, V. Functional ingredients from microalgae. Food Funct. 2014, 5, 1669–1685. [Google Scholar] [CrossRef]
- Bauer, L.M.; Costa, J.A.V.; da Rosa, A.P.C.; Santos, L.O. Growth stimulation and synthesis of lipids, pigments and antioxidants with magnetic fields in Chlorella kessleri cultivations. Bioresour. Technol. 2017, 244, 1425–1432. [Google Scholar] [CrossRef]
- Bengwayan, P.T.; Laygo, J.C.; Pacio, A.E.; Poyaoan, J.L.Z.; Rebugio, J.F.; Yuson, A.L.L. A comparative study on the antioxidant property of Chlorella (Chlorella sp.) tablet and glutathione tablet. E-Int. Sci. Res. J. 2010, 2, 25–35. [Google Scholar]
- Plaza, M.; Herrero, M.; Cifuentes, A.; Ibanez, E. Innovative natural functional ingredients from microalgae. J. Agric. Food Chem. 2009, 57, 7159–7170. [Google Scholar] [CrossRef]
- Sheih, I.-C.; Fang, T.J.; Wu, T.-K. Isolation and characterisation of a novel angiotensin I-converting enzyme (ACE) inhibitory peptide from the algae protein waste. Food Chem. 2009, 115, 279–284. [Google Scholar] [CrossRef]
- Ko, S.-C.; Kim, D.; Jeon, Y.-J. Protective effect of a novel antioxidative peptide purified from a marine Chlorella ellipsoidea protein against free radical-induced oxidative stress. Food Chem. Toxicol. 2012, 50, 2294–2302. [Google Scholar] [CrossRef]
- Cherng, J.-Y.; Shih, M.-F. Preventing dyslipidemia by Chlorella pyrenoidosa in rats and hamsters after chronic high fat diet treatment. Life Sci. 2005, 76, 3001–3013. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, X. Separation, antitumor activities, and encapsulation of polypeptide from Chlorella pyrenoidosa. Biotechnol. Prog. 2013, 29, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Cherng, J.-Y.; Shih, M.-F. Improving glycogenesis in Streptozocin (STZ) diabetic mice after administration of green algae Chlorella. Life Sci. 2006, 78, 1181–1186. [Google Scholar] [CrossRef]
- Peng, H.-Y.; Chu, Y.-C.; Chen, S.-J.; Chou, S.-T. Hepatoprotection of chlorella against carbon tetrachloride-induced oxidative damage in rats. In Vivo 2009, 23, 747–754. [Google Scholar] [PubMed]
- Guzman, S.; Gato, A.; Calleja, J. Antiinflammatory, analgesic and free radical scavenging activities of the marine microalgae Chlorella stigmatophora and Phaeodactylum tricornutum. Phytother. Res. 2001, 15, 224–230. [Google Scholar] [CrossRef]
- Teresa, V.-O.M.; Octavio, G.-F.; Nayelly, J.-S.; Mayumi, R.-V.; Alan, M.-S.; Yvonne, T.-J.; Edgar, M.-R.; Santos, B.-S.; Angel, G.-C.C. The administration of Chlorella vulgaris protects against nefrotoxicity caused by CCl4. RETEL Revista de Toxicologia 2011, 35, 1–13. [Google Scholar]
- Huang, Z.; Li, L.; Huang, G.; Yan, Q.; Shi, B.; Xu, X. Growth-inhibitory and metal-binding proteins in Chlorella vulgaris exposed to cadmium or zinc. Aquat. Toxicol. 2009, 91, 54–61. [Google Scholar] [CrossRef]
- Wang, L.; Liu, J.; Filipiak, M.; Mungunkhuyag, K.; Jedynak, P.; Burczyk, J.; Fu, P.; Malec, P. Fast and efficient cadmium biosorption by Chlorella vulgaris K-01 strain: The role of cell walls in metal sequestration. Algal Res. 2021, 60, 102497. [Google Scholar] [CrossRef]
- Yadav, M.; Kumar, V.; Sandal, N.; Chauhan, M.K. Quantitative evaluation of Chlorella vulgaris for removal of toxic metals from body. J. Appl. Phycol. 2022, 34, 2743–2754. [Google Scholar] [CrossRef]
- Dacie, J.V.; Lewis, S.M. Practical Haematology; Churchill Livingstone: London, UK; New York, NY, USA, 1984. [Google Scholar]
- Armstrong, W.D.; Carr, C.W. Physiological Chemistry Laboratory Direction, 3rd ed.; Burses Publishing Co.: Minneopollis, MN, USA, 1964. [Google Scholar]
- Wise, W.A. Determination of Serum Albumen; King: London, UK, 1965; p. 273. [Google Scholar]
- Deeg, R.; Ziegenohrm, J. Kinetic enzymatic method forautomated determination of total cholesterol in serum. J. Clin. Chem. 1983, 29, 1798–1802. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation ofthe concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Fossati, P.; Prencipe, L. Serum triglycerides determined colourimetrically with an enzyme that produces hydrogen peroxide. Clin. Chem. 1982, 28, 2077–2080. [Google Scholar] [CrossRef] [PubMed]
- Belfield, A.; Goldberg, D.M. Revised assay for serum phenylphosphatase activity using 4-amino-antipyrine. Enzyme 1971, 12, 561–573. [Google Scholar] [CrossRef]
- Reitman, S.; Frankel, S. A colorimetric method for thedetermination of serum glutamic oxaloacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957, 28, 56–63. [Google Scholar] [CrossRef]
- Bianchi, A.T.; Moonen-Leusen, H.W.; Van der Heijden, P.J.; Bokhout, B.A. The use of a double antibody sandwich ELISA and monoclonal antibodies for the assessment of porcine IgM, IgG and IgA concentrations. Vet. Immunol. Immunopathol. 1995, 44, 309–317. [Google Scholar] [CrossRef]
- Vidal-Vanaclocha, F.; Fantuzzi, G.; Mendoza, L.; Fuentes, A.M.; Anasagasti, M.J.; Martin, J.; Carrascal, T.; Walsh, P.; Reznikov, L.L.; Kim, S.-H.; et al. IL-18 regulates IL-1-beta-dependent hepatic melanoma metastasis via vascular cell adhesion molecule-1. Proc. Nat. Acad. Sci. USA 2000, 97, 734–739. [Google Scholar] [CrossRef]
- Koracevic, D.; Koracevic, G.; Djordjevic, V.; Andrejevic, S.; Cosic, V. Method for the measurement of antioxidant activity in human fluids. J. Clin. Pathol. 2001, 54, 356–361. [Google Scholar] [CrossRef]
- Preuss, H.G.; Jarrell, S.T.; Scheckenbach, R.; Lieberman, S.; Anderson, R.A. Comparative effects of chromium, vanadium andgymnema sylvestre on sugar-induced blood pressure elevations in SHR. J. Am. Coll. Nutr. 1998, 17, 116–123. [Google Scholar] [CrossRef]
- Khalil, S.R.; Awad, A.; Mohammed, H.H.; Nassan, M.A. Imidacloprid insecticide exposure induces stress and disrupts glucose homeostasis inmale rats. Environ. Toxicol. Pharmacol. 2017, 55, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Pakzad, M.; Fouladdel, S.; Nili-Ahmadabadi, A.; Pourkhalili, N.; Baeeri, M.; Azizi, E.; Sabzevari, O.; Ostad, S.N.; Abdollahi, M. Sublethal exposures of diazinon alters glucose homostasis in Wistar rats: Biochemical and molecular evidences of oxidative stress in adipose tissues. Pestic. Biochem. Physiol. 2013, 105, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, X.; Cheng, Y.; Abdelnasir, A.; Tang, S.; Kemper, N.; Hartung, J.; Bao, E. Association of heat shock protein 70 expression with rat myocardial cell damage during heat stress in vitro and in vivo. Genet. Mol. Res. 2015, 14, 1994–2005. [Google Scholar] [CrossRef] [PubMed]
- Sang, L.; Wang, X.-M.; Xu, D.-Y.; Sang, L.-X.; Han, Y.; Jiang, L.-Y. Morin enhances hepatic Nrf2 expression in a liver fibrosis rat model. World J. Gastroenterol. 2017, 23, 8334. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Shen, H.-H.; Huang, S.-Y.; Cheng, P.-Y.; Chu, Y.-J.; Chen, S.-Y.; Lam, K.-K.; Lee, Y.-M. Involvement of HSP70 and HO-1 in the protective effects of raloxifene on multiple organ dysfunction syndrome by endotoxemia in ovariectomized rats. Menopause 2017, 24, 959–969. [Google Scholar] [CrossRef]
- Luu Quoc, Q.; Cao Thi Bich, T.; Kim, S.H.; Park, H.S.; Shin, Y.S. Administration of vitamin E attenuates airway inflammation through restoration of Nrf2 in a mouse model of asthma. J. Cell. Mol. Med. 2021, 25, 6721–6732. [Google Scholar] [CrossRef]
- Saygi, A.; Deniz, G.; Kutsal, O.; Vural, N. Chronic effects of cadmium on kidney, liver, testis, and fertility of male rats. Biol. Trace Elem. Res. 1991, 31, 209–214. [Google Scholar] [CrossRef]
- Gonick, H. Nephrotoxicity of cadmium & lead. Indian J. Med. Res. 2008, 128, 335–352. [Google Scholar]
- Branca, J.J.V.; Morucci, G.; Pacini, A. Cadmium-induced neurotoxicity: Still much ado. Neural Regen. Res. 2018, 13, 1879. [Google Scholar]
- Huff, J.; Lunn, R.M.; Waalkes, M.P.; Tomatis, L.; Infante, P.F. Cadmium-induced cancers in animals and in humans. Int. J. Occup. Environ. Health 2007, 13, 202–212. [Google Scholar] [CrossRef]
- Hassoun, E.A.; Stohs, S.J. Cadmium-induced production of superoxide anion and nitric oxide, DNA single strand breaks and lactate dehydrogenase leakage in J774A. 1 cell cultures. Toxicology 1996, 112, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Hart, B.; Lee, C.; Shukla, G.; Shukla, A.; Osier, M.; Eneman, J.; Chiu, J.-F. Characterization of cadmium-induced apoptosis in rat lung epithelial cells: Evidence for the participation of oxidant stress. Toxicology 1999, 133, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Patra, R.; Rautray, A.K.; Swarup, D. Oxidative stress in lead and cadmium toxicity and its amelioration. Vet. Med. Int. 2011, 2011, 457327. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.A.; Zafar, F. Haematological study in response to varying doses of estrogen in broiler chicken. Int. J. Poult. Sci. 2005, 4, 748–751. [Google Scholar]
- Andjelkovic, M.; Buha Djordjevic, A.; Antonijevic, E.; Antonijevic, B.; Stanic, M.; Kotur-Stevuljevic, J.; Spasojevic-Kalimanovska, V.; Jovanovic, M.; Boricic, N.; Wallace, D. Toxic effect of acute cadmium and lead exposure in rat blood, liver, and kidney. Int. J. Environ. Res. Public Health 2019, 16, 274. [Google Scholar] [CrossRef]
- Dökmeci, A.H.; Karaboğa, İ.; Güzel, S.; Erboğa, Z.F.; Yılmaz, A. Toxicological assessment of low-dose bisphenol A, lead and endosulfan combination: Chronic toxicity study in male rats. Environ. Sci. Pollut. Res. 2021, 29, 10558–10574. [Google Scholar] [CrossRef]
- Farag, M.R.; Alagawany, M.; Abd El-Hack, M.E.; El-Sayed, S.A.; Ahmed, S.Y.; Samak, D.H. Yucca schidigera extract modulates the lead-induced oxidative damage, nephropathy and altered inflammatory response and glucose homeostasis in Japanese quails. Ecotoxicol. Environ. Saf. 2018, 156, 311–321. [Google Scholar] [CrossRef]
- Pawlikowska-Pawlęga, B.; Gruszecki, W.I.; Misiak, L.; Paduch, R.; Piersiak, T.; Zarzyka, B.; Pawelec, J.; Gawron, A. Modification of membranes by quercetin, a naturally occurring flavonoid, via its incorporation in the polar head group. Biochim. Biophys. Acta (BBA)-Biomembr. 2007, 1768, 2195–2204. [Google Scholar] [CrossRef]
- Safi, C.; Zebib, B.; Merah, O.; Pontalier, P.-Y.; Vaca-Garcia, C. Morphology, composition, production, processing and applications of Chlorella vulgaris: A review. Renew. Sustain. Energy Rev. 2014, 35, 265–278. [Google Scholar] [CrossRef]
- Mathew, B.; Sankaranarayanan, R.; Nair, P.P.; Varghese, C.; Somanathan, T.; Amma, B.P.; Amma, N.S.; Nair, M.K. Evaluation of chemoprevention of oral cancer with Spirulina fusiformis. Nutr. Cancer 1995, 24, 197–202. [Google Scholar] [CrossRef]
- Rana, S.; Singh, R.; Verma, S. Protective effects of few antioxidants on liver function in rats treated with cadmium and mercury. Indian. J. Exp. Biol. 1996, 34, 177–179. [Google Scholar] [PubMed]
- Patil, G. Role of ascorbic acid on mercuric chloride toxicity in vital organs of mice. Indian J. Environ. Toxicol. 1999, 9, 53–55. [Google Scholar]
- Xu, W.; Gao, Z.; Qi, Z.; Qiu, M.; Peng, J.-q.; Shao, R. Effect of dietary Chlorella on the growth performance and physiological parameters of gibel carp, Carassius auratus gibelio. Turk. J. Fish. Aquat. Sci. 2014, 14, 53–57. [Google Scholar]
- Kang, H.; Salim, H.; Akter, N.; Kim, D.; Kim, J.; Bang, H.; Kim, M.; Na, J.; Hwangbo, J.; Choi, H. Effect of various forms of dietary Chlorella supplementation on growth performance, immune characteristics, and intestinal microflora population of broiler chickens. J. Appl. Poult. Res. 2013, 22, 100–108. [Google Scholar] [CrossRef]
- Yap, C.Y.; Aw, T.C. Liver function tests (LFTs). Proc. Singap. Healthc. 2010, 19, 80–82. [Google Scholar] [CrossRef]
- Philip, G.; Reddy, P.; Sridevi, G. Cypermethrin-induced in vivo alterations in the carbohydrate metabolism of freshwater fish, Labeo rohita. Ecotoxicol. Environ. Saf. 1995, 31, 173–178. [Google Scholar] [CrossRef]
- Shimada, Y.; Wiget, P.; Gulli, M.P.; Bi, E.; Peter, M. The nucleotide exchange factor Cdc24p may be regulated by auto-inhibition. EMBO J. 2004, 23, 1051–1062. [Google Scholar] [CrossRef]
- Renugadevi, J.; Prabu, S.M. Cadmium-induced hepatotoxicity in rats and the protective effect of naringenin. Exp. Toxicol. Pathol. 2010, 62, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.R.; Alagawany, M. Erythrocytes as a biological model for screening of xenobiotics toxicity. Chem.-Biol. Interact. 2018, 279, 73–83. [Google Scholar] [CrossRef]
- Alagawany, M.; Abd El-Hack, M.; Farag, M.; Elnesr, S.; El-Kholy, M.; Saadeldin, I.; Swelum, A. Dietary supplementation of Yucca schidigera extract enhances productive and reproductive performances, blood profile, immune function, and antioxidant status in laying Japanese quails exposed to lead in the diet. Poult. Sci. 2018, 97, 3126–3137. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, Y.; Chai, L.; Wang, H. Histological changes, lipid metabolism and oxidative stress in the liver of Bufo gargarizans exposed to cadmium concentrations. Chemosphere 2017, 179, 337–346. [Google Scholar] [CrossRef]
- Theocharis, S.; Margeli, A.; Fasitsas, C.; Loizidou, M.; Deliconstantinos, G. Acute exposure to cadmium causes time-dependent liver injury in rats. Comp. Biochem. Physiol. Part C Comp. Pharmacol. 1991, 99, 127–130. [Google Scholar] [CrossRef]
- Sudo, J.-i.; Hayashi, T.; Kimura, S.-i.; Kakuno, K.; Terui, J.; Takashima, K.; Soyama, M. Mechanism of nephrotoxicity induced by repeated administration of cadmium chloride in rats. J. Toxicol. Environ. Health 1996, 48, 333–348. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, S.; Maringwa, J.; Faes, C.; Lambrichts, I.; Van Kerkhove, E. Chronic exposure of mice to environmentally relevant, low doses of cadmium leads to early renal damage, not predicted by blood or urine cadmium levels. Toxicology 2007, 229, 145–156. [Google Scholar] [CrossRef]
- Choi, J.-H.; Rhee, S.-J. Effects of vitamin E on renal dysfunction in chronic cadmium-poisoned rats. J. Med. Food 2003, 6, 209–215. [Google Scholar] [CrossRef]
- Damek-Poprawa, M.; Sawicka-Kapusta, K. Histopathological changes in the liver, kidneys, and testes of bank voles environmentally exposed to heavy metal emissions from the steelworks and zinc smelter in Poland. Environ. Res. 2004, 96, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Jemai, H.; Lachkar, H.A.; Messaoudi, I.; Kerkeni, A. Effects of zinc pre-treatment on blood glutathione, serum zinc and kidney histological organisation in male rats exposed to cadmium. J. Trace Elem. Med. Biol. 2010, 24, 277–282. [Google Scholar] [CrossRef]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr. Physiol. 2016, 6, 603. [Google Scholar] [PubMed]
- Queiroz, J.S.; Blasco, I.M.; Gagliano, H.; Daviu, N.; Román, A.G.; Belda, X.; Carrasco, J.; Rocha, M.C.; Neto, J.P.; Armario, A. Chlorella vulgaris reduces the impact of stress on hypothalamic–pituitary–adrenal axis and brain c-fos expression. Psychoneuroendocrinology 2016, 65, 1–8. [Google Scholar] [CrossRef]
- Sahin, K.; Orhan, C.; Akdemir, F.; Tuzcu, M.; Iben, C.; Sahin, N. Resveratrol protects quail hepatocytes against heat stress: Modulation of the Nrf2 transcription factor and heat shock proteins. J. Anim. Physiol. Anim. Nutr. 2012, 96, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Coronado-Reyes, J.A.; Acosta-Ramírez, E.; Martínez-Olguín, M.V.; González-Hernández, J.C. Antioxidant Activity and Kinetic Characterization of Chlorella vulgaris Growth under Flask-Level Photoheterotrophic Growth Conditions. Molecules 2022, 27, 6346. [Google Scholar] [CrossRef] [PubMed]
- Dodson, M.; Redmann, M.; Rajasekaran, N.S.; Darley-Usmar, V.; Zhang, J. KEAP1–NRF2 signalling and autophagy in protection against oxidative and reductive proteotoxicity. Biochem. J. 2015, 469, 347–355. [Google Scholar] [CrossRef]
- Zhao, P.; Piao, X.; Pan, L.; Zeng, Z.; Li, Q.; Xu, X.; Wang, H. Forsythia suspensa extract attenuates lipopolysaccharide-induced inflammatory liver injury in rats via promoting antioxidant defense mechanisms. Anim. Sci. J. 2017, 88, 873–881. [Google Scholar] [CrossRef]
- Na, H.-K.; Surh, Y.-J. Oncogenic potential of Nrf2 and its principal target protein heme oxygenase-1. Free. Radic. Biol. Med. 2014, 67, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Elsherbiny, N.M.; Eladl, M.A.; Al-Gayyar, M.M. Renal protective effects of arjunolic acid in a cisplatin-induced nephrotoxicity model. Cytokine 2016, 77, 26–34. [Google Scholar] [CrossRef]
- Oku, H.; Nakazato, H.; Horikawa, T.; Tsuruta, Y.; Suzuki, R. Pirfenidone suppresses tumor necrosis factor-α, enhances interleukin-10 and protects mice from endotoxic shock. Eur. J. Pharmacol. 2002, 446, 167–176. [Google Scholar] [CrossRef]
- Hsu, C.-M.; Wang, J.-S.; Liu, C.-H.; Chen, L.-W. Kupffer cells protect liver from ischemia-reperfusion injury by an inducible nitric oxide synthase-dependent mechanism. Shock 2002, 17, 280–285. [Google Scholar] [CrossRef]
- Hon, W.M.; Lee, K.H.; Khoo, H.E. Nitric oxide in liver diseases: Friend, foe, or just passerby? Ann. N. Y. Acad. Sci. 2002, 962, 275–295. [Google Scholar] [CrossRef]
- Tamura, Y.; Torigoe, T.; Kukita, K.; Saito, K.; Okuya, K.; Kutomi, G.; Hirata, K.; Sato, N. Heat-shock proteins as endogenous ligands building a bridge between innate and adaptive immunity. Immunotherapy 2012, 4, 841–852. [Google Scholar] [CrossRef]
- Ohashi, K.; Burkart, V.; Flohé, S.; Kolb, H. Cutting edge: Heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J. Immunol. 2000, 164, 558–561. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Khater, M.R. Evaluation of the protective potential of Ambrosia maritima extract on acetaminophen-induced liver damage. J. Ethnopharmacol. 2001, 75, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Garcia, I.; Guil-Guerrero, J.L. Evaluation of the antioxidant activity of three microalgal species for use as dietary supplements and in the preservation of foods. Food Chem. 2008, 108, 1023–1026. [Google Scholar] [CrossRef]
- Cheng, D.; Wan, Z.; Zhang, X.; Li, J.; Li, H.; Wang, C. Dietary Chlorella vulgaris ameliorates altered immunomodulatory functions in cyclophosphamide-induced immunosuppressive mice. Nutrients 2017, 9, 708. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Fanning, K.; Netzel, M.; Turner, W.; Li, Y.; Schenk, P.M. Profiling of carotenoids and antioxidant capacity of microalgae from subtropical coastal and brackish waters. Food Chem. 2014, 165, 300–306. [Google Scholar] [CrossRef]
- Machu, L.; Misurcova, L.; Vavra Ambrozova, J.; Orsavova, J.; Mlcek, J.; Sochor, J.; Jurikova, T. Phenolic content and antioxidant capacity in algal food products. Molecules 2015, 20, 1118–1133. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.; Barros, L.; Ferreira, I.C. In vivo antioxidant activity of phenolic compounds: Facts and gaps. Trends Food Sci. Technol. 2016, 48, 1–12. [Google Scholar] [CrossRef]
- Renugadevi, K.; Nachiyar, C.V.; Sowmiya, P.; Sunkar, S. Antioxidant activity of phycocyanin pigment extracted from marine filamentous cyanobacteria Geitlerinema sp TRV57. Biocatal. Agric. Biotechnol. 2018, 16, 237–242. [Google Scholar] [CrossRef]
- El Latif, A.A.; Assar, D.H.; Elkaw, E.M.; Hamza, H.A.; Alkhalifah, D.H.; Hozzein, W.N.; Hamouda, R.A. Protective role of Chlorella vulgaris with Thiamine against Paracetamol induced toxic effects on haematological, biochemical, oxidative stress parameters and histopathological changesin Wistar rats. Sci. Rep. 2021, 11, 3911. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).