Bipolar Androgen Therapy: When Excess Fuel Extinguishes the Fire
Abstract
1. Introduction
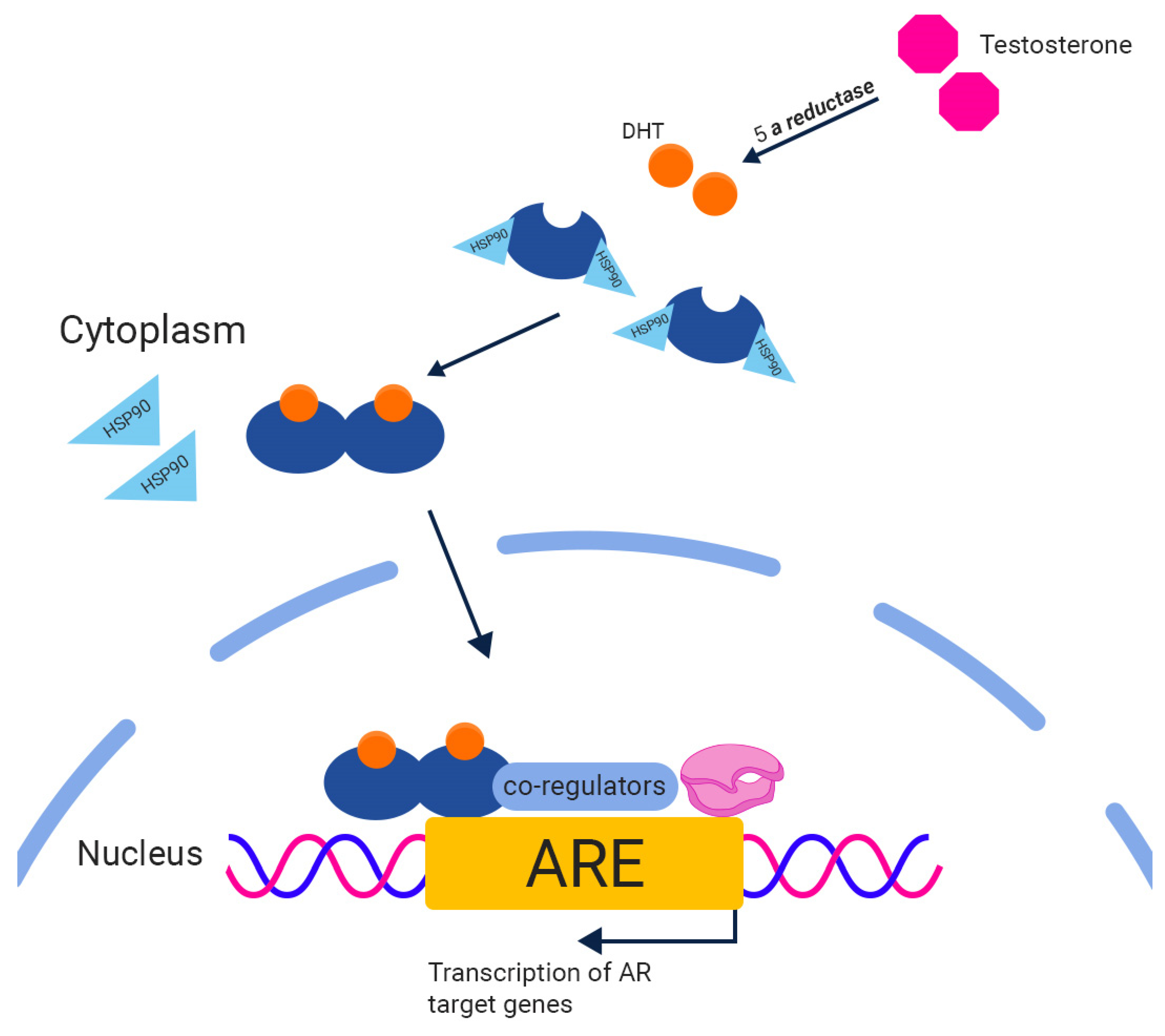
2. The Androgen Receptor (AR)
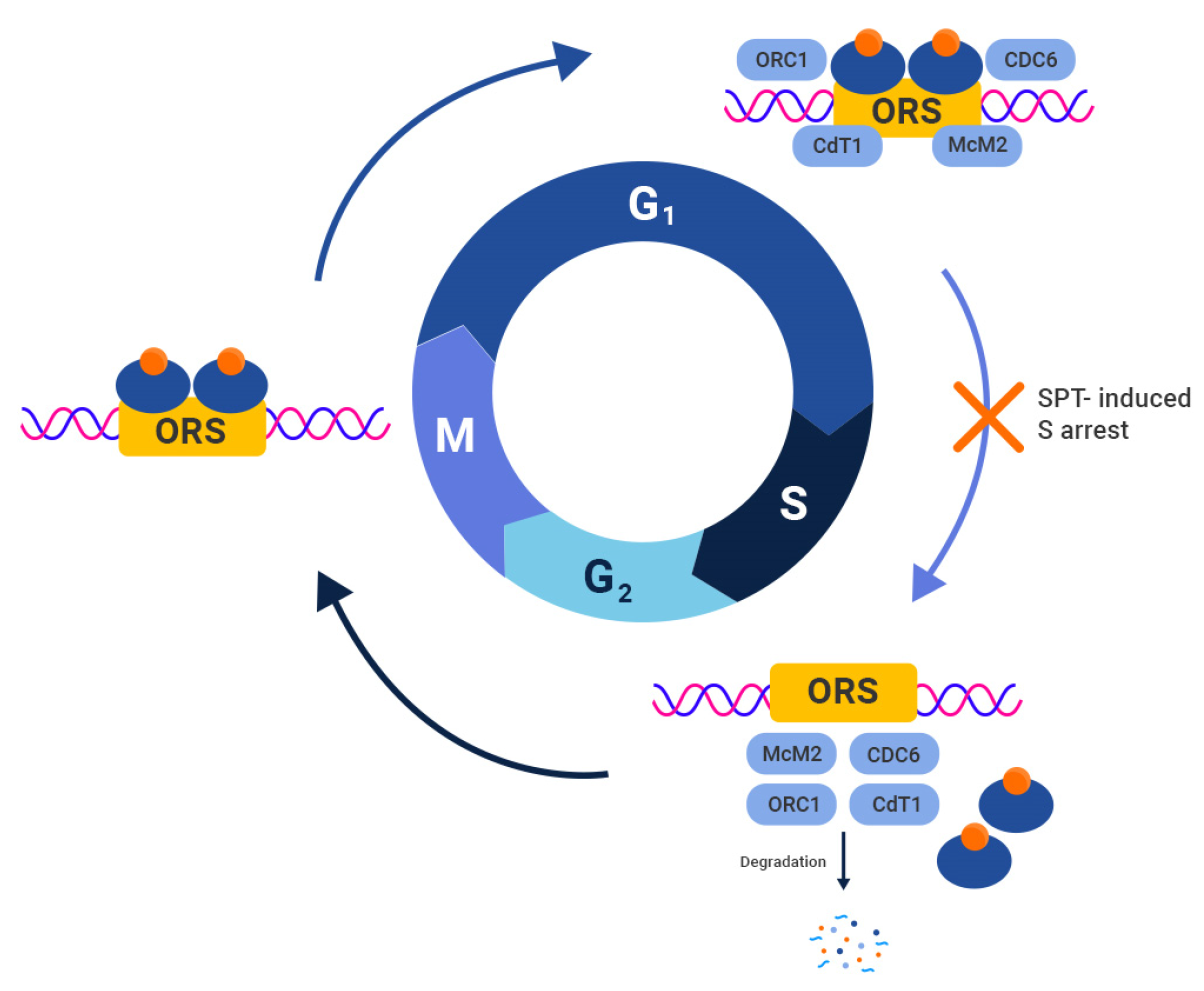
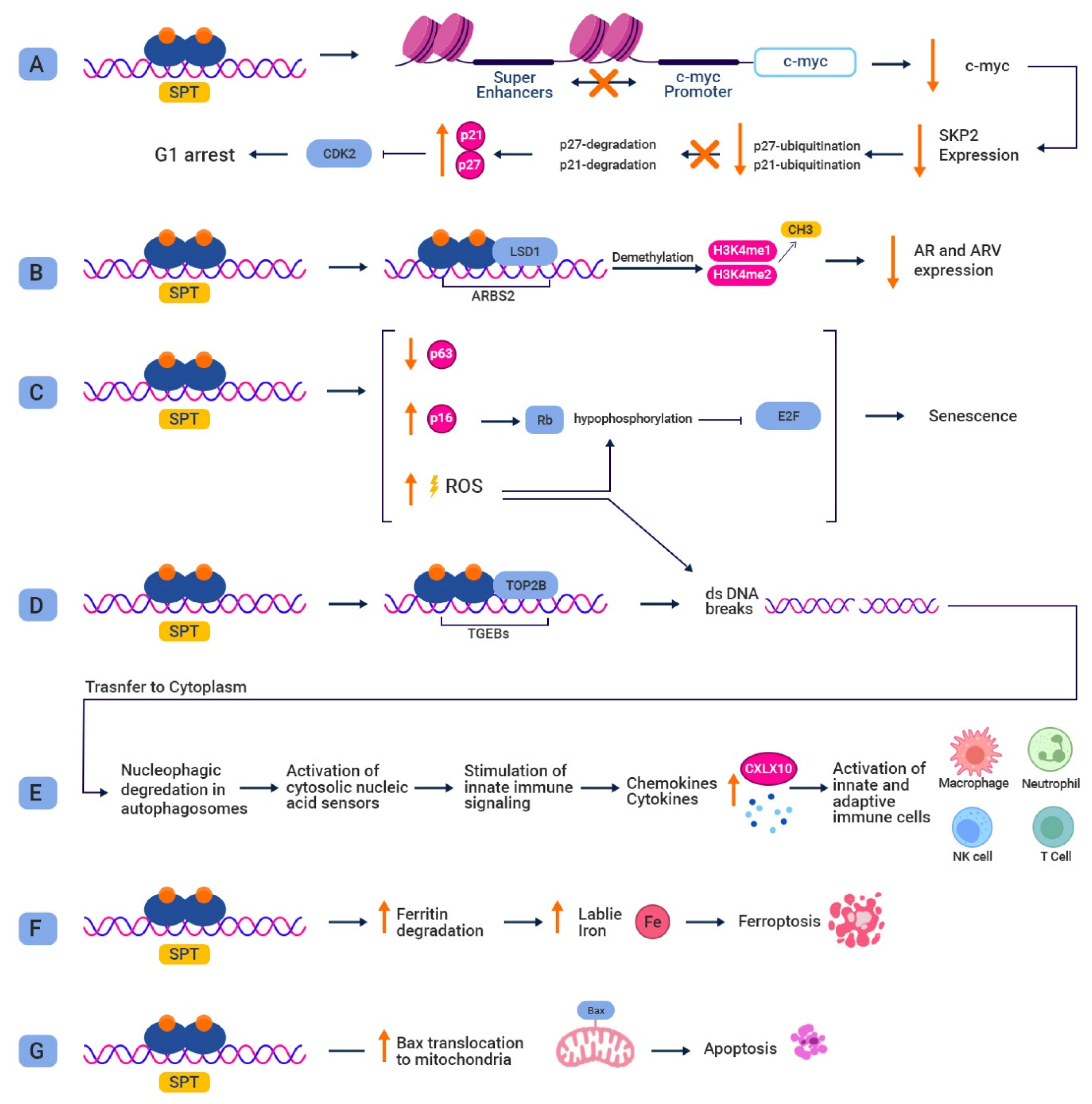
3. Preclinical Studies Utilizing SPT
4. Bipolar Androgen Therapy (BAT)
5. BAT Studies
6. Ongoing Clinical Studies
7. Safety
8. Biomarker Selection
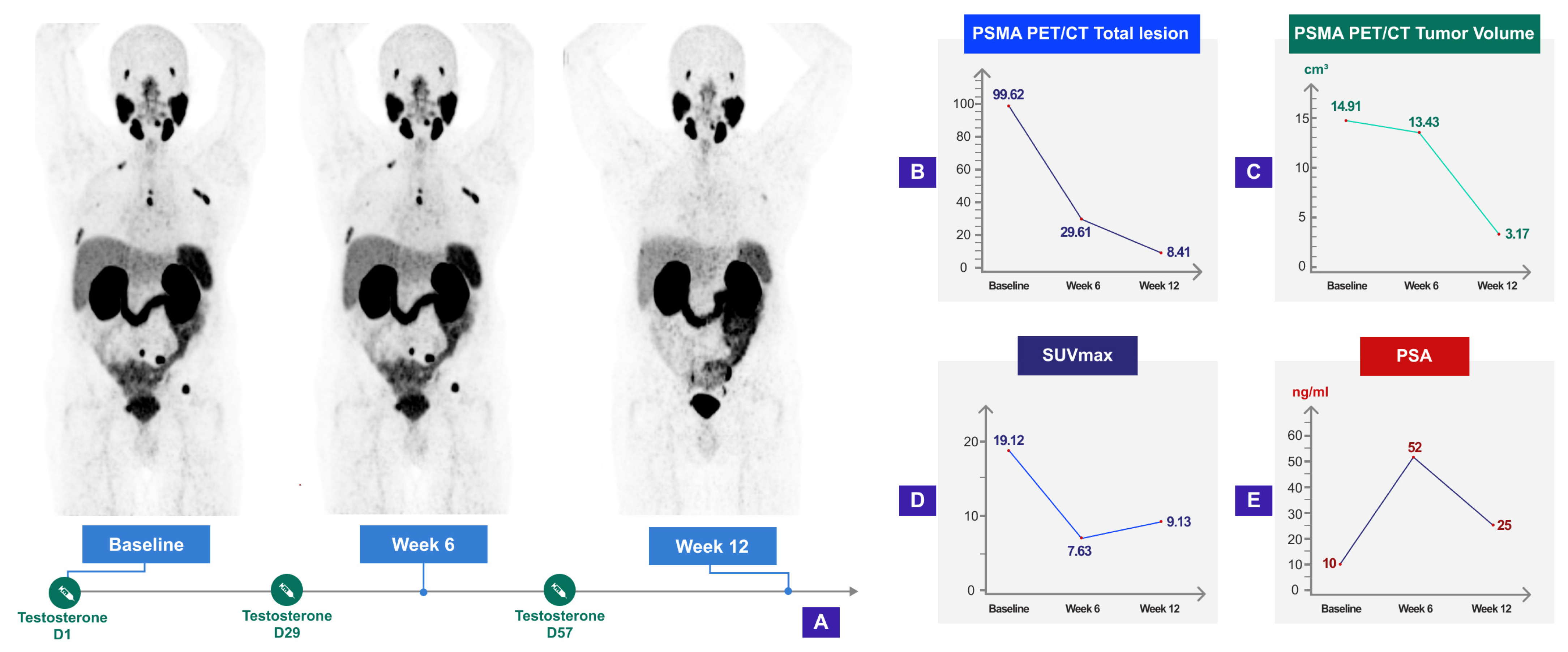
9. BAT and Molecular Imaging
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gandaglia, G.; Leni, R.; Bray, F.; Fleshner, N.; Freedland, S.J.; Kibel, A.; Stattin, P.; Van Poppel, H.; La Vecchia, C. Epidemiology and Prevention of Prostate Cancer. Eur. Urol. Oncol. 2021, 4, 877–892. [Google Scholar] [CrossRef] [PubMed]
- De Silva, F.; Alcorn, J. A Tale of Two Cancers: A Current Concise Overview of Breast and Prostate Cancer. Cancers 2022, 14, 2954. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Mohler, J.L.; Antonarakis, E.S.; Armstrong, A.J.; D’Amico, A.V.; Davis, B.J.; Dorff, T.; Eastham, J.A.; Enke, C.A.; Farrington, T.A.; Higano, C.S.; et al. Prostate Cancer, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2019, 17, 479–505. [Google Scholar] [CrossRef]
- Morote, J.; Aguilar, A.; Planas, J.; Trilla, E. Definition of Castrate Resistant Prostate Cancer: New Insights. Biomedicines 2022, 10, 689. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.B.; Mehra, R.; Chinnaiyan, A.M.; Shen, R.; Ghosh, D.; Zhou, M.; MacVicar, G.R.; Varambally, S.; Harwood, J.; Bismar, T.A.; et al. Androgen-Independent Prostate Cancer Is a Heterogeneous Group of Diseases: Lessons from a Rapid Autopsy Program. Cancer Res. 2004, 64, 9209–9216. [Google Scholar] [CrossRef] [PubMed]
- de Bono, J.S.; Logothetis, C.J.; Molina, A.; Fizazi, K.; North, S.; Chu, L.; Chi, K.N.; Jones, R.J.; Goodman, O.B., Jr.; Saad, F.; et al. Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med. 2011, 364, 1995–2005. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.I.; Fizazi, K.; Saad, F.; Taplin, M.E.; Sternberg, C.N.; Miller, K.; de Wit, R.; Mulders, P.; Chi, K.N.; Shore, N.D.; et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med. 2012, 367, 1187–1197. [Google Scholar] [CrossRef]
- Schrader, A.J.; Boegemann, M.; Ohlmann, C.H.; Schnoeller, T.J.; Krabbe, L.M.; Hajili, T.; Jentzmik, F.; Stoeckle, M.; Schrader, M.; Herrmann, E.; et al. Enzalutamide in castration-resistant prostate cancer patients progressing after docetaxel and abiraterone. Eur. Urol. 2014, 65, 30–36. [Google Scholar] [CrossRef]
- Noonan, K.L.; North, S.; Bitting, R.L.; Armstrong, A.J.; Ellard, S.L.; Chi, K.N. Clinical activity of abiraterone acetate in patients with metastatic castration-resistant prostate cancer progressing after enzalutamide. Ann. Oncol. 2013, 24, 1802–1807. [Google Scholar] [CrossRef]
- Loriot, Y.; Bianchini, D.; Ileana, E.; Sandhu, S.; Patrikidou, A.; Pezaro, C.; Albiges, L.; Attard, G.; Fizazi, K.; De Bono, J.S.; et al. Antitumour activity of abiraterone acetate against metastatic castration-resistant prostate cancer progressing after docetaxel and enzalutamide (MDV3100). Ann. Oncol. 2013, 24, 1807–1812. [Google Scholar] [CrossRef] [PubMed]
- Brasso, K.; Thomsen, F.B.; Schrader, A.J.; Schmid, S.C.; Lorente, D.; Retz, M.; Merseburger, A.S.; von Klot, C.A.; Boegemann, M.; de Bono, J. Enzalutamide Antitumour Activity Against Metastatic Castration-resistant Prostate Cancer Previously Treated with Docetaxel and Abiraterone: A Multicentre Analysis. Eur. Urol. 2015, 68, 317–324. [Google Scholar] [CrossRef] [PubMed]
- de Wit, R.; de Bono, J.; Sternberg, C.N.; Fizazi, K.; Tombal, B.; Wülfing, C.; Kramer, G.; Eymard, J.-C.; Bamias, A.; Carles, J.; et al. Cabazitaxel versus Abiraterone or Enzalutamide in Metastatic Prostate Cancer. N. Engl. J. Med. 2019, 381, 2506–2518. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, C.N.; Fizazi, K.; Saad, F.; Shore, N.D.; De Giorgi, U.; Penson, D.F.; Ferreira, U.; Efstathiou, E.; Madziarska, K.; Kolinsky, M.P.; et al. Enzalutamide and Survival in Nonmetastatic, Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020, 382, 2197–2206. [Google Scholar] [CrossRef]
- Smith, M.R.; Saad, F.; Chowdhury, S.; Oudard, S.; Hadaschik, B.A.; Graff, J.N.; Olmos, D.; Mainwaring, P.N.; Lee, J.Y.; Uemura, H.; et al. Apalutamide Treatment and Metastasis-free Survival in Prostate Cancer. N. Engl. J. Med. 2018, 378, 1408–1418. [Google Scholar] [CrossRef]
- Fizazi, K.; Shore, N.; Tammela, T.L.; Ulys, A.; Vjaters, E.; Polyakov, S.; Jievaltas, M.; Luz, M.; Alekseev, B.; Kuss, I.; et al. Darolutamide in Nonmetastatic, Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2019, 380, 1235–1246. [Google Scholar] [CrossRef]
- Fizazi, K.; Shore, N.; Tammela, T.L.; Ulys, A.; Vjaters, E.; Polyakov, S.; Jievaltas, M.; Luz, M.; Alekseev, B.; Kuss, I.; et al. Nonmetastatic, Castration-Resistant Prostate Cancer and Survival with Darolutamide. N. Engl. J. Med. 2020, 383, 1040–1049. [Google Scholar] [CrossRef]
- Fizazi, K.; Tran, N.; Fein, L.; Matsubara, N.; Rodriguez-Antolin, A.; Alekseev, B.Y.; Özgüroğlu, M.; Ye, D.; Feyerabend, S.; Protheroe, A.; et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2017, 377, 352–360. [Google Scholar] [CrossRef]
- James, N.D.; de Bono, J.S.; Spears, M.R.; Clarke, N.W.; Mason, M.D.; Dearnaley, D.P.; Ritchie, A.W.S.; Amos, C.L.; Gilson, C.; Jones, R.J.; et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N. Engl. J. Med. 2017, 377, 338–351. [Google Scholar] [CrossRef]
- Armstrong, A.J.; Szmulewitz, R.Z.; Petrylak, D.P.; Holzbeierlein, J.; Villers, A.; Azad, A.; Alcaraz, A.; Alekseev, B.; Iguchi, T.; Shore, N.D.; et al. ARCHES: A Randomized, Phase III Study of Androgen Deprivation Therapy with Enzalutamide or Placebo in Men with Metastatic Hormone-Sensitive Prostate Cancer. J. Clin. Oncol. 2019, 37, 2974–2986. [Google Scholar] [CrossRef]
- Davis, I.D.; Martin, A.J.; Stockler, M.R.; Begbie, S.; Chi, K.N.; Chowdhury, S.; Coskinas, X.; Frydenberg, M.; Hague, W.E.; Horvath, L.G.; et al. Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N. Engl. J. Med. 2019, 381, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Chi, K.N.; Agarwal, N.; Bjartell, A.; Chung, B.H.; Pereira de Santana Gomes, A.J.; Given, R.; Juárez Soto, Á.; Merseburger, A.S.; Özgüroğlu, M.; Uemura, H.; et al. Apalutamide for Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2019, 381, 13–24. [Google Scholar] [CrossRef]
- Teply, B.A.; Qiu, F.; Antonarakis, E.S.; Carducci, M.A.; Denmeade, S.R. Risk of development of visceral metastases subsequent to abiraterone vs. placebo: An analysis of mode of radiographic progression in COU-AA-302. Prostate 2019, 79, 929–933. [Google Scholar] [CrossRef] [PubMed]
- Velho, P.I.; Bastos, D.A.; Antonarakis, E.S. New approaches to targeting the androgen receptor pathway in prostate cancer. Clin. Adv. Hematol. Oncol. 2021, 19, 228–240. [Google Scholar] [PubMed]
- Denmeade, S.R.; Isaacs, J.T. Bipolar androgen therapy: The rationale for rapid cycling of supraphysiologic androgen/ablation in men with castration resistant prostate cancer. Prostate 2010, 70, 1600–1607. [Google Scholar] [CrossRef]
- Schweizer, M.T.; Antonarakis, E.S.; Wang, H.; Ajiboye, A.S.; Spitz, A.; Cao, H.; Luo, J.; Haffner, M.C.; Yegnasubramanian, S.; Carducci, M.A.; et al. Effect of bipolar androgen therapy for asymptomatic men with castration-resistant prostate cancer: Results from a pilot clinical study. Sci. Transl. Med. 2015, 7, 269ra. [Google Scholar] [CrossRef]
- Mohammad, O.S.; Nyquist, M.D.; Schweizer, M.T.; Balk, S.P.; Corey, E.; Plymate, S.; Nelson, P.S.; Mostaghel, E.A. Supraphysiologic Testosterone Therapy in the Treatment of Prostate Cancer: Models, Mechanisms and Questions. Cancers 2017, 9, 166. [Google Scholar] [CrossRef]
- Isaacs, J.T.; D’Antonio, J.M.; Chen, S.; Antony, L.; Dalrymple, S.P.; Ndikuyeze, G.H.; Luo, J.; Denmeade, S.R. Adaptive auto-regulation of androgen receptor provides a paradigm shifting rationale for bipolar androgen therapy (BAT) for castrate resistant human prostate cancer. Prostate 2012, 72, 1491–1505. [Google Scholar] [CrossRef]
- Isaacs, J.T.; Brennen, W.N.; Denmeade, S.R. Rationale for bipolar androgen therapy (BAT) for metastatic prostate cancer. Cell Cycle 2017, 16, 1639–1640. [Google Scholar] [CrossRef][Green Version]
- Schweizer, M.T.; Wang, H.; Luber, B.; Nadal, R.; Spitz, A.; Rosen, D.M.; Cao, H.; Antonarakis, E.S.; Eisenberger, M.A.; Carducci, M.A.; et al. Bipolar Androgen Therapy for Men with Androgen Ablation Naïve Prostate Cancer: Results from the Phase II BATMAN Study. Prostate 2016, 76, 1218–1226. [Google Scholar] [CrossRef]
- Denmeade, S.; Lim, S.J.; Isaaccson Velho, P.; Wang, H. PSA provocation by bipolar androgen therapy may predict duration of response to first-line androgen deprivation: Updated results from the BATMAN study. Prostate 2022, 82, 1529–1536. [Google Scholar] [CrossRef]
- Teply, B.A.; Wang, H.; Luber, B.; Sullivan, R.; Rifkind, I.; Bruns, A.; Spitz, A.; DeCarli, M.; Sinibaldi, V.; Pratz, C.F.; et al. Bipolar androgen therapy in men with metastatic castration-resistant prostate cancer after progression on enzalutamide: An open-label, phase 2, multicohort study. Lancet Oncol. 2018, 19, 76–86. [Google Scholar] [CrossRef]
- Denmeade, S.R.; Wang, H.; Agarwal, N.; Smith, D.C.; Schweizer, M.T.; Stein, M.N.; Assikis, V.; Twardowski, P.W.; Flaig, T.W.; Szmulewitz, R.Z.; et al. TRANSFORMER: A Randomized Phase II Study Comparing Bipolar Androgen Therapy Versus Enzalutamide in Asymptomatic Men with Castration-Resistant Metastatic Prostate Cancer. J. Clin. Oncol. 2021, 39, 1371–1382. [Google Scholar] [CrossRef] [PubMed]
- Markowski, M.C.; Wang, H.; Sullivan, R.; Rifkind, I.; Sinibaldi, V.; Schweizer, M.T.; Teply, B.A.; Ngomba, N.; Fu, W.; Carducci, M.A.; et al. A Multicohort Open-label Phase II Trial of Bipolar Androgen Therapy in Men with Metastatic Castration-resistant Prostate Cancer (RESTORE): A Comparison of Post-abiraterone Versus Post-enzalutamide Cohorts. Eur. Urol. 2021, 79, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Sena, L.A.; Wang, H.; Lim Sc, M.S.; Rifkind, I.; Ngomba, N.; Isaacs, J.T.; Luo, J.; Pratz, C.; Sinibaldi, V.; Carducci, M.A.; et al. Bipolar androgen therapy sensitizes castration-resistant prostate cancer to subsequent androgen receptor ablative therapy. Eur. J. Cancer 2021, 144, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Heemers, H.; Sharifi, N. Androgen Signaling in Prostate Cancer. Cold Spring Harb. Perspect. Med. 2017, 7, a030452. [Google Scholar] [CrossRef] [PubMed]
- Aurilio, G.; Cimadamore, A.; Mazzucchelli, R.; Lopez-Beltran, A.; Verri, E.; Scarpelli, M.; Massari, F.; Cheng, L.; Santoni, M.; Montironi, R. Androgen Receptor Signaling Pathway in Prostate Cancer: From Genetics to Clinical Applications. Cells 2020, 9, 2653. [Google Scholar] [CrossRef]
- Tan, M.H.; Li, J.; Xu, H.E.; Melcher, K.; Yong, E.L. Androgen receptor: Structure, role in prostate cancer and drug discovery. Acta Pharmacol. Sin. 2015, 36, 3–23. [Google Scholar] [CrossRef]
- Antony, L.; van der Schoor, F.; Dalrymple, S.L.; Isaacs, J.T. Androgen receptor (AR) suppresses normal human prostate epithelial cell proliferation via AR/β-catenin/TCF-4 complex inhibition of c-MYC transcription. Prostate 2014, 74, 1118–1131. [Google Scholar] [CrossRef]
- Lee, S.O.; Tian, J.; Huang, C.K.; Ma, Z.; Lai, K.P.; Hsiao, H.; Jiang, M.; Yeh, S.; Chang, C. Suppressor role of androgen receptor in proliferation of prostate basal epithelial and progenitor cells. J. Endocrinol. 2012, 213, 173–182. [Google Scholar] [CrossRef]
- Vander Griend, D.J.; Litvinov, I.V.; Isaacs, J.T. Conversion of androgen receptor signaling from a growth suppressor in normal prostate epithelial cells to an oncogene in prostate cancer cells involves a gain of function in c-Myc regulation. Int. J. Biol. Sci. 2014, 10, 627–642. [Google Scholar] [CrossRef]
- Sharma, M.; Li, X.; Wang, Y.; Zarnegar, M.; Huang, C.Y.; Palvimo, J.J.; Lim, B.; Sun, Z. hZimp10 is an androgen receptor co-activator and forms a complex with SUMO-1 at replication foci. EMBO J. 2003, 22, 6101–6114. [Google Scholar] [CrossRef]
- Huang, C.Y.; Beliakoff, J.; Li, X.; Lee, J.; Li, X.; Sharma, M.; Lim, B.; Sun, Z. hZimp7, a novel PIAS-like protein, enhances androgen receptor-mediated transcription and interacts with SWI/SNF-like BAF complexes. Mol. Endocrinol. 2005, 19, 2915–2929. [Google Scholar] [CrossRef] [PubMed]
- Litvinov, I.V.; Vander Griend, D.J.; Antony, L.; Dalrymple, S.; De Marzo, A.M.; Drake, C.G.; Isaacs, J.T. Androgen receptor as a licensing factor for DNA replication in androgen-sensitive prostate cancer cells. Proc. Natl. Acad. Sci. USA 2006, 103, 15085–15090. [Google Scholar] [CrossRef] [PubMed]
- Tomlins, S.A.; Rhodes, D.R.; Perner, S.; Dhanasekaran, S.M.; Mehra, R.; Sun, X.W.; Varambally, S.; Cao, X.; Tchinda, J.; Kuefer, R.; et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 2005, 310, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.K.; Young, D.; Yeboah, E.D.; Coburn, S.B.; Tettey, Y.; Biritwum, R.B.; Adjei, A.A.; Tay, E.; Niwa, S.; Truelove, A.; et al. TMPRSS2:ERG Gene Fusions in Prostate Cancer of West African Men and a Meta-Analysis of Racial Differences. Am. J. Epidemiol. 2017, 186, 1352–1361. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Xiao, L.; Sheng, L.; Xu, J.; Sun, Z.Q. TMPRSS2:ETS fusions and clinicopathologic characteristics of prostate cancer patients from Eastern China. Asian Pac. J. Cancer Prev. 2014, 15, 3099–3103. [Google Scholar] [CrossRef]
- Tu, J.J.; Rohan, S.; Kao, J.; Kitabayashi, N.; Mathew, S.; Chen, Y.T. Gene fusions between TMPRSS2 and ETS family genes in prostate cancer: Frequency and transcript variant analysis by RT-PCR and FISH on paraffin-embedded tissues. Mod. Pathol. 2007, 20, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, Y.; Sasaki, T.; Fujinami, K.; Sano, J.; Senga, Y.; Miura, T.; Kameda, Y.; Sakuma, Y.; Nakamura, Y.; Harada, M.; et al. ETS family-associated gene fusions in Japanese prostate cancer: Analysis of 194 radical prostatectomy samples. Mod. Pathol. 2010, 23, 1492–1498. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.S.; Schultz, N.; Hieronymus, H.; Gopalan, A.; Xiao, Y.; Carver, B.S.; Arora, V.K.; Kaushik, P.; Cerami, E.; Reva, B.; et al. Integrative genomic profiling of human prostate cancer. Cancer Cell 2010, 18, 11–22. [Google Scholar] [CrossRef]
- Marcelli, M.; Ittmann, M.; Mariani, S.; Sutherland, R.; Nigam, R.; Murthy, L.; Zhao, Y.; DiConcini, D.; Puxeddu, E.; Esen, A.; et al. Androgen receptor mutations in prostate cancer. Cancer Res. 2000, 60, 944–949. [Google Scholar] [PubMed]
- Korpal, M.; Korn, J.M.; Gao, X.; Rakiec, D.P.; Ruddy, D.A.; Doshi, S.; Yuan, J.; Kovats, S.G.; Kim, S.; Cooke, V.G.; et al. An F876L mutation in androgen receptor confers genetic and phenotypic resistance to MDV3100 (enzalutamide). Cancer Discov. 2013, 3, 1030–1043. [Google Scholar] [CrossRef] [PubMed]
- Azad, A.A.; Volik, S.V.; Wyatt, A.W.; Haegert, A.; Le Bihan, S.; Bell, R.H.; Anderson, S.A.; McConeghy, B.; Shukin, R.; Bazov, J.; et al. Androgen Receptor Gene Aberrations in Circulating Cell-Free DNA: Biomarkers of Therapeutic Resistance in Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2015, 21, 2315–2324. [Google Scholar] [CrossRef]
- Arora, V.K.; Schenkein, E.; Murali, R.; Subudhi, S.K.; Wongvipat, J.; Balbas, M.D.; Shah, N.; Cai, L.; Efstathiou, E.; Logothetis, C.; et al. Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell 2013, 155, 1309–1322. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.A.; Arora, V.K.; Sawyers, C.L. Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat. Rev. Cancer 2015, 15, 701–711. [Google Scholar] [CrossRef]
- Grindstad, T.; Andersen, S.; Al-Saad, S.; Donnem, T.; Kiselev, Y.; Nordahl Melbø-Jørgensen, C.; Skjefstad, K.; Busund, L.T.; Bremnes, R.M.; Richardsen, E. High progesterone receptor expression in prostate cancer is associated with clinical failure. PLoS ONE 2015, 10, e0116691. [Google Scholar] [CrossRef][Green Version]
- Karantanos, T.; Evans, C.P.; Tombal, B.; Thompson, T.C.; Montironi, R.; Isaacs, W.B. Understanding the mechanisms of androgen deprivation resistance in prostate cancer at the molecular level. Eur. Urol. 2015, 67, 470–479. [Google Scholar] [CrossRef]
- Lee, E.; Madar, A.; David, G.; Garabedian, M.J.; Dasgupta, R.; Logan, S.K. Inhibition of androgen receptor and β-catenin activity in prostate cancer. Proc. Natl. Acad. Sci. USA 2013, 110, 15710–15715. [Google Scholar] [CrossRef]
- Zhao, J.C.; Fong, K.W.; Jin, H.J.; Yang, Y.A.; Kim, J.; Yu, J. FOXA1 acts upstream of GATA2 and AR in hormonal regulation of gene expression. Oncogene 2016, 35, 4335–4344. [Google Scholar] [CrossRef]
- Chiang, Y.T.; Wang, K.; Fazli, L.; Qi, R.Z.; Gleave, M.E.; Collins, C.C.; Gout, P.W.; Wang, Y. GATA2 as a potential metastasis-driving gene in prostate cancer. Oncotarget 2014, 5, 451–461. [Google Scholar] [CrossRef]
- Linja, M.J.; Savinainen, K.J.; Saramäki, O.R.; Tammela, T.L.; Vessella, R.L.; Visakorpi, T. Amplification and overexpression of androgen receptor gene in hormone-refractory prostate cancer. Cancer Res. 2001, 61, 3550–3555. [Google Scholar] [PubMed]
- Ford, O.H., 3rd; Gregory, C.W.; Kim, D.; Smitherman, A.B.; Mohler, J.L. Androgen receptor gene amplification and protein expression in recurrent prostate cancer. J. Urol. 2003, 170, 1817–1821. [Google Scholar] [CrossRef] [PubMed]
- Efstathiou, E.; Titus, M.; Wen, S.; Hoang, A.; Karlou, M.; Ashe, R.; Tu, S.M.; Aparicio, A.; Troncoso, P.; Mohler, J.; et al. Molecular characterization of enzalutamide-treated bone metastatic castration-resistant prostate cancer. Eur. Urol. 2015, 67, 53–60. [Google Scholar] [CrossRef]
- Robinson, D.; Van Allen, E.M.; Wu, Y.M.; Schultz, N.; Lonigro, R.J.; Mosquera, J.M.; Montgomery, B.; Taplin, M.E.; Pritchard, C.C.; Attard, G.; et al. Integrative clinical genomics of advanced prostate cancer. Cell 2015, 161, 1215–1228. [Google Scholar] [CrossRef] [PubMed]
- Antonarakis, E.S.; Lu, C.; Wang, H.; Luber, B.; Nakazawa, M.; Roeser, J.C.; Chen, Y.; Mohammad, T.A.; Chen, Y.; Fedor, H.L.; et al. AR-V7 and Resistance to Enzalutamide and Abiraterone in Prostate Cancer. N. Engl. J. Med. 2014, 371, 1028–1038. [Google Scholar] [CrossRef]
- Khera, M.; Crawford, D.; Morales, A.; Salonia, A.; Morgentaler, A. A new era of testosterone and prostate cancer: From physiology to clinical implications. Eur. Urol. 2014, 65, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Huggins, C.; Hodges, C.V. Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J. Clin. 1972, 22, 232–240. [Google Scholar] [CrossRef]
- Huggins, C.; Stevens, R.E., Jr.; Hodges, C.V. Studies on Prostatic Cancer: II. The Effects of Castration on Advanced Carcinoma of the Prostate Gland. Arch. Surg. 1941, 43, 209–223. [Google Scholar] [CrossRef]
- Huggins, C.; Yang, N.C. Induction and extinction of mammary cancer. A striking effect of hydrocarbons permits analysis of mechanisms of causes and cure of breast cancer. Science 1962, 137, 257–262. [Google Scholar] [CrossRef]
- Abate-Shen, C.; Nunes de Almeida, F. Establishment of the LNCaP Cell Line—The Dawn of an Era for Prostate Cancer Research. Cancer Res. 2022, 82, 1689–1691. [Google Scholar] [CrossRef]
- Horoszewicz, J.S.; Leong, S.S.; Kawinski, E.; Karr, J.P.; Rosenthal, H.; Chu, T.M.; Mirand, E.A.; Murphy, G.P. LNCaP model of human prostatic carcinoma. Cancer Res. 1983, 43, 1809–1818. [Google Scholar]
- Berns, E.M.; de Boer, W.; Mulder, E. Androgen-dependent growth regulation of and release of specific protein(s) by the androgen receptor containing human prostate tumor cell line LNCaP. Prostate 1986, 9, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Kokontis, J.; Takakura, K.; Hay, N.; Liao, S. Increased androgen receptor activity and altered c-myc expression in prostate cancer cells after long-term androgen deprivation. Cancer Res. 1994, 54, 1566–1573. [Google Scholar] [PubMed]
- Wolf, D.A.; Schulz, P.; Fittler, F. Synthetic androgens suppress the transformed phenotype in the human prostate carcinoma cell line LNCaP. Br. J. Cancer 1991, 64, 47–53. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yuan, S.; Trachtenberg, J.; Mills, G.B.; Brown, T.J.; Xu, F.; Keating, A. Androgen-induced inhibition of cell proliferation in an androgen-insensitive prostate cancer cell line (PC-3) transfected with a human androgen receptor complementary DNA. Cancer Res. 1993, 53, 1304–1311. [Google Scholar]
- Heisler, L.E.; Evangelou, A.; Lew, A.M.; Trachtenberg, J.; Elsholtz, H.P.; Brown, T.J. Androgen-dependent cell cycle arrest and apoptotic death in PC-3 prostatic cell cultures expressing a full-length human androgen receptor. Mol. Cell. Endocrinol. 1997, 126, 59–73. [Google Scholar] [CrossRef]
- Umekita, Y.; Hiipakka, R.A.; Kokontis, J.M.; Liao, S. Human prostate tumor growth in athymic mice: Inhibition by androgens and stimulation by finasteride. Proc. Natl. Acad. Sci. USA 1996, 93, 11802–11807. [Google Scholar] [CrossRef]
- Chuu, C.P.; Hiipakka, R.A.; Fukuchi, J.; Kokontis, J.M.; Liao, S. Androgen causes growth suppression and reversion of androgen-independent prostate cancer xenografts to an androgen-stimulated phenotype in athymic mice. Cancer Res. 2005, 65, 2082–2084. [Google Scholar] [CrossRef]
- Nyquist, M.D.; Corella, A.; Mohamad, O.; Coleman, I.; Kaipainen, A.; Kuppers, D.A.; Lucas, J.M.; Paddison, P.J.; Plymate, S.R.; Nelson, P.S.; et al. Molecular determinants of response to high-dose androgen therapy in prostate cancer. JCI Insight 2019, 4, e129715. [Google Scholar] [CrossRef]
- Guo, H.; Wu, Y.; Nouri, M.; Spisak, S.; Russo, J.W.; Sowalsky, A.G.; Pomerantz, M.M.; Wei, Z.; Korthauer, K.; Seo, J.-H. Androgen receptor and MYC equilibration centralizes on developmental super-enhancer. Nat. Commun. 2021, 12, 7308. [Google Scholar] [CrossRef]
- Sena, L.A.; Kumar, R.; Sanin, D.E.; Thompson, E.A.; Rosen, D.M.; Dalrymple, S.L.; Antony, L.; Yang, Y.; Gomes-Alexandre, C.; Hicks, J.L.; et al. Androgen receptor activity in prostate cancer dictates efficacy of bipolar androgen therapy through MYC. J. Clin. Investig. 2022, 132, jci162396. [Google Scholar] [CrossRef]
- Polyak, K.; Lee, M.H.; Erdjument-Bromage, H.; Koff, A.; Roberts, J.M.; Tempst, P.; Massagué, J. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell 1994, 78, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Kokontis, J.M.; Hay, N.; Liao, S. Progression of LNCaP prostate tumor cells during androgen deprivation: Hormone-independent growth, repression of proliferation by androgen, and role for p27Kip1 in androgen-induced cell cycle arrest. Mol. Endocrinol. 1998, 12, 941–953. [Google Scholar] [CrossRef] [PubMed]
- Tsihlias, J.; Zhang, W.; Bhattacharya, N.; Flanagan, M.; Klotz, L.; Slingerland, J. Involvement of p27Kip1 in G1 arrest by high dose 5 alpha-dihydrotestosterone in LNCaP human prostate cancer cells. Oncogene 2000, 19, 670–679. [Google Scholar] [CrossRef]
- Chuu, C.P.; Kokontis, J.M.; Hiipakka, R.A.; Fukuchi, J.; Lin, H.P.; Lin, C.Y.; Huo, C.; Su, L.C.; Liao, S. Androgen suppresses proliferation of castration-resistant LNCaP 104-R2 prostate cancer cells through androgen receptor, Skp2, and c-Myc. Cancer Sci. 2011, 102, 2022–2028. [Google Scholar] [CrossRef]
- Wolf, D.A.; Kohlhuber, F.; Schulz, P.; Fittler, F.; Eick, D. Transcriptional down-regulation of c-myc in human prostate carcinoma cells by the synthetic androgen mibolerone. Br. J. Cancer 1992, 65, 376–382. [Google Scholar] [CrossRef]
- Cai, C.; Wang, H.; Xu, Y.; Chen, S.; Balk, S.P. Reactivation of androgen receptor-regulated TMPRSS2:ERG gene expression in castration-resistant prostate cancer. Cancer Res. 2009, 69, 6027–6032. [Google Scholar] [CrossRef]
- Wolf, D.A.; Herzinger, T.; Hermeking, H.; Blaschke, D.; Hörz, W. Transcriptional and posttranscriptional regulation of human androgen receptor expression by androgen. Mol. Endocrinol. 1993, 7, 924–936. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Henttu, P.; Vihko, P. Growth factor regulation of gene expression in the human prostatic carcinoma cell line LNCaP. Cancer Res. 1993, 53, 1051–1058. [Google Scholar]
- Thelen, P.; Heinrich, E.; Bremmer, F.; Trojan, L.; Strauss, A. Testosterone boosts for treatment of castration resistant prostate cancer: An experimental implementation of intermittent androgen deprivation. Prostate 2013, 73, 1699–1709. [Google Scholar] [CrossRef]
- Cai, C.; He, H.H.; Chen, S.; Coleman, I.; Wang, H.; Fang, Z.; Chen, S.; Nelson, P.S.; Liu, X.S.; Brown, M.; et al. Androgen receptor gene expression in prostate cancer is directly suppressed by the androgen receptor through recruitment of lysine-specific demethylase 1. Cancer Cell 2011, 20, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Nakata, D.; Nakayama, K.; Masaki, T.; Tanaka, A.; Kusaka, M.; Watanabe, T. Growth Inhibition by Testosterone in an Androgen Receptor Splice Variant-Driven Prostate Cancer Model. Prostate 2016, 76, 1536–1545. [Google Scholar] [CrossRef] [PubMed]
- Vander Griend, D.J.; Litvinov, I.V.; Isaacs, J.T. Stabilizing androgen receptor in mitosis inhibits prostate cancer proliferation. Cell Cycle 2007, 6, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Haffner, M.C.; Aryee, M.J.; Toubaji, A.; Esopi, D.M.; Albadine, R.; Gurel, B.; Isaacs, W.B.; Bova, G.S.; Liu, W.; Xu, J.; et al. Androgen-induced TOP2B-mediated double-strand breaks and prostate cancer gene rearrangements. Nat. Genet. 2010, 42, 668–675. [Google Scholar] [CrossRef]
- Haffner, M.C.; De Marzo, A.M.; Meeker, A.K.; Nelson, W.G.; Yegnasubramanian, S. Transcription-induced DNA double strand breaks: Both oncogenic force and potential therapeutic target? Clin. Cancer Res. 2011, 17, 3858–3864. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, P.; Schweizer, M.T.; Lucas, J.M.; Coleman, I.; Nyquist, M.D.; Frank, S.B.; Tharakan, R.; Mostaghel, E.; Luo, J.; Pritchard, C.C.; et al. Supraphysiological androgens suppress prostate cancer growth through androgen receptor-mediated DNA damage. J. Clin. Investig. 2019, 129, 4245–4260. [Google Scholar] [CrossRef]
- Cristini, A.; Géraud, M.; Sordet, O. Transcription-associated DNA breaks and cancer: A matter of DNA topology. Int. Rev. Cell Mol. Biol. 2021, 364, 195–240. [Google Scholar] [CrossRef]
- Joly-Pharaboz, M.O.; Ruffion, A.; Roch, A.; Michel-Calemard, L.; André, J.; Chantepie, J.; Nicolas, B.; Panaye, G. Inhibition of growth and induction of apoptosis by androgens of a variant of LNCaP cell line. J. Steroid Biochem. Mol. Biol. 2000, 73, 237–249. [Google Scholar] [CrossRef]
- Lin, Y.; Kokontis, J.; Tang, F.; Godfrey, B.; Liao, S.; Lin, A.; Chen, Y.; Xiang, J. Androgen and its receptor promote Bax-mediated apoptosis. Mol. Cell. Biol. 2006, 26, 1908–1916. [Google Scholar] [CrossRef]
- Lin, Y.; Lu, Z.; Kokontis, J.; Xiang, J. Androgen receptor primes prostate cancer cells to apoptosis through down-regulation of basal p21 expression. Biochem. Biophys. Res. Commun. 2013, 430, 289–293. [Google Scholar] [CrossRef]
- Collado, M.; Gil, J.; Efeyan, A.; Guerra, C.; Schuhmacher, A.J.; Barradas, M.; Benguría, A.; Zaballos, A.; Flores, J.M.; Barbacid, M.; et al. Tumour biology: Senescence in premalignant tumours. Nature 2005, 436, 642. [Google Scholar] [CrossRef]
- Schmitt, C.A. Cellular senescence and cancer treatment. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2007, 1775, 5–20. [Google Scholar] [CrossRef]
- Mirochnik, Y.; Veliceasa, D.; Williams, L.; Maxwell, K.; Yemelyanov, A.; Budunova, I.; Volpert, O.V. Androgen receptor drives cellular senescence. PLoS ONE 2012, 7, e31052. [Google Scholar] [CrossRef]
- Roediger, J.; Hessenkemper, W.; Bartsch, S.; Manvelyan, M.; Huettner, S.S.; Liehr, T.; Esmaeili, M.; Foller, S.; Petersen, I.; Grimm, M.O.; et al. Supraphysiological androgen levels induce cellular senescence in human prostate cancer cells through the Src-Akt pathway. Mol. Cancer 2014, 13, 214. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Mendonca, J.; Owoyemi, O.; Boyapati, K.; Thomas, N.; Kanacharoen, S.; Coffey, M.; Topiwala, D.; Gomes, C.; Ozbek, B.; et al. Supraphysiologic Testosterone Induces Ferroptosis and Activates Immune Pathways through Nucleophagy in Prostate Cancer. Cancer Res. 2021, 81, 5948–5962. [Google Scholar] [CrossRef]
- Deng, L.; Liang, H.; Xu, M.; Yang, X.; Burnette, B.; Arina, A.; Li, X.D.; Mauceri, H.; Beckett, M.; Darga, T.; et al. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity 2014, 41, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Eapen, V.V.; Waterman, D.P.; Bernard, A.; Schiffmann, N.; Sayas, E.; Kamber, R.; Lemos, B.; Memisoglu, G.; Ang, J.; Mazella, A.; et al. A pathway of targeted autophagy is induced by DNA damage in budding yeast. Proc. Natl. Acad. Sci. USA 2017, 114, E1158–E1167. [Google Scholar] [CrossRef] [PubMed]
- Fowler, J.E., Jr.; Whitmore, W.F., Jr. The response of metastatic adenocarcinoma of the prostate to exogenous testosterone. J. Urol. 1981, 126, 372–375. [Google Scholar] [CrossRef]
- Nguyen, L.; Chapdelaine, A.; Chevalier, S. Prostatic acid phosphatase in serum of patients with prostatic cancer is a specific phosphotyrosine acid phosphatase. Clin. Chem. 1990, 36, 1450–1455. [Google Scholar] [CrossRef]
- Fishman, W.H.; Bonner, C.D.; Homburger, F. Serum Prostatic Acid Phosphatase and Cancer of the Prostate. N. Engl. J. Med. 1956, 255, 925–933. [Google Scholar] [CrossRef]
- Tagnon, H.J.; Schulman, P.; Whitmore, W.F.; Leone, L.A. Prostatic fibrinolysin; study of a case illustrating role in hemorrhagic diathesis of cancer of the prostate. Am. J. Med. 1953, 15, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Brendler, H.; Chase, W.E.; Scott, W.W. Prostatic cancer; further investigation of hormonal relationships. Arch. Surg. 1950, 61, 433–440. [Google Scholar] [CrossRef]
- Trunnell, J.B.; Duffy, B.J., Jr. The Influence of Certain Steroids on the Behavior of Human Prostatic Cancer. Trans. N. Y. Acad. Sci. 1950, 12, 238–241. [Google Scholar] [CrossRef]
- Pearson, O.H. Discussion of Dr. Huggins’ paper: Control of cancers of man by endocrinological methods. Cancer Res. 1957, 17, 473–479. [Google Scholar]
- Prout, G.R., Jr.; Brewer, W.R. Response of men with advanced prostatic carcinoma to exogenous administration of testosterone. Cancer 1967, 20, 1871–1878. [Google Scholar] [CrossRef]
- Khera, M.; Grober, E.D.; Najari, B.; Colen, J.S.; Mohamed, O.; Lamb, D.J.; Lipshultz, L.I. Testosterone replacement therapy following radical prostatectomy. J. Sex. Med. 2009, 6, 1165–1170. [Google Scholar] [CrossRef]
- Morgentaler, A.; Lipshultz, L.I.; Bennett, R.; Sweeney, M.; Avila, D., Jr.; Khera, M. Testosterone therapy in men with untreated prostate cancer. J. Urol. 2011, 185, 1256–1260. [Google Scholar] [CrossRef]
- Pastuszak, A.W.; Pearlman, A.M.; Godoy, G.; Miles, B.J.; Lipshultz, L.I.; Khera, M. Testosterone replacement therapy in the setting of prostate cancer treated with radiation. Int. J. Impot. Res. 2013, 25, 24–28. [Google Scholar] [CrossRef]
- Pastuszak, A.W.; Pearlman, A.M.; Lai, W.S.; Godoy, G.; Sathyamoorthy, K.; Liu, J.S.; Miles, B.J.; Lipshultz, L.I.; Khera, M. Testosterone replacement therapy in patients with prostate cancer after radical prostatectomy. J. Urol. 2013, 190, 639–644. [Google Scholar] [CrossRef]
- Xie, T.; Song, X.L.; Wang, C.; Yu, Y.Z.; Wang, J.Q.; Chen, Z.S.; Zhao, S.C. The role of androgen therapy in prostate cancer: From testosterone replacement therapy to bipolar androgen therapy. Drug Discov. Today 2021, 26, 1293–1301. [Google Scholar] [CrossRef]
- Morris, M.J.; Huang, D.; Kelly, W.K.; Slovin, S.F.; Stephenson, R.D.; Eicher, C.; Delacruz, A.; Curley, T.; Schwartz, L.H.; Scher, H.I. Phase 1 trial of high-dose exogenous testosterone in patients with castration-resistant metastatic prostate cancer. Eur. Urol. 2009, 56, 237–244. [Google Scholar] [CrossRef]
- Szmulewitz, R.; Mohile, S.; Posadas, E.; Kunnavakkam, R.; Karrison, T.; Manchen, E.; Stadler, W.M. A randomized phase 1 study of testosterone replacement for patients with low-risk castration-resistant prostate cancer. Eur. Urol. 2009, 56, 97–103. [Google Scholar] [CrossRef]
- Schweizer, M.T.; Gulati, R.; Yezefski, T.; Cheng, H.H.; Mostaghel, E.; Haffner, M.C.; Patel, R.A.; De Sarkar, N.; Ha, G.; Dumpit, R.; et al. Bipolar androgen therapy plus olaparib in men with metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2023, 26, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Markowski, M.C.; Taplin, M.-E.; Aggarwal, R.R.; Wang, H.; Lalji, A.; Paller, C.J.; Marshall, C.H.; Carducci, M.A.; Eisenberger, M.A.; Marzo, A.M.D.; et al. COMBAT-CRPC: Concurrent administration of bipolar androgen therapy (BAT) and nivolumab in men with metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2021, 39, 5014. [Google Scholar] [CrossRef]
- Xiong, X.; Qiu, S.; Yi, X.; Xu, H.; Lei, H.; Liao, D.; Bai, S.; Peng, G.; Wei, Q.; Ai, J.; et al. Efficacy and safety of bipolar androgen therapy in mCRPC after progression on abiraterone or enzalutamide: A systematic review. Urol. Oncol. 2022, 40, 4.e19–4.e28. [Google Scholar] [CrossRef] [PubMed]
- Abufaraj, M.; Iwata, T.; Kimura, S.; Haddad, A.; Al-Ani, H.; Abusubaih, L.; Moschini, M.; Briganti, A.; Karakiewicz, P.I.; Shariat, S.F. Differential Impact of Gonadotropin-releasing Hormone Antagonist Versus Agonist on Clinical Safety and Oncologic Outcomes on Patients with Metastatic Prostate Cancer: A Meta-analysis of Randomized Controlled Trials. Eur. Urol. 2021, 79, 44–53. [Google Scholar] [CrossRef]
- Sciarra, A.; Busetto, G.M.; Salciccia, S.; Del Giudice, F.; Maggi, M.; Crocetto, F.; Ferro, M.; De Berardinis, E.; Scarpa, R.M.; Porpiglia, F.; et al. Does Exist a Differential Impact of Degarelix Versus LHRH Agonists on Cardiovascular Safety? Evidences from Randomized and Real-World Studies. Front. Endocrinol. 2021, 12, 695170. [Google Scholar] [CrossRef]
- Marshall, C.H.; Tunacao, J.; Danda, V.; Tsai, H.L.; Barber, J.; Gawande, R.; Weiss, C.R.; Denmeade, S.R.; Joshu, C. Reversing the effects of androgen-deprivation therapy in men with metastatic castration-resistant prostate cancer. BJU Int. 2021, 128, 366–373. [Google Scholar] [CrossRef]
- Antoun, S.; Bayar, A.; Ileana, E.; Laplanche, A.; Fizazi, K.; di Palma, M.; Escudier, B.; Albiges, L.; Massard, C.; Loriot, Y. High subcutaneous adipose tissue predicts the prognosis in metastatic castration-resistant prostate cancer patients in post chemotherapy setting. Eur. J. Cancer 2015, 51, 2570–2577. [Google Scholar] [CrossRef]
- Ohtaka, A.; Aoki, H.; Nagata, M.; Kanayama, M.; Shimizu, F.; Ide, H.; Tsujimura, A.; Horie, S. Sarcopenia is a poor prognostic factor of castration-resistant prostate cancer treated with docetaxel therapy. Prostate Int. 2019, 7, 9–14. [Google Scholar] [CrossRef]
- Stangl-Kremser, J.; Suarez-Ibarrola, R.; Andrea, D.; Korn, S.M.; Pones, M.; Kramer, G.; Marhold, M.; Krainer, M.; Enikeev, D.V.; Glybochko, P.V.; et al. Assessment of body composition in the advanced stage of castration-resistant prostate cancer: Special focus on sarcopenia. Prostate Cancer Prostatic Dis. 2020, 23, 309–315. [Google Scholar] [CrossRef]
- Markowski, M.C.; Kachhap, S.; De Marzo, A.M.; Sena, L.A.; Luo, J.; Denmeade, S.R.; Antonarakis, E.S. Molecular and Clinical Characterization of Patients with Metastatic Castration Resistant Prostate Cancer Achieving Deep Responses to Bipolar Androgen Therapy. Clin. Genitourin. Cancer 2022, 20, 97–101. [Google Scholar] [CrossRef]
- Caputo, S.A.; Hawkins, M.; Jaeger, E.B.; Fleming, W.; Casado, C.; Manogue, C.; Huang, M.; Lieberman, A.; Light, M.; Sussman, I.P.; et al. Clinical and molecular determinants of PSA response to bipolar androgen therapy in prostate cancer. Prostate 2023, 83, 879–885. [Google Scholar] [CrossRef]
- Lawhn-Heath, C.; Salavati, A.; Behr, S.C.; Rowe, S.P.; Calais, J.; Fendler, W.P.; Eiber, M.; Emmett, L.; Hofman, M.S.; Hope, T.A. Prostate-specific Membrane Antigen PET in Prostate Cancer. Radiology 2021, 299, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Hernes, E.; Revheim, M.E.; Hole, K.H.; Tulipan, A.J.; Strømme, H.; Lilleby, W.; Seierstad, T. Prostate-Specific Membrane Antigen PET for Assessment of Primary and Recurrent Prostate Cancer with Histopathology as Reference Standard: A Systematic Review and Meta-Analysis. PET Clin. 2021, 16, 147–165. [Google Scholar] [CrossRef] [PubMed]
- Vaz, S.; Hadaschik, B.; Gabriel, M.; Herrmann, K.; Eiber, M.; Costa, D. Influence of androgen deprivation therapy on PSMA expression and PSMA-ligand PET imaging of prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Kranzbühler, B.; Salemi, S.; Umbricht, C.A.; Müller, C.; Burger, I.A.; Sulser, T.; Eberli, D. Pharmacological upregulation of prostate-specific membrane antigen (PSMA) expression in prostate cancer cells. Prostate 2018, 78, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Lückerath, K.; Wei, L.; Fendler, W.P.; Evans-Axelsson, S.; Stuparu, A.D.; Slavik, R.; Mona, C.E.; Calais, J.; Rettig, M.; Reiter, R.E.; et al. Preclinical evaluation of PSMA expression in response to androgen receptor blockade for theranostics in prostate cancer. EJNMMI Res. 2018, 8, 96. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.; Wei, X.; Kim, W.; Small, E.J.; Ryan, C.J.; Carroll, P.; Cooperberg, M.; Evans, M.J.; Hope, T. Heterogeneous Flare in Prostate-specific Membrane Antigen Positron Emission Tomography Tracer Uptake with Initiation of Androgen Pathway Blockade in Metastatic Prostate Cancer. Eur. Urol. Oncol. 2018, 1, 78–82. [Google Scholar] [CrossRef]
- Leitsmann, C.; Thelen, P.; Schmid, M.; Meller, J.; Sahlmann, C.O.; Meller, B.; Trojan, L.; Strauss, A. Enhancing PSMA-uptake with androgen deprivation therapy—A new way to detect prostate cancer metastases? Int. Braz. J. Urol. 2019, 45, 459–467. [Google Scholar] [CrossRef]
- Ettala, O.; Malaspina, S.; Tuokkola, T.; Luoto, P.; Löyttyniemi, E.; Boström, P.J.; Kemppainen, J. Prospective study on the effect of short-term androgen deprivation therapy on PSMA uptake evaluated with (68)Ga-PSMA-11 PET/MRI in men with treatment-naïve prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Emmett, L.; Yin, C.; Crumbaker, M.; Hruby, G.; Kneebone, A.; Epstein, R.; Nguyen, Q.; Hickey, A.; Ihsheish, N.; O’Neill, G.; et al. Rapid Modulation of PSMA Expression by Androgen Deprivation: Serial (68)Ga-PSMA-11 PET in Men with Hormone-Sensitive and Castrate-Resistant Prostate Cancer Commencing Androgen Blockade. J. Nucl. Med. 2019, 60, 950–954. [Google Scholar] [CrossRef]
- Sayar, E.; Patel, R.A.; Coleman, I.M.; Roudier, M.P.; Zhang, A.; Mustafi, P.; Low, J.Y.; Hanratty, B.; Ang, L.S.; Bhatia, V.; et al. Reversible epigenetic alterations mediate PSMA expression heterogeneity in advanced metastatic prostate cancer. JCI Insight 2023, 8, e162907. [Google Scholar] [CrossRef] [PubMed]
- Jadvar, H.; Colletti, P.M. Molecular Imaging Assessment of Androgen Deprivation Therapy in Prostate Cancer. PET Clin. 2022, 17, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Esen, B.; Herrmann, K.; Bavbek, S.; Kordan, Y.; Tilki, D.; Esen, T. Prostate-specific Membrane Antigen Positron Emission Tomography as a Biomarker to Assess Treatment Response in Patients with Advanced Prostate Cancer. Eur. Urol. Focus. 2023; in press. [Google Scholar] [CrossRef]
| Study Name | Setting | Number of Patients | Design | Regimen | PSA 50 | ORR | rPFS | crPFS | PSAPFS |
|---|---|---|---|---|---|---|---|---|---|
| Pilot | CRPC with low to moderate metastasis burden | 16 | Single arm, pilot study | BAT + Oral etoposide | 4/14 (28.6%) | 5/10 (50%) | NR | NR | NR |
| BATMAN | Low volume mHSPC or nmHSPC | 29 | Single arm, Phase 2 | ADT followed by BAT-ADT alternation | PSA < 4: 17/29 (58.6%) | 8/10 (80%) | NR | NR | NR |
| RESTORE | Cohort A: mCRPC post-ENZ | 30 | Multi-cohort, Single center, phase2 | BAT | 9/30 (30%) | 6/12 (50%) | NR | 8.6 months | 3.3 months |
| Cohort B: mCRPC post-ABI | 29 | BAT | 5/29 (17.2%) | 2/7 (28.5%) | 5 months | 4.3 months | NR | ||
| Cohort C: CRPC (no prior NHA exposure) | 29 | BAT | 4/29 (13.7%) | 4/13 (30.7%) | NR | 8.5 months | 1 month | ||
| TRANSFORMER | mCRPC post-ABI | 195 | Multicenter, Randomized, Phase 2 | BAT or ENZ | 24/85 (28.2%) vs. 24/94 (25.5%) | 8/33 (28.2%) vs. 1/24 (4.2%) | 6.05 vs. 8.29 months | 5.6 vs. 5.7 months | 2.8 vs. 3.8 months |
| COMBAT | mCRPC post-ENZ/ABI ± Taxane chemo | 45 | Single arm, multi center, Phase 2 | BAT followed by BAT+Nivolumab | 18/45 (40%) | 10/42 (23.8%) | 5.7 months | NR | NR |
| BAT plus Olaparib | mCRPC post-ENZ and/or ABI | 36 | Single arm. Single center, phase 2 | BAT + Olaparib (300 mg P.O BID) | 16/36 (44%) | 7/13 (54.5%) | NR | 13 months | 7 months |
| Study | Protocol | Number of pts | Patient Population | Design | Primary Endpoint | CTC Identifier |
|---|---|---|---|---|---|---|
| BAT-RAD | BAT + Radium-223 | 47 | mCRPC | Single arm, Phase 2 | Median rPFS | NCT04704505 |
| HiTeCH | BAT + Carboplatin | 30 | mCRPC with confirmed HRR-mutations | Single arm, Phase 2 | PSA response rate | NCT035522064 |
| PSMA-BAT | BAT | 20 | mCRPC post-ABI/ENZ | Pilot, Single arm, Biomarker study | Ga-68-PSMA uptake and response to BAT | NCT04424654 |
| Ex-BAT | BAT + Darolutamide | 47 | mCRPC post-ABI | Single arm, Multi center, Phase 2 | rPFS rate | NCT04558866 |
| STEP-UP | Arm A: Continuous ENZ | 50 | mCRPC post-ABI ± ADT | Three-arm randomized, Phase 2 | crPFS | NCT04363164 |
| Arm B: Fixed cycles of Sequential BAT and ENZ | 50 | |||||
| Variable cycles of sequential BAT and ENZ | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nabavi, N.; Mahdavi, S.R.; Ardalan, M.A.; Chamanara, M.; Mosaed, R.; Lara, A.; Bastos, D.; Harsini, S.; Askari, E.; Velho, P.I.; et al. Bipolar Androgen Therapy: When Excess Fuel Extinguishes the Fire. Biomedicines 2023, 11, 2084. https://doi.org/10.3390/biomedicines11072084
Nabavi N, Mahdavi SR, Ardalan MA, Chamanara M, Mosaed R, Lara A, Bastos D, Harsini S, Askari E, Velho PI, et al. Bipolar Androgen Therapy: When Excess Fuel Extinguishes the Fire. Biomedicines. 2023; 11(7):2084. https://doi.org/10.3390/biomedicines11072084
Chicago/Turabian StyleNabavi, Nima, Seied Rabi Mahdavi, Mohammad Afshar Ardalan, Mohsen Chamanara, Reza Mosaed, Aline Lara, Diogo Bastos, Sara Harsini, Emran Askari, Pedro Isaacsson Velho, and et al. 2023. "Bipolar Androgen Therapy: When Excess Fuel Extinguishes the Fire" Biomedicines 11, no. 7: 2084. https://doi.org/10.3390/biomedicines11072084
APA StyleNabavi, N., Mahdavi, S. R., Ardalan, M. A., Chamanara, M., Mosaed, R., Lara, A., Bastos, D., Harsini, S., Askari, E., Velho, P. I., & Bagheri, H. (2023). Bipolar Androgen Therapy: When Excess Fuel Extinguishes the Fire. Biomedicines, 11(7), 2084. https://doi.org/10.3390/biomedicines11072084






