Assessment of Hyperosmolar Blood–Brain Barrier Opening in Glioblastoma via Histology with Evans Blue and DCE-MRI
Abstract
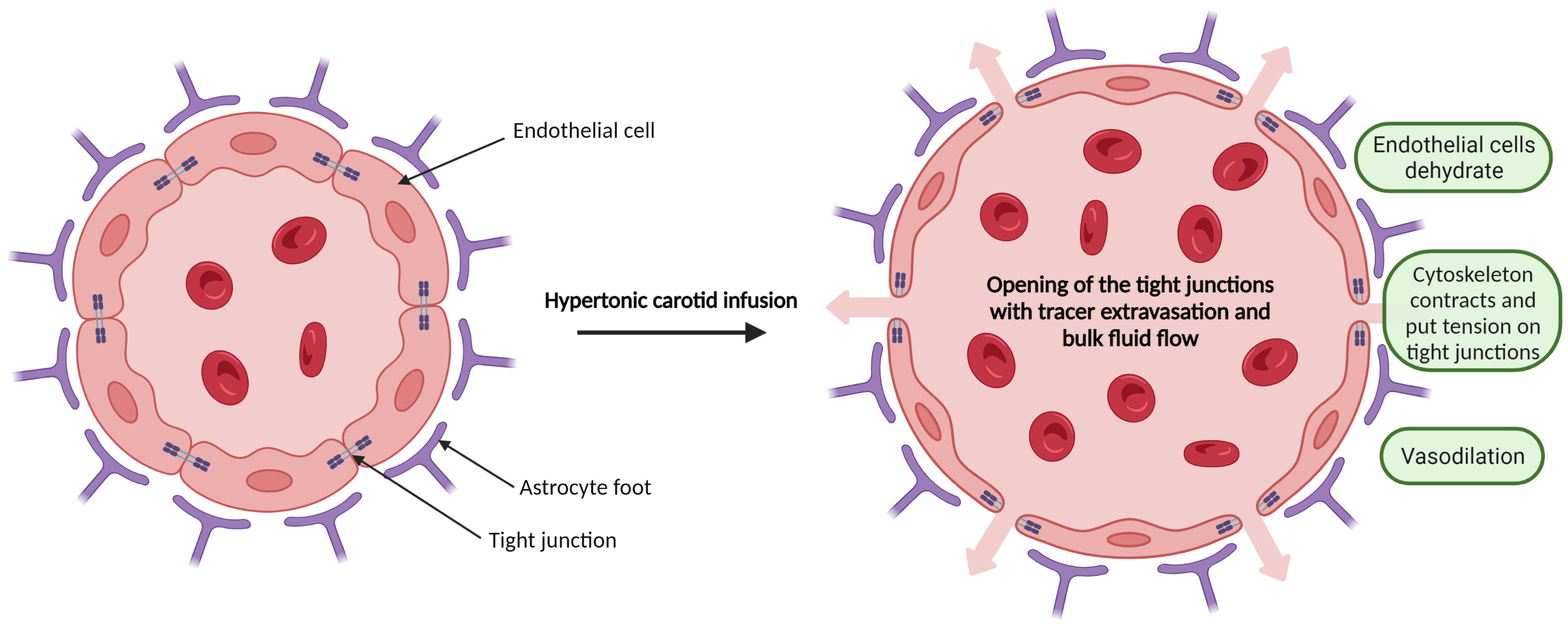
1. Introduction
2. Materials and Methods
2.1. Orthotopic U-87MG Mouse Model
2.2. Mannitol-Induced Hyperosmotic BBB Disruption
2.3. MRI
2.4. Histological Study
2.5. Image Processing and Statistical Analyses
3. Results
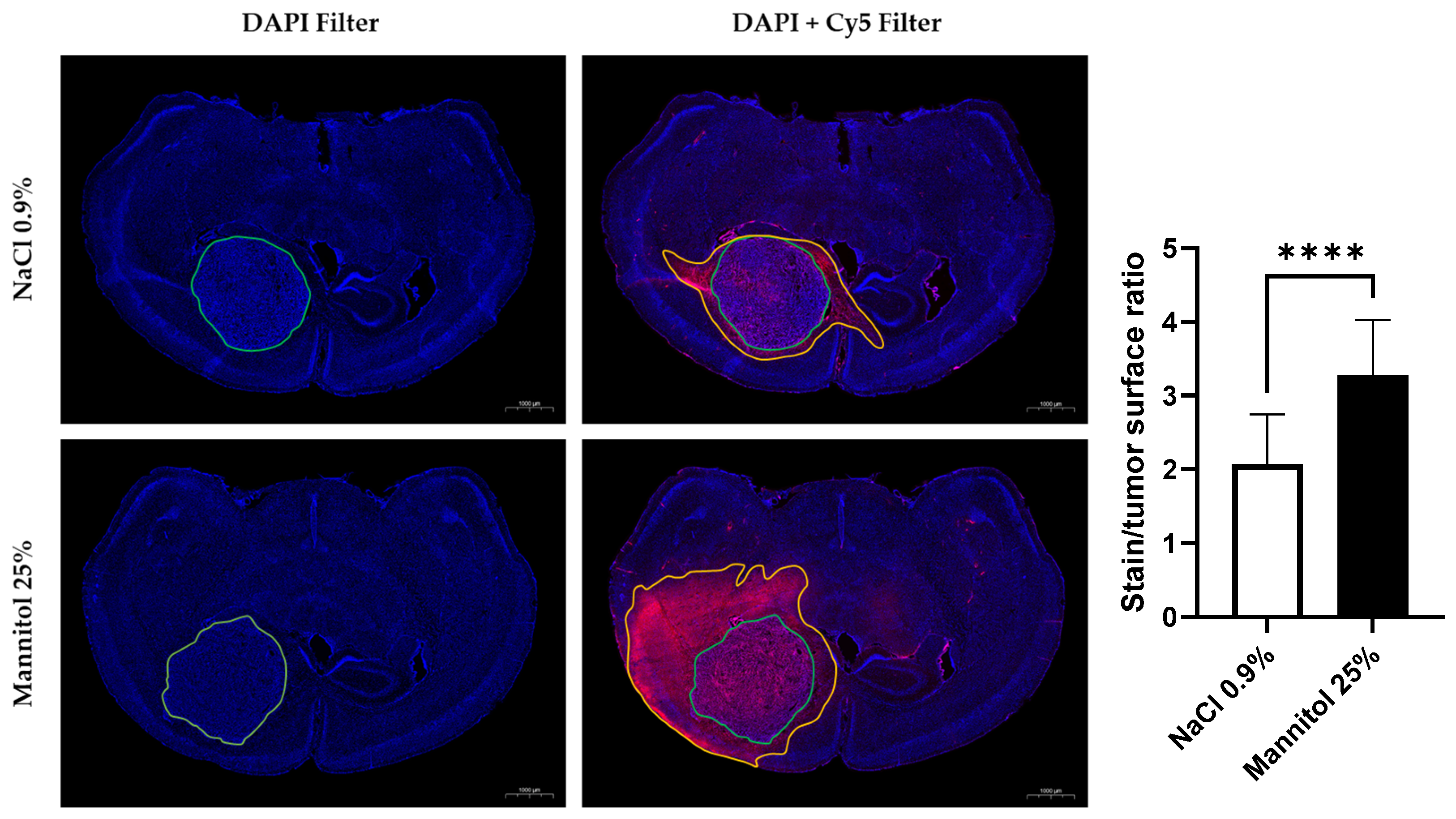
3.1. Histological Study
3.2. MRI Studies
3.2.1. T1/T2 Tumor Surface Ratio
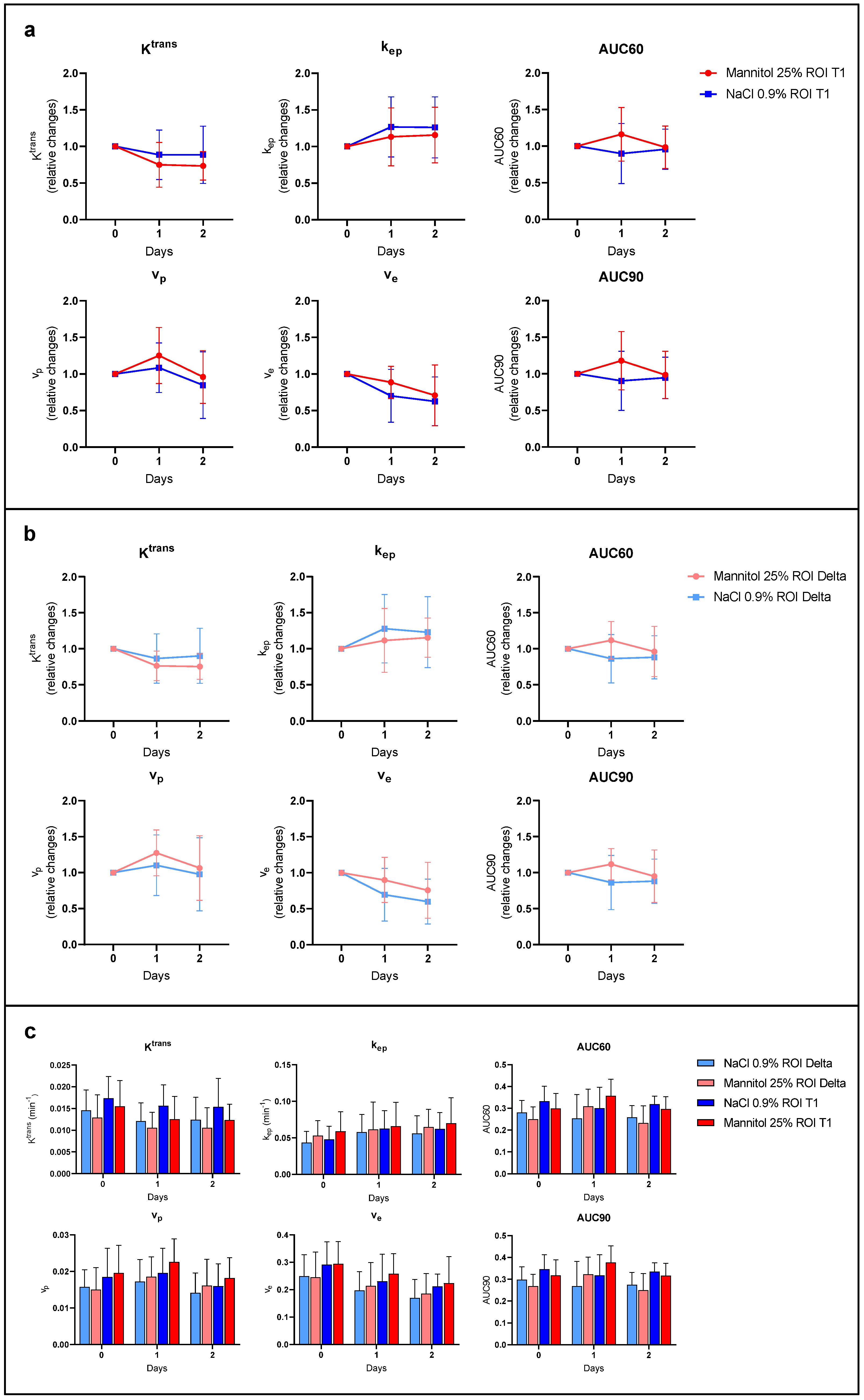
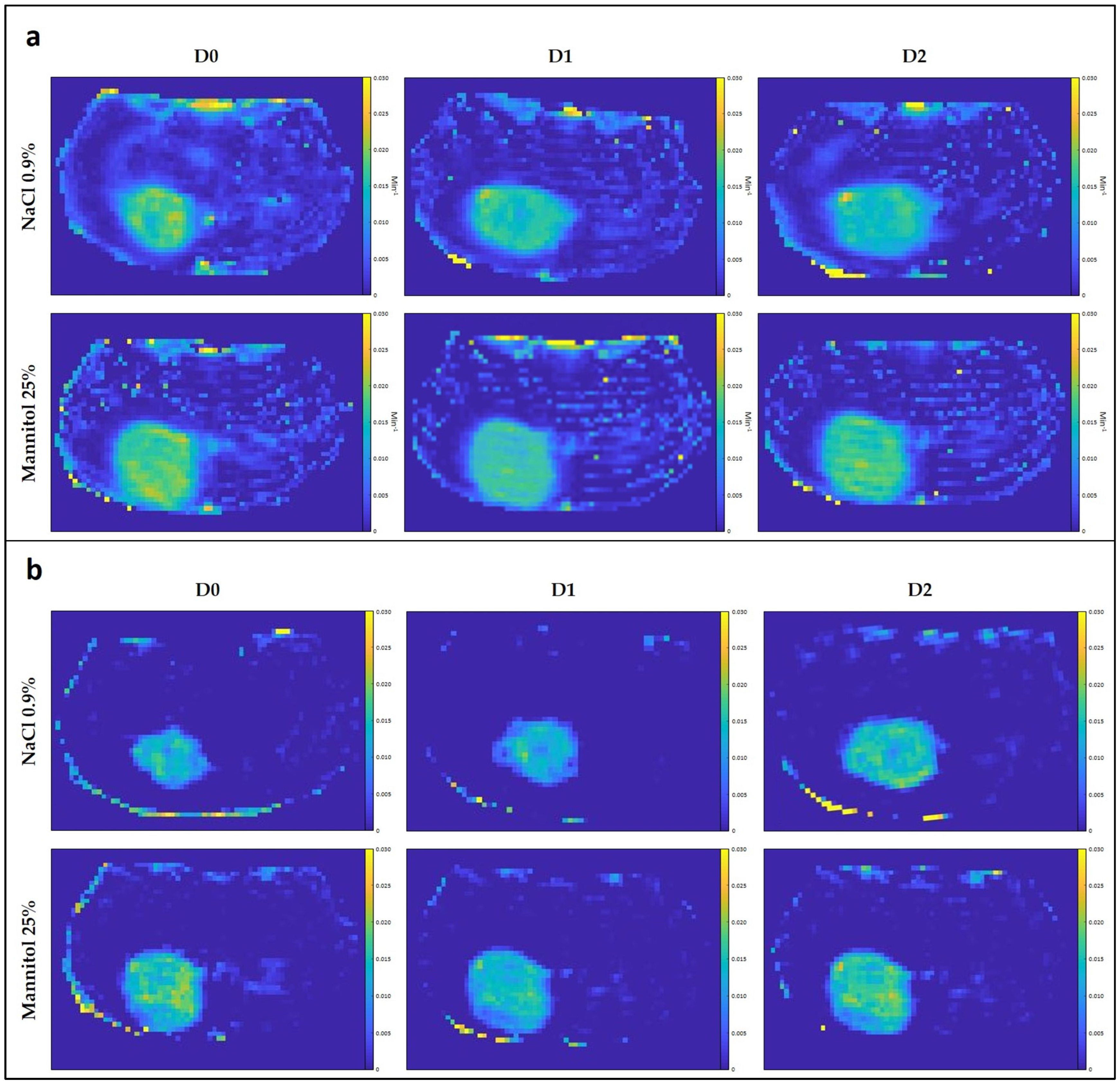
3.2.2. DCE-MRI
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cha, G.D.; Kang, T.; Baik, S.; Kim, D.; Choi, S.H.; Hyeon, T.; Kim, D.-H. Advances in drug delivery technology for the treatment of glioblastoma multiforme. J. Control. Release 2020, 328, 350–367. [Google Scholar] [CrossRef] [PubMed]
- Lukas, R.V.; Wainwright, D.A.; Ladomersky, E.; Sachdev, S.; Sonabend, A.M.; Stupp, R. Newly Diagnosed Glioblastoma: A Review on Clinical Management. Oncology 2019, 33, 91–100. [Google Scholar] [PubMed]
- Hottinger, A.F.; Stupp, R.; Homicsko, K. Standards of care and novel approaches in the management of glioblastoma multiforme. Chin. J. Cancer 2014, 33, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef] [PubMed]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 2020, 20, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Lockman, P.R.; Mittapalli, R.K.; Taskar, K.S.; Rudraraju, V.; Gril, B.; Bohn, K.A.; Adkins, C.E.; Roberts, A.; Thorsheim, H.R.; Gaasch, J.A.; et al. Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin. Cancer Res. 2010, 16, 5664–5678. [Google Scholar] [CrossRef]
- Osswald, M.; Blaes, J.; Liao, Y.; Solecki, G.; Gömmel, M.; Berghoff, A.S.; Salphati, L.; Wallin, J.J.; Phillips, H.S.; Wick, W.; et al. Impact of Blood-Brain Barrier Integrity on Tumor Growth and Therapy Response in Brain Metastases. Clin. Cancer Res. 2016, 22, 6078–6087. [Google Scholar] [CrossRef]
- Rapoport, S.I.; Hori, M.; Klatzo, I. Testing of a hypothesis for osmotic opening of the blood-brain barrier. Am. J. Physiol. 1972, 223, 323–331. [Google Scholar] [CrossRef]
- Rapoport, S.I. Advances in osmotic opening of the blood-brain barrier to enhance CNS chemotherapy. Expert Opin. Investig. Drugs 2001, 10, 1809–1818. [Google Scholar] [CrossRef]
- Shukla, G.; Alexander, G.S.; Bakas, S.; Nikam, R.; Talekar, K.; Palmer, J.D.; Shi, W. Advanced magnetic resonance imaging in glioblastoma: A review. Chin. Clin. Oncol. 2017, 6, 40. [Google Scholar] [CrossRef]
- Ahishali, B.; Kaya, M. Evaluation of Blood-Brain Barrier Integrity Using Vascular Permeability Markers: Evans Blue, Sodium Fluorescein, Albumin-Alexa Fluor Conjugates, and Horseradish Peroxidase. Methods Mol. Biol. 2021, 2367, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Bianco, J.; Bastiancich, C.; Joudiou, N.; Gallez, B.; des Rieux, A.; Danhier, F. Novel model of orthotopic U-87 MG glioblastoma resection in athymic nude mice. J. Neurosci. Methods 2017, 284, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Liu, G.; Janowski, M.; Bulte, J.W.M.; Li, S.; Pearl, M.; Walczak, P. Real-Time MRI Guidance for Reproducible Hyperosmolar Opening of the Blood-Brain Barrier in Mice. Front. Neurol. 2018, 9, 921. [Google Scholar] [CrossRef] [PubMed]
- Nicaise, C.; Mitrecic, D.; Demetter, P.; De Decker, R.; Authelet, M.; Boom, A.; Pochet, R. Impaired blood-brain and blood-spinal cord barriers in mutant SOD1-linked ALS rat. Brain Res. 2009, 1301, 152–162. [Google Scholar] [CrossRef] [PubMed]
- del Valle, J.; Camins, A.; Pallàs, M.; Vilaplana, J.; Pelegrí, C. A new method for determining blood-brain barrier integrity based on intracardiac perfusion of an Evans Blue-Hoechst cocktail. J. Neurosci. Methods 2008, 174, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Sourbron, S.P.; Buckley, D.L. Classic models for dynamic contrast-enhanced MRI. NMR Biomed. 2013, 26, 1004–1027. [Google Scholar] [CrossRef]
- Tofts, P.S.; Brix, G.; Buckley, D.L.; Evelhoch, J.L.; Henderson, E.; Knopp, M.V.; Larsson, H.B.; Lee, T.Y.; Mayr, N.A.; Parker, G.J.; et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: Standardized quantities and symbols. J. Magn. Reson. Imaging 1999, 10, 223–232. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]
- Van Tellingen, O.; Yetkin-Arik, B.; de Gooijer, M.C.; Wesseling, P.; Wurdinger, T.; de Vries, H.E. Overcoming the blood-brain tumor barrier for effective glioblastoma treatment. Drug Resist. Updates 2015, 19, 1–12. [Google Scholar] [CrossRef]
- Kane, J.R. The Role of Brain Vasculature in Glioblastoma. Mol. Neurobiol. 2019, 56, 6645–6653. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Dmello, C.; Chen, L.; Arrieta, V.A.; Gonzalez-Buendia, E.; Kane, J.R.; Magnusson, L.P.; Baran, A.; James, C.D.; Horbinski, C.; et al. Ultrasound-mediated Delivery of Paclitaxel for Glioma: A Comparative Study of Distribution, Toxicity, and Efficacy of Albumin-bound Versus Cremophor Formulations. Clin. Cancer Res. 2020, 26, 477–486. [Google Scholar] [CrossRef]
- Gould, A.; Zhang, D.; Arrieta, V.A.; Stupp, R.; Sonabend, A.M. Delivering albumin-bound paclitaxel across the blood-brain barrier for gliomas. Oncotarget 2021, 12, 2474–2475. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, B.J.; Dobelbower, M.C.; Ennis, W.H.; Bag, A.K.; Markert, J.M.; Fiveash, J.B. Patterns of failure for glioblastoma multiforme following limited-margin radiation and concurrent temozolomide. Radiat. Oncol. 2014, 9, 130. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; He, L.; Chen, L.; Deng, Q. Opening the Blood-Brain Barrier and Improving the Efficacy of Temozolomide Treatments of Glioblastoma Using Pulsed, Focused Ultrasound with a Microbubble Contrast Agent. BioMed Res. Int. 2018, 2018, 6501508. [Google Scholar] [CrossRef] [PubMed]
- Stegmayr, C.; Oliveira, D.; Niemietz, N.; Willuweit, A.; Lohmann, P.; Galldiks, N.; Shah, N.J.; Ermert, J.; Langen, K.J. Influence of Bevacizumab on Blood-Brain Barrier Permeability and O-(2-(18)F-Fluoroethyl)-l-Tyrosine Uptake in Rat Gliomas. J. Nucl. Med. 2017, 58, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Leten, C.; Struys, T.; Dresselaers, T.; Himmelreich, U. In vivo and ex vivo assessment of the blood brain barrier integrity in different glioblastoma animal models. J. Neurooncology 2014, 119, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Rhine, W.D.; Benaron, D.A.; Enzmann, D.R.; Chung, C.; Gonzales-Mendez, R.; Sayre, J.R.; Stevenson, D.K. Gd-DTPA MR detection of blood-brain barrier opening in rats after hyperosmotic shock. J. Comput. Assist. Tomogr. 1993, 17, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Blanchette, M.; Pellerin, M.; Tremblay, L.; Lepage, M.; Fortin, D. Real-time monitoring of gadolinium diethylenetriamine penta-acetic acid during osmotic blood-brain barrier disruption using magnetic resonance imaging in normal wistar rats. Neurosurgery 2009, 65, 344–350; discussion 350-341. [Google Scholar] [CrossRef]
- Ahmed, M.H.; Hernández-Verdin, I.; Quissac, E.; Lemaire, N.; Guerin, C.; Guyonnet, L.; Zahr, N.; Mouton, L.; Santin, M.; Petiet, A.; et al. Low-Intensity Pulsed Ultrasound-Mediated Blood-Brain Barrier Opening Increases Anti-Programmed Death-Ligand 1 Delivery and Efficacy in Gl261 Mouse Model. Pharmaceutics 2023, 15, 455. [Google Scholar] [CrossRef]
- Wang, J.; Li, Z.; Pan, M.; Fiaz, M.; Hao, Y.; Yan, Y.; Sun, L.; Yan, F. Ultrasound-mediated blood-brain barrier opening: An effective drug delivery system for theranostics of brain diseases. Adv. Drug Deliv. Rev. 2022, 190, 114539. [Google Scholar] [CrossRef]
- Coluccia, D.; Figueiredo, C.A.; Wu, M.Y.; Riemenschneider, A.N.; Diaz, R.; Luck, A.; Smith, C.; Das, S.; Ackerley, C.; O’Reilly, M.; et al. Enhancing glioblastoma treatment using cisplatin-gold-nanoparticle conjugates and targeted delivery with magnetic resonance-guided focused ultrasound. Nanomedicine 2018, 14, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Brady, M.; Raghavan, R.; Sampson, J. Determinants of Intraparenchymal Infusion Distributions: Modeling and Analyses of Human Glioblastoma Trials. Pharmaceutics 2020, 12, 895. [Google Scholar] [CrossRef] [PubMed]
- Cavagna, F.M.; Maggioni, F.; Castelli, P.M.; Daprà, M.; Imperatori, L.G.; Lorusso, V.; Jenkins, B.G. Gadolinium chelates with weak binding to serum proteins. A new class of high-efficiency, general purpose contrast agents for magnetic resonance imaging. Investig. Radiol. 1997, 32, 780–796. [Google Scholar] [CrossRef] [PubMed]
- Sourbron, S.P.; Buckley, D.L. On the scope and interpretation of the Tofts models for DCE-MRI. Magn. Reson. Med. 2011, 66, 735–745. [Google Scholar] [CrossRef]
- Oghabian, M.A.; Fatemidokht, A.; Haririchian, M.H. Quantification of Blood-Brain-Barrier Permeability Dysregulation and Inflammatory Activity in MS Lesions by Dynamic-Contrast Enhanced MR Imaging. Basic Clin. Neurosci. 2022, 13, 117–128. [Google Scholar] [CrossRef]
- Oh, S.S.; Lee, E.H.; Kim, J.H.; Seo, Y.B.; Choo, Y.J.; Park, J.; Chang, M.C. The Use of Dynamic Contrast-Enhanced Magnetic Resonance Imaging for the Evaluation of Blood-Brain Barrier Disruption in Traumatic Brain Injury: What Is the Evidence? Brain Sci. 2021, 11, 775. [Google Scholar] [CrossRef]
- Yoen, H.; Yoo, R.E.; Choi, S.H.; Kim, E.; Oh, B.M.; Yang, D.; Hwang, I.; Kang, K.M.; Yun, T.J.; Kim, J.H.; et al. Blood-Brain Barrier Disruption in Mild Traumatic Brain Injury Patients with Post-Concussion Syndrome: Evaluation with Region-Based Quantification of Dynamic Contrast-Enhanced MR Imaging Parameters Using Automatic Whole-Brain Segmentation. Korean J. Radiol. 2021, 22, 118–130. [Google Scholar] [CrossRef]
- Inglese, M.; Ordidge, K.L.; Honeyfield, L.; Barwick, T.D.; Aboagye, E.O.; Waldman, A.D.; Grech-Sollars, M. Reliability of dynamic contrast-enhanced magnetic resonance imaging data in primary brain tumours: A comparison of Tofts and shutter speed models. Neuroradiology 2019, 61, 1375–1386. [Google Scholar] [CrossRef]
- Keil, V.C.; Gielen, G.H.; Pintea, B.; Baumgarten, P.; Datsi, A.; Hittatiya, K.; Simon, M.; Hattingen, E. DCE-MRI in Glioma, Infiltration Zone and Healthy Brain to Assess Angiogenesis: A Biopsy Study. Clin. Neuroradiol. 2021, 31, 1049–1058. [Google Scholar] [CrossRef]
- Heye, A.K.; Thrippleton, M.J.; Armitage, P.A.; Valdés Hernández, M.D.C.; Makin, S.D.; Glatz, A.; Sakka, E.; Wardlaw, J.M. Tracer kinetic modelling for DCE-MRI quantification of subtle blood-brain barrier permeability. Neuroimage 2016, 125, 446–455. [Google Scholar] [CrossRef]
- Heye, A.K.; Culling, R.D.; Valdés Hernández Mdel, C.; Thrippleton, M.J.; Wardlaw, J.M. Assessment of blood-brain barrier disruption using dynamic contrast-enhanced MRI. A systematic review. NeuroImage Clin. 2014, 6, 262–274. [Google Scholar] [CrossRef] [PubMed]
- Manning, C.; Stringer, M.; Dickie, B.; Clancy, U.; Valdés Hernandez, M.C.; Wiseman, S.J.; Garcia, D.J.; Sakka, E.; Backes, W.H.; Ingrisch, M.; et al. Sources of systematic error in DCE-MRI estimation of low-level blood-brain barrier leakage. Magn. Reson. Med. 2021, 86, 1888–1903. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, G.A.; Wallin, A.; Wardlaw, J.M.; Markus, H.S.; Montaner, J.; Wolfson, L.; Iadecola, C.; Zlokovic, B.V.; Joutel, A.; Dichgans, M.; et al. Consensus statement for diagnosis of subcortical small vessel disease. J. Cereb. Blood Flow Metab. 2016, 36, 6–25. [Google Scholar] [CrossRef] [PubMed]
- Thrippleton, M.J.; Backes, W.H.; Sourbron, S.; Ingrisch, M.; van Osch, M.J.P.; Dichgans, M.; Fazekas, F.; Ropele, S.; Frayne, R.; van Oostenbrugge, R.J.; et al. Quantifying blood-brain barrier leakage in small vessel disease: Review and consensus recommendations. Alzheimer’s Dement. J. 2019, 15, 840–858. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.M.; Jansen, J.F.A.; Zhang, C.E.; Staals, J.; Hofman, P.A.M.; van Oostenbrugge, R.J.; Jeukens, C.; Backes, W.H. Measuring subtle leakage of the blood-brain barrier in cerebrovascular disease with DCE-MRI: Test-retest reproducibility and its influencing factors. J. Magn. Reson. Imaging 2017, 46, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Baudelet, C.; Gallez, B. Effect of anesthesia on the signal intensity in tumors using BOLD-MRI: Comparison with flow measurements by Laser Doppler flowmetry and oxygen measurements by luminescence-based probes. Magn. Reson. Imaging 2004, 22, 905–912. [Google Scholar] [CrossRef]
- Gallez, B. Oxygenation Status in Normal Tissues, Pathological Tissues and Malignant Tumors: A pO2 Database Based on Electron Paramagnetic Resonance (EPR) Oximetry Measurements. Appl. Magn. Reson. 2021, 52, 1395–1450. [Google Scholar] [CrossRef]
- Schroeter, A.; Schlegel, F.; Seuwen, A.; Grandjean, J.; Rudin, M. Specificity of stimulus-evoked fMRI responses in the mouse: The influence of systemic physiological changes associated with innocuous stimulation under four different anesthetics. Neuroimage 2014, 94, 372–384. [Google Scholar] [CrossRef]
- Grandjean, J.; Schroeter, A.; Batata, I.; Rudin, M. Optimization of anesthesia protocol for resting-state fMRI in mice based on differential effects of anesthetics on functional connectivity patterns. Neuroimage 2014, 102 Pt 2, 838–847. [Google Scholar] [CrossRef]
- Wu, T.; Grandjean, J.; Bosshard, S.C.; Rudin, M.; Reutens, D.; Jiang, T. Altered regional connectivity reflecting effects of different anaesthesia protocols in the mouse brain. Neuroimage 2017, 149, 190–199. [Google Scholar] [CrossRef]
- Munting, L.P.; Derieppe, M.P.P.; Suidgeest, E.; Denis de Senneville, B.; Wells, J.A.; van der Weerd, L. Influence of different isoflurane anesthesia protocols on murine cerebral hemodynamics measured with pseudo-continuous arterial spin labeling. NMR Biomed. 2019, 32, e4105. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conq, J.; Joudiou, N.; Ucakar, B.; Vanvarenberg, K.; Préat, V.; Gallez, B. Assessment of Hyperosmolar Blood–Brain Barrier Opening in Glioblastoma via Histology with Evans Blue and DCE-MRI. Biomedicines 2023, 11, 1957. https://doi.org/10.3390/biomedicines11071957
Conq J, Joudiou N, Ucakar B, Vanvarenberg K, Préat V, Gallez B. Assessment of Hyperosmolar Blood–Brain Barrier Opening in Glioblastoma via Histology with Evans Blue and DCE-MRI. Biomedicines. 2023; 11(7):1957. https://doi.org/10.3390/biomedicines11071957
Chicago/Turabian StyleConq, Jérôme, Nicolas Joudiou, Bernard Ucakar, Kevin Vanvarenberg, Véronique Préat, and Bernard Gallez. 2023. "Assessment of Hyperosmolar Blood–Brain Barrier Opening in Glioblastoma via Histology with Evans Blue and DCE-MRI" Biomedicines 11, no. 7: 1957. https://doi.org/10.3390/biomedicines11071957
APA StyleConq, J., Joudiou, N., Ucakar, B., Vanvarenberg, K., Préat, V., & Gallez, B. (2023). Assessment of Hyperosmolar Blood–Brain Barrier Opening in Glioblastoma via Histology with Evans Blue and DCE-MRI. Biomedicines, 11(7), 1957. https://doi.org/10.3390/biomedicines11071957





