MALDI-TOF MS Analysis of Serum Peptidome Patterns in Cervical Cancer
Abstract
1. Introduction
2. Methodology
2.1. Characteristics of Participants
2.2. Serum Sample Preparation
2.3. MALDI-TOF MS Processing
2.4. MALDI-TOF MS Data Analysis
3. Results
3.1. Peptide Profile Detection
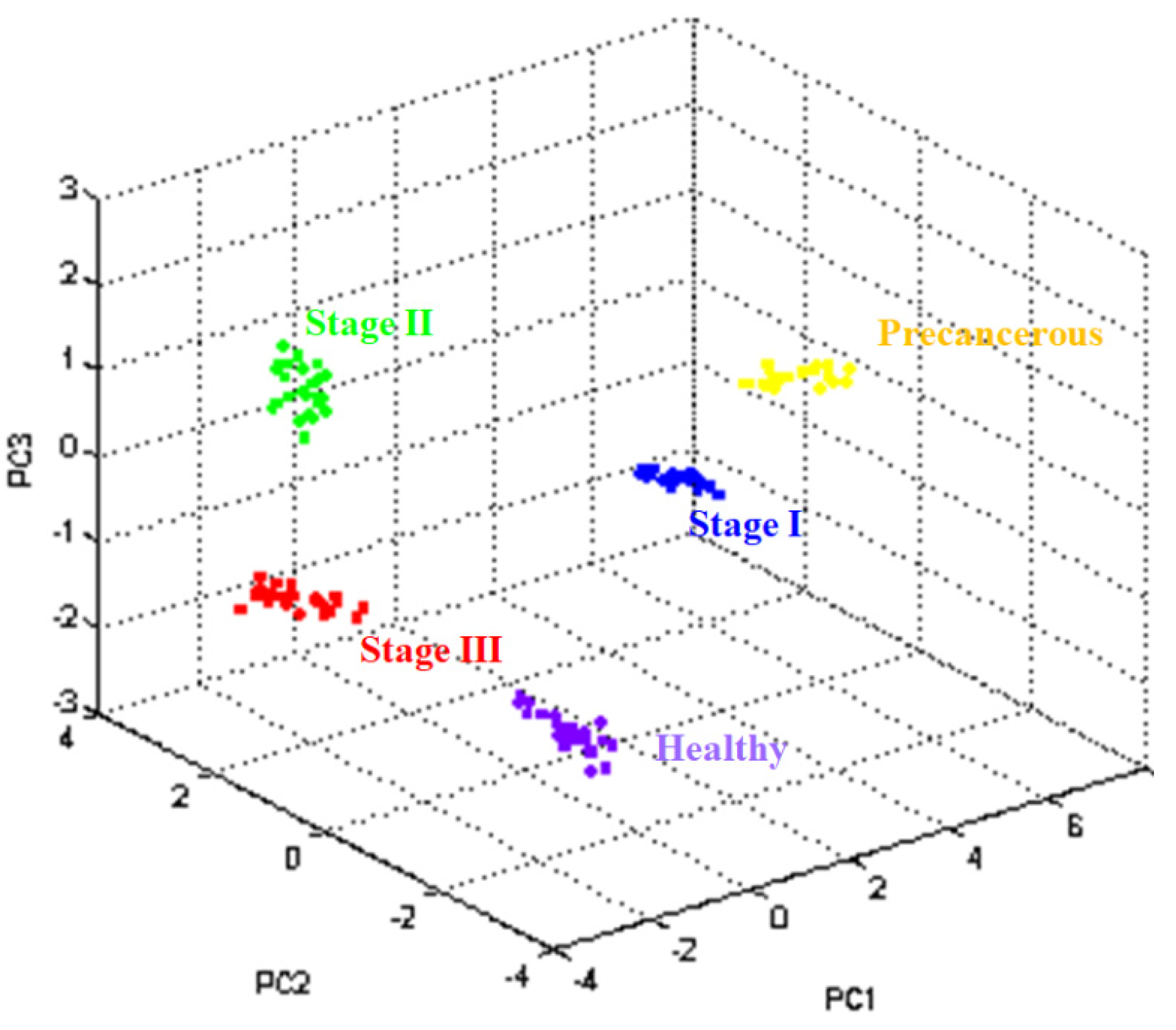
3.2. Cluster Analysis
3.3. The Prominent Expression of Peptide Peaks among the Investigated Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Sankaranarayanan, R.; Ferlay, J. Worldwide burden of gynaecological cancer: The size of the problem. Best Prac. Res. Clin. Obstet. Gynaecol. 2006, 20, 207–225. [Google Scholar] [CrossRef] [PubMed]
- Catarino, R.; Petignat, P.; Dongui, G.; Vassilakos, P. Cervical cancer screening in developing countries at a crossroad: Emerging technologies and policy choices. World J. Clin. Oncol. 2015, 6, 281–290. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Tewari, K.S.; Sill, M.W.; Long, I.I.I.H.J.; Penson, R.T.; Huang, H.; Ramondetta, L.M.; Landrum, L.M.; Oaknin, A.; Reid, T.J.; Leitao, M.M.; et al. Faculty Opinions recommendation of Improved survival with bevacizumab in advanced cervical cancer. N. Engl. J. Med. 2014, 370, 734–743. [Google Scholar] [CrossRef]
- Chadza, E.; Chirwa, E.; Maluwa, A.; Malata, A.; Kazembe, A.; Chimwaza, A. Factors that contribute to delay in seeking cervical cancer diagnosis and treatment among women in Malawi. Health 2012, 4, 1015–1022. [Google Scholar] [CrossRef]
- Matsuo, K.; Machida, H.; Mandelbaum, R.S.; Konishi, I.; Mikami, M. Validation of the 2018 FIGO cervical cancer staging system. Gynecol. Oncol. 2018, 152, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Kelly-Spratt, K.S.; Kasarda, A.E.; Igra, M.; Kemp, C.J. A Mouse Model Repository for Cancer Biomarker Discovery. J. Proteome Res. 2008, 7, 3613–3618. [Google Scholar] [CrossRef][Green Version]
- Shin, S.H.; Bode, A.M.; Dong, Z. Precision medicine: The foundation of future cancer therapeutics. NPJ Precis. Oncol. 2017, 1, 12–14. [Google Scholar] [CrossRef]
- Kosugi, S.-I.; Nishimaki, T.; Kanda, T.; Nakagawa, S.; Ohashi, M.; Hatakeyama, K. Clinical Significance of Serum Carcinoembryonic Antigen, Carbohydrate Antigen 19-9, and Squamous Cell Carcinoma Antigen Levels in Esophageal Cancer Patients. World J. Surg. 2004, 28, 680–685. [Google Scholar] [CrossRef]
- Petrelli, N.J.; Palmer, M.; Herrera, L.; Bhargava, A. The utility of squamous cell carcinoma antigen for the follow-up of patients with squamous cell carcinoma of the anal canal. Cancer 1992, 70, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.D.; Jackowski, G.; Bayer, N.; Lawrence, M.; Jaeschke, R. Biochemical markers in acute ischemic stroke. Can. Med. Assoc. J. 2000, 162, 1139–1140. [Google Scholar]
- Mazzone, P.J.; Wang, X.-F.; Han, X.; Choi, H.; Seeley, M.; Scherer, R.; Doseeva, V. Evaluation of a Serum Lung Cancer Biomarker Panel. Biomark. Insights 2018, 13, 1–5. [Google Scholar] [CrossRef]
- Guo, J.; Yang, J.; Zhang, X.; Feng, X.; Zhang, H.; Chen, L.; Johnson, H.; Persson, J.L.; Xiao, K. A Panel of Biomarkers for Diagnosis of Prostate Cancer Using Urine Samples. Anticancer Res. 2018, 38, 1471–1477. [Google Scholar] [CrossRef]
- Wang, Q.-T.; Li, Y.-Z.; Liang, Y.-F.; Hu, C.-J.; Zhai, Y.-H.; Zhao, G.-F.; Zhang, J.; Li, N.; Ni, A.-P.; Chen, W.-M.; et al. Construction of A Multiple Myeloma Diagnostic Model by Magnetic Bead-Based MALDI-TOF Mass Spectrometry of Serum and Pattern Recognition Software. Anat. Rec. 2009, 292, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Yigitbasi, T.; Calibasi-Kocal, G.; Buyukuslu, N.; Atahan, M.K.; Kupeli, H.; Yigit, S.; Tarcan, E.; Baskin, Y. An efficient biomarker panel for diagnosis of breast cancer using surface-enhanced laser desorption ionization time-of-flight mass spectrometry. Biomed. Rep. 2018, 8, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Tirumalai, R.S.; Chan, K.C.; Prieto, D.A.; Issaq, H.J.; Conrads, T.P.; Veenstra, T.D. Characterization of the Low Molecular Weight Human Serum Proteome. Mol. Cell. Proteom. 2003, 2, 1096–1103. [Google Scholar] [CrossRef]
- Ying, X.; Han, S.-X.; Wang, J.-L.; Zhou, X.; Jin, G.-H.; Jin, L.; Wang, H.; Wu, L.; Zhang, J.; Zhu, Q. Serum peptidome patterns of hepatocellular carcinoma based on magnetic bead separation and mass spectrometry analysis. Diagn. Pathol. 2013, 8, 130. [Google Scholar] [CrossRef]
- Zhang, Z.; Bast, R.; Yu, Y.; Li, J.; Sokoll, L.J.; Rai, A.J.; Rosenzweig, J.M.; Cameron, B.; Wang, Y.Y.; Meng, X.-Y.; et al. Three Biomarkers Identified from Serum Proteomic Analysis for the Detection of Early Stage Ovarian Cancer. Cancer Res. 2004, 64, 5882–5890. [Google Scholar] [CrossRef]
- Li, Y.-Z.; Hu, C.-J.; Leng, X.-M.; Zhao, G.-F.; Li, N.; Xu, Y. Promising Diagnostic Biomarkers for Primary Biliary Cirrhosis Identified with Magnetic Beads and MALDI-TOF-MS. Anat. Rec. 2009, 292, 455–460. [Google Scholar] [CrossRef]
- Wu, S.; Xu, K.; Chen, G.; Zhang, J.; Liu, Z.; Xie, X. Identification of serum biomarkers for ovarian cancer using MALDI–TOF-MS combined with magnetic beads. Int. J. Clin. Oncol. 2012, 17, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Mahboob, S.; Mohamedali, A.; Ahn, S.; Schulz-Knappe, P.; Nice, E.; Baker, M. Is isolation of comprehensive human plasma peptidomes an achievable quest? J. Proteom. 2015, 127, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Petricoin, E.F.; Belluco, C.; Araujo, R.P.; Liotta, L.A. The blood peptidome: A higher dimension of information content for cancer biomarker discovery. Nat. Rev. Cancer 2006, 6, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.-H.; Xu, X.-H.; Wang, Y.; Linq, Q.-X.; Bi, Y.-T.; Miao, X.-J.; Ye, C.-F.; Gao, S.-X.; Gong, C.-Y.; Xiang, H.; et al. A Prognostic Biomarker for Gastric Cancer with Lymph Node Metastases. Anat. Rec. 2013, 296, 590–594. [Google Scholar] [CrossRef]
- Ahmed, N.; Oliva, K.T.; Barker, G.; Hoffmann, P.; Reeve, S.; Smith, I.A.; Quinn, M.A.; Rice, G.E. Proteomic tracking of serum protein isoforms as screening biomarkers of ovarian cancer. Proteomics 2005, 5, 4625–4636. [Google Scholar] [CrossRef]
- Veenstra, T.D.; Prieto, D.A.; Conrads, T.P. Proteomic patterns for early cancer detection. Drug Discov. Today 2004, 9, 889–897. [Google Scholar] [CrossRef]
- Valerio, A.; Basso, D.; Mazza, S.; Baldo, G.; Tiengo, A.; Pedrazzoli, S.; Seraglia, R.; Plebani, M. Serum protein profiles of patients with pancreatic cancer and chronic pancreatitis: Searching for a diagnostic protein pattern. Rapid Commun. Mass Spectrom. 2001, 15, 2420–2425. [Google Scholar] [CrossRef]
- Lavine, B.K.; White, C.G.; DeNoyer, L.; Mechref, Y. Multivariate classification of disease phenotypes of esophageal adenocarcinoma by pattern recognition analysis of MALDI-TOF mass spectra of serum N-linked glycans. Microchem. J. 2017, 132, 83–88. [Google Scholar] [CrossRef]
- Schwamborn, K.; Krieg, R.C.; Uhlig, S.; Ikenberg, H.; Wellmann, A. MALDI imaging as a specific diagnostic tool for routine cervical cytology specimens. Int. J. Mol. Med. 2011, 27, 417–421. [Google Scholar] [CrossRef][Green Version]
- Li, Z.; Tang, C.; Li, X.; Li, J.; Wang, W.; Qin, H.; Xu, B.; Chen, J.; Gao, H.; He, K.; et al. Detection and significance of small-cell lung cancer serum protein markers using MALDI-TOF-MS. Int. J. Clin. Exp. Med. 2017, 10, 929–936. [Google Scholar]
- Zhou, J.-H.; Ma, Y.-Y.; Lu, H.L.; Tang, Y.L.; Zhang, Q.-Y.; Zhao, C.-H. Five Serum Proteins Identified Using SELDI-TOF-MS as Potential Biomarkers of Gastric Cancer. Jpn. J. Clin. Oncol. 2010, 40, 336–342. [Google Scholar] [CrossRef]
- Li, P.; Ma, D.; Zhu, S.T.; Tang, X.D.; Zhang, S.T. Serum peptide mapping in gastric precancerous lesion and cancer. J. Dig. Dis. 2014, 15, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Chaiyarit, P.; Taweechaisupapong, S.; Jaresitthikunchai, J.; Phaonakrop, N.; Roytrakul, S. Comparative evaluation of 5–15-kDa salivary proteins from patients with different oral diseases by MALDI-TOF/TOF mass spectrometry. Clin. Oral Investig. 2015, 19, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Sandanayake, N.S.; Camuzeaux, S.; Sinclair, J.; Blyuss, O.; Andreola, F.; Chapman, M.H.; Webster, G.J.; Smith, R.C.; Timms, J.F.; Pereira, S.P. Identification of potential serum peptide bi-omarkers of biliary tract cancer using MALDI MS profiling. BMC Clin. Pathol. 2014, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Pan, C.; Shen, J.; Wang, H.; Yong, L.; Zhang, R. Discrimination analysis of mass spectrometry proteomics for cervical cancer detection. Med. Oncol. 2011, 28, S553–S559. [Google Scholar] [CrossRef]
- Lai, X.; Witzmann, F.A.; Liangpunsakul, S. Characterization of peptides and low molecular weight proteins in plasma from subjects with hepatocellular carcinoma. Proteomics 2014, 1, 6. [Google Scholar]
- Thiede, B.; Wittmann-Liebold, B.; Bienert, M.; Krause, E. MALDI-MS for C-terminal sequence determination of peptides and proteins degraded by carboxypeptidase Y and P. FEBS Lett. 1995, 357, 65–69. [Google Scholar] [CrossRef]
- Hajduk, J.; Matysiak, J.; Kokot, Z.J. Challenges in biomarker discovery with MALDI-TOF MS. Clin. Chim. Acta 2016, 458, 84–98. [Google Scholar] [CrossRef]
- Villanueva, J.; Shaffer, D.R.; Philip, J.; Chaparro, C.A.; Erdjument-Bromage, H.; Olshen, A.B.; Fleisher, M.; Lilja, H.; Brogi, E.; Boyd, J.; et al. Faculty Opinions recommendation of Differential exoprotease activities confer tumor-specific serum peptidome patterns. J. Clin. Investig. 2006, 116, 271–284. [Google Scholar] [CrossRef]
- Villanueva, J.; Martorella, A.J.; Lawlor, K.; Philip, J.; Fleisher, M.; Robbins, R.J.; Tempst, P. Serum Peptidome Patterns That Distinguish Metastatic Thyroid Carcinoma from Cancer-free Controls Are Unbiased by Gender and Age. Mol. Cell. Proteom. 2006, 5, 1840–1852. [Google Scholar] [CrossRef]
- Karas, M.; Glückmann, M.; Schäfer, J. Ionization in matrix-assisted laser desorption/ionization: Singly charged molecular ions are the lucky survivors. J. Mass Spectrom. 2000, 35, 1–12. [Google Scholar] [CrossRef]
- Siuzdak, G. An introduction to mass spectrometry ionization: An excerpt from the expanding role of mass spectrometry in biotechnology. JALA J. Assoc. Lab. Autom. 2004, 9, 50–63. [Google Scholar] [CrossRef]
- Aebersold, R.; Mann, M. Mass spectrometry-based proteomics. Nature 2003, 422, 198–207. [Google Scholar] [CrossRef] [PubMed]
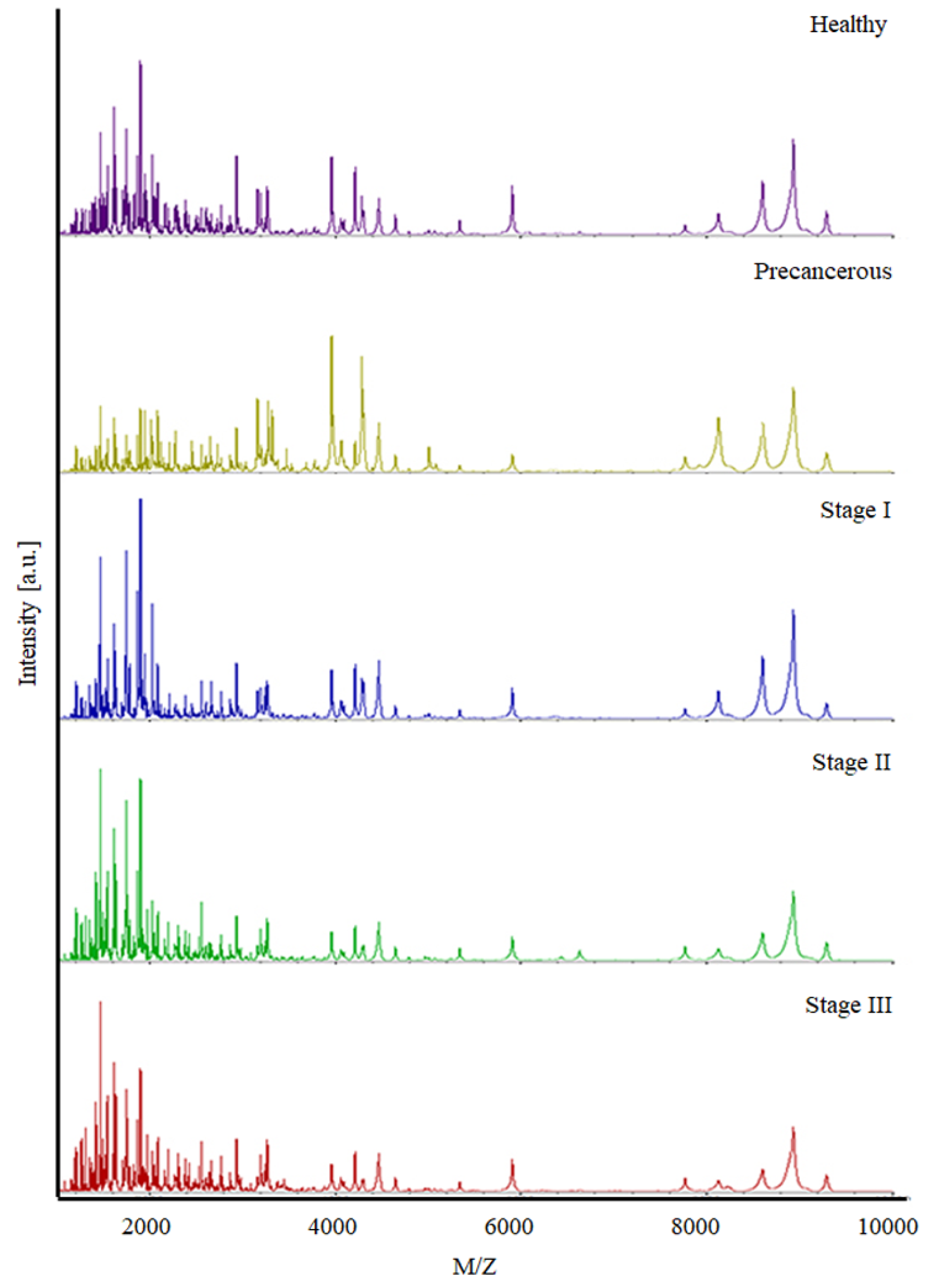

| Sample Group | Pathological Stage | Overall No. of Cases | Training Set | Validation Set | ||
|---|---|---|---|---|---|---|
| No. of Cases | Age (Median/Range) | No. of Cases | Age (Median/Range) | |||
| Healthy controls | - | 83 | 30 | 42/(31–60) | 53 | 42/(30–59) |
| Patients with cervical cancer | Precancerous | 51 | 30 | 44/(30–61) | 21 | 45/(30–61) |
| Stage I | 24 | 15 | 45/(32–54) | 9 | 48/(32–65) | |
| Stage II | 37 | 15 | 45/(31–65) | 22 | 51/(39–61) | |
| Stage III | 27 | 15 | 44/(32–61) | 12 | 52/(43–63) | |
| Average Mass | Mass Range (m/z) | Predominated Group | Statistical Analysis |
|---|---|---|---|
| 1466.91 | 1460.72–1481.63 | Stage III | Different average, pWK, p = 0.0001 |
| 1488.72 | 1481.63–1498.28 | Stage III | GA |
| 1741.72 | 1436.11–1747.68 | Stage I | GA |
| 1898.01 | 1892.44–1912.26 | Stage I | SNN, ANOVA, p = 0.000008 |
| 2044.73 | 2038.47–2052.65 | Healthy subjects | SNN |
| 2307.55 | 2300.88–2316.39 | Stage II | GA |
| 3159.09 | 3148.93–3176.56 | Precancerous | ANOVA, p = 0.000008 |
| 3242.1 | 3231.43–3252.41 | Stage II | GA |
| 4299.4 | 4293.28–4336.07 | Stage III | ANOVA, p-value = 0.000002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rungkamoltip, P.; Roytrakul, S.; Navakanitworakul, R. MALDI-TOF MS Analysis of Serum Peptidome Patterns in Cervical Cancer. Biomedicines 2023, 11, 2327. https://doi.org/10.3390/biomedicines11082327
Rungkamoltip P, Roytrakul S, Navakanitworakul R. MALDI-TOF MS Analysis of Serum Peptidome Patterns in Cervical Cancer. Biomedicines. 2023; 11(8):2327. https://doi.org/10.3390/biomedicines11082327
Chicago/Turabian StyleRungkamoltip, Phetploy, Sittiruk Roytrakul, and Raphatphorn Navakanitworakul. 2023. "MALDI-TOF MS Analysis of Serum Peptidome Patterns in Cervical Cancer" Biomedicines 11, no. 8: 2327. https://doi.org/10.3390/biomedicines11082327
APA StyleRungkamoltip, P., Roytrakul, S., & Navakanitworakul, R. (2023). MALDI-TOF MS Analysis of Serum Peptidome Patterns in Cervical Cancer. Biomedicines, 11(8), 2327. https://doi.org/10.3390/biomedicines11082327






