Automatic Detection and Classification of Epileptic Seizures from EEG Data: Finding Optimal Acquisition Settings and Testing Interpretable Machine Learning Approach
Abstract
1. Introduction
- 1.
- Select the optimal system architecture for seizure detection and classification, train the corresponding models, and test their performance on an open-source dataset;
- 2.
- Compare the accuracy of ML-based seizure detection and classification according to the EEG sampling frequency;
- 3.
- Determine if the accuracy of seizure detection and classification depends on the number of EEG electrodes;
- 4.
- Apply the activation maximization technique to interpretable ML for seizure detection and classification;
- 5.
- Differentiate among seizure types according to the location of electrophysiologic activity in the brain through the scalp.
2. Materials and Methods
2.1. Methodology
2.2. Dataset Description
2.3. Data Preprocessing
2.4. CNN Model Architecture
2.4.1. Binary Classification
2.4.2. Multigroup Seizure Classification
2.5. Source Reconstruction Technique
3. Results
3.1. Model Performance
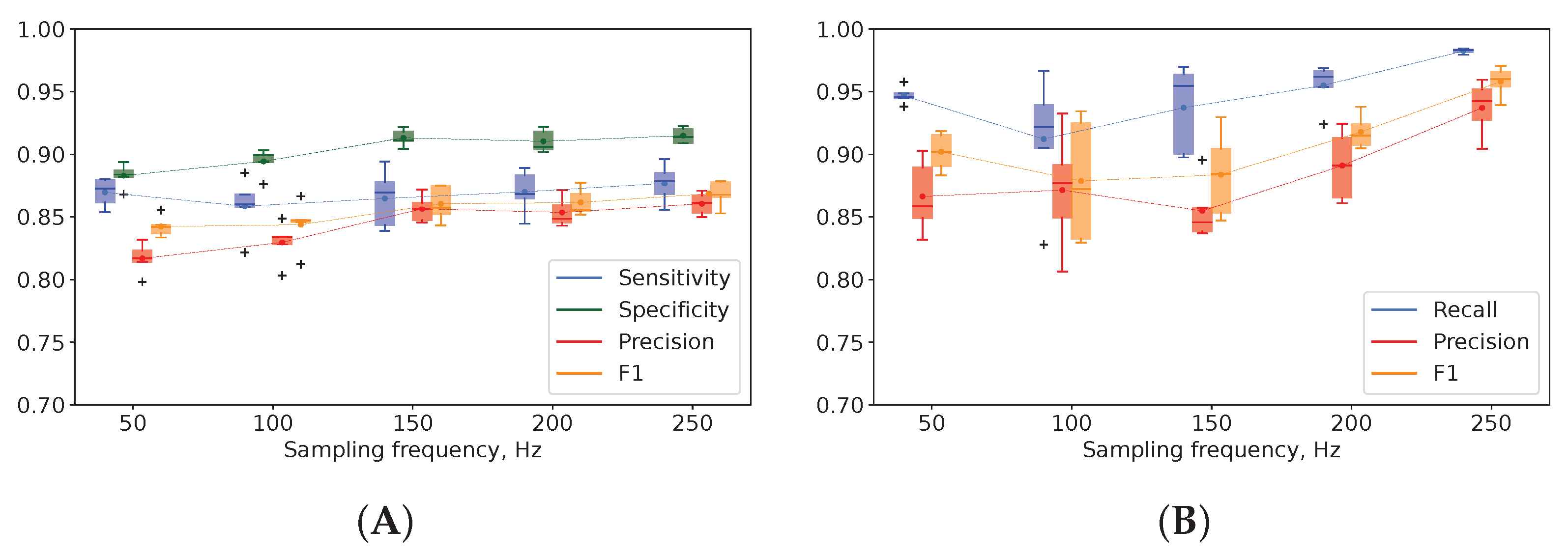
3.2. EEG Sampling Frequency in Seizure Detection and Classification
3.3. Number of EEG Electrodes in Automatic Detection and Classification of Epileptic Episodes
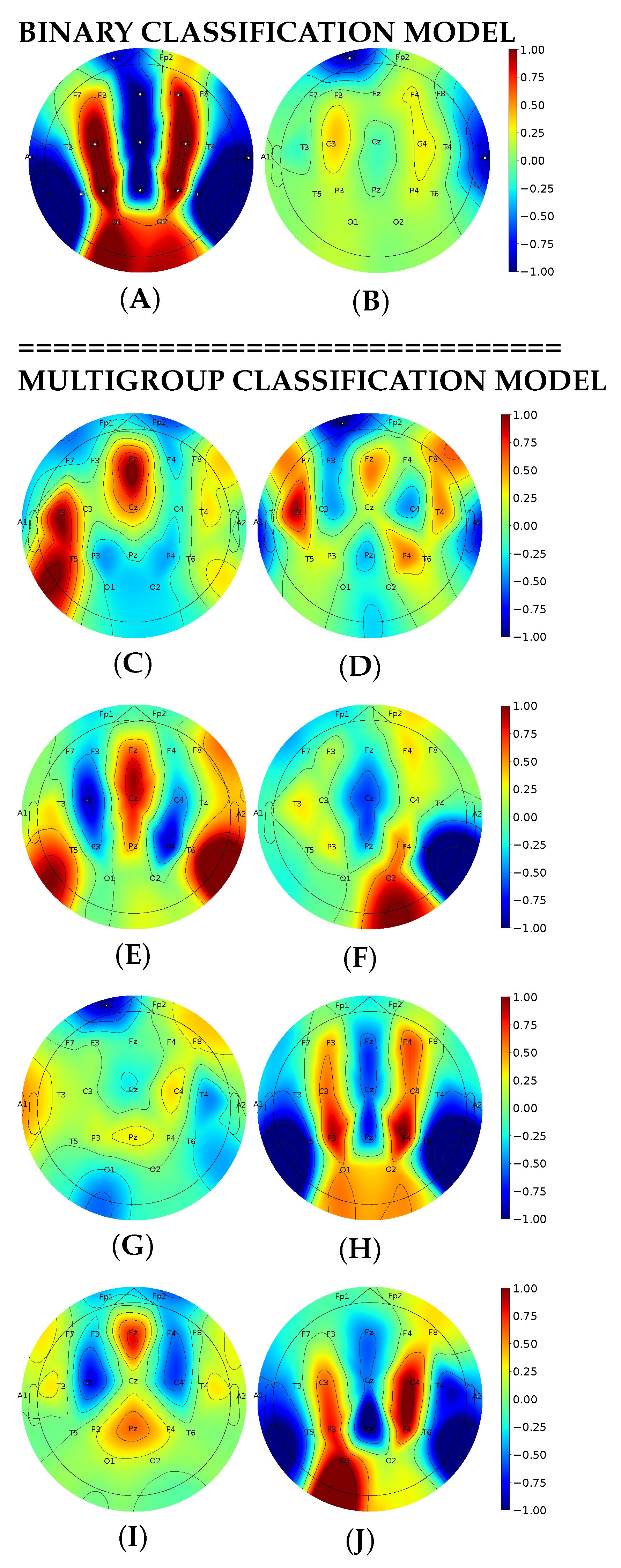
3.4. Interpretable Machine Learning for Seizure Detection and Classification with Activation Maximization
3.5. Brain Electrophysiologic Activity in Seizure Differentiation with Source Reconstruction
4. Discussion
4.1. Performance of Models for EEG-Based Seizure Detection and Classification
4.2. EEG Sampling Frequency in Seizure Detection and Classification
4.3. Number of EEG Electrodes in Automatic Detection and Classification of Epileptic Episodes
4.4. Activation Maximization for Seizure Detection and Classification
4.5. Brain Electrophysiologic Activity in Seizure Differentiation with Source Reconstruction Technique
4.6. Prospects of Interpretable Machine Learning for Medicine
5. Strengths and Limitations
6. Conclusions
- In the available literature, we looked for models that optimally fit the following criteria: reliable performance, ability to generalize on a broad set of EEG recordings, quick processing of the input data, high explainability and interpretability. We selected the top-performing models to construct a system for automatic seizure detection and classification.The system accurately detects seizure episodes (87.7% Sn, 91.16% Sp) and carefully distinguishes eight seizure types (95–100% Acc).
- An increase in EEG sampling rate from 50 to 250 Hz boosted model performance: the precision of seizure detection rose by 5% and seizure differentiation by 7%. A low sampling rate is a reasonable solution for training reliable models with EEG data.
- Decreasing the number of EEG electrodes from 21 to 8 did not affect seizure detection but significantly worsened seizure differentiation. Different electrodes were equally informative in detecting epileptic episodes but not as effective in differentiating among seizure types. However, the optimal number and location of sensors depends on individual clinical signs.
- We improved model explainability with interpretable ML: topoplots displaying neuron maximization showed evident differences between seizure and non-seizure classes. Activation maximization highlighted the presence of EEG patterns specific to eight seizure types. With interpretable ML, we justified that our system recognizes biologically meaningful features as indicators of epileptic activity in EEG.
- The source reconstruction technique further improved the model explainability by supplying us with the cortical projection of epileptic sources in the brain. The received cortical projections depicted EEG source differences between the onset activity in generalized and focal seizures. New studies are required to test whether seizure onset analysis will improve classification into types.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABSZ | absence seizure |
| Acc | accuracy |
| AM | activation maximization |
| ATSZ | atonic seizure |
| CNN | convolutional neural network |
| CNSZ | clonic seizure |
| CPSZ | complex partial seizure |
| DL | deep learning |
| EEG | electroencephalography |
| FNSZ | focal non-specific seizure |
| GNSZ | generalized non-specific seizure |
| LSTM | long short-term memory |
| MI | mutual information |
| ML | machine learning |
| MRI | magnetic resonance imaging |
| MYSZ | myoclonic seizure |
| NESZ | non-epileptic seizure |
| RNN | recurrent neural network |
| Sn | sensitivity |
| Sp | specificity |
| SPSZ | simple partial seizure |
| SR | sampling rate |
| STFT | short time Fourier transform |
| TCSZ | tonic-clonic seizure |
| TNSZ | tonic seizure |
| TUSZ | Temple University Hospital EEG Seizure Corpus |
References
- World Health Organization. Epilepsy: A Public Health Imperative: Summary; World Health Organization: Geneva, Switzerland, 2019; 12p.
- World Health Organization. Atlas: Country Resources for Neurological Disorders; World Health Organization: Geneva, Switzerland, 2017; 71p.
- Benbadis, S.R.; Tatum, W.O. Overintepretation of EEGs and misdiagnosis of epilepsy. J. Clin. Neurophysiol. 2003, 20, 42–44. [Google Scholar] [CrossRef]
- Benbadis, S.R.; Lin, K. Errors in EEG interpretation and misdiagnosis of epilepsy. Eur. Neurol. 2008, 59, 267–271. [Google Scholar] [CrossRef]
- Michel, C.M.; Brunet, D. EEG Source Imaging: A Practical Review of the Analysis Steps. Front. Neurol. 2019, 10, 325. [Google Scholar] [CrossRef]
- Kotte, S.; Dabbakuti, J.K. Methods for removal of artifacts from EEG signal: A review. J. Physics Conf. Ser. 2020, 1706, 012093. [Google Scholar] [CrossRef]
- Baud, M.O.; Schindler, K.; Rao, V.R. Under-sampling in epilepsy: Limitations of conventional EEG. Clin. Neurophysiol. Pract. 2021, 6, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Gao, J.; Zhang, Z.; Huang, Q.; Wu, Y.; Xu, B. Distinguishing epileptiform discharges from normal electroencephalograms using adaptive fractal and network analysis: A clinical perspective. Front. Physiol. 2020, 11, 828. [Google Scholar] [CrossRef] [PubMed]
- Roy, Y.; Banville, H.; Albuquerque, I.; Gramfort, A.; Falk, T.H.; Faubert, J. Deep learning-based electroencephalography analysis: A systematic review. J. Neural Eng. 2019, 16, 051001. [Google Scholar] [CrossRef]
- The Lancet Respiratory Medicine. Opening the black box of machine learning, 2018. Lancet Resp. Med. 2018, 6, 801. [Google Scholar] [CrossRef]
- Jemal, I.; Mezghani, N.; Abou-Abbas, L.; Mitiche, A. An interpretable deep learning classifier for epileptic seizure prediction using EEG data. IEEE Access 2022, 10, 60141–60150. [Google Scholar] [CrossRef]
- Lawhern, V.J.; Solon, A.J.; Waytowich, N.R.; Gordon, S.M.; Hung, C.P.; Lance, B.J. EEGNet: A compact convolutional neural network for EEG-based brain–computer interfaces. J. Neural Eng. 2018, 15, 056013. [Google Scholar] [CrossRef]
- Selvaraju, R.R.; Cogswell, M.; Das, A.; Vedantam, R.; Parikh, D.; Batra, D. Grad-cam: Visual explanations from deep networks via gradient-based localization. In Proceedings of the IEEE International Conference on Computer Vision, Venice, Italy, 22–29 October 2017; pp. 618–626. [Google Scholar]
- Uyttenhove, T.; Maes, A.; Van Steenkiste, T.; Deschrijver, D.; Dhaene, T. Interpretable epilepsy detection in routine, interictal eeg data using deep learning. In Proceedings of the Machine Learning for Health. PMLR, Virtual Conference, 11 December 2020; pp. 355–366. [Google Scholar]
- Cui, J.; Lan, Z.; Sourina, O.; Müller-Wittig, W. EEG-Based Cross-Subject Driver Drowsiness Recognition with an Interpretable Convolutional Neural Network. IEEE Trans. Neural Netw. Learn. Syst. 2022. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.; Golmohammadi, M.; Ziyabari, S.; Von Weltin, E.; Obeid, I.; Picone, J. Optimizing channel selection for seizure detection. In Proceedings of the 2017 IEEE Signal Processing in Medicine and Biology Symposium (SPMB), Philadelphia, PA, USA, 2 December 2017; pp. 1–5. [Google Scholar]
- Erhan, D.; Bengio, Y.; Courville, A.; Vincent, P. Visualizing Higher-Layer Features of a Deep Network; Technical Report; Univeristé de Montréal: Montréal, QC, Canada, 2009. [Google Scholar]
- Bilal, A.; Jourabloo, A.; Ye, M.; Liu, X.; Ren, L. Do Convolutional Neural Networks Learn Class Hierarchy? IEEE Trans. Vis. Comput. Graph. 2018, 24, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.H.; Usman, S.M.; Khalid, S.; Anwar, A.; Alroobaea, R.; Hussain, S.; Almotiri, J.; Ullah, S.S.; Yasin, A. Classification of EEG signals for prediction of epileptic seizures. Appl. Sci. 2022, 12, 7251. [Google Scholar] [CrossRef]
- Liu, T.; Truong, N.D.; Nikpour, A.; Zhou, L.; Kavehei, O. Epileptic Seizure Classification with Symmetric and Hybrid Bilinear Models. IEEE J. Biomed. Health Inform. 2020, 24, 2844–2851. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Cole, A.; Pechmann, D.; Wakeman, D.; Hämäläinen, M.; Liu, H.; Madsen, J.; Blaise, D.; Stufflebeam, S. Dynamic statistical parametric mapping for analyzing ictal magnetoencephalographic spikes in patients with intractable frontal lobe epilepsy. Epilepsy Res. 2009, 85, 279–286. [Google Scholar] [CrossRef]
- Jmail, N.; Hadriche, A.; Ichrak, B.; Necibi, A.; Ben Amar, C. A comparison of inverse problem methods for source localization of epileptic MEG spikes. In Proceedings of the 2019 IEEE 19th International Conference on Bioinformatics and Bioengineering (BIBE), Athens, Greece, 28–30 October 2019. [Google Scholar] [CrossRef]
- Gramfort, A.; Luessi, M.; Larson, E.; Engemann, D.A.; Strohmeier, D.; Brodbeck, C.; Goj, R.; Jas, M.; Brooks, T.; Parkkonen, L.; et al. MEG and EEG Data Analysis with MNE-Python. Front. Neurosci. 2013, 7, 267. [Google Scholar] [CrossRef]
- Selvakumari, R.S.; Mahalakshmi, M.; Prashalee, P. Patient-specific seizure detection method using hybrid classifier with optimized electrodes. J. Med. Syst. 2019, 43, 121. [Google Scholar] [CrossRef]
- Li, M.; Chen, W.; Zhang, T. Automatic epileptic EEG detection using DT-CWT-based non-linear features. Biomed. Signal Process. Control 2017, 34, 114–125. [Google Scholar] [CrossRef]
- Acharya, U.R.; Molinari, F.; Subbhuraam, V.S.; Chattopadhyay, S.; Kh, N.; Suri, J. Automated diagnosis of epileptic EEG using entropies. Biomed. Signal Process. Control 2012, 7, 401–408. [Google Scholar] [CrossRef]
- Wang, D.; Ren, D.; Li, K.; Feng, Y.; Ma, D.; Yan, X.; Wang, G. Epileptic seizure detection in long-term EEG recordings by using wavelet-based directed transfer function. IEEE Trans. Biomed. Eng. 2018, 65, 2591–2599. [Google Scholar] [CrossRef]
- Mutlu, A.Y. Detection of epileptic dysfunctions in EEG signals using Hilbert vibration decomposition. Biomed. Signal Process. Control 2018, 40, 33–40. [Google Scholar] [CrossRef]
- Sargolzaei, S.; Cabrerizo, M.; Goryawala, M.; Eddin, A.S.; Adjouadi, M. Scalp EEG brain functional connectivity networks in pediatric epilepsy. Comput. Biol. Med. 2015, 56, 158–166. [Google Scholar] [CrossRef]
- Sood, M.; Bhooshan, S.V. Prognosis of epileptic seizures using EEG signals. In Proceedings of the 2015 Third International Conference on Image Information Processing (ICIIP), Waknaghat, India, 21–24 December 2015; pp. 12–16. [Google Scholar]
- Chen, S.; Zhang, X.; Chen, L.; Yang, Z. Automatic diagnosis of epileptic seizure in electroencephalography signals using nonlinear dynamics features. IEEE Access 2019, 7, 61046–61056. [Google Scholar] [CrossRef]
- Jacobs, D.; Hilton, T.; Del Campo, M.; Carlen, P.L.; Bardakjian, B.L. Classification of pre-clinical seizure states using scalp EEG cross-frequency coupling features. IEEE Trans. Biomed. Eng. 2018, 65, 2440–2449. [Google Scholar] [CrossRef] [PubMed]
- Zabihi, M.; Kiranyaz, S.; Rad, A.B.; Katsaggelos, A.K.; Gabbouj, M.; Ince, T. Analysis of high-dimensional phase space via Poincaré section for patient-specific seizure detection. IEEE Trans. Neural Syst. Rehabil. Eng. 2015, 24, 386–398. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Amin, S.; Alsulaiman, M.; Muhammad, G. Applying Deep Learning for Epilepsy Seizure Detection and Brain Mapping Visualization. ACM Trans. Multimed. Comput. Commun. Appl. 2019, 15, 1–17. [Google Scholar] [CrossRef]
- Thara, D.K.; PremaSudha, B.G.; Xiong, F. Auto-detection of epileptic seizure events using deep neural network with different feature scaling techniques. Pattern Recognit. Lett. 2019, 128, 544–550. [Google Scholar] [CrossRef]
- Akyol, K. Stacking ensemble based deep neural networks modeling for effective epileptic seizure detection. Expert Syst. Appl. 2020, 148, 113239. [Google Scholar] [CrossRef]
- Thuwajit, P.; Rangpong, P.; Sawangjai, P.; Autthasan, P.; Chaisaen, R.; Banluesombatk, N. EEGWaveNet: Multiscale CNN-Based Spatiotemporal Feature Extraction for EEG Seizure Detection. IEEE Trans. Ind. Inform. 2021, 18, 5547–5557. [Google Scholar] [CrossRef]
- Hussein, R.; Palangi, H.; Ward, R.K.; Wang, Z.J. Optimized deep neural network architecture for robust detection of epileptic seizures using EEG signals. Clin. Neurophysiol. 2019, 130, 25–37. [Google Scholar] [CrossRef]
- Ullah, I.; Hussain, M.; Qazi, E.-u.-H.; Aboalsamh, H. An automated system for epilepsy detection using EEG brain signals based on deep learning approach. Expert Syst. Appl. 2018, 107, 61–71. [Google Scholar] [CrossRef]
- Wei, X.; Zhou, L.; Zhang, Z.; Chen, Z.; Zhou, Y. Early prediction of epileptic seizures using a long-term recurrent convolutional network. J. Neurosci. Methods 2019, 327, 108395. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Deng, Z.; Ying, W.; Choi, K.S.; Wu, D.; Qin, B.; Wang, J.; Shen, H.; Wang, S. Deep multi-view feature learning for EEG-based epileptic seizure detection. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 1962–1972. [Google Scholar] [CrossRef] [PubMed]
- Covert, I.C.; Krishnan, B.; Najm, I.; Zhan, J.; Shore, M.; Hixson, J.; Po, M.J. Temporal graph convolutional networks for automatic seizure detection. In Proceedings of the Machine Learning for Healthcare Conference, Ann Arbor, MI, USA, 8–10 August 2019; pp. 160–180. [Google Scholar]
- Minxing, G.; Zhou, W.; Liu, G.; Li, C.; Zhang, Y. Epileptic Seizure Detection Based on Stockwell Transform and Bidirectional Long Short-Term Memory. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 573–580. [Google Scholar] [CrossRef]
- Kane, N.; Acharya, J.; Beniczky, S.; Caboclo, L.; Finnigan, S.; Kaplan, P.W.; Shibasaki, H.; Pressler, R.; van Putten, M.J. A revised glossary of terms most commonly used by clinical electroencephalographers and updated proposal for the report format of the EEG findings. Revision 2017. Clin. Neurophysiol. Pract. 2017, 2, 170. [Google Scholar] [CrossRef]
- Ramírez-Fuentes, C.; Barrera-Figueroa, V.; Tovar-Corona, B.; Silva-Ramírez, M.; Garay-Jiménez, L. Epileptic focus location in the cerebral cortex using linear techniques and complex networks. Nonlinear Dyn. 2021, 104, 2687–2710. [Google Scholar] [CrossRef]
- Wendling, F.; Bartolomei, F.; Bellanger, J.J.; Bourien, J.; Chauvel, P. Epileptic fast intracerebral EEG activity: Evidence for spatial decorrelation at seizure onset. Brain 2003, 126, 1449–1459. [Google Scholar] [CrossRef]
- Meeren, H.; van Luijtelaar, G.; da Silva, F.L.; Coenen, A. Evolving concepts on the pathophysiology of absence seizures: The cortical focus theory. Arch. Neurol. 2005, 62, 371–376. [Google Scholar] [CrossRef]
- Behrens, T.E.; Johansen-Berg, H.; Woolrich, M.; Smith, S.; Wheeler-Kingshott, C.; Boulby, P.; Barker, G.; Sillery, E.; Sheehan, K.; Ciccarelli, O.; et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat. Neurosci. 2003, 6, 750–757. [Google Scholar] [CrossRef]
- Pavone, A.; Niedermeyer, E. Absence seizures and the frontal lobe. Clin. Electroencephalogr. 2000, 31, 153–156. [Google Scholar] [CrossRef]
- Clay Reid, R.; Alonso, J.M. Specificity of monosynaptic connections from thalamus to visual cortex. Nature 1995, 378, 281–284. [Google Scholar] [CrossRef]
- Jan, M.M.; Girvin, J.P. Seizure semiology: Value in identifying seizure origin. Can. J. Neurol. Sci. 2008, 35, 22–30. [Google Scholar] [CrossRef]
- Bonini, F.; McGonigal, A.; Wendling, F.; Régis, J.; Scavarda, D.; Carron, R.; Chauvel, P.; Bartolomei, F. Epileptogenic networks in seizures arising from motor systems. Epilepsy Res. 2013, 106, 92–102. [Google Scholar] [CrossRef]
- Siuly, S.; Li, Y.; Zhang, Y. Electroencephalogram (eeg) and its background. In EEG Signal Analysis and Classification; Springer: Cham, Switzerland, 2016; pp. 3–21. [Google Scholar]
- Wang, L.; Xue, W.; Li, Y.; Luo, M.; Huang, J.; Cui, W.; Huang, C. Automatic epileptic seizure detection in EEG signals using multi-domain feature extraction and nonlinear analysis. Entropy 2017, 19, 222. [Google Scholar] [CrossRef]
- Craley, J.; Johnson, E.; Venkataraman, A. Integrating convolutional neural networks and probabilistic graphical modeling for epileptic seizure detection in multichannel EEG. In Proceedings of the International Conference on Information Processing in Medical Imaging, Hong Kong, China, 2–7 June 2019; Springer: Cham, Switzerland, 2019; pp. 291–303. [Google Scholar]
- Zhang, Z.; Ren, Y.; Sabor, N.; Pan, J.; Luo, X.; Li, Y.; Chen, Y.; Wang, G. DWT-Net: Seizure Detection System with Structured EEG Montage and Multiple Feature Extractor in Convolution Neural Network. J. Sens. 2020, 2020, 3083910. [Google Scholar] [CrossRef]
- Vanabelle, P.; De Handschutter, P.; El Tahry, R.; Benjelloun, M.; Boukhebouze, M. Epileptic seizure detection using EEG signals and extreme gradient boosting. J. Biomed. Res. 2020, 34, 228. [Google Scholar] [CrossRef]
- Khan, I.D.; Farooq, O.; Khan, Y.U. Automatic Seizure Detection Using Modified CNN Architecture and Activation Layer. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2022; Volume 2318, p. 012013. [Google Scholar]
- Fraiwan, M.A.; Alafeef, M. Multiclass Epilepsy Classification Using Wavelet Decomposition, Direct Quadrature, and Shannon Entropy. J. Eng. Sci. Technol. 2022, 17, 781–797. [Google Scholar]
- Subasi, A. EEG signal classification using wavelet feature extraction and a mixture of expert model. Expert Syst. Appl. 2007, 32, 1084–1093. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, G.; Xiao, R.; Cui, W.; Cai, J.; Hu, X.; Sun, Y.; Qiu, J.; Qi, Y. A combination of statistical parameters for epileptic seizure detection and classification using VMD and NLTWSVM. Biocybern. Biomed. Eng. 2022, 42, 258–272. [Google Scholar] [CrossRef]
- Andrzejak, R.G.; Lehnertz, K.; Mormann, F.; Rieke, C.; David, P.; Elger, C.E. Indications of nonlinear deterministic and finite-dimensional structures in time series of brain electrical activity: Dependence on recording region and brain state. Phys. Rev. E 2001, 64, 061907. [Google Scholar] [CrossRef]
- Wijayanto, I.; Hartanto, R.; Nugroho, H.A.; Winduratna, B. Seizure type detection in epileptic EEG signal using empirical mode decomposition and support vector machine. In Proceedings of the 2019 International Seminar on Intelligent Technology and Its Applications (ISITIA), Surabaya, Indonesia, 28–29 August 2019; pp. 314–319. [Google Scholar]
- Rahmani, A.; Venkitaraman, A.; Frossard, P. A Meta-GNN approach to personalized seizure detection and classification. In Proceedings of the ICASSP 2023-2023 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), Rhodes Island, Greece, 4–10 June 2023; pp. 1–5. [Google Scholar]
- Zhu, Y.; Saqib, M.; Ham, E.; Belhareth, S.; Hoffman, R.; Wang, M.D. Mitigating patient-to-patient variation in EEG seizure detection using meta transfer learning. In Proceedings of the 2020 IEEE 20th International Conference on Bioinformatics and Bioengineering (BIBE), Cincinnati, OH, USA, 26–28 October 2020; pp. 548–555. [Google Scholar]
- Jacobs, J.; LeVan, P.; Châtillon, C.É.; Olivier, A.; Dubeau, F.; Gotman, J. High frequency oscillations in intracranial EEGs mark epileptogenicity rather than lesion type. Brain 2009, 132, 1022–1037. [Google Scholar] [CrossRef] [PubMed]
- Bragin, A.; Engel, J., Jr.; Staba, R.J. High-frequency oscillations in epileptic brain. Curr. Opin. Neurol. 2010, 23, 151. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Valenca, L.; Dubeau, F.; Mari, F.; Zelmann, R.; Gotman, J. Interictal scalp fast oscillations as a marker of the seizure onset zone. Neurology 2011, 77, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Jouny, C.C.; Franaszczuk, P.J.; Bergey, G.K. Improving early seizure detection. Epilepsy Behav. 2011, 22, S44–S48. [Google Scholar] [CrossRef]
- Tzallas, A.T.; Tsipouras, M.G.; Fotiadis, D.I. Automatic seizure detection based on time-frequency analysis and artificial neural networks. Comput. Intell. Neurosci. 2007, 2007, 080510. [Google Scholar] [CrossRef]
- Patidar, S.; Panigrahi, T. Detection of epileptic seizure using Kraskov entropy applied on tunable-Q wavelet transform of EEG signals. Biomed. Signal Process. Control 2017, 34, 74–80. [Google Scholar] [CrossRef]
- Elhosary, H.; Zakhari, M.H.; Elgammal, M.A.; Kelany, K.A.H.; Abd El Ghany, M.A.; Salama, K.N.; Mostafa, H. Hardware acceleration of high sensitivity power-aware epileptic seizure detection system using dynamic partial reconfiguration. IEEE Access 2021, 9, 75071–75081. [Google Scholar] [CrossRef]
- Davis, K.A.; Devries, S.P.; Krieger, A.; Mihaylova, T.; Minecan, D.; Litt, B.; Wagenaar, J.B.; Stacey, W.C. The effect of increased intracranial EEG sampling rates in clinical practice. Clin. Neurophysiol. 2018, 129, 360–367. [Google Scholar] [CrossRef]
- Birjandtalab, J.; Pouyan, M.B.; Cogan, D.; Nourani, M.; Harvey, J. Automated seizure detection using limited-channel EEG and non-linear dimension reduction. Comput. Biol. Med. 2017, 82, 49–58. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Pachori, R.B. A multivariate approach for patient-specific EEG seizure detection using empirical wavelet transform. IEEE Trans. Biomed. Eng. 2017, 64, 2003–2015. [Google Scholar] [CrossRef]
- Maher, C.F.; Yang, Y.; Truong, D.; Wang, C.; Nikpour, A.; Kavehei, O. Towards long term monitoring: Seizure detection with reduced electroencephalogram channels. medRxiv 2021. [Google Scholar] [CrossRef]
- Kjaer, T.W.; Sorensen, H.B.; Groenborg, S.; Pedersen, C.R.; Duun-Henriksen, J. Detection of paroxysms in long-term, single-channel EEG-monitoring of patients with typical absence seizures. IEEE J. Transl. Eng. Health Med. 2017, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pisano, F.; Sias, G.; Fanni, A.; Cannas, B.; Dourado, A.; Pisano, B.; Teixeira, C.A. Convolutional neural network for seizure detection of nocturnal frontal lobe epilepsy. Complexity 2020, 2020, 4825767. [Google Scholar] [CrossRef]
- Herta, J.; Koren, J.; Fürbass, F.; Hartmann, M.; Gruber, A.; Baumgartner, C. Reduced electrode arrays for the automated detection of rhythmic and periodic patterns in the intensive care unit: Frequently tried, frequently failed? Clin. Neurophysiol. 2017, 128, 1524–1531. [Google Scholar] [CrossRef]
- Zanetti, R.; Aminifar, A.; Atienza, D. Robust epileptic seizure detection on wearable systems with reduced false-alarm rate. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 4248–4251. [Google Scholar]
- Saab, M.; Gotman, J. A system to detect the onset of epileptic seizures in scalp EEG. Clin. Neurophysiol. 2005, 116, 427–442. [Google Scholar] [CrossRef]
- Teng, Q.; Liu, Z.; Song, Y.; Han, K.; Lu, Y. A survey on the interpretability of deep learning in medical diagnosis. Multimed. Syst. 2022, 28, 2335–2355. [Google Scholar] [CrossRef]
- Ellis, C.A.; Sendi, M.S.; Miller, R.; Calhoun, V. A Novel Activation Maximization-based Approach for Insight into Electrophysiology Classifiers. In Proceedings of the 2021 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), Houston, TX, USA, 9–12 December 2021; pp. 3358–3365. [Google Scholar]
- Mahendran, A.; Vedaldi, A. Visualizing deep convolutional neural networks using natural pre-images. Int. J. Comput. Vis. 2016, 120, 233–255. [Google Scholar] [CrossRef]
- Kuang, D.; Michoski, C. SEER-net: Simple EEG-based Recognition network. Biomed. Signal Process. Control 2023, 83, 104620. [Google Scholar] [CrossRef]
- Truong, D.; Makeig, S.; Delorme, A. Assessing learned features of Deep Learning applied to EEG. In Proceedings of the 2021 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), Houston, TX, USA, 9–12 December 2021; pp. 3667–3674. [Google Scholar]
- Aslan, Z.; Akin, M. A deep learning approach in automated detection of schizophrenia using scalogram images of EEG signals. Phys. Eng. Sci. Med. 2022, 45, 83–96. [Google Scholar] [CrossRef]
- Xu, M.; Yao, J.; Zhang, Z.; Li, R.; Yang, B.; Li, C.; Li, J.; Zhang, J. Learning EEG topographical representation for classification via convolutional neural network. Pattern Recognit. 2020, 105, 107390. [Google Scholar] [CrossRef]
- Priyasad, D.; Fernando, T.; Denman, S.; Sridharan, S.; Fookes, C. Interpretable seizure classification using unprocessed EEG with multi-channel attentive feature fusion. IEEE Sens. J. 2021, 21, 19186–19197. [Google Scholar] [CrossRef]
- Strawbridge, L.M.; Schultz, A.M.; Liverman, C.T.; England, M.J. Epilepsy across the Spectrum: Promoting Health and Understanding; National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Kelly, K.M.; Chung, S.S. Surgical treatment for refractory epilepsy: Review of patient evaluation and surgical options. Epilepsy Res. Treat. 2011, 2011, 303624. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ambati, R.; Raja, S.; Al-Hameed, M.; John, T.; Arjoune, Y.; Shekhar, R. Neuromorphic Architecture Accelerated Automated Seizure Detection in Multi-Channel Scalp EEG. Sensors 2022, 22, 1852. [Google Scholar] [CrossRef]
- Lüders, H.O.; Najm, I.; Nair, D.; Widdess-Walsh, P.; Bingman, W. The epileptogenic zone: General principles. Epileptic Disord. 2006, 8, 1–9. [Google Scholar]
- Myers, M.H.; Padmanabha, A.; Bidelman, G.M.; Wheless, J.W. Seizure localization using EEG analytical signals. Clin. Neurophysiol. 2020, 131, 2131–2139. [Google Scholar] [CrossRef] [PubMed]
- Santiuste, M.; Nowak, R.; Russi, A.; Tarancon, T.; Oliver, B.; Ayats, E.; Scheler, G.; Graetz, G. Simultaneous magnetoencephalography and intracranial EEG registration: Technical and clinical aspects. J. Clin. Neurophysiol. 2008, 25, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Michel, C.M.; Lantz, G.; Spinelli, L.; De Peralta, R.G.; Landis, T.; Seeck, M. 128-channel EEG source imaging in epilepsy: Clinical yield and localization precision. J. Clin. Neurophysiol. 2004, 21, 71–83. [Google Scholar] [CrossRef]
- Brodbeck, V.; Spinelli, L.; Lascano, A.M.; Wissmeier, M.; Vargas, M.I.; Vulliemoz, S.; Pollo, C.; Schaller, K.; Michel, C.M.; Seeck, M. Electroencephalographic source imaging: A prospective study of 152 operated epileptic patients. Brain 2011, 134, 2887–2897. [Google Scholar] [CrossRef]
- Wang, G.; Worrell, G.; Yang, L.; Wilke, C.; He, B. Interictal spike analysis of high-density EEG in patients with partial epilepsy. Clin. Neurophysiol. 2011, 122, 1098–1105. [Google Scholar] [CrossRef]
- Pascual-Marqui, R.D.; Lehmann, D.; Koukkou, M.; Kochi, K.; Anderer, P.; Saletu, B.; Tanaka, H.; Hirata, K.; John, E.R.; Prichep, L.; et al. Assessing interactions in the brain with exact low-resolution electromagnetic tomography. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2011, 369, 3768–3784. [Google Scholar] [CrossRef]
- Cannon, R.L.; Baldwin, D.R.; Shaw, T.L.; Diloreto, D.J.; Phillips, S.M.; Scruggs, A.M.; Riehl, T.C. Reliability of quantitative EEG (qEEG) measures and LORETA current source density at 30 days. Neurosci. Lett. 2012, 518, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, S.; Ishii, R.; Pascual-Marqui, R.D.; Canuet, L.; Yoshimura, M.; Nishida, K.; Kitaura, Y.; Katsura, K.; Kinoshita, T. Automated source estimation of scalp EEG epileptic activity using eLORETA kurtosis analysis. Neuropsychobiology 2019, 77, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Craley, J.; Johnson, E.; Jouny, C.; Venkataraman, A. Automated noninvasive seizure detection and localization using switching markov models and convolutional neural networks. In Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention; Springer: Cham, Switzerland, 2019; pp. 253–261. [Google Scholar]
- Ochal, D.; Rahman, S.; Ferrell, S.; Elseify, T.; Obeid, I.; Picone, J. The temple university hospital eeg corpus: Annotation guidelines. Inst. Signal Inf. Process. Rep. 2020, 1, 1–28. [Google Scholar]
- Spencer, S.S. Neural networks in human epilepsy: Evidence of and implications for treatment. Epilepsia 2002, 43, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Gabeff, V.; Teijeiro, T.; Zapater, M.; Cammoun, L.; Rheims, S.; Ryvlin, P.; Atienza, D. Interpreting deep learning models for epileptic seizure detection on EEG signals. Artif. Intell. Med. 2021, 117, 102084. [Google Scholar] [CrossRef]
- Avcu, M.T.; Zhang, Z.; Chan, D.W.S. Seizure detection using least eeg channels by deep convolutional neural network. In Proceedings of the ICASSP 2019-2019 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), Brighton, UK, 12–17 May 2019; pp. 1120–1124. [Google Scholar]
- Qiu, S.; Wang, W.; Jiao, H. LightSeizureNet: A Lightweight Deep Learning Model for Real-Time Epileptic Seizure Detection. IEEE J. Biomed. Health Inform. 2022, 27, 1845–1856. [Google Scholar] [CrossRef]
- Vergara, J.; Estevez, P. A Review of Feature Selection Methods Based on Mutual Information. Neural Comput. Appl. 2014, 24, 175–186. [Google Scholar] [CrossRef]
- Beraha, M.; Metelli, A.M.; Papini, M.; Tirinzoni, A.; Restelli, M. A Feature Selection via Mutual Information: New Theoretical Insights. In Proceedings of the 2019 International Joint Conference on Neural Networks (IJCNN), Budapest, Hungary, 14–19 July 2019; pp. 1–9. [Google Scholar] [CrossRef]
- Fawcett, T. ROC graphs: Notes and practical considerations for researchers. Mach. Learn. 2004, 31, 1–38. [Google Scholar]
- Saito, T.; Rehmsmeier, M. The precision-recall plot is more informative than the ROC plot when evaluating binary classifiers on imbalanced datasets. PLoS ONE 2015, 10, e0118432. [Google Scholar] [CrossRef]






| Reference | Year | Database | Method | Sampling Rate, Hz | Metrics | ||
|---|---|---|---|---|---|---|---|
| Accuracy, % | Sensitivity, % | Specificity, % | |||||
| Conventional Machine Learning | |||||||
| [24] | 2019 | CHB-MIT | SVM Naive Bayes | 256 | 95.63 | 95.7 | 96.55 |
| [25] | 2017 | UoB | DT-CWT | 173.6 | 98.87 | 98.20 | 100 |
| [26] | 2012 | UoB | FSC | 173.6 | 98.1 | 99.4 | 100 |
| SVM | 95.9 | 97.2 | 100 | ||||
| kNN | 93 | 97.8 | 97.8 | ||||
| PNN | 93 | 97.8 | 97.8 | ||||
| DT | 88.5 | 98.3 | 91.1 | ||||
| GMM | 95.9 | 98.3 | 95.6 | ||||
| DBC | 97.8 | 94.4 | 97.8 | ||||
| [27] | 2018 | XJU | WDTF | 256 | 99.4 | 92.1 | 99.5 |
| [28] | 2018 | UoB | HVD | 173.6 | 97.66 | 98 | 98 |
| [29] | 2015 | MCH | GLM | 200 500 512 | 87.5 | 88.8 | 85.7 |
| [30] | 2015 | UoB | MLPNN SVM kNN+NLFV | 173.6 | 96.5 94.8 98.4 | 96.5 96.5 99 | 96.5 93 97.9 |
| [31] | 2019 | UoB | LS-SVM | 173.6 | 99.5 | 100 | 99.4 |
| [32] | 2018 | TWH | CFC Morlet | 500 1024 | 82.4 | 87.9 | 82.4 |
| [33] | 2015 | CHB-MIT | LDA | 256 | 94.69 | 89.1 | 94.8 |
| Deep Learning | |||||||
| [34] | 2019 | CHB-MIT | 2D CNN | 256 | 98.05 | 90 | 91.65 |
| [35] | 2019 | UoB | DNN | 173.6 | 97.21 | 98.59 | 91.47 |
| [36] | 2020 | UoB | SEA-based DNN | 173.6 | 97.17 | 93.11 | 98.18 |
| [37] | 2021 | TUSZ CHB-MIT UoB | EEGWaveNet | 256 256 173.6 | 67.68 96.17 99.89 | 59.21 65.83 99.80 | 75.30 96.96 99.97 |
| [38] | 2019 | UoB | ESD-LSTM | 173.6 | 100 | 100 | 100 |
| [39] | 2018 | UoB | P-1D-CNN | 173.6 | 99.1 | 96 | 98 |
| [40] | 2019 | XMUH | LRCN | 500 | 93.4 | 91.88 | 86.13 |
| [41] | 2019 | CHB-MIT | 2D CNN 3D CNN | 256 | 98.33 | 96.66 | 99.14 |
| [42] | 2019 | CC | TGCN | 200 | 98.05 | 90 | 91.65 |
| [43] | 2020 | FED | BILSTM | 256 | 98.69 | 98.09 | 98.69 |
| Binary Classification | Multiclass Classification | ||
|---|---|---|---|
| Electrode | MI Significance Score | Electrode | MI Significance Score |
| C4 | 0.006894 | Fz | 0.178724 |
| F4 | 0.006632 | Pz | 0.178065 |
| T3 | 0.006631 | Fp2 | 0.177629 |
| Fp2 | 0.006592 | T4 | 0.176386 |
| P4 | 0.006588 | O2 | 0.176262 |
| F3 | 0.006522 | C4 | 0.176049 |
| O1 | 0.006444 | F4 | 0.175952 |
| A1 | 0.006419 | F8 | 0.175667 |
| C3 | 0.006405 | F3 | 0.175562 |
| T6 | 0.006347 | T5 | 0.175543 |
| T5 | 0.006268 | O1 | 0.175284 |
| P3 | 0.006251 | A2 | 0.175005 |
| T4 | 0.006234 | Cz | 0.174934 |
| Fp1 | 0.006216 | P4 | 0.174509 |
| O2 | 0.006126 | P3 | 0.174291 |
| A2 | 0.006085 | A1 | 0.174280 |
| Cz | 0.006038 | F7 | 0.173992 |
| F8 | 0.006025 | T3 | 0.173690 |
| Pz | 0.006023 | C3 | 0.173577 |
| Fz | 0.005865 | T6 | 0.173267 |
| F7 | 0.005654 | Fp1 | 0.172294 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Statsenko, Y.; Babushkin, V.; Talako, T.; Kurbatova, T.; Smetanina, D.; Simiyu, G.L.; Habuza, T.; Ismail, F.; Almansoori, T.M.; Gorkom, K.N.-V.; et al. Automatic Detection and Classification of Epileptic Seizures from EEG Data: Finding Optimal Acquisition Settings and Testing Interpretable Machine Learning Approach. Biomedicines 2023, 11, 2370. https://doi.org/10.3390/biomedicines11092370
Statsenko Y, Babushkin V, Talako T, Kurbatova T, Smetanina D, Simiyu GL, Habuza T, Ismail F, Almansoori TM, Gorkom KN-V, et al. Automatic Detection and Classification of Epileptic Seizures from EEG Data: Finding Optimal Acquisition Settings and Testing Interpretable Machine Learning Approach. Biomedicines. 2023; 11(9):2370. https://doi.org/10.3390/biomedicines11092370
Chicago/Turabian StyleStatsenko, Yauhen, Vladimir Babushkin, Tatsiana Talako, Tetiana Kurbatova, Darya Smetanina, Gillian Lylian Simiyu, Tetiana Habuza, Fatima Ismail, Taleb M. Almansoori, Klaus N.-V. Gorkom, and et al. 2023. "Automatic Detection and Classification of Epileptic Seizures from EEG Data: Finding Optimal Acquisition Settings and Testing Interpretable Machine Learning Approach" Biomedicines 11, no. 9: 2370. https://doi.org/10.3390/biomedicines11092370
APA StyleStatsenko, Y., Babushkin, V., Talako, T., Kurbatova, T., Smetanina, D., Simiyu, G. L., Habuza, T., Ismail, F., Almansoori, T. M., Gorkom, K. N.-V., Szólics, M., Hassan, A., & Ljubisavljevic, M. (2023). Automatic Detection and Classification of Epileptic Seizures from EEG Data: Finding Optimal Acquisition Settings and Testing Interpretable Machine Learning Approach. Biomedicines, 11(9), 2370. https://doi.org/10.3390/biomedicines11092370






