Chronic Kidney Disease Has No Impact on Tear Film Substance P Concentration in Type 2 Diabetes
Abstract
:1. Introduction
2. Methods
- All participants recruited into the study were above 18 years of age and gave written informed consent.
- Recruitment was restricted to persons with type 2 diabetes.
2.1. Ocular Surface Assessment
2.2. Sample Size Calculation
2.3. Indicators of Kidney Function
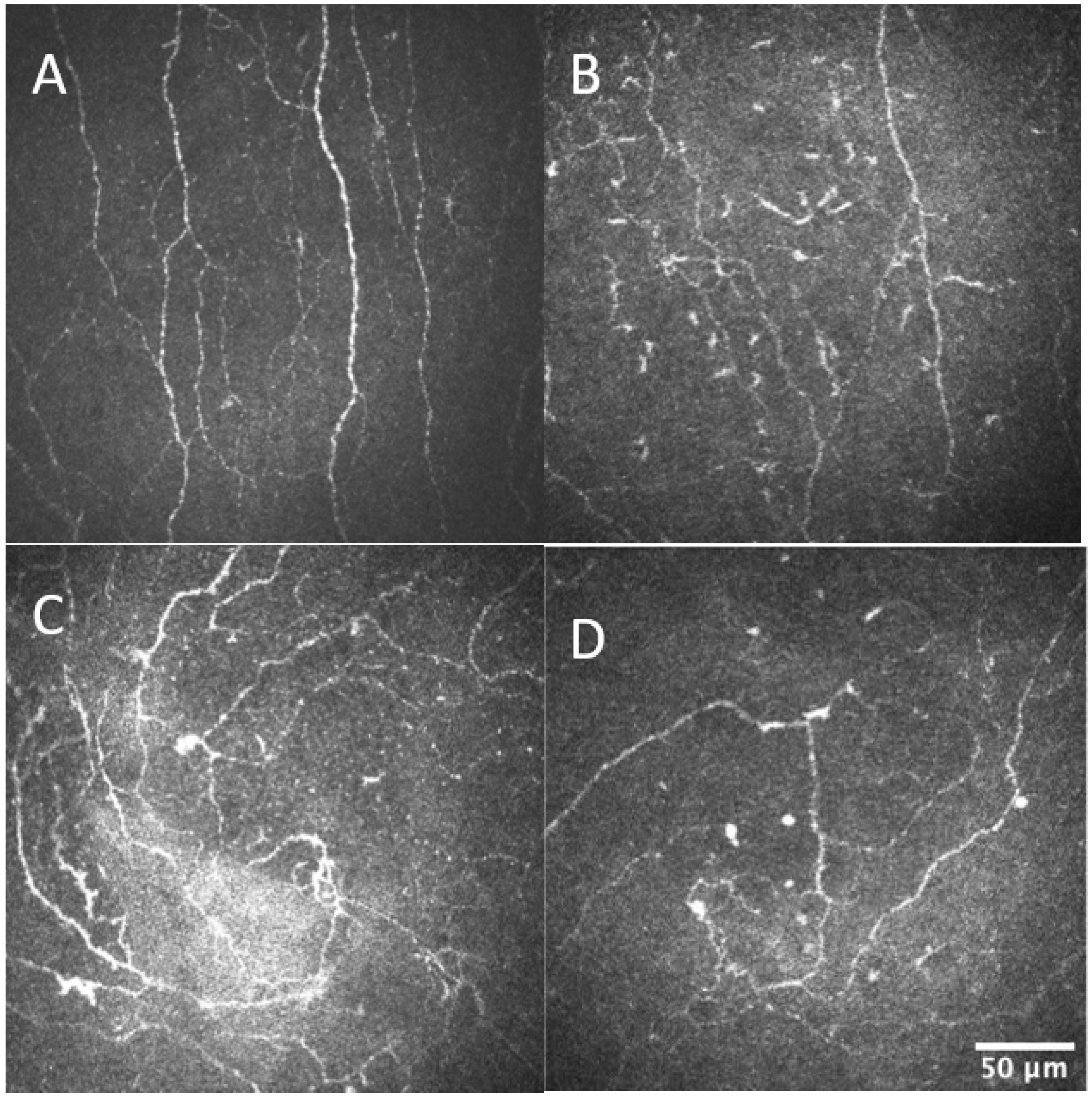
2.4. Corneal Confocal Microscopy
2.5. Tear Sample Collection
2.6. ELISA of Substance P Concentration
2.7. Peripheral Neuropathy Assessment and Diagnosis
2.8. Data Analysis
3. Results
3.1. Participant Demographic Data and Metabolic Parameters
3.2. Tear Film Substance P Concentration
3.3. Corneal Nerve Morphological Parameters
3.4. Correlational and Hierarchical Multiple Regression Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arnold, R.; Pianta, T.J.; Issar, T.; Kirby, A.; Scales, C.M.; Kwai, N.C.; Endre, Z.; Krishnan, A.V. Peripheral neuropathy: An important contributor to physical limitation and morbidity in stages 3 and 4 chronic kidney disease. Nephrol. Dial. Transplant. 2022, 37, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, S.; Boulton, A.J.; Dyck, P.J.; Freeman, R.; Horowitz, M.; Kempler, P.; Lauria, G.; Malik, R.A.; Spallone, V.; Vinik, A. Diabetic neuropathies: Update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010, 33, 2285. [Google Scholar] [CrossRef]
- Asiedu, K.; Markoulli, M.; Tummanapalli, S.; Chiang, J.; Alotaibi, S.; Wang, L.; Dhanapalaratnam, R.; Kwai, N.; Poynten, A.; Krishnan, A. Impact of Chronic Kidney Disease on Corneal Neuroimmune Features in Type 2 Diabetes. J. Clin. Med. 2022, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, F.; Di Zazzo, A.; De Simone, L.; Bonini, S. The intriguing role of neuropeptides at the ocular surface. Ocul. Surf. 2017, 15, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Lasagni Vitar, R.M.; Rama, P.; Ferrari, G. The two-faced effects of nerves and neuropeptides in corneal diseases. Prog. Retin Eye Res. 2022, 86, 100974. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.B.; Naderi, A.; Cho, W.; Ortiz, G.; Musayeva, A.; Dohlman, T.H.; Chen, Y.; Ferrari, G.; Dana, R. Modulating the tachykinin: Role of substance P and neurokinin receptor expression in ocular surface disorders. Ocul. Surf. 2022, 25, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Tummanapalli, S.S.; Willcox, M.D.P.; Issar, T.; Yan, A.; Pisarcikova, J.; Kwai, N.; Poynten, A.M.; Krishnan, A.V.; Markoulli, M. Tear film substance P: A potential biomarker for diabetic peripheral neuropathy. Ocul. Surf. 2019, 17, 690–698. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, T.M.; O’Connell, J.; O’Brien, D.I.; Goode, T.; Bredin, C.P.; Shanahan, F. The role of substance P in inflammatory disease. J. Cell. Physiol. 2004, 201, 167–180. [Google Scholar] [CrossRef]
- Yang, T.; Zhou, Y.; Ma, B.; Duan, H.; Liu, R.; Zhao, L.; Qi, H. Elevated Neuropeptides in Dry Eye Disease and Their Clinical Correlations. Cornea 2023, 42, 557–564. [Google Scholar] [CrossRef]
- Yu, M.; Lee, S.M.; Lee, H.; Amouzegar, A.; Nakao, T.; Chen, Y.; Dana, R. Neurokinin-1 Receptor Antagonism Ameliorates Dry Eye Disease by Inhibiting Antigen-Presenting Cell Maturation and T Helper 17 Cell Activation. Am. J. Pathol. 2020, 190, 125–133. [Google Scholar] [CrossRef]
- Kim, D.-J.; Moon, J.-Y.; Kim, S.-M.; Seo, J.-W.; Lee, Y.H.; Jung, S.W.; Kim, K.; Kim, Y.G.; Lim, S.-J.; Lee, S. Substance P improves renal ischemia reperfusion injury through modulating immune response. Front. Immunol. 2020, 11, 600. [Google Scholar] [CrossRef] [PubMed]
- Kramer, H.J.; Klingmüller, D.; Flachskampf, F.A.; Düsing, R. Substance P-induced changes in kidney function in the conscious rat: Relation to the renal prostaglandin system. Kidney Blood Press. Res. 1983, 6, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Qazi, Y.; Hurwitz, S.; Khan, S.; Jurkunas, U.V.; Dana, R.; Hamrah, P. Validity and Reliability of a Novel Ocular Pain Assessment Survey (OPAS) in Quantifying and Monitoring Corneal and Ocular Surface Pain. Ophthalmology 2016, 123, 1458–1468. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, R.M.; Christianson, M.D.; Jacobsen, G.; Hirsch, J.D.; Reis, B.L. Reliability and validity of the Ocular Surface Disease Index. Arch. Ophthalmol. 2000, 118, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Bron, A.J.; Evans, V.E.; Smith, J.A. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea 2003, 22, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Eom, Y.; Lee, J.-S.; Lee, H.; Kim, H.; Song, J.S. Comparison of conjunctival staining between lissamine green and yellow filtered fluorescein sodium. Can. J. Ophthalmol. J. Can. d’Ophtalmol. 2015, 50, 273–277. [Google Scholar] [CrossRef]
- Markoulli, M.; You, J.; Kim, J.; Duong, C.L.; Tolentino, J.B.; Karras, J.; Lum, E. Corneal Nerve Morphology and Tear Film Substance P in Diabetes. Optom. Vis. Sci. 2017, 94, 726–731. [Google Scholar] [CrossRef]
- Levin, A.; Stevens, P.E.; Bilous, R.W.; Coresh, J.; De Francisco, A.L.; De Jong, P.E.; Griffith, K.E.; Hemmelgarn, B.R.; Iseki, K.; Lamb, E.J. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 2013, 3, 1–150. [Google Scholar]
- Misra, S.; Craig, J.P.; McGhee, C.N.J.; Patel, D.V. Interocular Comparison by In Vivo Confocal Microscopy of the 2-Dimensional Architecture of the Normal Human Corneal Subbasal Nerve Plexus. Cornea 2012, 31, 1376–1380. [Google Scholar] [CrossRef]
- Petropoulos, I.N.; Alam, U.; Fadavi, H.; Asghar, O.; Green, P.; Ponirakis, G.; Marshall, A.; Boulton, A.J.; Tavakoli, M.; Malik, R.A. Corneal nerve loss detected with corneal confocal microscopy is symmetrical and related to the severity of diabetic polyneuropathy. Diabetes Care 2013, 36, 3646–3651. [Google Scholar] [CrossRef]
- Vagenas, D.; Pritchard, N.; Edwards, K.; Shahidi, A.M.; Sampson, G.P.; Russell, A.W.; Malik, R.A.; Efron, N. Optimal image sample size for corneal nerve morphometry. Optom. Vis. Sci. 2012, 89, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Dabbah, M.A.; Graham, J.; Petropoulos, I.; Tavakoli, M.; Malik, R.A. Dual-model automatic detection of nerve-fibres in corneal confocal microscopy images. Med. Image Comput. Comput. Assist Interv. 2010, 13, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Cornblath, D.; Chaudhry, V.; Carter, K.; Lee, D.; Seysedadr, M.; Miernicki, M.; Joh, T. Total neuropathy score: Validation and reliability study. Neurology 1999, 53, 1660. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, V.; Rowinsky, E.K.; Sartorius, S.E.; Donehower, R.C.; Cornblath, D.R. Peripheral neuropathy from taxol and cisplatin combination chemotherapy: Clinical and electrophysiological studies. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 1994, 35, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Yang, L.; Wang, Q.; Li, Y.; Wei, C.; Xie, L. Mechanistic investigations of diabetic ocular surface diseases. Front. Endocrinol. 2022, 13, 1079541. [Google Scholar] [CrossRef] [PubMed]
- Dozio, E.; Vettoretti, S.; Lungarella, G.; Messa, P.; Corsi Romanelli, M.M. Sarcopenia in chronic kidney disease: Focus on advanced glycation end products as mediators and markers of oxidative stress. Biomedicines 2021, 9, 405. [Google Scholar] [CrossRef] [PubMed]
- El-Saeed, G.S.; Fadel, F.; Elshamaa, M.F.; Galal, R.E.; Elghoroury, E.A.; Nasr, S.A.; Thabet, E.H.; Abdelrahman, S.M. Advanced glycation end products and soluble receptor as markers of oxidative stress in children on hemodialysis. Ren. Fail. 2015, 37, 1452–1456. [Google Scholar] [CrossRef] [PubMed]
- Arnold, R.; Pussell, B.A.; Howells, J.; Grinius, V.; Kiernan, M.C.; Lin, C.S.-Y.; Krishnan, A.V. Evidence for a causal relationship between hyperkalaemia and axonal dysfunction in end-stage kidney disease. Clin. Neurophysiol. 2014, 125, 179–185. [Google Scholar] [CrossRef]
- Krishnan, A.V.; Lin, C.S.-Y.; Kiernan, M.C. Activity-dependent excitability changes suggest Na+/K+ pump dysfunction in diabetic neuropathy. Brain 2008, 131, 1209–1216. [Google Scholar] [CrossRef]
- Abu, E.K.; Ofori, A.O.; Boadi-Kusi, S.B.; Ocansey, S.; Yankah, R.K.; Kyei, S.; Awuku, A.Y. Dry eye disease and meibomian gland dysfunction among a clinical sample of type 2 diabetes patients in Ghana. Afr. Health Sci. 2022, 22, 293–302. [Google Scholar] [CrossRef]
- Byambajav, M.; Collier, A.; Shu, X.; Hagan, S. Tear Fluid Biomarkers and Quality of Life in People with Type 2 Diabetes and Dry Eye Disease. Metabolites 2023, 13, 733. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.; Golebiowski, B.; Zhao, X.; Chen, S.; Zhou, S.; Stapleton, F. Long-term effects of LASIK on corneal innervation and tear neuropeptides and the associations with dry eye. J. Refract. Surg. 2016, 32, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-C.; Yam, G.H.-F.; Lin, M.T.-Y.; Teo, E.; Koh, S.-K.; Deng, L.; Zhou, L.; Tong, L.; Mehta, J.S. Comparison of tear proteomic and neuromediator profiles changes between small incision lenticule extraction (SMILE) and femtosecond laser-assisted in-situ keratomileusis (LASIK). J. Adv. Res. 2021, 29, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, M.F.; Soto, L.F.; Requena, D.; Ruiz-Alejos, A.O.; Huaylinos, Y.; Velasquez, R.; Bernabe-Ortiz, A.; Gilman, R.H. Tear biomarkers and corneal sensitivity as an indicator of neuropathy in type 2 diabetes. Diabetes Res. Clin. Pract. 2020, 163, 108143. [Google Scholar] [CrossRef]
- Moulton, E.A.; Borsook, D. C-Fiber Assays in the Cornea vs. Skin. Brain Sci. 2019, 9, 320. [Google Scholar] [CrossRef] [PubMed]
- Belmonte, C.; Acosta, M.C.; Gallar, J. Neural basis of sensation in intact and injured corneas. Exp. Eye Res. 2004, 78, 513–525. [Google Scholar] [CrossRef] [PubMed]
- MacIver, M. Structural and functional specialization of A delta and C fiber free nerve endings innervating rabbit corneal epithelium. J. Neurosci. 1993, 13, 4511–4524. [Google Scholar] [CrossRef] [PubMed]
- Waddell, P.J.; Lawson, S.N. Electrophysiological properties of subpopulations of rat dorsal root ganglion neurons in vitro. Neuroscience 1990, 36, 811–822. [Google Scholar] [CrossRef]
- Bae, J.Y.; Kim, J.H.; Cho, Y.S.; Mah, W.; Bae, Y.C. Quantitative analysis of afferents expressing substance P, calcitonin gene-related peptide, isolectin B4, neurofilament 200, and Peripherin in the sensory root of the rat trigeminal ganglion. J. Comp. Neurol. 2015, 523, 126–138. [Google Scholar] [CrossRef]
- Byun, Y.S.; Mok, J.W.; Chung, S.H.; Kim, H.S.; Joo, C.K. Ocular surface inflammation induces de novo expression of substance P in the trigeminal primary afferents with large cell bodies. Sci. Rep. 2020, 10, 15210. [Google Scholar] [CrossRef]
- Tavakoli, M.; Quattrini, C.; Abbott, C.; Kallinikos, P.; Marshall, A.; Finnigan, J.; Morgan, P.; Efron, N.; Boulton, A.J.; Malik, R.A. Corneal confocal microscopy: A novel noninvasive test to diagnose and stratify the severity of human diabetic neuropathy. Diabetes Care 2010, 33, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, I.N.; Ponirakis, G.; Ferdousi, M.; Azmi, S.; Kalteniece, A.; Khan, A.; Gad, H.; Bashir, B.; Marshall, A.; Boulton, A.J.M.; et al. Corneal Confocal Microscopy: A Biomarker for Diabetic Peripheral Neuropathy. Clin. Ther. 2021, 43, 1457–1475. [Google Scholar] [CrossRef] [PubMed]
- Angus-Leppan, H.; Burke, D. The function of large and small nerve fibers in renal failure. Muscle Nerve 1992, 15, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.C.B.; Goldstein, D.; Trinh, T.; Au, K.; Park, S.B.; Krishnan, A.V.; Markoulli, M. Tear film substance P in patients treated with neurotoxic chemotherapy. Exp. Eye Res. 2022, 224, 109253. [Google Scholar] [CrossRef] [PubMed]
- Burgess, J.; Ferdousi, M.; Gosal, D.; Boon, C.; Matsumoto, K.; Marshall, A.; Mak, T.; Marshall, A.; Frank, B.; Malik, R.A.; et al. Chemotherapy-Induced Peripheral Neuropathy: Epidemiology, Pathomechanisms and Treatment. Oncol. Ther. 2021, 9, 385–450. [Google Scholar] [CrossRef]
| Parameter | T2DM-CKD (n = 25) | T2DM-no CKD (n = 25) | p-Value |
|---|---|---|---|
| Age, years | 70.8 ± 8.5 | 68.2 ± 8.5 | p = 0.29 |
| Sex, % male | 64 | 72 | p = 0.54 |
| Body mass index, kg/m2 | 31.7 ± 6.9 | 32.1 ± 6.7 | p = 0.98 |
| Duration of diagnosis, years | 20.7 ± 8.8 | 14.7 ± 12.5 | p = 0.06 |
| HbA1c, % | 8.1 ± 1.8 | 8.7 ± 2.1 | p = 0.25 |
| Serum urea, mg/dL | 10.8 ± 4.6 | 6.4 ± 1.9 | p < 0.001 |
| Creatinine, mg/dL | 171.2 ±118.5 | 76.3 ± 16.1 | p < 0.001 |
| Estimated glomerular filtration rate, mL/min/1.73 m2 | 41.3 ± 19.2 | 80.4 ± 10.9 | p < 0.001 |
| Urine ACR, mg/mmol | 44.3 ± 88.1 | 3.8 ±3.9 | p = 0.03 |
| Serum potassium, mmol/l | 4.5 ± 0.3 | 4.3 ± 0.5 | p = 0.04 |
| Total cholesterol, mmol/L | 3.8 ± 1.1 | 3.8 ± 1.0 | p = 0.71 |
| High-density lipoprotein, mmol/L | 1.1 ± 0.4 | 1.3 ± 0.4 | p = 0.24 |
| Low-density lipoprotein, mmol/L | 1.8 ± 0.8 | 1.7 ± 0.9 | p = 0.80 |
| Triglycerides, mmol/L | 2.2 ± 2.1 | 1.7 ± 1.4 | p = 0.40 |
| Total Neuropathy Score (scores) | 6.9 ± 5.4 | 6.4 ± 5.2 | p = 0.74 |
| Ocular surface staining (scores) | 3.6 ± 1.5 | 2.7 ± 2.3 | p = 0.09 |
| Ocular Surface Disease Index (scores) | 13.0 ± 11.4 | 12.6 ± 11.6 | p = 0.76 |
| Ocular Pain Assessment Survey (scores) | 3.5 ± 5.6 | 3.6 ± 4.9 | p = 0.96 |
| Parameter | High-Severity Neuropathy Group (TNS Grade 3–4) (n = 25) | Low-Severity Neuropathy Group (TNS Grade 0–2) (n = 25) | p-Value |
|---|---|---|---|
| Age, years | 68.8 ± 10.9 | 69.3 ± 5.9 | p = 0.86 |
| Duration of disease, years | 17.5 ± 11.0 | 15.0 ± 9.4 | p = 0.61 |
| Estimated glomerular filtration rate, mL/min/1.73 m2 | 59.5 ± 23.2 | 64.9 ± 21.3 | p = 0.44 |
| Corneal nerve fiber density (no./mm2) | 15.8 ± 7.7 | 21.0 ± 8.1 | p = 0.04 |
| Corneal nerve fiber length (mm/mm2) | 9.9 ± 4.0 | 12.9 ± 4.2 | p = 0.03 |
| Inferior whorl length (IWL) (mm/mm2) | 8.6 ± 2.8 | 10.0 ± 5.0 | p = 0.29 |
| Substance P concentration (ng/mL) | 3.3 (0.3–40.7) * | 4.4 (0.2–50.4) * | p = 0.80 |
| Ocular surface staining (scores) | 3.6 ± 1.7 | 3.0 ± 2.1 | p = 0.33 |
| Ocular surface disease index (scores) | 13.2 ± 13.0 | 9.2 ± 9.2 | p = 0.21 |
| Parameter | T2DM-CKD | T2DM-No CKD | p-Value |
|---|---|---|---|
| Corneal nerve fiber density (no./mm2) | 14.7 ± 8.5 | 21.1 ± 7.0 | p < 0.01 |
| Corneal nerve fiber length (mm/mm2) | 9.8 ± 4.6 | 12.4 ± 3.8 | p = 0.04 |
| Inferior whorl length (mm/mm2) | 8.1 ± 4.0 | 9.7 ± 4.9 | p = 0.21 |
| Substance P (ng/mL) | 4.4 (0.6–40.8) * | 5.9 (0.2–47.2) * | p = 0.54 |
| Parameter | β Coefficient | Standard Error | p-Value | 95% Lower | 95% Upper |
|---|---|---|---|---|---|
| Dependent Variable: Tear Film Substance P Concentration | |||||
| Corneal nerve fiber density (no./mm2) | 0.04 | 0.06 | 0.42 | −0.07 | 0.16 |
| Corneal nerve fiber length (mm/mm2) | −0.11 | 0.10 | 0.28 | −0.32 | 0.10 |
| Inferior whorl length (mm/mm2) | 0.02 | 0.04 | 0.63 | −0.06 | 0.10 |
| Estimated glomerular filtration rate (mL/min/1.73 m2) | −0.01 | 0.007 | 0.50 | −0.02 | 0.01 |
| Urinary albumin/ creatinine ratio (mg/mmol) | −0.001 | 0.002 | 0.69 | −0.06 | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asiedu, K.; Alotaibi, S.; Krishnan, A.V.; Kwai, N.; Poynten, A.; Markoulli, M.; Dhanapalaratnam, R. Chronic Kidney Disease Has No Impact on Tear Film Substance P Concentration in Type 2 Diabetes. Biomedicines 2023, 11, 2368. https://doi.org/10.3390/biomedicines11092368
Asiedu K, Alotaibi S, Krishnan AV, Kwai N, Poynten A, Markoulli M, Dhanapalaratnam R. Chronic Kidney Disease Has No Impact on Tear Film Substance P Concentration in Type 2 Diabetes. Biomedicines. 2023; 11(9):2368. https://doi.org/10.3390/biomedicines11092368
Chicago/Turabian StyleAsiedu, Kofi, Sultan Alotaibi, Arun V. Krishnan, Natalie Kwai, Ann Poynten, Maria Markoulli, and Roshan Dhanapalaratnam. 2023. "Chronic Kidney Disease Has No Impact on Tear Film Substance P Concentration in Type 2 Diabetes" Biomedicines 11, no. 9: 2368. https://doi.org/10.3390/biomedicines11092368
APA StyleAsiedu, K., Alotaibi, S., Krishnan, A. V., Kwai, N., Poynten, A., Markoulli, M., & Dhanapalaratnam, R. (2023). Chronic Kidney Disease Has No Impact on Tear Film Substance P Concentration in Type 2 Diabetes. Biomedicines, 11(9), 2368. https://doi.org/10.3390/biomedicines11092368







