Melatonin as a Coadjuvant in the Treatment of Patients with Fibromyalgia
Abstract
1. Introduction
2. Role of Melatonin in Fibromyalgia
2.1. Receptor-Mediated Effects of Melatonin and Its Involvement in Fibromyalgia
2.2. Interaction of Melatonin with Receptors Implicated in the Pain in FMS
2.3. Implication of Melatonin in Improving the Alteration of Circadian Rhythms in FMS
2.4. Effects of Melatonin on Pain and Sleep Quality in FMS
2.5. Relationship between Melatonin and Cortisol in Fibromyalgia Symptoms
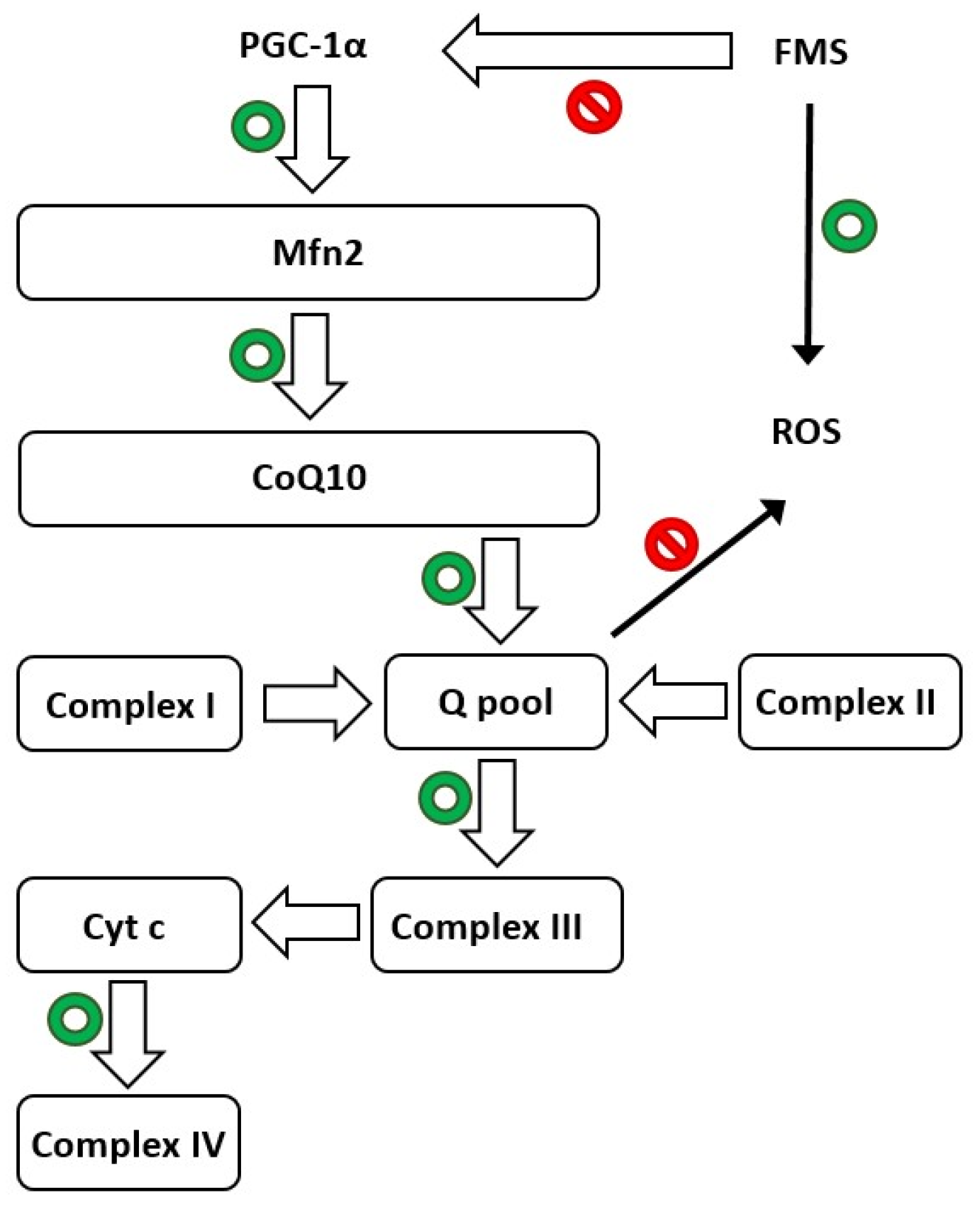
2.6. Melatonin and Its Significant Antioxidant Role in FMS
2.7. Oxidative Stress and Neuroinflammation in FMS
2.8. Novel Findings in Therapies According to Animal Models of FMS
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borchers, A.T.; Gershwin, M.E. Fibromyalgia: A Critical and Comprehensive Review. Clin. Rev. Allergy Immunol. 2015, 49, 100–151. [Google Scholar] [CrossRef]
- Sifuentes-Giraldo, W.A.; Morell-Hita, J.L. Fibromialgia. Medicine 2017, 12, 1586–1595. [Google Scholar] [CrossRef]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.-A.; Goldenberg, D.L.; Katz, R.S.; Mease, P.; Russell, A.S.; Russell, I.J.; Winfield, J.B.; Yunus, M.B. The American College of Rheumatology Preliminary Diagnostic Criteria for Fibromyalgia and Measurement of Symptom Severity. Arthritis Care Res. 2010, 62, 600–610. [Google Scholar] [CrossRef]
- Bagis, S.; Tamer, L.; Sahin, G.; Bilgin, R.; Guler, H.; Ercan, B.; Erdogan, C. Free radicals and antioxidants in primary fibromyalgia: An oxidative stress disorder? Rheumatol. Int. 2005, 25, 188–190. [Google Scholar] [CrossRef] [PubMed]
- Sartori, J.E.; Cireia, L.F.; Martins, D.P.; Rocha, G.P.; Cintra, J.R. Association of Markers of Oxidative Stress, Medication Therapy and Life Habits in Fibromyalgia. J. Rheum. Dis. Treat. 2016, 2, 035. [Google Scholar] [CrossRef]
- Ozgocmen, S.; Ozyurt, H.; Sogut, S.; Akyol, O.; Ardicoglu, O.; Yildizhan, H. Antioxidant status, lipid peroxidation and nitric oxide in fibromyalgia: Etiologic and therapeutic concerns. Rheumatol. Int. 2006, 26, 598–603. [Google Scholar] [CrossRef]
- Altindag, O.; Çelik, H. Total antioxidant capacity and the severity of the pain in patients with fibromyalgia. Redox Rep. 2006, 11, 131–135. [Google Scholar] [CrossRef]
- La Rubia, M.; Rus, A.; Molina, F.; Del Moral, M.L. Is fibromyalgia-related oxidative stress implicated in the decline of physical and mental health status? Clin. Exp. Rheumatol. 2013, 31 (Suppl. S79), S121–S127. [Google Scholar]
- Sendur, O.F.; Turan, Y.; Tastaban, E.; Yenisey, C.; Serter, M. Serum antioxidants and nitric oxide levels in fibromyalgia: A controlled study. Rheumatol. Int. 2009, 29, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Shukla, V.; Das, S.K.; Mahdi, A.A.; Agarwal, S.; Alok, R.; Ansari, J.A.; Khandpur, S. Metal-induced oxidative stress level in patients with fibromyalgia syndrome and its contribution to the severity of the disease: A correlational study. J. Back Musculoskelet. Rehabil. 2021, 34, 319–326. [Google Scholar] [CrossRef]
- Cordero, M.D.; De-Miguel, M.; Carmona-López, I.; Bonal, P.; Campa, F.; Moreno-Fernández, A.M. Oxidative stress and mitochondrial dysfunction in fibromyalgia. Neuro Endocrinol. Lett. 2010, 31, 101–105. [Google Scholar]
- Lawson, K. Is there a role for melatonin in fibromyalgia? AIMS Mol. Sci. 2019, 6, 73–86. [Google Scholar] [CrossRef]
- Alcocer-Gómez, E.; Culic, O.; Navarro-Pando, J.M.; Sánchez-Alcázar, J.A.; Bullón, P. Effect of Coenzyme Q10 on Psychopathological Symptoms in Fibromyalgia Patients. CNS Neurosci. Ther. 2017, 23, 188–189. [Google Scholar] [CrossRef]
- Basso, V.; Marchesan, E.; Peggion, C.; Chakraborty, J.; von Stockum, S.; Giacomello, M.; Ottolini, D.; Debattisti, V.; Caicci, F.; Tasca, E.; et al. Regulation of ER-mitochondria contacts by Parkin via Mfn2. Pharmacol. Res. 2018, 138, 43–56. [Google Scholar] [CrossRef]
- Tur, J.; Pereira-Lopes, S.; Vico, T.; Marín, E.A.; Muñoz, J.P.; Hernández-Alvarez, M.; Cardona, P.-J.; Zorzano, A.; Lloberas, J.; Celada, A. Mitofusin 2 in Macrophages Links Mitochondrial ROS Production, Cytokine Release, Phagocytosis, Autophagy, and Bactericidal Activity. Cell Rep. 2020, 32, 108079. [Google Scholar] [CrossRef]
- Lloberas, J.; Muñoz, J.P.; Hernández-Álvarez, M.I.; Cardona, P.-J.; Zorzano, A.; Celada, A. Macrophage mitochondrial MFN2 (mitofusin 2) links immune stress and immune response through reactive oxygen species (ROS) production. Autophagy 2020, 16, 2307–2309. [Google Scholar] [CrossRef] [PubMed]
- Mourier, A.; Motori, E.; Brandt, T.; Lagouge, M.; Atanassov, I.; Galinier, A.; Rappl, G.; Brodesser, S.; Hultenby, K.; Dieterich, C.; et al. Mitofusin 2 is required to maintain mitochondrial coenzyme Q levels. J. Cell Biol. 2015, 208, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Alcocer-Gómez, E.; Sánchez-Alcázar, J.A.; Cordero, M.D. Coenzyme Q10 Regulates Serotonin Levels and Depressive Symptoms in Fibromyalgia Patients. J. Clin. Psychopharmacol. 2014, 34, 277–278. [Google Scholar] [CrossRef]
- Procaccio, V.; Bris, C.; de la Barca, J.C.; Oca, F.; Chevrollier, A.; Amati-Bonneau, P.; Bonneau, D.; Reynier, P. Perspectives of drug-based neuroprotection targeting mitochondria. Rev. Neurol. 2014, 170, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Acuna-Castroviejo, D.; Tan, D.-X. Melatonin therapy in fibromyalgia. Curr. Pain Headache Rep. 2007, 11, 339–342. [Google Scholar] [CrossRef]
- Liu, J.; Clough, S.J.; Dubocovich, M.L. Role of the MT1 and MT2 melatonin receptors in mediating depressive- and anxiety-like behaviors in C3H/HeN mice. Genes Brain Behav. 2017, 16, 546–553. [Google Scholar] [CrossRef]
- Kamal, M.; Gbahou, F.; Guillaume, J.-L.; Daulat, A.M.; Benleulmi-Chaachoua, A.; Luka, M.; Chen, P.; Anaraki, D.K.; Baroncini, M.; la Cour, C.M.; et al. Convergence of Melatonin and Serotonin (5-HT) Signaling at MT2/5-HT2C Receptor Heteromers. J. Biol. Chem. 2015, 290, 11537–11546. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Jiang, Y.-J.; Zou, M.-S.; Liu, J.; Zhao, H.-Q.; Wang, Y.-H. Antidepressant actions of melatonin and melatonin receptor agonist: Focus on pathophysiology and treatment. Behav. Brain Res. 2022, 420, 113724. [Google Scholar] [CrossRef]
- Mease, P.J.; Farmer, M.V.; Palmer, R.H.; Gendreau, R.M.; Trugman, J.M.; Wang, Y. Milnacipran combined with pregabalin in fibromyalgia: A randomized, open-label study evaluating the safety and efficacy of adding milnacipran in patients with incomplete response to pregabalin. Ther. Adv. Musculoskelet. Dis. 2013, 5, 113–126. [Google Scholar] [CrossRef]
- Ghini, M.; Carpenito, G.; Mascia, M.T. Effects of a paracetamol and tramadol fixed-dose combination on pain, asthenia, cognitive disorders and sleep quality in fibromyalgia. Clin. Exp. Rheumatol. 2016, 34, 152. [Google Scholar]
- De Zanette, S.A.; Vercelino, R.; Laste, G.; Rozisky, J.R.; Schwertner, A.; Machado, C.B.; Xavier, F.; de Souza, I.C.; Deitos, A.; Torres, I.L.S.; et al. Melatonin analgesia is associated with improvement of the descending endogenous pain-modulating system in fibromyalgia: A phase II, randomized, double-dummy, controlled trial. BMC Pharmacol. Toxicol. 2014, 15, 40. [Google Scholar] [CrossRef] [PubMed]
- de Bodinat, C.; Guardiola-Lemaitre, B.; Mocaër, E.; Renard, P.; Muñoz, C.; Millan, M.J. Agomelatine, the first melatonergic antidepressant: Discovery, characterization and development. Nat. Rev. Drug Discov. 2010, 9, 628–642. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, A.A.; Fatima, G.; Das, S.K.; Verma, N.S. Abnormality of circadian rhythm of serum melatonin and other biochemical parameters in fibromyalgia syndrome. Indian J. Biochem. Biophys. 2011, 48, 82–87. [Google Scholar]
- Bonnefont-Rousselot, D.; Collin, F. Melatonin: Action as antioxidant and potential applications in human disease and aging. Toxicology 2010, 278, 55–67. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.-X.; Manchester, L.C.; Paredes, S.D.; Mayo, J.C.; Sainz, R.M. Melatonin and Reproduction Revisited. Biol. Reprod. 2009, 81, 445–456. [Google Scholar] [CrossRef]
- Guerrero, J.M.; Reiter, R.J. Melatonin-Immune System Relationships. Curr. Top. Med. Chem. 2002, 2, 167–179. [Google Scholar] [CrossRef]
- Pin, G.; Merino, M.; de la Calle, T.; Hidalgo, M.I.; Rodríguez, P.J.; Soto, V.; Madrid, J.A. Consenso sobre el uso de melatonina en niños y adolescentes con dificultades para iniciar el sueño. Pediatr. Integral 2014, 18, 577–585. [Google Scholar]
- Danilov, A.; Kurganova, J. Melatonin in Chronic Pain Syndromes. Pain Ther. 2016, 5, 1–17. [Google Scholar] [CrossRef]
- Terrón, M.P.; Delgado, J.; Paredes, S.D.; Barriga, C.; Reiter, R.J.; Rodríguez, A.B. Effect of melatonin and tryptophan on humoral immunity in young and old ringdoves (Streptopelia risoria). Exp. Gerontol. 2009, 44, 653–658. [Google Scholar] [CrossRef]
- Nabavi, S.M.; Nabavi, S.F.; Sureda, A.; Xiao, J.; Dehpour, A.R.; Shirooie, S.; Silva, A.S.; Baldi, A.; Khan, H.; Daglia, M. Anti-inflammatory effects of Melatonin: A mechanistic review. Crit. Rev. Food Sci. Nutr. 2018, 59, S4–S16. [Google Scholar] [CrossRef]
- Jin, X.; von Gall, C.; Pieschl, R.L.; Gribkoff, V.K.; Stehle, J.H.; Reppert, S.M.; Weaver, D.R. Targeted Disruption of the Mouse Mel1b Melatonin Receptor. Mol. Cell. Biol. 2003, 23, 1054–1060. [Google Scholar] [CrossRef]
- Masana, M.I.; Doolen, S.; Ersahin, C.; Al-Ghoul, W.M.; Duckles, S.P.; Dubocovich, M.L.; Krause, D.N. MT2 Melatonin Receptors Are Present and Functional in Rat Caudal Artery. Experiment 2002, 302, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Drazen, D.L.; Nelson, R.J. Melatonin Receptor Subtype MT2 (Mel 1b) and Not mt1 (Mel 1a) Is Associated with Melatonin-Induced Enhancement of Cell-Mediated and Humoral Immunity. Neuroendocrinology 2001, 74, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Von Gall, C.; Weaver, D.R.; Moek, J.; Jilg, A.; Stehle, J.H.; Korf, H.W. Melatonin Plays a Crucial Role in the Regulation of Rhythmic Clock Gene Expression in the Mouse Pars Tuberalis. Ann. N. Y. Acad. Sci. 2005, 1040, 508–511. [Google Scholar] [CrossRef] [PubMed]
- Missbach, M.; Jagher, B.; Sigg, I.; Nayeri, S.; Carlberg, C.; Wiesenberg, I. Thiazolidine Diones, Specific Ligands of the Nuclear Receptor Retinoid Z Receptor/Retinoid Acid Receptor-related Orphan Receptor α with Potent Antiarthritic Activity. J. Biol. Chem. 1996, 271, 13515–13522. [Google Scholar] [CrossRef]
- Hirose, T.; Smith, R.J.; Jetten, A. ROR-γ: The Third Member of ROR/RZR Orphan Receptor Subfamily That Is Highly Expressed in Skeletal Muscle. Biochem. Biophys. Res. Commun. 1994, 205, 1976–1983. [Google Scholar] [CrossRef]
- Steinhilber, D.; Brungs, M.; Werz, O.; Wiesenberg, I.; Danielsson, C.; Kahlen, J.-P.; Nayeri, S.; Schräder, M.; Carlberg, C. The Nuclear Receptor for Melatonin Represses 5-Lipoxygenase Gene Expression in Human B Lymphocytes. J. Biol. Chem. 1995, 270, 7037–7040. [Google Scholar] [CrossRef] [PubMed]
- Pozo, D.; Reiter, R.J.; Calvo, J.R.; Guerrero, J.M. Inhibition of cerebellar nitric oxide synthase and cyclic GMP production by melatonin via complex formation with calmodulin. J. Cell. Biochem. 1997, 65, 430–442. [Google Scholar] [CrossRef]
- Allegra, M.; Reiter, R.; Tan, D.-X.; Gentile, C.; Tesoriere, L.; Livrea, M. The chemistry of melatonin’s interaction with reactive species. J. Pineal Res. 2004, 34, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Manchester, L.; Tan, D.-X. Melatonin in walnuts: Influence on levels of melatonin and total antioxidant capacity of blood. Nutrition 2005, 21, 920–924. [Google Scholar] [CrossRef]
- Reiter, R.J. Functional diversity of the pineal hormone melatonin: Its role as an antioxidant. Exp. Clin. Endocrinol. Diabetes 1996, 104, 10–16. [Google Scholar] [CrossRef]
- Beyer, C.E.; Steketee, J.D.; Saphier, D. Antioxidant properties of melatonin—An emerging mystery. Biochem. Pharmacol. 1998, 56, 1265–1272. [Google Scholar] [CrossRef]
- Mahdi, A.A.; Fatima, G. A Quest for Better Understanding of Biochemical Changes in Fibromyalgia Syndrome. Indian J. Clin. Biochem. 2013, 29, 1. [Google Scholar] [CrossRef]
- Chen, W.-W.; Zhang, X.; Huang, W.-J. Pain control by melatonin: Physiological and pharmacological effects (Review). Exp. Ther. Med. 2016, 12, 1963–1968. [Google Scholar] [CrossRef]
- Posa, L.; De Gregorio, D.; Lopez-Canul, M.; He, Q.; Darcq, E.; Rullo, L.; Pearl-Dowler, L.; Luongo, L.; Candeletti, S.; Romualdi, P.; et al. Supraspinal melatonin MT 2 receptor agonism alleviates pain via a neural circuit that recruits mu opioid receptors. J. Pineal Res. 2022, 73, e12825. [Google Scholar] [CrossRef]
- Lopez-Canul, M.; Palazzo, E.; Dominguez-Lopez, S.; Luongo, L.; Lacoste, B.; Comai, S.; Angeloni, D.; Fraschini, F.; Boccella, S.; Spadoni, G.; et al. Selective melatonin MT2 receptor ligands relieve neuropathic pain through modulation of brainstem descending antinociceptive pathways. Pain 2015, 156, 305–317. [Google Scholar] [CrossRef]
- Marseglia, L.; D’Angelo, G.; Manti, S.; Aversa, S.; Arrigo, T.; Reiter, R.J.; Gitto, E. Analgesic, Anxiolytic and Anaesthetic Effects of Melatonin: New Potential Uses in Pediatrics. Int. J. Mol. Sci. 2015, 16, 1209–1220. [Google Scholar] [CrossRef]
- Wilhelmsen, M.; Amirian, I.; Reiter, R.J.; Rosenberg, J.; Gögenur, I. Analgesic effects of melatonin: A review of current evidence from experimental and clinical studies. J. Pineal Res. 2011, 51, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Li, J.C.; Ma, K.C.; Wang, M. Effects of Melatonin on Hypothalamic γ-Aminobutyric Acid, Aspartic Acid, Glutamic Acid, β-Endorphin and Serotonin Levels in Male Mice. Neurosignals 1995, 4, 225–231. [Google Scholar] [CrossRef]
- Yu, C.-X.; Zhu, C.-B.; Xu, S.-F.; Cao, X.-D.; Wu, G.-C. The analgesic effects of peripheral and central administration of melatonin in rats. Eur. J. Pharmacol. 2000, 403, 49–53. [Google Scholar] [CrossRef]
- Shavali, S.; Ho, B.; Govitrapong, P.; Sawlom, S.; Ajjimaporn, A.; Klongpanichapak, S.; Ebadi, M. Melatonin exerts its analgesic actions not by binding to opioid receptor subtypes but by increasing the release of β-endorphin an endogenous opioid. Brain Res. Bull. 2005, 64, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Posa, L.; Lopez-Canul, M.; Rullo, L.; De Gregorio, D.; Dominguez-Lopez, S.; Aboud, M.K.; Caputi, F.F.; Candeletti, S.; Romualdi, P.; Gobbi, G. Nociceptive responses in melatonin MT2 receptor knockout mice compared to MT1 and double MT1/MT2 receptor knockout mice. J. Pineal Res. 2020, 69, e12671. [Google Scholar] [CrossRef] [PubMed]
- Lakin, M.L.; Miller, C.H.; Stott, M.L.; Winters, W.D. Involvement of the pineal gland and melatonin in murine analgesia. Life Sci. 1981, 29, 2543–2551. [Google Scholar] [CrossRef]
- El-Shenawy, S.M.; Abdel-Salam, O.M.; Baiuomy, A.R.; El-Batran, S.; Arbid, M.S. Studies on the anti-inflammatory and anti-nociceptive effects of melatonin in the rat. Pharmacol. Res. 2002, 46, 235–243. [Google Scholar] [CrossRef]
- Hernández-Pacheco, A.; Araiza-Saldaña, C.I.; Granados-Soto, V.; Mixcoatl-Zecuatl, T. Possible participation of the nitric oxide-cyclic GMP-protein kinase G-K+ channels pathway in the peripheral antinociception of melatonin. Eur. J. Pharmacol. 2008, 596, 70–76. [Google Scholar] [CrossRef]
- Srinivasan, V.; Lauterbach, E.C.; Ho, K.Y.; Acuña-Castroviejo, D.; Zakaria, R.; Brzezinski, A. Melatonin in Antinociception: Its Therapeutic Applications. Curr. Neuropharmacol. 2012, 10, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Jiang, H.; Liu, X.; Hu, S.; Li, H.; Feng, Y.; Ke, J.; Long, X. Melatonin Abates TMJOA Chronic Pain by MT2R in Trigeminal Ganglion Neurons. J. Dent. Res. 2022, 101, 111–119. [Google Scholar] [CrossRef]
- Benlidayi, I.C. Role of inflammation in the pathogenesis and treatment of fibromyalgia. Rheumatol. Int. 2019, 39, 781–791. [Google Scholar] [CrossRef]
- Serfaty, M.A.; Osborne, D.; Buszewicz, M.J.; Blizard, R.; Raven, P.W. A randomized double-blind placebo-controlled trial of treatment as usual plus exogenous slow-release melatonin (6 mg) or placebo for sleep disturbance and depressed mood. Int. Clin. Psychopharmacol. 2010, 25, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Vacas, M.I.; Del Zar, M.M.; Martinuzzo, M.; Falcon, C.; Carreras, L.O.; Cardinali, D.P. Inhibition of human platelet aggregation and thromboxane B2 production by melatonin. Correlation with plasma melatonin levels. J. Pineal Res. 1991, 11, 135–139. [Google Scholar] [CrossRef]
- Cardinali, D.P.; Del Zar, M.M.; Vacas, M.I. The effects of melatonin in human platelets. Acta Physiol. Pharmacol. Ther. Latinoam. 1993, 43, 1–13. [Google Scholar]
- Aktürk, S.; Büyükavcı, R. Evaluation of blood neutrophil-lymphocyte ratio and platelet distribution width as inflammatory markers in patients with fibromyalgia. Clin. Rheumatol. 2017, 36, 1885–1889. [Google Scholar] [CrossRef] [PubMed]
- Ferracioli-Oda, E.; Qawasmi, A.; Bloch, M.H. Meta-Analysis: Melatonin for the Treatment of Primary Sleep Disorders. PLoS ONE 2013, 8, e63773. [Google Scholar] [CrossRef]
- Castro-Marrero, J.; Zaragozá, M.-C.; López-Vílchez, I.; Galmés, J.L.; Cordobilla, B.; Maurel, S.; Domingo, J.C.; Alegre-Martín, J. Effect of Melatonin Plus Zinc Supplementation on Fatigue Perception in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Antioxidants 2021, 10, 1010. [Google Scholar] [CrossRef]
- Yunus, M.B.; Dailey, J.W.; Aldag, J.C.; Masi, A.T.; Jobe, P.C. Plasma tryptophan and other amino acids in primary fibromyalgia: A controlled study. J. Rheumatol. 1992, 19, 90–94. [Google Scholar]
- Di Franco, M.; Iannuccelli, C.; Valesini, G. Neuroendocrine immunology of fibromyalgia. Ann. N. Y. Acad. Sci. 2010, 1193, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Riva, R.; Mork, P.J.; Westgaard, R.H.; Lundberg, U. Comparison of the cortisol awakening response in women with shoulder and neck pain and women with fibromyalgia. Psychoneuroendocrinology 2012, 37, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, L.S.; Correa, H.; Silva, G.C.; Campos, F.S.; Baião, F.R.; Ribeiro, L.S.; Faria, A.M.; d’Avila Reis, D. May genetic factors in fibromyalgia help to identify patients with differentially altered frequencies of immune cells? Clin. Exp. Immunol. 2018, 154, 346–352. [Google Scholar] [CrossRef]
- Pernambuco, A.P.; Schetino, L.P.; Alvim, C.C.; Murad, C.M.; Viana, R.S.; Carvalho, L.S.; Reis, D.A. Increased levels of IL-17A in patients with fibromyalgia. Ann. Rheum. Dis. 2013, 31, 60–63. [Google Scholar]
- Martin, J.L.; Hakim, A.D. Wrist Actigraphy. Chest 2011, 139, 1514–1527. [Google Scholar] [CrossRef] [PubMed]
- Sadeh, A. The role and validity of actigraphy in sleep medicine: An update. Sleep Med. Rev. 2011, 15, 259–267. [Google Scholar] [CrossRef]
- Castaño, M.Y.; Garrido, M.; Delgado-Adámez, J.; Martillanes, S.; Gómez, M.; Rodríguez, A.B. Oral melatonin administration improves the objective and subjective sleep quality, increases 6-sulfatoxymelatonin levels and total antioxidant capacity in patients with fibromyalgia. J. Appl. Biomed. 2018, 16, 186–191. [Google Scholar] [CrossRef]
- Castaño, M.Y.; Garrido, M.; Rodríguez, A.B.; Gómez, M.Á. Melatonin Improves Mood Status and Quality of Life and Decreases Cortisol Levels in Fibromyalgia. Biol. Res. Nurs. 2019, 21, 22–29. [Google Scholar] [CrossRef]
- Littner, M.; Kushida, C.A.; Anderson, W.M.; Bailey, D.; Berry, R.B.; Davila, D.G.; Hirshkowitz, M.; Kapen, S.; Kramer, M.; Loube, D.; et al. Practice Parameters for the Role of Actigraphy in the Study of Sleep and Circadian Rhythms: An Update for 2002. Sleep 2003, 26, 337–341. [Google Scholar] [CrossRef]
- Mcloughlin, M.J.; Colbert, L.H.; Stegner, A.J.; Cook, D.B. Are Women with Fibromyalgia Less Physically Active than Healthy Women? Med. Sci. Sport. Exerc. 2011, 43, 905–912. [Google Scholar] [CrossRef]
- Okifuji, A.; Hare, B.D. Nightly Analyses of Subjective and Objective (Actigraphy) Measures of Sleep in Fibromyalgia Syndrome: What Accounts for the Discrepancy? Clin. J. Pain 2011, 27, 289–296. [Google Scholar] [CrossRef]
- Segura-Jiménez, V.; Camiletti-Moirón, D.; Munguía-Izquierdo, D.; Álvarez-Gallardo, I.C.; Ruiz, J.R.; Ortega, F.B.; Delgado-Fernández, M. Agreement between self-reported sleep patterns and actigraphy in fibromyalgia and healthy women. Clin. Exp. Rheumatol. 2015, 33, 58–67. [Google Scholar]
- Chinn, S.; Caldwell, W.; Gritsenko, K. Fibromyalgia Pathogenesis and Treatment Options Update. Curr. Pain Headache Rep. 2016, 20, 25. [Google Scholar] [CrossRef] [PubMed]
- Pernambuco, A.P.; Schetino, L.P.L.; Viana, R.S.; Carvalho, L.S.C.; Reis, D. The involvement of melatonin in the clinical status of patients with fibromyalgia syndrome. Clin. Exp. Rheumatol. 2014, 33, 14–19. [Google Scholar]
- Citera, G.; Arias, M.A.; Maldonado-Cocco, J.A.; La´zaro, M.A.; Rosemffet, M.G.; Brusco, L.I.; Scheines, E.J.; Cardinalli, D.P. The Effect of Melatonin in Patients with Fibromyalgia: A Pilot Study. Clin. Rheumatol. 2000, 19, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Acuna-Castroviejo, D.; Escames, G.; Reiter, R.J. Melatonin therapy in fibromyalgia. J. Pineal Res. 2006, 40, 98–99. [Google Scholar] [CrossRef]
- Hussain, S.A.-R.; Al-Khalifa, I.I.; Jasim, N.A.; Gorial, F.I. Adjuvant use of melatonin for treatment of fibromyalgia. J. Pineal Res. 2011, 50, 267–271. [Google Scholar] [CrossRef]
- Bigatti, S.M.; Hernandez, A.M.; Cronan, T.A.; Rand, K.L. Sleep disturbances in fibromyalgia syndrome: Relationship to pain and depression. Arthritis Care Rheum. 2008, 59, 961–967. [Google Scholar] [CrossRef]
- Ulus, Y.; Akyol, Y.; Tander, B.; Durmus, D.; Bilgici, A.; Kuru, O. Sleep quality in fibromyalgia and rheumatoid arthritis: Associations with pain, fatigue, depression, and disease activity. Clin. Exp. Rheumatol. 2012, 29, 92–96. [Google Scholar]
- Hita-Contreras, F.; Martínez-López, E.; Latorre-Román, P.A.; Garrido, F.; Santos, M.A.; Martínez-Amat, A. Reliability and validity of the Spanish version of the Pittsburgh Sleep Quality Index (PSQI) in patients with fibromyalgia. Rheumatol. Int. 2014, 34, 929–936. [Google Scholar] [CrossRef]
- Wikner, J.; Hirsch, U.; Wetterberg, L.; Röjdmark, S. Fibromyalgia-a syndrome associated with decreased nocturnal melatonin secretion. Clin. Endocrinol. 1998, 49, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Finan, P.H.; Goodin, B.R.; Smith, M.T. The Association of Sleep and Pain: An Update and a Path Forward. J. Pain 2013, 14, 1539–1552. [Google Scholar] [CrossRef] [PubMed]
- Dolberg, O.T.; Hirschmann, S.; Grunhaus, L. Melatonin for the Treatment of Sleep Disturbances in Major Depressive Disorder. Am. J. Psychiatry 1998, 155, 1119–1121. [Google Scholar] [CrossRef] [PubMed]
- Gross, P.K.; Nourse, R.; Wasser, T.E. Ramelteon for insomnia symptoms in a community sample of adults with generalized anxiety disorder: An open label study. J. Clin. Sleep Med. 2009, 15, 28–33. [Google Scholar] [CrossRef]
- Lewy, A.J.; Lefler, B.J.; Emens, J.S.; Bauer, V.K. The circadian basis of winter depression. Proc. Natl. Acad. Sci. USA 2006, 103, 7414–7419. [Google Scholar] [CrossRef]
- González-Flores, D.; Gamero, E.; Garrido, M.; Ramirez, R.; Moreno, D.; Delgado, J.; Valdés, E.; Barriga, C.; Rodríguez, A.B.; Paredes, S.D. Urinary 6-sulfatoxymelatonin and total antioxidant capacity increase after the intake of a grape juice cv. Tempranillo stabilized with HHP. Food Funct. 2012, 3, 34–39. [Google Scholar] [CrossRef]
- McLean, S.A.; Williams, D.A.; Harris, R.E.; Kop, W.J.; Groner, K.H.; Ambrose, K.; Lyden, A.K.; Gracely, R.H.; Crofford, L.J.; Geisser, M.E.; et al. Momentary relationship between cortisol secretion and symptoms in patients with fibromyalgia. Arthritis Rheum. 2005, 52, 3660–3669. [Google Scholar] [CrossRef]
- Fischer, S.; Doerr, J.M.; Strahler, J.; Mewes, R.; Thieme, K.; Nater, U.M. Stress exacerbates pain in the everyday lives of women with fibromyalgia syndrome—The role of cortisol and alpha-amylase. Psychoneuroendocrinology 2016, 63, 68–77. [Google Scholar] [CrossRef]
- Uçar, M.; Sarp, Ü.; Karaaslan, Ö.; Gül, A.I.; Tanik, N.; Arik, H.O. Health anxiety and depression in patients with fibromyalgia syndrome. J. Int. Med. Res. 2015, 43, 679–685. [Google Scholar] [CrossRef]
- Kalsbeek, A.; van der Spek, R.; Lei, J.; Endert, E.; Buijs, R.M.; Fliers, E. Circadian rhythms in the hypothalamo–pituitary–adrenal (HPA) axis. Mol. Cell. Endocrinol. 2012, 349, 20–29. [Google Scholar] [CrossRef]
- De Oliveira, C.; Scarabelot, V.L.; de Souza, A.; de Oliveira, C.M.; Medeiros, L.F.; de Macedo, I.C.; Filho, P.R.M.; Cioato, S.G.; Caumo, W.; Torres, I.L. Obesity and chronic stress are able to desynchronize the temporal pattern of serum levels of leptin and triglycerides. Peptides 2014, 51, 46–53. [Google Scholar] [CrossRef]
- Kara, O.; Polo, O. Autonomic and central stress-regulation disintegration in stress-related anxiety disorders. Acta Neuropsychol. 2014, 1, 1–25. [Google Scholar]
- Wingenfeld, K.; Heim, C.; Schmidt, I.; Wagner, D.; Meinlschmidt, G.; Hellhammer, D.H. HPA Axis Reactivity and Lymphocyte Glucocorticoid Sensitivity in Fibromyalgia Syndrome and Chronic Pelvic Pain. Psychosom. Med. 2008, 70, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Wingenfeld, K.; Nutzinger, D.; Kauth, J.; Hellhammer, D.H.; Lautenbacher, S. Salivary Cortisol Release and Hypothalamic Pituitary Adrenal Axis Feedback Sensitivity in Fibromyalgia Is Associated with Depression But Not With Pain. J. Pain 2010, 11, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Curtis, K.; Osadchuk, A.; Katz, J. An eight-week yoga intervention is associated with improvements in pain, psychological functioning and mindfulness, and changes in cortisol levels in women with fibromyalgia. J. Pain Res. 2011, 4, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Riva, R.; Mork, P.J.; Westgaard, R.H.; Rø, M.; Lundberg, U. Fibromyalgia Syndrome is Associated with Hypocortisolism. Int. J. Behav. Med. 2010, 17, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, A.S.; Bancalero, F.J.; García, P.M.; Serrano, O.E.; Alegre, D.M.; Bocos, T.J. Evaluation of urinary cortisol levels in women with fibromyalgia. Med. Clin. 2009, 133, 255–257. [Google Scholar] [CrossRef]
- Semiz, E.A.; Hizmetli, S.; Semiz, M.; Karadağ, A.; Adalı, M.; Tuncay, M.S.; Alim, B.; Hayta, E.; Uslu, A.U. Serum cortisol and dehydroepiandrosterone-sulfate levels after balneotherapy and physical therapy in patients with fibromyalgia. Saudi Med. J. 2016, 37, 544–550. [Google Scholar] [CrossRef]
- Torres-Farfán, C.; Richter, H.G.; Rojas-García, P.; Vergara, M.; Forcelledo, M.L.; Valladares, L.E.; Torrealba, F.; Valenzuela, G.J.; Serón-Ferré, M. mt1 Melatonin Receptor in the Primate Adrenal Gland: Inhibition of Adrenocorticotropin-Stimulated Cortisol Production by Melatonin. J. Clin. Endocrinol. Metab. 2003, 88, 450–458. [Google Scholar] [CrossRef]
- Geiss, A.; Rohleder, N.; Anton, F. Evidence for an association between an enhanced reactivity of interleukin-6 levels and reduced glucocorticoid sensitivity in patients with fibromyalgia. Psychoneuroendocrinology 2012, 37, 671–684. [Google Scholar] [CrossRef]
- Goudman, L.; De Smedt, A.; Roggeman, S.; Fernández-de-las-Peñas, C.; Hatem, S.M.; Schiltz, M.; Billot, M.; Roulaud, M.; Rigoard, P.; Moens, M. Association between Experimental Pain Measurements and the Central Sensitization Inventory in Patients at Least 3 Months after COVID-19 Infection: A Cross-Sectional Pilot Study. J. Clin. Med. 2023, 12, 661. [Google Scholar] [CrossRef]
- Yunus, M.B. Editorial review: An update on central sensitivity syndromes and the issues of nosology and psychobiology. Curr. Rheumatol. Rev. 2015, 11, 70–85. [Google Scholar] [CrossRef]
- Bierle, D.M.; Aakre, C.A.; Grach, S.L.; Salonen, B.R.; Croghan, I.T.; Hurt, R.T.; Ganesh, R. Central Sensitization Phenotypes in Post Acute Sequelae of SARS-CoV-2 Infection (PASC): Defining the Post COVID Syndrome. J. Prim. Care Community Health 2021, 12, 21501327211030826. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, D.P.; Brown, G.M.; Pandi-Perumal, S.R. Possible Application of Melatonin in Long COVID. Biomolecules 2022, 12, 1646. [Google Scholar] [CrossRef]
- Bjurstrom, M.F.; Giron, S.E.; Griffis, C.A. Cerebrospinal fluid cytokines and neurotrophic factors in human chronic pain populations: A comprehensive review. Pain Pr. 2016, 16, 183–203. [Google Scholar] [CrossRef]
- Christidis, N.; Ghafouri, B.; Larsson, A.; Palstam, A.; Mannerkorpi, K.; Bileviciute-Ljungar, I.; Löfgren, M.; Bjersing, J.; Kosek, E.; Gerdle, B.; et al. Comparison of the Levels of Pro-Inflammatory Cytokines Released in the Vastus Lateralis Muscle of Patients with Fibromyalgia and Healthy Controls during Contractions of the Quadriceps Muscle–A Microdialysis Study. PLoS ONE 2015, 10, e0143856. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, Z.; Wu, X.; Mao, A.; Chang, F.; Deng, X.; Gao, H.; Ouyang, C.; Dery, K.J.; Le, K.; et al. Discovery of Potential New Gene Variants and Inflammatory Cytokine Associations with Fibromyalgia Syndrome by Whole Exome Sequencing. PLoS ONE 2013, 8, e65033. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.B.; Maixner, D.W.; Fillingim, R.B.; Slade, G.; Gracely, R.H.; Ambrose, K.; Zaykin, D.V.; Hyde, C.; John, S.; Tan, K.; et al. Large candidate gene association study reveals genetic risk factors and therapeutic targets for fibromyalgia. Arthritis Rheumatol. 2012, 64, 584–593. [Google Scholar] [CrossRef]
- Iacob, E.; Light, A.R.; Donaldson, G.W.; Okifuji, A.; Hughen, R.W.; White, A.T.; Light, K.C. Gene Expression Factor Analysis to Differentiate Pathways Linked to Fibromyalgia, Chronic Fatigue Syndrome, and Depression in a Diverse Patient Sample. Arthritis Care Res. 2016, 68, 132–140. [Google Scholar] [CrossRef]
- Bjørklund, G.; Dadar, M.; Chirumbolo, S.; Aaseth, J. Fibromyalgia and nutrition: Therapeutic possibilities? Biomed. Pharmacother. 2018, 103, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.P.; Titova, D.; Oeser, A.; Randels, M.; Avalos, I.; Milne, G.L.; Morrow, J.D.; Stein, C.M. Oxidative stress in fibromyalgia and its relationship to symptoms. Clin. Rheumatol. 2009, 28, 435–438. [Google Scholar] [CrossRef]
- Nosjean, O.; Ferro, M.; Cogé, F.; Beauverger, P.; Henlin, J.-M.; Lefoulon, F.; Fauchère, J.-L.; Delagrange, P.; Canet, E.; Boutin, J.A. Identification of the Melatonin-binding SiteMT 3 as the Quinone Reductase. J. Biol. Chem. 2000, 275, 31311–31317. [Google Scholar] [CrossRef]
- Legros, C.; Dupuis, P.; Ferry, G.; Boutin, J.A. Measuring Binding at the Putative Melatonin Receptor MT3. Methods Mol. Biol. 2022, 2550, 283–289. [Google Scholar] [CrossRef]
- Calamini, B.; Ferry, G.; Boutin, J.A. Melatonin Binding to Human NQO2 by Isothermal Titration Calorimetry. Methods Mol. Biol. 2022, 2550, 305–314. [Google Scholar] [CrossRef]
- Calamini, B.; Ferry, G.; Boutin, J.A. Cloning, Expression, Purification, Crystallization, and X-Ray Structural Determination of the Human NQO2 in Complex with Melatonin. Methods Mol. Biol. 2022, 2550, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Fratter, A. New Development in Melatonin Research; Nova Science Publisher, Inc.: New York, NY, USA, 2013. [Google Scholar]
- Schmidt-Wilcke, T.; Kairys, A.; Ichesco, E.; Fernandez-Sanchez, M.L.; Barjola, P.; Heitzeg, M.; Harris, R.E.; Clauw, D.J.; Glass, J.; Williams, D.A. Changes in Clinical Pain in Fibromyalgia Patients Correlate with Changes in Brain Activation in the Cingulate Cortex in a Response Inhibition Task. Pain Med. 2014, 15, 1346–1358. [Google Scholar] [CrossRef]
- Alcocer-Gómez, E.; Garrido-Maraver, J.; Bullón, P.; Marín-Aguilar, F.; Cotán, D.; Carrión, A.M.; Alvarez-Suarez, J.M.; Giampieri, F.; Sánchez-Alcazar, J.A.; Battino, M.; et al. Metformin and caloric restriction induce an AMPK-dependent restoration of mitochondrial dysfunction in fibroblasts from Fibromyalgia patients. Biochim. Biophys. Acta 2015, 1852, 1257–1267. [Google Scholar] [CrossRef] [PubMed]
- Cordero, M.D.; Díaz-Parrado, E.; Carrión, A.M.; Alfonsi, S.; Sánchez Alcazar, J.A.; Bullón, P.; Battino, M.; de Miguel, M. Is inflammation a mitochondrial dysfunction-dependent event in fibromyalgia? Antioxid. Redox Signal. 2013, 18, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Kadetoff, D.; Lampa, J.; Westman, M.; Andersson, M.; Kosek, E. Evidence of central inflammation in fibromyalgia—Increased cerebrospinal fluid interleukin-8 levels. J. Neuroimmunol. 2012, 242, 33–38. [Google Scholar] [CrossRef]
- Kosek, E.; Altawil, R.; Kadetoff, D.; Finn, A.; Westman, M.; Le Maître, E.; Andersson, M.; Jensen-Urstad, M.; Lampa, J. Evidence of different mediators of central inflammation in dysfunctional and inflammatory pain-interleukin-8 in fibromyalgia and interleukin-1 β in rheumatoid arthritis. J. Neuroimmunol. 2015, 280, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Bhutani, S.; Kim, C.H.; Irwin, M.R. Anti-inflammatory effects of melatonin: A systematic review and meta-analysis of clinical trials. Brain Behav. Immun. 2021, 93, 245–253. [Google Scholar] [CrossRef]
- Higgs, J.B. Fibromyalgia in Primary Care. Prim. Care Clin. Off. Pr. 2018, 45, 325–341. [Google Scholar] [CrossRef]
- Suofu, Y.; Li, W.; Jean-Alphonse, F.G.; Jia, J.; Khattar, N.K.; Li, J.; Baranov, S.V.; Leronni, D.; Mihalik, A.C.; He, Y.; et al. Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc. Natl. Acad. Sci. USA 2017, 114, E7997–E8006. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Manchester, L.C.; El-Sawi, M.R. Melatonin Reduces Oxidant Damage and Promotes Mitochondrial Respiration: Implications for aging. Ann. N. Y. Acad. Sci. 2002, 959, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhu, S.; Pei, Z.; Drozda, M.; Stavrovskaya, I.G.; Del Signore, S.J.; Cormier, K.; Shimony, E.M.; Wang, H.; Ferrante, R.J.; et al. Inhibitors of Cytochrome c Release with Therapeutic Potential for Huntington’s Disease. J. Neurosci. 2008, 28, 9473–9485. [Google Scholar] [CrossRef]
- Wang, X.; Sirianni, A.; Pei, Z.; Cormier, K.; Smith, K.; Jiang, J.; Zhou, S.; Wang, H.; Zhao, R.; Yano, H.; et al. The Melatonin MT1 Receptor Axis Modulates Mutant Huntingtin-Mediated Toxicity. J. Neurosci. 2011, 31, 14496–14507. [Google Scholar] [CrossRef]
- Favero, G.; Bonomini, F.; Franco, C.; Rezzani, R. Mitochondrial Dysfunction in Skeletal Muscle of a Fibromyalgia Model: The Potential Benefits of Melatonin. Int. J. Mol. Sci. 2019, 20, 765. [Google Scholar] [CrossRef]
- Favero, G.; Trapletti, V.; Bonomini, F.; Stacchiotti, A.; Lavazza, A.; Rodella, L.F.; Rezzani, R. Oral Supplementation of Melatonin Protects against Fibromyalgia-Related Skeletal Muscle Alterations in Reserpine-Induced Myalgia Rats. Int. J. Mol. Sci. 2017, 18, 1389. [Google Scholar] [CrossRef] [PubMed]
- Blasco-Serra, A.; Escrihuela-Vidal, F.; González-Soler, E.M.; Martínez-Expósito, F.; Blasco-Ausina, M.C.; Martínez-Bellver, S.; Cervera-Ferri, A.; Teruel-Martí, V.; Valverde-Navarro, A.A. Depressive-like symptoms in a reserpine-induced model of fibromyalgia in rats. Physiol. Behav. 2015, 151, 456–462. [Google Scholar] [CrossRef]
- Maeda, T.; Kudo, Y.; Horiuchi, T.; Makino, N. Clinical and anti-aging effect of mud-bathing therapy for patients with fibromyalgia. Mol. Cell. Biochem. 2018, 444, 87–92. [Google Scholar] [CrossRef]
- Segura-Jiménez, V.; Borges-Cosic, M.; Soriano-Maldonado, A.; Estévez-López, F.; Álvarez-Gallardo, I.C.; Herrador-Colmenero, M.; Delgado-Fernández, M.; Ruiz, J.R. Association of sedentary time and physical activity with pain, fatigue, and impact of fibromyalgia: The al-Ándalus study. Scand. J. Med. Sci. Sport. 2017, 27, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Cannavino, J.; Brocca, L.; Sandri, M.; Bottinelli, R.; Pellegrino, M.A. PGC1-α over-expression prevents metabolic alterations and soleus muscle atrophy in hindlimb unloaded mice. J. Physiol. 2014, 592, 4575–4589. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.L.; Robinson, M.M.; Nair, K.S. Skeletal muscle aging and the mitochondrion. Trends Endocrinol. Metab. 2013, 24, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Asfour, H.A.; Allouh, M.Z.; Said, R.S. Myogenic regulatory factors: The orchestrators of myogenesis after 30 years of discovery. Exp. Biol. Med. 2018, 243, 118–128. [Google Scholar] [CrossRef]
- Bazgir, B.; Fathi, R.; Valojerdi, M.R.; Mozdziak, P.; Asgari, A. Satellite Cells Contribution to Exercise Mediated Muscle Hypertrophy and Repair. Cell J. 2017, 18, 473–484. [Google Scholar] [CrossRef]
- Gammazza, A.M.; Macaluso, F.; Di Felice, V.; Cappello, F.; Barone, R. Hsp60 in Skeletal Muscle Fiber Biogenesis and Homeostasis: From Physical Exercise to Skeletal Muscle Pathology. Cells 2018, 7, 224. [Google Scholar] [CrossRef]
- Cordero, M.D.; Alcocer-Gómez, E.; de Miguel, M.; Cano-García, F.J.; Luque, C.M.; Fernández-Riejo, P.; Fernández, A.M.; Sánchez-Alcazar, J.A. Coenzyme Q10: A novel therapeutic approach for Fibromyalgia? Case series with 5 patients. Mitochondrion 2011, 11, 623–625. [Google Scholar] [CrossRef]
- Cordero, M.D.; Cotán, D.; Del-Pozo-Martín, Y.; Carrión, A.M.; de Miguel, M.; Bullón, P.; Sánchez-Alcazar, J.A. Oral coenzyme Q10 supplementation improves clinical symptoms and recovers pathologic alterations in blood mononuclear cells in a fibromyalgia patient. Nutrition 2012, 28, 1200–1203. [Google Scholar] [CrossRef]
- Miyamae, T.; Seki, M.; Naga, T.; Uchino, S.; Asazuma, H.; Yoshida, T.; Iizuka, Y.; Kikuchi, M.; Imagawa, T.; Natsumeda, Y.; et al. Increased oxidative stress and coenzyme Q10 deficiency in juvenile fibromyalgia: Amelioration of hypercholesterolemia and fatigue by ubiquinol-10 supplementation. Redox Rep. 2013, 18, 12–19. [Google Scholar] [CrossRef]
- Cordero, M.D.; Alcocer-Gómez, E.; Culic, O.; Carrión, A.M.; de Miguel, M.; Díaz-Parrado, E.; Pérez-Villegas, E.M.; Bullón, P.; Battino, M.; Sánchez-Alcazar, J.A.; et al. NLRP3 Inflammasome Is Activated in Fibromyalgia: The Effect of Coenzyme Q10. Antioxid. Redox Signal. 2014, 20, 1169–1180. [Google Scholar] [CrossRef]
- Sawaddiruk, P.; Apaijai, N.; Paiboonworachat, S.; Kaewchur, T.; Kasitanon, N.; Jaiwongkam, T.; Kerdphoo, S.; Chattipakorn, N.; Chattipakorn, S.C. Coenzyme Q10 supplementation alleviates pain in pregabalin-treated fibromyalgia patients via reducing brain activity and mitochondrial dysfunction. Free Radic. Res. 2019, 53, 901–909. [Google Scholar] [CrossRef]
- Yu, B.P. Cellular defenses against damage from reactive oxygen species. Physiol. Rev. 1994, 74, 139–162. [Google Scholar] [CrossRef] [PubMed]
- Urata, Y.; Honma, S.; Goto, S.; Todoroki, S.; Iida, T.; Cho, S.; Honma, K.; Kondo, T. Melatonin induces γ-glutamylcysteine synthetase mediated by activator protein-1 in human vascular endothelial cells. Free Radic. Biol. Med. 1999, 27, 838–847. [Google Scholar] [CrossRef]
- Castroviejo, D.A.; Lopez, L.C.; Escames, G.; Lopez, A.; Garcia, J.A.; Reiter, R.J. Melatonin-mitochondria Interplay in Health and Disease. Curr. Top. Med. Chem. 2011, 11, 221–240. [Google Scholar] [CrossRef] [PubMed]
- Deker, J.F.; Quay, W.B. Stimulatory effects of melatonin on ependymal epithelium of choroid plexuses in Golden Hamsters. J. Neural Transm. 1982, 55, 53–67. [Google Scholar] [CrossRef]
- Poon, A.M.S.; Pang, S.F. 2-[125I] iodomelatonin binding sites in spleens of guinea pig. Life Sci. 1992, 50, 1719–1726. [Google Scholar] [CrossRef]
- Wakatsuki, A.; Okatani, Y.; Shinohara, K.; Ikenoue, N.; Kaneda, C.; Fukaya, T. Melatonin protects fetal rat brain against oxidative mitochondrial damage. J. Pineal Res. 2001, 30, 22–28. [Google Scholar] [CrossRef]
- Okatani, Y.; Wakatsuki, A.; Reiter, R.J. Melatonin protects hepatic mitochondrial respiratory chain activity in senescence-accelerated mice. J. Pineal Res. 2002, 23, 143–148. [Google Scholar] [CrossRef]
- Ceraulo, L.; Ferrugia, M.; Tesoriere, L.; Segreto, S.; Livrea, M.A.; Liveri, V.T. Interactions of melatonin with membrane models: Portioning of melatonin in AOT and lecithin reversed micelles. J. Pineal Res. 1999, 26, 108–112. [Google Scholar] [CrossRef]
- Rodriguez, A.B.; Nogales, G.; Marchena, J.M.; Ortega, E.; Barriga, C. Suppression of both basal and antigen-induced lipid peroxidation in ring dove heterophils by melatonin. Biochem. Pharmacol. 1999, 58, 1301–1306. [Google Scholar] [CrossRef]
- Terrón, M.P.; Paredes, S.D.; Barriga, C.; Ortega, E.; Reiter, R.J.; Rodríguez, A.B. Melatonin, lipid peroxidation, and age in heterophils from the ring dove (Streptopelia risoria). Free Radic. Res. 2005, 39, 613–619. [Google Scholar] [CrossRef]
- García, J.J.; López-Pingarrón, L.; Almeida-Souza, P.; Tres, A.; Escudero, P.; García-Gil, F.A.; Tan, D.-X.; Reiter, R.J.; Ramírez, J.M.; Bernal-Pérez, M. Protective effects of melatonin in reducing oxidative stress and in preserving the fluidity of biological membranes: A review. J. Pineal Res. 2014, 56, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Siracusa, R.; D’amico, R.; Peritore, A.F.; Cordaro, M.; Gugliandolo, E.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; Di Paola, R. Melatonin Plus Folic Acid Treatment Ameliorates Reserpine-Induced Fibromyalgia: An Evaluation of Pain, Oxidative Stress, and Inflammation. Antioxidants 2019, 8, 628. [Google Scholar] [CrossRef] [PubMed]
- Ramis, M.R.; Esteban, S.; Miralles, A.; Tan, D.-X.; Reiter, R.J. Protective Effects of Melatonin and Mitochondria-targeted Antioxidants Against Oxidative Stress: A Review. Curr. Med. Chem. 2015, 22, 2690–2711. [Google Scholar] [CrossRef] [PubMed]
| Study | n | Dose | Study Design | Results |
|---|---|---|---|---|
| Citera et al., 2000 [85] | 19 | 3 mg | Pilot | Tender points, pain intensity, and sleep quality improved after 4 weeks |
| Acuña-Castroviejo et al., 2006 [86] | 4 | 6 mg daily | Pilot | Pain and fatigue improved. Sleep–wake cycle normalized after 15 days |
| Hussain et al., 2011 [87] | 101 | 5 mg daily/ 3 mg + 20 mg fluoxetine daily/5 mg + 20 mg fluoxetine daily | Double-blind RCT 1 | In every case, FIQ 2 score improved. After 8 weeks, combination with fluoxetine was more effective |
| De Zanette et al., 2014 [26] | 63 | 10 mg daily/10 mg + 25 mg amitriptyline daily | Double-blind RCT 1 | On pain and threshold, melatonin was more helpful than amitriptyline alone. Tender points and sleep quality were improved after 6 weeks |
| Castaño et al., 2018 [77] | 33 | 3, 6, 9, 12, and 15 mg daily | Longitudinal placebo-controlled design | Melatonin doses of 12–15 mg and 6–15 mg enhanced sleep quality, respectively. Melatonin (9–15 mg) for 10 days improved total antioxidant capacity |
| Castaño et al., 2019 [78] | 36 | 3, 6, 9, 12, and 15 mg daily | Longitudinal placebo-controlled design | After 10 days, pain, happiness, quality of life, anxiety, and FIQ 2 improved |
| Questionnaire | Melatonin (mg) | ||||
|---|---|---|---|---|---|
| 3 | 6 | 9 | 12 | 15 | |
| PSQI 1 | n.s. | + | + | ++ | + |
| NPS 2 | n.s. | n.s. | + | + | + |
| FIQ 3 | n.s. | n.s. | + | + | + |
| SF-36 4 | n.s. | n.s. | + | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Flores, D.; López-Pingarrón, L.; Castaño, M.Y.; Gómez, M.Á.; Rodríguez, A.B.; García, J.J.; Garrido, M. Melatonin as a Coadjuvant in the Treatment of Patients with Fibromyalgia. Biomedicines 2023, 11, 1964. https://doi.org/10.3390/biomedicines11071964
González-Flores D, López-Pingarrón L, Castaño MY, Gómez MÁ, Rodríguez AB, García JJ, Garrido M. Melatonin as a Coadjuvant in the Treatment of Patients with Fibromyalgia. Biomedicines. 2023; 11(7):1964. https://doi.org/10.3390/biomedicines11071964
Chicago/Turabian StyleGonzález-Flores, David, Laura López-Pingarrón, María Yolanda Castaño, María Ángeles Gómez, Ana B. Rodríguez, Joaquín J. García, and María Garrido. 2023. "Melatonin as a Coadjuvant in the Treatment of Patients with Fibromyalgia" Biomedicines 11, no. 7: 1964. https://doi.org/10.3390/biomedicines11071964
APA StyleGonzález-Flores, D., López-Pingarrón, L., Castaño, M. Y., Gómez, M. Á., Rodríguez, A. B., García, J. J., & Garrido, M. (2023). Melatonin as a Coadjuvant in the Treatment of Patients with Fibromyalgia. Biomedicines, 11(7), 1964. https://doi.org/10.3390/biomedicines11071964









