Effects of Progestin on Modulation of the Expression of Biomarkers in Endometriosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection and Serum and Tissue Samples
2.1.1. Inclusion Criteria
2.1.2. Exclusion Criteria
2.2. Enzyme-Linked Immunosorbent Assay (ELISA)
2.3. Immunohistochemistry
2.4. Statistical Analysis
3. Results
3.1. Serum OPN Levels
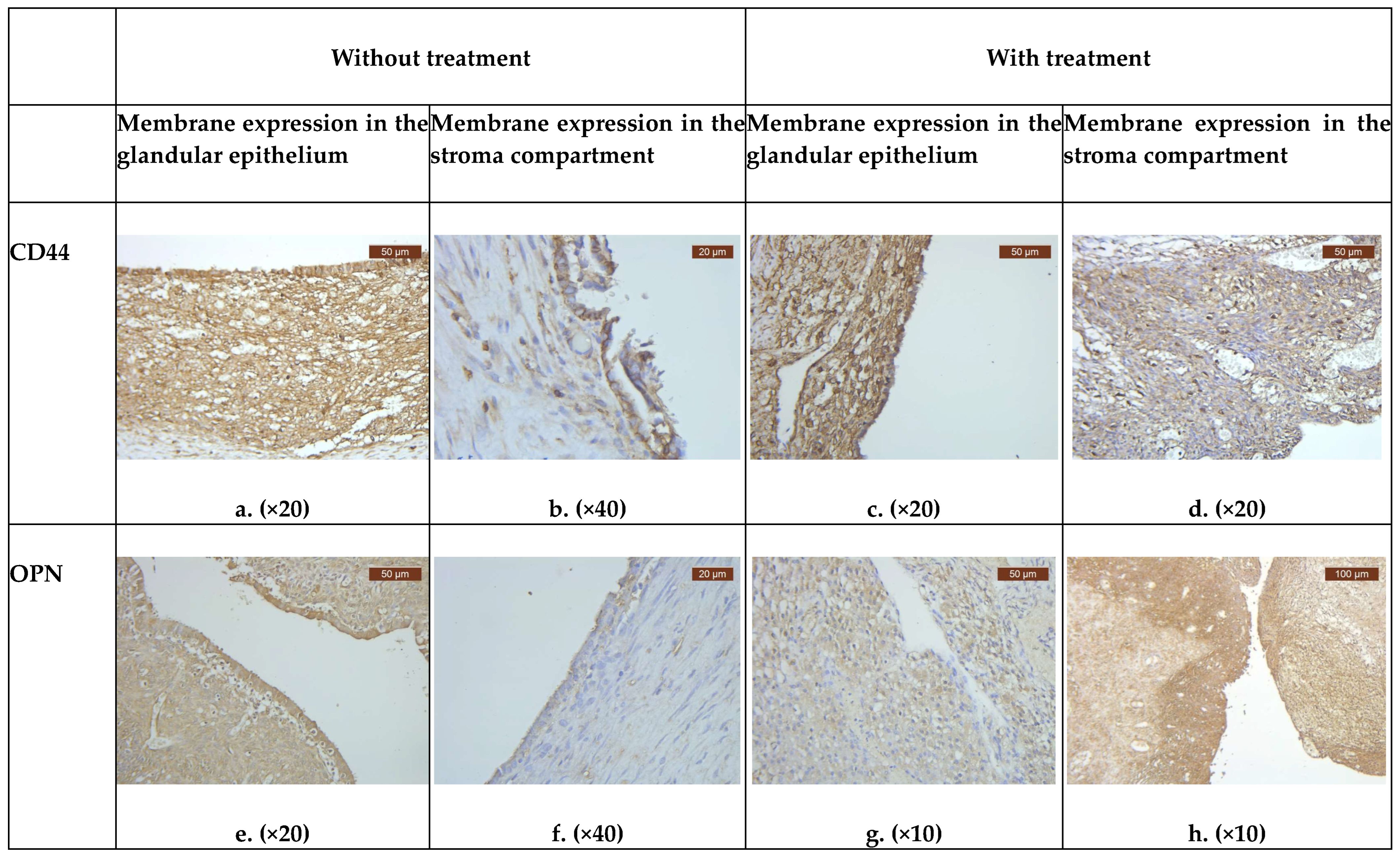
3.2. Evaluation of CD44 and OPN Endometriotic Sample Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poncelet, C.; Leblanc, M.; Walker-Combrouze, F.; Soriano, D.; Feldmann, G.; Madelenat, P.; Scoazec, J.Y.; Daraï, E. Expression of cadherins and CD44 isoforms in human endometrium and peritoneal endometriosis. Acta Obstet. Gynecol. Scand. 2002, 81, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Sancakli Usta, C.; Turan, G.; Bulbul, C.B.; Usta, A.; Adali, E. Differential expression of Oct-4, CD44, and E-cadherin in eutopic and ectopic endometrium in ovarian endometriomas and their correlations with clinicopathological variables. Reprod. Biol. Endocrinol. 2020, 18, 116. [Google Scholar] [CrossRef] [PubMed]
- Mignemi, G.; Facchini, C.; Raimondo, D.; Montanari, G.; Ferrini, G.; Seracchioli, R. A case report of nasal endometriosis in a patient affected by Behcet’s disease. J. Minim. Invasive Gynecol. 2012, 19, 514–516. [Google Scholar] [CrossRef] [PubMed]
- Sourial, S.; Tempest, N.; Hapangama, D.K. Theories on the pathogenesis of endometriosis. Int. J. Reprod. Med. 2014, 2014, 179515. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, M.; Yu, Q.; Fei, W.; Li, T.; Zhu, L.; Yao, Y.; Zheng, C.; Zhang, X. Hyaluronic Acid-Modified Nanoplatforms as a Vector for Targeted Delivery of Autophagy-Related Gene to the Endometriotic Lesions in Mice. Front. Bioeng. Biotechnol. 2022, 10, 918368. [Google Scholar] [CrossRef]
- Gadducci, A.; Multinu, F.; Cosio, S.; Carinelli, S.; Ghioni, M.; Aletti, G.D. Clear cell carcinoma of the ovary: Epidemiology, pathological and biological features, treatment options and clinical outcomes. Gynecol. Oncol. 2021, 162, 741–750. [Google Scholar] [CrossRef]
- Lin, S.; Xie, X.; Guo, Y.; Zhang, H.; Liu, C.; Yi, J.; Su, Y.; Deng, Q.; Zhu, W. Clinical characteristics and pregnancy outcomes of infertile patients with endometriosis and endometrial polyps: A retrospective cohort study. Taiwan. J. Obstet. Gynecol. 2020, 59, 916–921. [Google Scholar] [CrossRef]
- Mehdizadehkashi, A.; Tahermanesh, K.; Fazel Anvari-Yazdi, A.; Chaichian, S.; Azarpira, N.; Nobakht, M.; Abed, S.M.; Hashemi, N. Ultrastructural Investigation of Pelvic Peritoneum in Patients with Chronic Pelvic Pain and Subtle Endometriosis in Association with Chromoendoscopy. J. Minim. Invasive Gynecol. 2017, 24, 114–123. [Google Scholar] [CrossRef]
- Siufi Neto, J.; Kho, R.M.; Siufi, D.F.; Baracat, E.C.; Anderson, K.S.; Abrão, M.S. Cellular, histologic, and molecular changes associated with endometriosis and ovarian cancer. J. Minim. Invasive Gynecol. 2014, 21, 55–63. [Google Scholar] [CrossRef]
- Kurose, S.; Nakayama, K.; Razia, S.; Ishikawa, M.; Ishibashi, T.; Yamashita, H.; Sato, S.; Sakiyama, A.; Yoshioka, S.; Kobayashi, M.; et al. Whole-Exome Sequencing of Rare Site Endometriosis-Associated Cancer. Diseases 2021, 9, 14. [Google Scholar] [CrossRef]
- Mechsner, S. Endometriose: Eine oft verkannte Schmerzerkrankung [Endometriosis: An often unrecognized pain disorder]. Schmerz 2016, 30, 477–490. [Google Scholar] [CrossRef] [PubMed]
- Paravati, R.; De Mello, N.; Onyido, E.K.; Francis, L.W.; Brüsehafer, K.; Younas, K.; Spencer-Harty, S.; Conlan, R.S.; Gonzalez, D.; Margarit, L. Differential regulation of osteopontin and CD44 correlates with infertility status in PCOS patients. J. Mol. Med. 2020, 98, 1713–1725. [Google Scholar] [CrossRef] [PubMed]
- Kiyama, R. Nutritional implications of ginger: Chemistry, biological activities and signaling pathways. J. Nutr. Biochem. 2020, 86, 108486. [Google Scholar] [CrossRef]
- Wojciechowski, M.; Krawczyk, T.; Śmigielski, J.; Malinowski, A. CD44 expression in curettage and postoperative specimens of endometrial cancer. Arch. Gynecol. Obstet. 2015, 291, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Elbasateeny, S.S.; Salem, A.A.; Abdelsalam, W.A.; Salem, R.A. Immunohistochemical expression of cancer stem cell related markers CD44 and CD133 in endometrial cancer. Pathol. Res. Pract. 2016, 212, 10–16. [Google Scholar] [CrossRef]
- El-Sahwi, K.; Bellone, S.; Cocco, E.; Casagrande, F.; Bellone, M.; Abu-Khalaf, M.; Buza, N.; Tavassoli, F.A.; Hui, P.; Rüttinger, D.; et al. Overexpression of EpCAM in uterine serous papillary carcinoma: Implications for EpCAM-specific immunotherapy with human monoclonal antibody adecatumumab (MT201). Mol. Cancer Ther. 2010, 9, 57–66. [Google Scholar] [CrossRef]
- Torres, A.; Pac-Sosińska, M.; Wiktor, K.; Paszkowski, T.; Maciejewski, R.; Torres, K. CD44, TGM2 and EpCAM as novel plasma markers in endometrial cancer diagnosis. BMC Cancer 2019, 19, 401. [Google Scholar] [CrossRef]
- Zhao, M.D.; Cheng, J.L.; Yan, J.J.; Chen, F.Y.; Sheng, J.Z.; Sun, D.L.; Chen, J.; Miao, J.; Zhang, R.J.; Zheng, C.H.; et al. Hyaluronic acid reagent functional chitosan-PEI conjugate with AQP2-siRNA suppressed endometriotic lesion formation. Int. J. Nanomed. 2016, 11, 1323–1336. [Google Scholar] [CrossRef]
- Iwase, A.; Kotani, T.; Goto, M.; Kobayashi, H.; Takikawa, S.; Nakahara, T.; Nakamura, T.; Kondo, M.; Bayasula; Nagatomo, Y.; et al. Possible involvement of CD10 in the development of endometriosis due to its inhibitory effects on CD44-dependent cell adhesion. Reprod. Sci. 2014, 21, 82–88. [Google Scholar] [CrossRef]
- Heidari-Keshel, S.; Rezaei-Tavirani, M.; Ai, J.; Soleimani, M.; Baradaran-Rafii, A.; Ebrahimi, M.; Roozafzoon, R.; Rahmanzadeh, S.; Raeisossadati, R.; Omidi, R.; et al. Tissue-specific somatic stem-cell isolation and characterization from human endometriosis. Key roles in the initiation of endometrial proliferative disorders. Minerva Med. 2015, 106, 95–108. [Google Scholar]
- Niiro, E.; Kawahara, N.; Yamada, Y.; Yoshimoto, C.; Shimada, K.; Sudo, T.; Kobayashi, H. Immunohistochemical expression of CD44v9 and 8-OHdG in ovarian endometrioma and the benign endometriotic lesions adjacent to clear cell carcinoma. J. Obstet. Gynaecol. Res. 2019, 45, 2260–2266. [Google Scholar] [CrossRef] [PubMed]
- Pazhohan, A.; Amidi, F.; Akbari-Asbagh, F.; Seyedrezazadeh, E.; Aftabi, Y.; Abdolalizadeh, J.; Khodarahmian, M.; Khanlarkhani, N.; Sobhani, A. Expression and shedding of CD44 in the endometrium of women with endometriosis and modulating effects of vitamin D: A randomized exploratory trial. J. Steroid Biochem. Mol. Biol. 2018, 178, 150–158. [Google Scholar] [CrossRef]
- Olivares, C.N.; Alaniz, L.D.; Menger, M.D.; Barañao, R.I.; Laschke, M.W.; Meresman, G.F. Inhibition of Hyaluronic Acid Synthesis Suppresses Angiogenesis in Developing Endometriotic Lesions. PLoS ONE 2016, 11, e0152302. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Kong, L.; Hou, Z.; Ji, H. CD44 is a prognostic biomarker and correlated with immune infiltrates in gastric cancer. BMC Med. Genom. 2022, 15, 225. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Fu, C.; Zhang, Q.; He, C.; Zhang, F.; Wei, Q. The role of CD44 in pathological angiogenesis. FASEB J. 2020, 34, 13125–13139. [Google Scholar] [CrossRef] [PubMed]
- Ho, N.T.; Lin, S.W.; Lee, Y.R.; Tzeng, C.R.; Kao, S.H. Osteopontin Splicing Isoforms Contribute to Endometriotic Proliferation, Migration, and Epithelial-Mesenchymal Transition in Endometrial Epithelial Cells. Int. J. Mol. Sci. 2022, 23, 15328. [Google Scholar] [CrossRef]
- Wang, W.; Li, P.N.; Li, W.; Jiang, J.; Cui, Y.; Li, S.; Wang, Z. Osteopontin activates mesenchymal stem cells to repair skin wound. PLoS ONE 2017, 12, e0185346. [Google Scholar] [CrossRef]
- Konno, R.; Fujiwara, H.; Netsu, S.; Odagiri, K.; Shimane, M.; Nomura, H.; Suzuki, M. Gene Expression Profiling of the Rat Endometriosis Model. Am. J. Reprod. Immunol. 2007, 58, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Goodison, S.; Urquidi, V.; Tarin, D. CD44 cell adhesion molecules. Mol. Pathol. 1999, 52, 189–196. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, Q.; Alam, A.; Cui, J.; Suen, K.C.; Soo, A.P.; Eguchi, S.; Gu, J.; Ma, D. The role of osteopontin in the progression of solid organ tumour. Cell Death Dis. 2018, 9, 356. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, X.; Guo, S.W. Plasma High Mobility Group Box 1 (HMGB1), Osteopontin (OPN), and Hyaluronic Acid (HA) as Admissible Biomarkers for Endometriosis. Sci. Rep. 2019, 9, 9272. [Google Scholar] [CrossRef]
- Lin, E.Y.; Xi, W.; Aggarwal, N.; Shinohara, M.L. Osteopontin (OPN)/SPP1: From its biochemistry to biological functions in the innate immune system and the central nervous system (CNS). Int. Immunol. 2023, 35, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Rittling, S.R.; Singh, R. Osteopontin in Immune-mediated Diseases. J. Dent. Res. 2015, 94, 1638–1645. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Yao, M.; Ye, C.; Fang, T.; Wu, R. Osteopontin Regulates Endometrial Stromal Cell Migration in Endometriosis through the PI3K Pathway: Osteopontin Regulates Endometrial Cell Migration in Endometriosis. Reprod. Sci. 2021, 28, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Subraman, V.; Thiyagarajan, M.; Malathi, N.; Rajan, S.T. OPN—Revisited. J. Clin. Diagn. Res. 2015, 9, ZE10–ZE13. [Google Scholar] [CrossRef]
- Allaire, C.; Bedaiwy, M.A.; Yong, P.J. Diagnosis and management of endometriosis. CMAJ 2023, 195, E363–E371. [Google Scholar] [CrossRef]
- Bouquet de Joliniere, J.; Fruscalzo, A.; Khomsi, F.; Stochino Loi, E.; Cherbanyk, F.; Ayoubi, J.M.; Feki, A. Antiangiogenic Therapy as a New Strategy in the Treatment of Endometriosis? The First Case Report. Front. Surg. 2021, 8, 791686. [Google Scholar] [CrossRef] [PubMed]
- Malvezzi, H.; Marengo, E.B.; Podgaec, S.; de Azevedo Piccinato, C. Endometriosis: Current challenges in modeling a multifactorial disease of unknown etiology. J. Transl. Med. 2020, 18, 311. [Google Scholar] [CrossRef]
- Brichant, G.; Laraki, I.; Henry, L.; Munaut, C.; Nisolle, M. New Therapeutics in Endometriosis: A Review of Hormonal, Non-Hormonal, and Non-Coding RNA Treatments. Int. J. Mol. Sci. 2021, 22, 10498. [Google Scholar] [CrossRef]
- Vercellini, P.; Buggio, L.; Berlanda, N.; Barbara, G.; Somigliana, E.; Bosari, S. Estrogen-progestins and progestins for the management of endometriosis. Fertil. Steril. 2016, 106, 1552–1571.e2. [Google Scholar] [CrossRef]
- Vercellini, P.; Buggio, L.; Frattaruolo, M.P.; Borghi, A.; Dridi, D.; Somigliana, E. Medical treatment of endometriosis-related pain. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 51, 68–91. [Google Scholar] [CrossRef] [PubMed]
- Surrey, E.S.; Soliman, A.M.; Johns, B.; Vora, J.B.; Taylor, H.S.; Agarwal, S.K. Real-World Characterization of Women with Diagnosed Endometriosis Initiating Therapy with Elagolix Using a US Claims Database. Clin. Outcomes Res. 2020, 12, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.M.; Coyne, K.S.; Zaiser, E.; Castelli-Haley, J.; Fuldeore, M.J. The burden of endometriosis symptoms on health-related quality of life in women in the United States: A cross-sectional study. J. Psychosom. Obstet. Gynecol. 2017, 38, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.M.; Yang, H.; Du, E.X.; Kelley, C.; Winkel, C. The direct and indirect costs associated with endometriosis: A systematic literature review. Hum. Reprod. 2016, 31, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Giudice, L.C.; Kao, L.C. Endometriosis . Lancet 2004, 364, 1789–1799. [Google Scholar] [CrossRef]
- Koo, Y.H.; Na, Y.J.; Ahn, M.Y.; Jeon, H.N.; Yeom, J.I.; Lee, K.S. Expression of CD44 in endometrial stromal cells from women with and without endometriosis and its effect on the adherence to peritoneal mesothelial cells. Obstet. Gynecol. Sci. 2013, 56, 102–109. [Google Scholar] [CrossRef]
- Yang, M.; Jiang, C.; Chen, H.; Nian, Y.; Bai, Z.; Ha, C. The involvement of osteopontin and matrix metalloproteinase- 9 in the migration of endometrial epithelial cells in patients with endometriosis. Reprod. Biol. Endocrinol. 2015, 13, 95. [Google Scholar] [CrossRef]
- Nisenblat, V.; Bossuyt, P.M.; Shaikh, R.; Farquhar, C.; Jordan, V.; Scheffers, C.S.; Mol, B.W.; Johnson, N.; Hull, M.L. Blood biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst. Rev. 2016, 2016, CD012179. [Google Scholar] [CrossRef]
- D’Amico, F.; Skarmoutsou, E.; Quaderno, G.; Malaponte, G.; La Corte, C.; Scibilia, G.; D’Agate, G.; Scollo, P.; Fraggetta, F.; Spandidos, D.A.; et al. Expression and localisation of osteopontin and prominin-1 (CD133) in patients with endometriosis. Int. J. Mol. Med. 2013, 31, 1011–1016. [Google Scholar] [CrossRef]
- Cho, S.; Ahn, Y.S.; Choi, Y.S.; Seo, S.K.; Nam, A.; Kim, H.Y.; Kim, J.H.; Park, K.H.; Cho, D.J.; Lee, B.S. Endometrial osteopontin mRNA expression and plasma osteopontin levels are increased in patients with endometriosis. Am. J. Reprod. Immunol. 2009, 61, 286–293. [Google Scholar] [CrossRef]
- Streuli, I.; Santulli, P.; Chouzenoux, S.; Chapron, C.; Batteux, F. Serum Osteopontin Levels Are Decreased in Focal Adenomyosis. Reprod. Sci. 2017, 24, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.O.; Yang, K.M.; Kang, I.S.; Koong, M.K.; Kim, H.S.; Zhang, X.; Kim, I. Expression of CD44s, vascular endothelial growth factor, matrix metalloproteinase-2 and Ki-67 in peritoneal, rectovaginal and ovarian endometriosis. J. Reprod. Med. 2007, 52, 207–213. [Google Scholar] [PubMed]
- Knudtson, J.F.; Tekmal, R.R.; Santos, M.T.; Binkley, P.A.; Krishnegowda, N.; Valente, P.; Schenken, R.S. Impaired Development of Early Endometriotic Lesions in CD44 Knockout Mice. Reprod. Sci. 2016, 23, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Darcha, C.; Maleysson, E.; Canis, M.; Mage, G. Impaired down-regulation of E-cadherin and beta-catenin protein expression in endometrial epithelial cells in the mid-secretory endometrium of infertile patients with endometriosis. J. Clin. Endocrinol. Metab. 2010, 95, 3437–3445. [Google Scholar] [CrossRef]
- Nothnick, W.B.; Fan, F.; Iczkowski, K.A.; Ashwell, R.; Thomas, P.; Tawfik, O.W. CD44s expression is reduced in endometriotic lesions compared to eutopic endometrium in women with endometriosis. Int. J. Gynecol. Pathol. 2001, 20, 140–146. [Google Scholar] [CrossRef]
- Esfandiari, F.; Heidari Khoei, H.; Saber, M.; Favaedi, R.; Piryaei, A.; Moini, A.; Shahhoseini, M.; Ramezanali, F.; Ghaffari, F.; Baharvand, H. Disturbed progesterone signalling in an advanced preclinical model of endometriosis. Reprod. Biomed. Online 2021, 43, 139–147. [Google Scholar] [CrossRef]
- Tremaine, T.D.; Fouladi-Nashta, A.A. Steroid regulation of secreted phosphoprotein 1 (SPP1) expression in ovine endometrium. Reprod. Fertil. Dev. 2021, 33, 257–269. [Google Scholar] [CrossRef]
| Antibody | Clone, Manufacturer | Dilution | Expression |
|---|---|---|---|
| Anti—CD44 | Rabbit polyclonal IgG isotype, Abcam (ab157107) | 1:250 | Nuclear |
| Anti—OPN | Rabbit polyclonal IgG isotype, Abcam (ab8448) | 1:200 | Nuclear |
| Group | N | Mean | Std. Deviation | Std Error | 95% Confidence Interval for Mean | Min | Max | p | |
|---|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||||||
| M1 before treatment | <0.01 | ||||||||
| endometriosis group | 60 | 210.061 | 219.088 | 28.284 | 153.465/266.658 | 74 | 1070.92 | ||
| control group | 30 | 390.101 | 303.458 | 55.403 | 276.788/503.414 | 80.92 | 1086 | ||
| M2 | 0.053 | ||||||||
| without treatment | 36 | 201.918 | 204.253 | 34.042 | 132.809/271.028 | 74 | 1070.92 | ||
| with treatment | 24 | 318.943 | 235.269 | 48.024 | 219.597/418.289 | 75.20 | 893.810 | ||
| Group | N | CD44 | OPN | ||
|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | ||
| With treatment | 24 | 21 (40%) | 3 (43%) | 14 (50%) | 10 (31.25%) |
| Without treatment | 36 | 32 (60%) | 4 (57%) | 14 (50%) | 22 (68.75%) |
| p-value | 0.99 F | 0.14 | |||
| CD44 | OPN | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | N | Epithelial | Stromal | Epithelial | Stromal | ||||
| Positive | Negative | Positive | Negative | Positive | Negative | Positive | Negative | ||
| With treatment | 24 | 22 (39.28%) | 2 (50%) | 17 (38.63%) | 7 (43.75%) | 14 (41.17%) | 10 (38.46%) | 13 (14.81%) | 11 (33.33%) |
| Without treatment | 36 | 34 (60.71%) | 2 (50%) | 27 (61.36%) | 9 (56.25%) | 20 (58.82%) | 16 (61.53%) | 14 (51.85%) | 22 (66.66%) |
| p-value | 56 | 4 | 44 | 16 | 34 | 26 | 27 | 33 | |
| 0.99 F | 0.72 | 0.83 | 0.24 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matasariu, D.R.; Bausic, A.I.G.; Mandici, C.E.; Bujor, I.E.; Cristofor, A.E.; Bratila, E.; Lozneanu, L.; Boiculese, L.V.; Grigore, M.; Ursache, A. Effects of Progestin on Modulation of the Expression of Biomarkers in Endometriosis. Biomedicines 2023, 11, 2036. https://doi.org/10.3390/biomedicines11072036
Matasariu DR, Bausic AIG, Mandici CE, Bujor IE, Cristofor AE, Bratila E, Lozneanu L, Boiculese LV, Grigore M, Ursache A. Effects of Progestin on Modulation of the Expression of Biomarkers in Endometriosis. Biomedicines. 2023; 11(7):2036. https://doi.org/10.3390/biomedicines11072036
Chicago/Turabian StyleMatasariu, Daniela Roxana, Alexandra Irma Gabriela Bausic, Cristina Elena Mandici, Iuliana Elena Bujor, Alexandra Elena Cristofor, Elvira Bratila, Ludmila Lozneanu, Lucian Vasile Boiculese, Mihaela Grigore, and Alexandra Ursache. 2023. "Effects of Progestin on Modulation of the Expression of Biomarkers in Endometriosis" Biomedicines 11, no. 7: 2036. https://doi.org/10.3390/biomedicines11072036
APA StyleMatasariu, D. R., Bausic, A. I. G., Mandici, C. E., Bujor, I. E., Cristofor, A. E., Bratila, E., Lozneanu, L., Boiculese, L. V., Grigore, M., & Ursache, A. (2023). Effects of Progestin on Modulation of the Expression of Biomarkers in Endometriosis. Biomedicines, 11(7), 2036. https://doi.org/10.3390/biomedicines11072036






