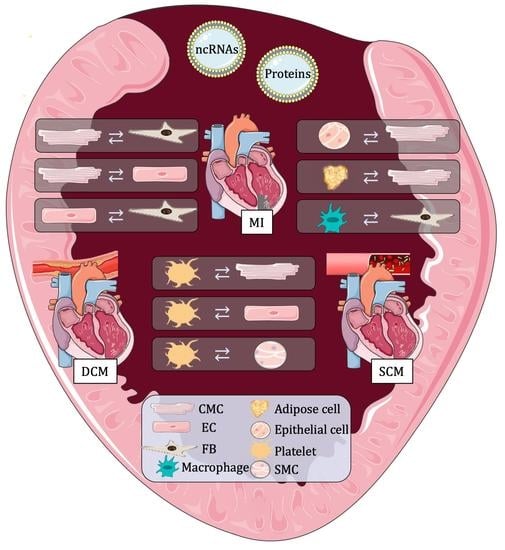
Therapeutic Potential of EVs: Targeting Cardiovascular Diseases
Abstract
1. Introduction
2. Extracellular Vesicles: Exosomes and Microvesicles
3. Different Roles of Extracellular Vesicles in Cardiovascular Research and Diagnosis
3.1. Extracellular Vesicles Used as Biomarkers
3.1.1. Myocardial Infarction
3.1.2. Diabetic Cardiomyopathy
3.1.3. Sepsis-Induced Cardiomyopathy
3.2. Extracellular Vesicles Used as Communication Molecules
3.2.1. Myocardial Infarction
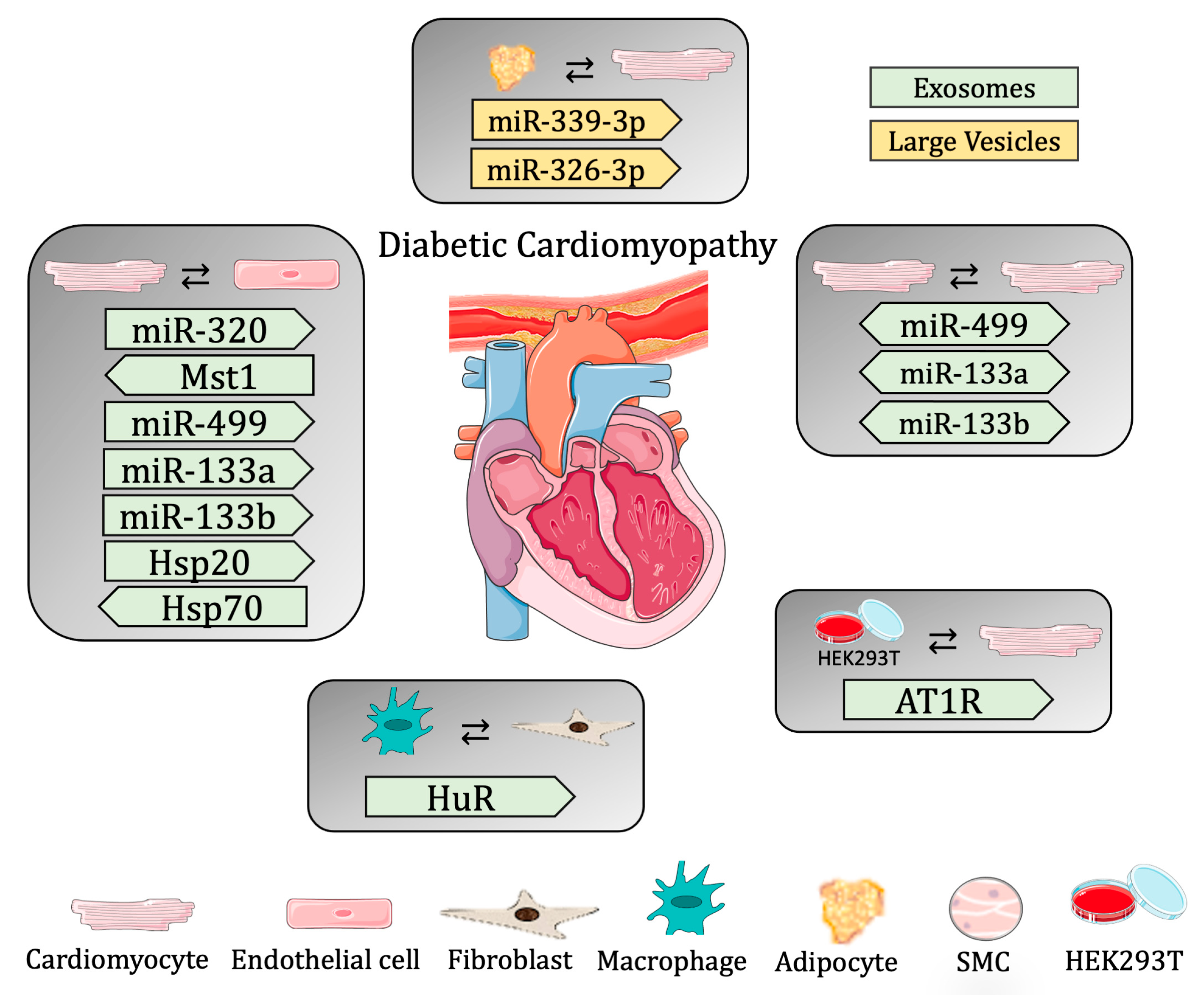
3.2.2. Diabetic Cardiomyopathy
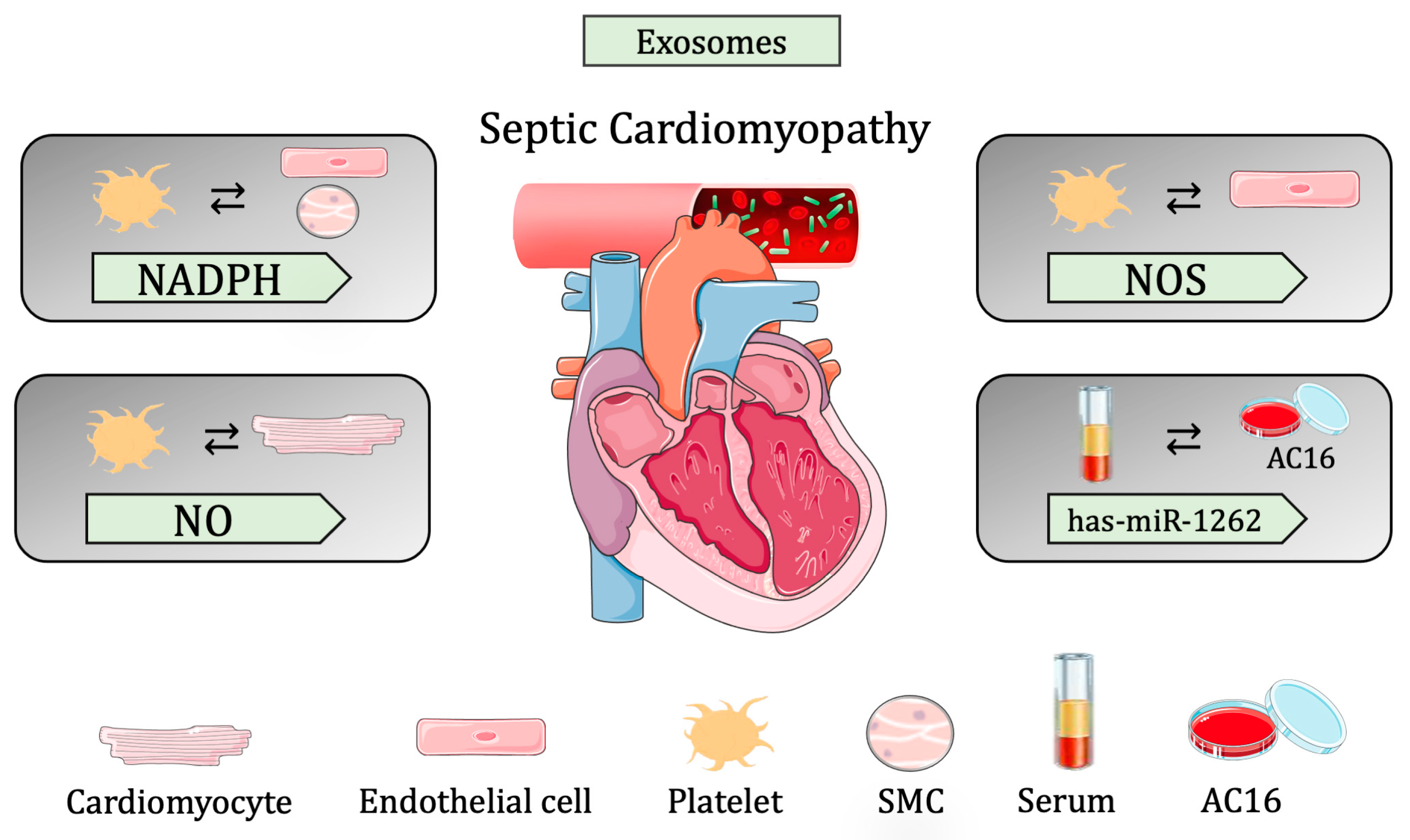
3.2.3. Sepsis-Induced Cardiomyopathy
3.3. Extracellular Vesicles Used as Carriers
3.3.1. Myocardial Infarction
3.3.2. Diabetic Cardiomyopathy
3.3.3. Sepsis-Induced Cardiomyopathy
4. Discussion and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patil, M.; Henderson, J.; Luong, H.; Annamalai, D.; Sreejit, G.; Krishnamurthy, P. The Art of Intercellular Wireless Communications: Exosomes in Heart Disease and Therapy. Front. Cell Dev. Biol. 2019, 7, 315. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Zhang, Y.; Li, Y.; Luo, L.; Zhao, Y.; Yao, Y. Extracellular vesicles in cardiovascular diseases. Cell Death Discov. 2020, 6, 68. [Google Scholar] [CrossRef] [PubMed]
- Jadli, A.S.; Parasor, A.; Gomes, K.P.; Shandilya, R.; Patel, V.B. Exosomes in Cardiovascular Diseases: Pathological Potential of Nano-Messenger. Front. Cardiovasc. Med. 2021, 8, 767488. [Google Scholar] [CrossRef] [PubMed]
- Sluijter, J.P.G.; Verhage, V.; Deddens, J.C.; Van Den Akker, F.; Doevendans, P.A. Microvesicles and exosomes for intracardiac communication. Cardiovasc. Res. 2014, 102, 302–311. [Google Scholar] [CrossRef]
- Yuan, M.J.; Maghsoudi, T.; Wang, T. Exosomes mediate the intercellular communication after myocardial infarction. Int. J. Med. Sci. 2016, 13, 113–116. [Google Scholar] [CrossRef]
- Bheri, S.; Kassouf, B.P.; Park, H.J.; Hoffman, J.R.; Davis, M.E. Engineering cardiac small extracellular vesicle-derived vehicles with thin-film hydration for customized microRNA loading. J. Cardiovasc. Dev. Dis. 2021, 8, 135. [Google Scholar] [CrossRef]
- Riaud, M.; Martinez, M.C.; Montero-Menei, C.N. Scaffolds and extracellular vesicles as a promising approach for cardiac regeneration after myocardial infarction. Pharmaceutics 2020, 12, 1195. [Google Scholar] [CrossRef]
- Yin, Z.; Chen, C. Biological Functions and Clinical Prospects of Extracellular Non-Coding RNAs in Diabetic Cardiomyopathy: An Updated Review. J. Cardiovasc. Transl. Res. 2022, 15, 469–476. [Google Scholar] [CrossRef]
- Yang, M.; Chen, J.; Chen, L. The roles of mesenchymal stem cell-derived exosomes in diabetes mellitus and its related complications. Front. Endocrinol. 2022, 13, 1027686. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, H.; Xu, H.; Tan, Y.; Zhang, C.; Zeng, Q.; Liu, L.; Qu, S. Targeting the microRNAs in exosome: A potential therapeutic strategy for alleviation of diabetes-related cardiovascular complication. Pharmacol. Res. 2021, 173, 105868. [Google Scholar] [CrossRef]
- Ahn, Y.; Jun, Y. Measurement of pain-like response to various NICU stimulants for high-risk infants. Early Hum. Dev. 2007, 83, 255–262. [Google Scholar] [CrossRef]
- Goldin, A.; Beckman, J.A.; Schmidt, A.M.; Creager, M.A. Advanced glycation end products: Sparking the development of diabetic vascular injury. Circulation 2006, 114, 597–605. [Google Scholar] [CrossRef]
- Gilbert, R.E.; Krum, H. Heart failure in diabetes: Effects of anti-hyperglycaemic drug therapy. Lancet 2015, 385, 2107–2117. [Google Scholar] [CrossRef]
- Mittal, A.; Garg, R.; Bahl, A.; Khullar, M. Molecular Mechanisms and Epigenetic Regulation in Diabetic Cardiomyopathy. Front. Cardiovasc. Med. 2021, 8, 725532. [Google Scholar] [CrossRef]
- Wang, X.; Gu, H.; Huang, W.; Peng, J.; Li, Y.; Yang, L.; Qin, D.; Essandoh, K.; Wang, Y.; Peng, T.; et al. Hsp20-mediated activation of exosome biogenesis in cardiomyocytes improves cardiac function and angiogenesis in diabetic mice. Diabetes 2016, 65, 3111–3128. [Google Scholar] [CrossRef]
- Muthu, S.; Sukumaran, V.; Sundararajan, V. Editorial: Understanding Molecular Mechanisms in Diabetic Cardiomyopathy (DCM). Front. Cardiovasc. Med. 2022, 9, 965650. [Google Scholar] [CrossRef]
- Cecconi, M.; Evans, L.; Levy, M.; Rhodes, A. Sepsis and septic shock. Lancet 2018, 392, 75–87. [Google Scholar] [CrossRef]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef]
- Fernando, S.M.; Rochwerg, B.; Seely, A.J.E. Clinical implications of the third international consensus definitions for sepsis and septic shock (Sepsis-3). Cmaj 2018, 190, E1058–E1059. [Google Scholar] [CrossRef]
- Lin, H.; Wang, W.; Lee, M.; Meng, Q.; Ren, H. Current Status of Septic Cardiomyopathy: Basic Science and Clinical Progress. Front. Pharmacol. 2020, 11, 210. [Google Scholar] [CrossRef]
- Jarczak, D.; Kluge, S.; Nierhaus, A. Sepsis—Pathophysiology and Therapeutic Concepts. Front. Med. 2021, 8, 628302. [Google Scholar] [CrossRef] [PubMed]
- Zanotti-Cavazzonia, S.L.; Hollenberg, S.M. Cardiac dysfunction in severe sepsis and septic shock. Curr. Opin. Crit. Care 2009, 15, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Heureux, M.L.; Sternberg, M.; Brath, L.; Turlington, J.; Kashiouris, M.G. Sepsis-Induced Cardiomyopathy: A Comprehensive Review. Curr. Cardiol. Rep. 2020, 22, 35. [Google Scholar] [PubMed]
- Hollenberg, S.M.; Singer, M. Pathophysiology of sepsis-induced cardiomyopathy. Nat. Rev. Cardiol. 2021, 18, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Ginini, L.; Billan, S.; Fridman, E.; Gil, Z. Insight into Extracellular Vesicle-Cell Communication: From Cell Recognition to Intracellular Fate. Cells 2022, 11, 1375. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Y.; Cheng, Q.; Bai, L.; Huang, S.; Gao, J. Extracellular Vesicles in Cardiovascular Diseases: Diagnosis and Therapy. Front. Cell Dev. Biol. 2022, 10, 875376. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, Z.; Liu, X.; Tyler, B.M. Biogenesis and Biological Functions of Extracellular Vesicles in Cellular and Organismal Communication With Microbes. Front. Microbiol. 2022, 13, 817844. [Google Scholar] [CrossRef]
- Alenquer, M.; Amorim, M.J. Exosome biogenesis, regulation, and function in viral infection. Viruses 2015, 7, 5066–5083. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Zhang, J.; Cui, X.; Guo, J.; Cao, C.; Zhang, Z.; Wang, B.; Zhang, L.; Shen, D.; Lim, K.; Woodfield, T.; et al. Small but significant: Insights and new perspectives of exosomes in cardiovascular disease. J. Cell. Mol. Med. 2020, 24, 8291–8303. [Google Scholar] [CrossRef]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal. 2021, 19, 47. [Google Scholar] [CrossRef]
- El Andaloussi, S.; Mäger, I.; Breakefield, X.O.; Wood, M.J.A. Extracellular vesicles: Biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 2013, 12, 347–357. [Google Scholar] [CrossRef]
- Dreyer, F.; Baur, A. Biogenesis and functions of exosomes and extracellular vesicles. Methods Mol. Biol. 2016, 1448, 201–216. [Google Scholar]
- Anakor, E.; Le Gall, L.; Dumonceaux, J.; Duddy, W.J.; Duguez, S. Exosomes in ageing and motor neurone disease: Biogenesis, uptake mechanisms, modifications in disease and uses in the development of biomarkers and therapeutics. Cells 2021, 10, 2930. [Google Scholar] [CrossRef]
- Sheta, M.; Taha, E.A.; Lu, Y.; Eguchi, T. Extracellular Vesicles: New Classification and Tumor Immunosuppression. Biology 2023, 12, 110. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Zhang, Q.; Franklin, J.L.; Coffey, R.J. Extracellular vesicles and nanoparticles: Emerging complexities. Trends Cell Biol. 2023; in press. [Google Scholar] [CrossRef]
- Tricarico, C.; Clancy, J.; D’Souza-Schorey, C. Biology and biogenesis of shed microvesicles. Small GTPases 2017, 8, 220–232. [Google Scholar] [CrossRef]
- Plawinski, L.; Cras, A.; Hernández Lopez, J.R.; de la Peña, A.; Van der Heyden, A.; Belle, C.; Toti, F.; Anglés-Cano, E. Distinguishing Plasmin-Generating Microvesicles: Tiny Messengers Involved in Fibrinolysis and Proteolysis. Int. J. Mol. Sci. 2023, 24, 1571. [Google Scholar] [CrossRef]
- Dhingra, R.; Vasan, R.S. Biomarkers in cardiovascular disease: Statistical assessment and section on key novel heart failure biomarkers. Trends Cardiovasc. Med. 2017, 27, 123–133. [Google Scholar] [CrossRef]
- Thupakula, S.; Nimmala, S.S.R.; Ravula, H.; Chekuri, S.; Padiya, R. Emerging biomarkers for the detection of cardiovascular diseases. Egypt. Hear. J. 2022, 74, 77. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.; Morris, P.; Fazlanie, A.L.; Vijayan, S.; Dancso, B.; Dastidar, A.G.; Plein, S.; Mueller, C.; Haaf, P. Cardiac biomarkers of acute coronary syndrome: From history to high-sensitivity cardiac troponin. Intern. Emerg. Med. 2017, 12, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.-K.; Tse, H.-F. Circulating Biomarkers for Cardiovascular Disease Risk Prediction in Patients With Cardiovascular Disease. Front. Cardiovasc. Med. 2021, 8, 713191. [Google Scholar] [CrossRef] [PubMed]
- Gilca, G.E.; Stefanescu, G.; Badulescu, O.; Tanase, D.M.; Bararu, I.; Ciocoiu, M. Diabetic Cardiomyopathy: Current Approach and Potential Diagnostic and Therapeutic Targets. J. Diabetes Res. 2017, 2017, 1310265. [Google Scholar] [CrossRef]
- Roncarati, R.; Viviani Anselmi, C.; Losi, M.A.; Papa, L.; Cavarretta, E.; Da Costa Martins, P.; Contaldi, C.; Saccani Jotti, G.; Franzone, A.; Galastri, L.; et al. Circulating miR-29a, among other up-regulated microRNAs, is the only biomarker for both hypertrophy and fibrosis in patients with hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2014, 63, 920–927. [Google Scholar] [CrossRef]
- Borga, C.; Meeran, S.M.; Fassan, M. Non-coding RNAs, a real Next-Gen Class of Biomarkers? Non-Coding RNA Res. 2019, 4, 80–81. [Google Scholar] [CrossRef]
- Hall, I.F.; Climent, M.; Viviani Anselmi, C.; Papa, L.; Tragante, V.; Lambroia, L.; Farina, F.M.; Kleber, M.E.; März, W.; Biguori, C.; et al. rs41291957 controls miR-143 and miR-145 expression and impacts coronary artery disease risk. EMBO Mol. Med. 2021, 13, e14060. [Google Scholar] [CrossRef]
- Dickhout, A.; Koenen, R.R. Extracellular Vesicles as Biomarkers in Cardiovascular Disease; Chances and Risks. Front. Cardiovasc. Med. 2018, 5, 113. [Google Scholar] [CrossRef]
- Anselmo, A.; Frank, D.; Papa, L.; Viviani Anselmi, C.; DI Pasquale, E.; Mazzola, M.; Panico, C.; Clemente, F.; Soldani, C.; Pagiatakis, C.; et al. Myocardial hypoxic stress mediates functional cardiac extracellular vesicle release. Eur. Heart J. 2021, 42, 2780–2792. [Google Scholar] [CrossRef]
- Jalaludin, I.; Lubman, D.M.; Kim, J. A guide to mass spectrometric analysis of extracellular vesicle proteins for biomarker discovery. Mass Spectrom. Rev. 2023, 42, 844–872. [Google Scholar] [CrossRef]
- de Freitas, R.C.C.; Hirata, R.D.C.; Hirata, M.H.; Aikawa, E. Circulating extracellular vesicles as biomarkers and drug delivery vehicles in cardiovascular diseases. Biomolecules 2021, 11, 388. [Google Scholar] [CrossRef]
- Pagiatakis, C.; Di Mauro, V. The emerging role of epigenetics in therapeutic targeting of cardiomyopathies. Int. J. Mol. Sci. 2021, 22, 8721. [Google Scholar] [CrossRef]
- Kenneweg, F.; Bang, C.; Xiao, K.; Boulanger, C.M.; Loyer, X.; Mazlan, S.; Schroen, B.; Hermans-Beijnsberger, S.; Foinquinos, A.; Hirt, M.N.; et al. Long Noncoding RNA-Enriched Vesicles Secreted by Hypoxic Cardiomyocytes Drive Cardiac Fibrosis. Mol. Ther.-Nucleic Acids 2019, 18, 363–374. [Google Scholar] [CrossRef]
- Matsumoto, S.; Sakata, Y.; Suna, S.; Nakatani, D.; Usami, M.; Hara, M.; Kitamura, T.; Hamasaki, T.; Nanto, S.; Kawahara, Y.; et al. Circulating p53-responsive MicroRNAs are predictive indicators of heart failure after acute myocardial infarction. Circ. Res. 2013, 113, 322–326. [Google Scholar] [CrossRef]
- Hermann, D.M.; Xin, W.; Bähr, M.; Giebel, B.; Doeppner, T.R. Emerging roles of extracellular vesicle-associated non-coding RNAs in hypoxia: Insights from cancer, myocardial infarction and ischemic stroke. Theranostics 2022, 12, 5776–5802. [Google Scholar] [CrossRef]
- Li, N.; Rochette, L.; Wu, Y.; Rosenblatt-Velin, N. New Insights into the Role of Exosomes in the Heart After Myocardial Infarction. J. Cardiovasc. Transl. Res. 2019, 12, 18–27. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, X.; Yang, J.; Duan, X.; Yao, Y.; Shi, X.; Chen, Z.; Fan, Z.; Liu, X.; Qin, S.; et al. A translational study of urine miRNAs in acute myocardial infarction. J. Mol. Cell. Cardiol. 2012, 53, 668–676. [Google Scholar] [CrossRef]
- Yu, S.; Tang, X.; Zheng, T.; Li, S.; Ren, H.; Wu, H.; Peng, F.; Gong, L. Plasma-derived extracellular vesicles transfer microRNA-130a-3p to alleviate myocardial ischemia/reperfusion injury by targeting ATG16L1. Cell Tissue Res. 2022, 389, 99–114. [Google Scholar] [CrossRef]
- Henning, R.J. Cardiovascular Exosomes and MicroRNAs in Cardiovascular Physiology and Pathophysiology. J. Cardiovasc. Transl. Res. 2021, 14, 195–212. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, M.; Liang, Y.; Wang, C.; Naruse, K.; Takahashi, K. Treatment of oxidative stress with exosomes in myocardial ischemia. Int. J. Mol. Sci. 2021, 22, 1729. [Google Scholar] [CrossRef]
- Otero-Ortega, L.; Alonso-López, E.; Pérez-Mato, M.; Laso-García, F.; De Frutos, M.C.G.; Diekhorst, L.; García-Bermejo, M.L.; Conde-Moreno, E.; Fuentes, B.; de Leciñana, M.A.; et al. Similarities and differences in extracellular vesicle profiles between ischaemic stroke and myocardial infarction. Biomedicines 2021, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Femminò, S.; Penna, C.; Margarita, S.; Comità, S.; Brizzi, M.F.; Pagliaro, P. Extracellular vesicles and cardiovascular system: Biomarkers and Cardioprotective Effectors. Vascul. Pharmacol. 2020, 135, 106790. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, N.; Waissi, F.; Dekker, M.; de Borst, G.J.; van Bennekom, J.; de Winter, R.J.; Hilvo, M.; Jylhä, A.; Pasterkamp, G.; de Kleijn, D.P.V.; et al. Ceramides and phospholipids in plasma extracellular vesicles are associated with high risk of major cardiovascular events after carotid endarterectomy. Sci. Rep. 2022, 12, 5521. [Google Scholar] [CrossRef] [PubMed]
- Burrello, J.; Biemmi, V.; Dei Cas, M.; Amongero, M.; Bolis, S.; Lazzarini, E.; Bollini, S.; Vassalli, G.; Paroni, R.; Barile, L. Sphingolipid composition of circulating extracellular vesicles after myocardial ischemia. Sci. Rep. 2020, 10, 16182. [Google Scholar] [CrossRef]
- Sharma, S.; Adrogue, J.V.; Golfman, L.; Uray, I.; Lemm, J.; Youker, K.; Noon, G.P.; Frazier, O.H.; Taegtmeyer, H. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J. 2004, 18, 1692–1700. [Google Scholar] [CrossRef]
- De Gonzalo-Calvo, D.; Van Der Meer, R.W.; Rijzewijk, L.J.; Smit, J.W.A.; Revuelta-Lopez, E.; Nasarre, L.; Escola-Gil, J.C.; Lamb, H.J.; Llorente-Cortes, V. Serum microRNA-1 and microRNA-133a levels reflect myocardial steatosis in uncomplicated type 2 diabetes. Sci. Rep. 2017, 7, 47. [Google Scholar] [CrossRef]
- Huang, J.P.; Chang, C.C.; Kuo, C.Y.; Huang, K.J.; Sokal, E.M.; Chen, K.H.; Hung, L.M. Exosomal microRNAs miR-30d-5p and miR-126a-5p Are Associated with Heart Failure with Preserved Ejection Fraction in STZ-Induced Type 1 Diabetic Rats. Int. J. Mol. Sci. 2022, 23, 7514. [Google Scholar] [CrossRef]
- Chen, F.-C.; Xu, Y.-C.; Zhan, Z.-C. Multi-biomarker strategy for prediction of myocardial dysfunction and mortality in sepsis. J. Zhejiang Univ. Sci. B 2020, 21, 537–548. [Google Scholar] [CrossRef]
- Ye, R.; Lin, Q.; Xiao, W.; Mao, L.; Zhang, P.; Zhou, L.; Wu, X.; Jiang, N.; Zhang, X.; Zhang, Y.; et al. miR-150-5p in neutrophil-derived extracellular vesicles associated with sepsis-induced cardiomyopathy in septic patients. Cell Death Discov. 2023, 9, 19. [Google Scholar] [CrossRef]
- Hegyesi, H.; Pallinger, É.; Mecsei, S.; Hornyák, B.; Kovácsházi, C.; Brenner, G.B.; Giricz, Z.; Pálóczi, K.; Kittel, Á.; Tóvári, J.; et al. Circulating cardiomyocyte-derived extracellular vesicles reflect cardiac injury during systemic inflammatory response syndrome in mice. Cell. Mol. Life Sci. 2022, 79, 84. [Google Scholar] [CrossRef]
- Laura Francés, J.; Musolino, E.; Papait, R.; Pagiatakis, C. Non-Coding RNAs in Cell-to-Cell Communication: Exploiting Physiological Mechanisms as Therapeutic Targets in Cardiovascular Pathologies. Int. J. Mol. Sci. 2023, 24, 2205. [Google Scholar] [CrossRef]
- Herrera-Zelada, N.; Zuñiga-Cuevas, U.; Ramirez-Reyes, A.; Lavandero, S.; Riquelme, J.A. Targeting the Endothelium to Achieve Cardioprotection. Front. Pharmacol. 2021, 12, 636134. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshevy, Y.V. Cardiac extracellular vesicles in normal and infarcted heart. Int. J. Mol. Sci. 2016, 17, 63. [Google Scholar] [CrossRef]
- Yu, X.; Deng, L.; Wang, D.; Li, N.; Chen, X.; Cheng, X.; Yuan, J.; Gao, X.; Liao, M.; Wang, M.; et al. Mechanism of TNF-α autocrine effects in hypoxic cardiomyocytes: Initiated by hypoxia inducible factor 1α, presented by exosomes. J. Mol. Cell. Cardiol. 2012, 53, 848–857. [Google Scholar] [CrossRef]
- Zhou, D.; Dai, Z.; Ren, M.; Yang, M. Adipose-Derived Stem Cells-Derived Exosomes with High Amounts of Circ_0001747 Alleviate Hypoxia/Reoxygenation-Induced Injury in Myocardial Cells by Targeting MiR-199b-3p/MCL1 Axis. Int. Heart J. 2022, 63, 356–366. [Google Scholar] [CrossRef]
- Morelli, M.B.; Shu, J.; Sardu, C.; Matarese, A.; Santulli, G. Cardiosomal microRNAs are essential in post-infarction myofibroblast phenoconversion. Int. J. Mol. Sci. 2020, 21, 201. [Google Scholar] [CrossRef]
- Lassen, T.R.; Just, J.; Hjortbak, M.V.; Jespersen, N.R.; Stenz, K.T.; Gu, T.; Yan, Y.; Su, J.; Hansen, J.; Bæk, R.; et al. Cardioprotection by remote ischemic conditioning is transferable by plasma and mediated by extracellular vesicles. Basic Res. Cardiol. 2021, 116, 16. [Google Scholar] [CrossRef]
- Terriaca, S.; Fiorelli, E.; Scioli, M.G.; Fabbri, G.; Storti, G.; Cervelli, V.; Orlandi, A. Endothelial progenitor cell-derived extracellular vesicles: Potential therapeutic application in tissue repair and regeneration. Int. J. Mol. Sci. 2021, 22, 6375. [Google Scholar] [CrossRef]
- Wei, G.; Li, C.; Jia, X.; Xie, J.; Tang, Z.; Jin, M.; Chen, Q.; Sun, Y.; He, S.; Li, X.; et al. Extracellular vesicle-derived CircWhsc1 promotes cardiomyocyte proliferation and heart repair by activating TRIM59/STAT3/Cyclin B2 pathway. J. Adv. Res. 2023; in press. [Google Scholar] [CrossRef]
- Ribeiro-Rodrigues, T.M.; Laundos, T.L.; Pereira-Carvalho, R.; Batista-Almeida, D.; Pereira, R.; Coelho-Santos, V.; Silva, A.P.; Fernandes, R.; Zuzarte, M.; Enguita, F.J.; et al. Exosomes secreted by cardiomyocytes subjected to ischaemia promote cardiac angiogenesis. Cardiovasc. Res. 2017, 113, 1338–1350. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Zhu, H.; Kranias, E.G.; Tang, Y.; Peng, T.; Chang, J.; Fan, G.C. Hsp20 functions as a novel cardiokine in promoting angiogenesis via activation of VEGFR2. PLoS ONE 2012, 7, e32765. [Google Scholar] [CrossRef] [PubMed]
- Wendt, S.; Goetzenich, A.; Goettsch, C.; Stoppe, C.; Bleilevens, C.; Kraemer, S.; Benstoem, C. Evaluation of the cardioprotective potential of extracellular vesicles—A systematic review and meta-analysis. Sci. Rep. 2018, 8, 15702. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Wang, Y.; Thomas, M.; McLaughlin, K.A.; Oguljahan, B.; Henderson, J.; Yang, Q.; Chen, Y.E.; Liu, D. Exosomes from adipose-derived stem cells alleviate myocardial infarction via microRNA-31/FIH1/HIF-1α pathway. J. Mol. Cell. Cardiol. 2022, 162, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Rezaie, J.; Rahbarghazi, R.; Pezeshki, M.; Mazhar, M.; Yekani, F.; Khaksar, M.; Shokrollahi, E.; Amini, H.; Hashemzadeh, S.; Sokullu, S.E.; et al. Cardioprotective role of extracellular vesicles: A highlight on exosome beneficial effects in cardiovascular diseases. J. Cell. Physiol. 2019, 234, 21732–21745. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lu, Y.; Mao, Y.; Yu, Y.; Wu, T.; Zhao, W.; Zhu, Y.; Zhao, P.; Zhang, F. IFN-γ enhances the efficacy of mesenchymal stromal cell-derived exosomes via miR-21 in myocardial infarction rats. Stem Cell Res. Ther. 2022, 13, 333. [Google Scholar] [CrossRef]
- Del Campo, C.V.; Liaw, N.Y.; Gunadasa-Rohling, M.; Matthaei, M.; Braga, L.; Kennedy, T.; Salinas, G.; Voigt, N.; Giacca, M.; Zimmermann, W.H.; et al. Regenerative potential of epicardium-derived extracellular vesicles mediated by conserved miRNA transfer. Cardiovasc. Res. 2022, 118, 597–611. [Google Scholar] [CrossRef]
- Maring, J.A.; Lodder, K.; Mol, E.; Verhage, V.; Wiesmeijer, K.C.; Dingenouts, C.K.E.; Moerkamp, A.T.; Deddens, J.C.; Vader, P.; Smits, A.M.; et al. Cardiac Progenitor Cell–Derived Extracellular Vesicles Reduce Infarct Size and Associate with Increased Cardiovascular Cell Proliferation. J. Cardiovasc. Transl. Res. 2019, 12, 5–17. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, Y.; Chen, D.; Zheng, Y.; Jiang, J. Exosomes and Exosomal Cargos: A Promising World for Ventricular Remodeling Following Myocardial Infarction. Int. J. Nanomed. 2022, 17, 4699–4719. [Google Scholar] [CrossRef]
- Duan, S.; Wang, C.; Xu, X.; Zhang, X.; Su, G.; Li, Y.; Fu, S.; Sun, P.; Tian, J. Peripheral Serum Exosomes Isolated from Patients with Acute Myocardial Infarction Promote Endothelial Cell Angiogenesis via the miR-126-3p/TSC1/mTORC1/HIF-1α Pathway. Int. J. Nanomed. 2022, 17, 1577–1592. [Google Scholar] [CrossRef]
- Wang, X.; Huang, W.; Liu, G.; Cai, W.; Millard, R.W.; Wang, Y.; Chang, J.; Peng, T.; Fan, G.C. Cardiomyocytes mediate anti-angiogenesis in type 2 diabetic rats through the exosomal transfer of miR-320 into endothelial cells. J. Mol. Cell. Cardiol. 2014, 74, 139–150. [Google Scholar] [CrossRef]
- Bitirim, C.V.; Ozer, Z.B.; Aydos, D.; Genc, K.; Demirsoy, S.; Akcali, K.C.; Turan, B. Cardioprotective effect of extracellular vesicles derived from ticagrelor-pretreated cardiomyocyte on hyperglycemic cardiomyocytes through alleviation of oxidative and endoplasmic reticulum stress. Sci. Rep. 2022, 12, 5651. [Google Scholar] [CrossRef]
- Vicencio, J.M.; Yellon, D.M.; Sivaraman, V.; Das, D.; Boi-Doku, C.; Arjun, S.; Zheng, Y.; Riquelme, J.A.; Kearney, J.; Sharma, V.; et al. Plasma exosomes protect the myocardium from ischemia-reperfusion injury. J. Am. Coll. Cardiol. 2015, 65, 1525–1536. [Google Scholar] [CrossRef]
- Davidson, S.M.; Riquelme, J.A.; Takov, K.; Vicencio, J.M.; Boi-Doku, C.; Khoo, V.; Doreth, C.; Radenkovic, D.; Lavandero, S.; Yellon, D.M. Cardioprotection mediated by exosomes is impaired in the setting of type II diabetes but can be rescued by the use of non-diabetic exosomes in vitro. J. Cell. Mol. Med. 2018, 22, 141–151. [Google Scholar] [CrossRef]
- Wang, D.; He, J.; Huang, B.; Liu, S.; Zhu, H.; Xu, T. Emerging role of the Hippo pathway in autophagy. Cell Death Dis. 2020, 11, 880. [Google Scholar] [CrossRef]
- Hu, J.; Wang, S.; Xiong, Z.; Cheng, Z.; Yang, Z.; Lin, J.; Wang, T.; Feng, X.; Gao, E.; Wang, H.; et al. Exosomal Mst1 transfer from cardiac microvascular endothelial cells to cardiomyocytes deteriorates diabetic cardiomyopathy. Biochim. Biophys. Acta-Mol. Basis Dis. 2018, 1864, 3639–3649. [Google Scholar] [CrossRef]
- Daiber, A.; Münzel, T. Organic nitrate therapy, nitrate tolerance, and nitrate-induced endothelial dysfunction: Emphasis on redox biology and oxidative stress. Antioxid. Redox Signal. 2015, 23, 899–942. [Google Scholar] [CrossRef]
- Zhao, K.; Zhang, J.; Li, P.; Yan, R. Isosorbide mononitrate inhibits myocardial fibrosis in diabetic rats by up-regulating exosomal MiR-378. Trop. J. Pharm. Res. 2022, 21, 1227–1235. [Google Scholar] [CrossRef]
- Kloc, M.; Uosef, A.; Leśniak, M.; Kubiak, J.Z.; Ghobrial, R.M. Reciprocal interactions between mesenchymal stem cells and macrophages. Int. J. Dev. Biol. 2020, 64, 465–469. [Google Scholar] [CrossRef]
- Krishnamurthy, P.; Rajasingh, J.; Lambers, E.; Qin, G.; Losordo, D.W.; Kishore, R. IL-10 inhibits inflammation and attenuates left ventricular remodeling after myocardial infarction via activation of STAT3 and suppression of HuR. Circ. Res. 2009, 104, 9–18. [Google Scholar] [CrossRef]
- Govindappa, P.K.; Patil, M.; Garikipati, V.N.S.; Verma, S.K.; Saheera, S.; Narasimhan, G.; Zhu, W.; Kishore, R.; Zhang, J.; Krishnamurthy, P. Targeting exosome-associated human antigen R attenuates fibrosis and inflammation in diabetic heart. FASEB J. 2020, 34, 2238–2251. [Google Scholar] [CrossRef]
- Pironti, G.; Strachan, R.T.; Abraham, D.; Mon-Wei Yu, S.; Chen, M.; Chen, W.; Hanada, K.; Mao, L.; Watson, L.J.; Rockman, H.A. Circulating exosomes induced by cardiac pressure overload contain functional angiotensin II type 1 receptors. Circulation 2015, 131, 2120–2130. [Google Scholar] [CrossRef] [PubMed]
- Gordan, R.; Gwathmey, J.K.; Xie, L.-H. Autonomic and endocrine control of cardiovascular function. World J. Cardiol. 2015, 7, 204. [Google Scholar] [CrossRef] [PubMed]
- Singla, R.; Garner, K.H.; Samsam, M.; Cheng, Z.; Singla, D.K. Exosomes derived from cardiac parasympathetic ganglionic neurons inhibit apoptosis in hyperglycemic cardiomyoblasts. Mol. Cell. Biochem. 2019, 462, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Burton, D.G.A.; Faragher, R.G.A. Obesity and type-2 diabetes as inducers of premature cellular senescence and ageing. Biogerontology 2018, 19, 447–459. [Google Scholar] [CrossRef]
- Lin, H.; Chen, X.; Pan, J.; Ke, J.; Zhang, A.; Liu, Y.; Wang, C.; Chang, A.C.Y.; Gu, J. Secretion of miRNA-326-3p by senescent adipose exacerbates myocardial metabolism in diabetic mice. J. Transl. Med. 2022, 20, 278. [Google Scholar] [CrossRef]
- Fernandes, C.J.; De Assuncao, M.S.C. Myocardial dysfunction in sepsis: A large, unsolved puzzle. Crit. Care Res. Pract. 2012, 2012, 896430. [Google Scholar] [CrossRef]
- Essandoh, K.; Yang, L.; Wang, X.; Huang, W.; Qin, D.; Hao, J.; Wang, Y.; Zingarelli, B.; Peng, T.; Fan, G.C. Blockade of exosome generation with GW4869 dampens the sepsis-induced inflammation and cardiac dysfunction. Biochim. Biophys. Acta-Mol. Basis Dis. 2015, 1852, 2362–2371. [Google Scholar] [CrossRef]
- Mahidhara, R.; Billiar, T.R. Apoptosis in sepsis. Crit. Care Med. 2000, 28, N105–N113. [Google Scholar] [CrossRef]
- Janiszewski, M.; Do Carmo, A.O.; Pedro, M.A.; Silva, E.; Knobel, E.; Laurindo, F.R.M. Platelet-derived exosomes of septic individuals possess proapoptotic NAD(P)H oxidase activity: A novel vascular redox pathway. Crit. Care Med. 2004, 32, 818–825. [Google Scholar] [CrossRef]
- Azevedo, L.C.P.; Janiszewski, M.; Pontieri, V.; de Almeida Pedro, M.; Bassi, E.; Tucci, P.J.F.; Laurindo, F.R.M. Platelet-derived exosomes from septic shock patients induce myocardial dysfunction. Crit. Care 2007, 11, 10. [Google Scholar] [CrossRef]
- Gambim, M.H.; de Oliveira do Carmo, A.; Marti, L.; Veríssimo-Filho, S.; Lopes, L.R.; Janiszewski, M. Platelet-derived exosomes induce endothelial cell apoptosis through peroxynitrite generation: Experimental evidence for a novel mechanism of septic vascular dysfunction. Crit. Care 2007, 11, R107. [Google Scholar] [CrossRef]
- Hu, G.; Tang, J.; Zhang, B.; Lin, Y.; Hanai, J.; Galloway, J.; Galloway, V.; Bahary, N.; Han, Z.; Ramchandran, R.; et al. A novel endothelial-specific heat shock protein HspA12B is required in both zebrafish development and endothelial functions in vitro. J. Cell Sci. 2006, 88, 4117–4126. [Google Scholar] [CrossRef]
- Agudo, J.; Ruzo, A.; Tung, N.; Salmon, H.; Leboeuf, M.; Hashimoto, D.; Becker, C.; Garrett-Sinha, L.A.; Baccarini, A.; Merad, M.; et al. The miR-126-VEGFR2 axis controls the innate response to pathogen-associated nucleic acids. Nat. Immunol. 2014, 15, 54–62. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Fan, M.; Tu, F.; Yang, K.; Ha, T.; Liu, L.; Kalbfleisch, J.; Williams, D.; Li, C. Endothelial HSPA12B Exerts Protection Against Sepsis-Induced Severe Cardiomyopathy via Suppression of Adhesion Molecule Expression by miR-126. Front. Immunol. 2020, 11, 566. [Google Scholar] [CrossRef]
- Tu, G.W.; Ma, J.F.; Li, J.K.; Su, Y.; Luo, J.C.; Hao, G.W.; Luo, M.H.; Cao, Y.R.; Zhang, Y.; Luo, Z. Exosome-Derived From Sepsis Patients’ Blood Promoted Pyroptosis of Cardiomyocytes by Regulating miR-885-5p/HMBOX1. Front. Cardiovasc. Med. 2022, 9, 774193. [Google Scholar] [CrossRef]
- Afanasyeva, E.A.; Mestdagh, P.; Kumps, C.; Vandesompele, J.; Ehemann, V.; Theissen, J.; Fischer, M.; Zapatka, M.; Brors, B.; Savelyeva, L.; et al. MicroRNA miR-885-5p targets CDK2 and MCM5, activates p53 and inhibits proliferation and survival. Cell Death Differ. 2011, 18, 974–984. [Google Scholar] [CrossRef]
- Ma, H.; Su, L.; Yue, H.; Yin, X.; Zhao, J.; Zhang, S.; Kung, H.; Xu, Z.; Miao, J. HMBOX1 interacts with MT2A to regulate autophagy and apoptosis in vascular endothelial cells. Sci. Rep. 2015, 5, 15121. [Google Scholar] [CrossRef]
- Sun, F.; Geng, H.; Sun, Y.; Feng, W.; Tian, T.; Ye, L.; Lei, M. Exosomes derived from the blood of patients with sepsis regulate apoptosis and aerobic glycolysis in human myocardial cells via the hsa-miR-1262/SLC2A1 signaling pathway. Mol. Med. Rep. 2022, 25, 12635. [Google Scholar] [CrossRef]
- Tewabe, A.; Abate, A.; Tamrie, M.; Seyfu, A.; Siraj, E.A. Targeted drug delivery—From magic bullet to nanomedicine: Principles, challenges, and future perspectives. J. Multidiscip. Healthc. 2021, 14, 1711–1724. [Google Scholar] [CrossRef]
- Svenson, S. Clinical translation of nanomedicines. Curr. Opin. Solid State Mater. Sci. 2012, 16, 287–294. [Google Scholar] [CrossRef]
- van der Koog, L.; Gandek, T.B.; Nagelkerke, A. Liposomes and Extracellular Vesicles as Drug Delivery Systems: A Comparison of Composition, Pharmacokinetics, and Functionalization. Adv. Healthc. Mater. 2022, 11, 2100639. [Google Scholar] [CrossRef] [PubMed]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal formulations in clinical use: An updated review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Kamaly, N.; He, J.C.; Ausiello, D.A.; Farokhzad, O.C. Nanomedicines for renal disease: Current status and future applications. Nat. Rev. Nephrol. 2016, 12, 738–753. [Google Scholar] [CrossRef] [PubMed]
- Meazza, C.; Asaftei, S.D. State-of-the-art, approved therapeutics for the pharmacological management of osteosarcoma. Expert Opin. Pharmacother. 2021, 22, 1995–2006. [Google Scholar] [CrossRef] [PubMed]
- Koudelka, Š.; Turánek, J. Liposomal paclitaxel formulations. J. Control. Release 2012, 163, 322–334. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, W.; Shen, Q.; Wang, Y.; Tseng, Y.; Luo, Z.; Wang, X.; Shi, L.; Li, C.; Liu, J. Identification and construction of a novel biomimetic delivery system of paclitaxel and its targeting therapy for cancer. Signal Transduct. Target. Ther. 2021, 6, 2020–2022. [Google Scholar] [CrossRef]
- Witwer, K.W.; Wolfram, J. Extracellular vesicles versus synthetic nanoparticles for drug delivery. Nat. Rev. Mater. 2021, 6, 103–106. [Google Scholar] [CrossRef]
- Qi, H.; Wang, Y.; Fa, S.; Yuan, C.; Yang, L. Extracellular Vesicles as Natural Delivery Carriers Regulate Oxidative Stress Under Pathological Conditions. Front. Bioeng. Biotechnol. 2021, 9, 752019. [Google Scholar] [CrossRef]
- Meng, W.; He, C.; Hao, Y.; Wang, L.; Li, L.; Zhu, G. Prospects and challenges of extracellular vesicle-based drug delivery system: Considering cell source. Drug Deliv. 2020, 27, 585–598. [Google Scholar] [CrossRef]
- Kamerkar, S.; Lebleu, V.S.; Sugimoto, H.; Yang, S.; Ruivo, C.F.; Melo, S.A.; Lee, J.J.; Kalluri, R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 2017, 546, 498–503. [Google Scholar] [CrossRef]
- Estes, S.; Konstantinov, K.; Young, J.D. Manufactured extracellular vesicles as human therapeutics: Challenges, advances, and opportunities. Curr. Opin. Biotechnol. 2022, 77, 102776. [Google Scholar] [CrossRef]
- Piffoux, M.; Volatron, J.; Silva, A.K.A.; Gazeau, F. Thinking quantitatively of rna-based information transfer via extracellular vesicles: Lessons to learn for the design of rna-loaded evs. Pharmaceutics 2021, 13, 1931. [Google Scholar] [CrossRef]
- Kooijmans, S.A.A.; de Jong, O.G.; Schiffelers, R.M. Exploring interactions between extracellular vesicles and cells for innovative drug delivery system design. Adv. Drug Deliv. Rev. 2021, 173, 252–278. [Google Scholar] [CrossRef]
- Wang, Z.; Mo, H.; He, Z.; Chen, A.; Cheng, P. Extracellular vesicles as an emerging drug delivery system for cancer treatment: Current strategies and recent advances. Biomed. Pharmacother. 2022, 153, 113480. [Google Scholar] [CrossRef]
- Thakur, A.; Sidu, R.K.; Zou, H.; Alam, M.K.; Yang, M.; Lee, Y. Inhibition of Glioma Cells’ proliferation by doxorubicin-loaded Exosomes via microfluidics. Int. J. Nanomed. 2020, 15, 8331–8343. [Google Scholar] [CrossRef]
- Pezzana, C.; Cras, A.; Simelière, F.; Guesdon, R.; Desgres, M.; Correa, B.L.; Peuffier, A.; Bellamy, V.; Gouarderes, S.; Alberdi, A.; et al. Biomaterial-embedded extracellular vesicles improve recovery of the dysfunctional myocardium. Biomaterials 2022, 291, 121877. [Google Scholar] [CrossRef]
- Davidson, S.M.; Andreadou, I.; Barile, L.; Birnbaum, Y.; Cabrera-Fuentes, H.A.; Cohen, M.V.; Downey, J.M.; Girao, H.; Pagliaro, P.; Penna, C.; et al. Circulating blood cells and extracellular vesicles in acute cardioprotection. Cardiovasc. Res. 2019, 115, 1156–1166. [Google Scholar] [CrossRef]
- Garbayo, E.; Ruiz-Villalba, A.; Hernandez, S.C.; Saludas, L.; Abizanda, G.; Pelacho, B.; Roncal, C.; Sanchez, B.; Palacios, I.; Prósper, F.; et al. Delivery of cardiovascular progenitors with biomimetic microcarriers reduces adverse ventricular remodeling in a rat model of chronic myocardial infarction. Acta Biomater. 2021, 126, 394–407. [Google Scholar] [CrossRef]
- Adamiak, M.; Cheng, G.; Bobis-Wozowicz, S.; Zhao, L.; Kedracka-Krok, S.; Samanta, A.; Karnas, E.; Xuan, Y.T.; Skupien-Rabian, B.; Chen, X.; et al. Induced Pluripotent Stem Cell (iPSC)-derived extracellular vesicles are safer and more effective for cardiac repair than iPSCs. Circ. Res. 2018, 122, 296–309. [Google Scholar] [CrossRef]
- Okamura, A.; Yoshioka, Y.; Saito, Y.; Ochiya, T. Can Extracellular Vesicles as Drug Delivery Systems Be a Game Changer in Cardiac Disease? Pharm. Res. 2023, 40, 889–908. [Google Scholar] [CrossRef]
- Zhao, J.; Li, X.; Hu, J.; Chen, F.; Qiao, S.; Sun, X.; Gao, L.; Xie, J.; Xu, B. Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovasc. Res. 2019, 115, 1205–1216. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Zhou, X.; Ge, Z.; Song, Y.; Wang, H.; Liu, X.; Zhang, D. Exosomes from adipose-derived mesenchymal stem cells ameliorate cardiac damage after myocardial infarction by activating S1P/SK1/S1PR1 signaling and promoting macrophage M2 polarization. Int. J. Biochem. Cell Biol. 2019, 114, 105564. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yang, Y.; Guo, Q.; Gao, Q.; Ding, Y.; Wang, H.; Xu, W.; Yu, B.; Wang, M.; Zhao, Y.; et al. Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Promote Fibroblast-to-Myofibroblast Differentiation in Inflammatory Environments and Benefit Cardioprotective Effects. Stem Cells Dev. 2019, 28, 799–811. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Nickoloff, E.; Abramova, T.; Johnson, J.; Verma, S.K.; Krishnamurthy, P.; Mackie, A.R.; Vaughan, E.; Garikipati, V.N.S.; Benedict, C.; et al. Embryonic Stem Cell-Derived Exosomes Promote Endogenous Repair Mechanisms and Enhance Cardiac Function Following Myocardial Infarction. Circ. Res. 2015, 117, 52–64. [Google Scholar] [CrossRef]
- Alam, P.; Maliken, B.D.; Jones, S.M.; Ivey, M.J.; Wu, Z.; Wang, Y.; Kanisicak, O. Cardiac remodeling and repair: Recent approaches, advancements, and future perspective. Int. J. Mol. Sci. 2021, 22, 3104. [Google Scholar] [CrossRef]
- Shafei, S.; Khanmohammadi, M.; Ghanbari, H.; Nooshabadi, V.T.; Tafti, S.H.A.; Rabbani, S.; Kasaiyan, M.; Basiri, M.; Tavoosidana, G. Effectiveness of exosome mediated miR-126 and miR-146a delivery on cardiac tissue regeneration. Cell Tissue Res. 2022, 390, 71–92. [Google Scholar] [CrossRef]
- Jiao, W.; Hao, J.; Xie, Y.; Meng, M.; Gao, W. EZH2 mitigates the cardioprotective effects of mesenchymal stem cell-secreted exosomes against infarction via HMGA2-mediated PI3K/AKT signaling. BMC Cardiovasc. Disord. 2022, 22, 1–13. [Google Scholar] [CrossRef]
- Ciullo, A.; Biemmi, V.; Milano, G.; Bolis, S.; Cervio, E.; Fertig, E.T.; Gherghiceanu, M.; Moccetti, T.; Camici, G.G.; Vassalli, G.; et al. Exosomal expression of CXCR4 targets cardioprotective vesicles to myocardial infarction and improves outcome after systemic administration. Int. J. Mol. Sci. 2019, 20, 468. [Google Scholar] [CrossRef]
- Zhu, D.; Liu, S.; Huang, K.; Wang, Z.; Hu, S.; Li, J.; Li, Z.; Cheng, K. Intrapericardial Exosome Therapy Dampens Cardiac Injury via Activating Foxo3. Circ. Res. 2022, 131, E135–E150. [Google Scholar] [CrossRef]
- Ibrahim, A.G.E.; Li, C.; Rogers, R.; Fournier, M.; Li, L.; Vaturi, S.D.; Antes, T.; Sanchez, L.; Akhmerov, A.; Moseley, J.J.; et al. Augmenting canonical Wnt signalling in therapeutically inert cells converts them into therapeutically potent exosome factories. Nat. Biomed. Eng. 2019, 3, 695–705. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, X.; Dou, L.; Huang, X.; Zhu, K.; Guo, J.; Yan, M.; Wang, S.; Man, Y.; Tang, W.; et al. MIR-154-5p functions as an important regulator of angiotensin II-mediated heart remodeling. Oxid. Med. Cell. Longev. 2019, 2019, 8768164. [Google Scholar] [CrossRef]
- Chen, Y.; Hua, Y.; Li, X.; Arslan, I.M.; Zhang, W.; Meng, G. Distinct types of cell death and the implication in diabetic cardiomyopathy. Front. Pharmacol. 2020, 11, 42. [Google Scholar] [CrossRef]
- Dewanjee, S.; Vallamkondu, J.; Kalra, R.S.; John, A.; Reddy, P.H.; Kandimalla, R. Autophagy in the diabetic heart: A potential pharmacotherapeutic target in diabetic cardiomyopathy. Ageing Res. Rev. 2021, 68, 101338. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, L.; Chen, X.; Qin, Y.; Tian, C.; Dai, X.; Meng, R.; Zhong, Y.; Liang, W.; Shen, C.; et al. Exosomes derived from human umbilical cord mesenchymal stem cells (HUCMSC-EXO) regulate autophagy through AMPK-ULK1 signaling pathway to ameliorate diabetic cardiomyopathy. Biochem. Biophys. Res. Commun. 2022, 632, 195–203. [Google Scholar] [CrossRef]
- Weil, B.R.; Herrmann, J.L.; Abarbanell, A.M.; Manukyan, M.C.; Poynter, J.A.; Meldrum, D.R. Intravenous infusion of mesenchymal stem cells is associated with improved myocardial function during endotoxemia. Shock 2011, 36, 235–241. [Google Scholar] [CrossRef]
- Sun, D.; Zhuang, X.; Xiang, X.; Liu, Y.; Zhang, S.; Liu, C.; Barnes, S.; Grizzle, W.; Miller, D.; Zhang, H.G. A novel nanoparticle drug delivery system: The anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol. Ther. 2010, 18, 1606–1614. [Google Scholar] [CrossRef]
- Thakur, A.; Ke, X.; Chen, Y.W.; Motallebnejad, P.; Zhang, K.; Lian, Q.; Chen, H.J. The mini player with diverse functions: Extracellular vesicles in cell biology, disease, and therapeutics. Protein Cell 2022, 13, 631–654. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, F.; Lian, X.F.; Peng, W.Q.; Yin, C.Y. Mesenchymal stem cell-derived exosomes improve diabetes mellitus-induced myocardial injury and fibrosis via inhibition of TGF-β1/Smad2 signaling pathway. Cell. Mol. Biol. 2019, 65, 123–126. [Google Scholar] [CrossRef]
- Lian, Q.; Zhang, Y.; Zhang, J.; Zhang, H.K.; Wu, X.; Zhang, Y.; Lam, F.F.Y.; Kang, S.; Xia, J.C.; Lai, W.H.; et al. Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation 2010, 121, 1113–1123. [Google Scholar] [CrossRef]
- Sun, S.J.; Lai, W.H.; Jiang, Y.; Zhen, Z.; Wei, R.; Lian, Q.; Liao, S.Y.; Tse, H.F. Immunomodulation by systemic administration of human-induced pluripotent stem cell-derived mesenchymal stromal cells to enhance the therapeutic efficacy of cell-based therapy for treatment of myocardial infarction. Theranostics 2021, 11, 1641–1654. [Google Scholar] [CrossRef]
- Gnecchi, M. Mesencymal Stem Cells; Springer: New York, NY, USA, 2016; Volume 1416, ISBN 9781493935826. [Google Scholar]
- Bloor, A.J.C.; Patel, A.; Griffin, J.E.; Gilleece, M.H.; Radia, R.; Yeung, D.T.; Drier, D.; Larson, L.S.; Uenishi, G.I.; Hei, D.; et al. Production, safety and efficacy of iPSC-derived mesenchymal stromal cells in acute steroid-resistant graft versus host disease: A phase I, multicenter, open-label, dose-escalation study. Nat. Med. 2020, 26, 1720–1725. [Google Scholar] [CrossRef] [PubMed]
- Ribichini, F.; Wijns, W. Acute myocardial infarction: Reperfusion treatment. Heart 2002, 88, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Kou, M.; Huang, L.; Yang, J.; Chiang, Z.; Chen, S.; Liu, J.; Guo, L.; Zhang, X.; Zhou, X.; Xu, X.; et al. Mesenchymal stem cell-derived extracellular vesicles for immunomodulation and regeneration: A next generation therapeutic tool? Cell Death Dis. 2022, 13, 580. [Google Scholar] [CrossRef] [PubMed]
- De Matos, B.M.; Stimamiglio, M.A.; Correa, A.; Robert, A.W. Human pluripotent stem cell-derived extracellular vesicles: From now to the future. World J. Stem Cells 2023, 15, 453–465. [Google Scholar] [CrossRef]
- Cade, W.T. Diabetes-related microvascular and macrovascular diseases in the physical therapy setting. Phys. Ther. 2008, 88, 1322–1335. [Google Scholar] [CrossRef]
- Burgelman, M.; Vandendriessche, C.; Vandenbroucke, R.E. Extracellular vesicles: A double-edged sword in sepsis. Pharmaceuticals 2021, 14, 829. [Google Scholar] [CrossRef]
- Perner, A.; Gordon, A.C.; De Backer, D.; Dimopoulos, G.; Russell, J.A.; Lipman, J.; Jensen, J.U.; Myburgh, J.; Singer, M.; Bellomo, R.; et al. Sepsis: Frontiers in diagnosis, resuscitation and antibiotic therapy. Intensive Care Med. 2016, 42, 1958–1969. [Google Scholar] [CrossRef]
- Russell, J.A.; Walley, K.R. Update in Sepsis 2012. Am. J. Respir. Crit. Care Med. 2013, 187, 1303–1307. [Google Scholar] [CrossRef]

| Myocardial Infarction | |||
|---|---|---|---|
| Cargo | Origin | EV Classification | Biological Function/Correlation |
| not specified | plasma | increase endothelial-derived EVs | increased mortality in HF patients |
| lncRNA Neat1 | conditioned medium CMs | increased in EVs | CMs and FBs survival |
| miR-126 | plasma | reduced in EVs | high-risk of CVD |
| miR-192, miR-194, miR-34a | plasma | increased in EVs | correlate with acute MI |
| miR-1915-3p, miR-457, miR-3656 | serum | decreased in EVs | correlate with MI |
| miR-1, miR-208 | serum and urine | increased in exosomes from damaged myocardium | correlate with post-acute MI |
| miR-130a | plasma | increased in EVs | attenuation of cardiac remodeling post-MI |
| miR-30a | serum and CMs conditioned medium | increased in exosomes | correlated with AMI and regulation of hypoxic response |
| miR-340, miR-424/miR-29b | serum | decreased/increased in EVs | correlate with MI |
| CD172a | plasma | increased in cardiac EVs | correlated in hypoxia conditions, as MI |
| HIF-1α and TGF-β | FB | increase exosome | increased vascular expression of collagens and fibronectin |
| APOD, APOC3, C1Q1A, C5, GP1BA, PPBP | plasma | EVs | predictive of MI and myocardial damage |
| not specified | plasma | platelet- and leukocyte-derived EVs | decreased during P2Y12 treatment |
| ceramides, dihydroceramides, sphingomyelins | plasma | increased in EVs | correlation with MI |
| Diabetic Cardiomyopathy | |||
| Cargo | Origin | EV classification | Biological function/correlation |
| miR-1 and miR-133 | CMs conditioned medium | increased in exosomes | correlation with lipid accumulation in CMs, diabetic model |
| miR-30d-5p and miR-126-5p | plasma | reduction in exosomes | correlation with HFpEF in diabetic rats |
| Sepsis-induced cardiomyopathy | |||
| Cargo | Origin | EV classification | Biological function/correlation |
| miR-150-5p | neutrophils | decreased in EVs | contributes to the worsening of SIC |
| troponin I and muscle-associated glycogen phosphorylase | blood | CM-derived small- and medium EVs | LPS-induced systemic inflammatory response syndrome |
| Myocardial Infarction | ||||
|---|---|---|---|---|
| Cargo | Donor Cell | Recipient Cell | EV Classification | Biological Conditions |
| TNF-α | CMs | CMs | exosomes | induced by hypoxia in vitro, promotes inflammation |
| miR-126 and miR-210 | ECs | CPCs | exosomes | under hypoxia, increasing cardiac progenitor cells resistance to hypoxic stress |
| ENSMUST00000122745/Neat1 | CMs | FBs | small/large EVs | regulation FB survival, under hypoxic conditions |
| circ_0001747 | adipose-derived stem cells | CMs | exosomes | protective effects against H/R |
| miR-195 | CMs | FBs | exosomes | maintenance of cardiac homeostasis |
| miR-144 | plasma | myocardium | EVs | cardioprotection, promoting cell survival during reperfusion |
| miR-133 | EPC | FBs | EVs | regulation of cardiac fibrosis under hypoxia |
| circRNA Whsc1 | CMs | ECs | EVs | cardiac regeneration post-MI |
| miR-222 and miR-143 | CMs | ECs | EVs | protection against oxidative stress by enhancing angiogenesis |
| Hsp20 | CMs | ECs | exosomes | cardioprotection by myocardial angiogenesis |
| miR-31 | ASC | ECs | exosomes | enhance angiogenesis during ischemia |
| miR-30a | CMs | not specified | exosomes | regulate autophagy in a paracrine way, myocardium protection after MI |
| miR-21 | Mesenchymal stromal cells | CMs and ECs | exosomes | INFg treatment, improved cardiac function in MI conditions |
| miR-30a, miR-100, miR-27a, and miR-30e | epicardial | CMs | exosomes | enhance proliferation of CMs in vitro and in vivo |
| not specified | cardiac progenitor cells | infarct side | EVs | CPCs transplantation enhances cardiac recovery post-MI |
| miR-155 | immune cells | FBs and ECs | exosomes | repressing FB proliferation and EC angiogenesis |
| miR-126 | serum | ECs | exosomes | promote angiogenesis in AMI patients |
| Diabetic Cardiomyopathy | ||||
| Cargo | Donor cell | Recipient cell | EV classification | Biological Conditions |
| miR-320 (up) and miR-126 (down) | CMs | ECs | exosomes | impairment of myocardial angiogenesis in vitro |
| Hsp20 | CMs overexpressing Hsp20 | ECs and CMs | exosomes | cardioprotective and increase angiogenesis in vitro and in vivo |
| miR-499, miR-133a and miR-133b | CMs | ECs and CMs | EVs | cardioprotective effects after ticagrelor treatment in vitro |
| Hsp70 | ECs | CMs | exosomes | cardioprotective effects of exo-derived from healthy subjects, not diabetic |
| Mst1 | ECs overexpressing Mst1 | CM | exosomes | worsening of cardiac function and aggravated insulin resistance, in vitro and in vivo |
| miR-378 | serum | FBs | exosomes | inhibition of FBs proliferation in DCM rats treated with isosorbide mononitrate |
| HuR (down) | MOs | FB | exosomes | increase expression of inflammatory genes and fibrogenesis, in vitro and in vivo |
| AT1R | serum or hypotonic cells overexpressing AT1R | CMs | exosomes | increase systolic blood pressure, maintain cardiac homeostasis |
| not specified | parasympathetic ganglionic neurons | CMs | exosomes | cardioprotective effect in vitro |
| miR-339-3p and-326-3p | adipose tissue | CMs | LEV | worsen cardiac function when diabetic exosomes are used |
| Sepsis-induced cardiomyopathy | ||||
| Cargo | Donor cell | Recipient cell | EV classification | Biological Conditions |
| not specified | MOs | exosomes | enhance cardiac inflammation | |
| NADPH | Platelets | ECs and SMCs | exosomes | vascular dysfunction by increases apoptosis |
| NO | Platelets | CMs | exosomes | reduced myocardial contractility |
| NOS | Platelets | ECs | exosomes | increased ROS production |
| miR-126 | ECs | Myocardium | exosomes | regulation of adhesion molecules and immune cell infiltration |
| not specified | Serum | CMs | exosomes | increases secreted cytokines and pyroptosis proteins |
| has-miR-1262 | Serum | CMs | exosomes | reduces glycolysis activity and increased apoptosis |
| Myocardial Infarction | |||
|---|---|---|---|
| Cargo | Origin | EV Classification | Therapeutic Outcome |
| not specified | HUCMSC | HA-embedded EVs | improved angiogenesis, decreased apoptosis and fibrosis and maintained cardiac function |
| endoglin | cardiac progenitor cell | ECs | promoting angiogenesis |
| miR-146a-3p, miR-132 and miR-201 | cardiac progenitor cell | EVs | cardioprotective by promoting angiogenesis |
| not specified | cardiac progenitor cell | biomimetic EVs | higher CPC retention in the infarct area in chronic MI, increasing cardiac function |
| let-7, miR-145, miR-17–92 cluster, and miR-302a-5p | mouse fibroblast-derived iPSC | EVs | improved cardiac repair induction of angiogenesis, adaptation capacity to hypoxic stress |
| lncRNA MALAT1 | hESC-CVPs | EVs | angiogenesis and cell viability through a miR-497-dependent mechanism |
| miR-182 | bone marrow | EVs | improving cardiac repair through inhibition of TLR4 |
| not specified | adipose tissue-derived MSC | EVs | improving cardiac function, reducing serum levels of IL-6, IL-1β, TNF-α, and IFN-γ |
| not specified | HUCMSC | EVs | anti-inflammatory properties post-MI |
| miR-294 | ESC-derived | exosomes | angiogenesis and cell survival |
| let-7b-5p | human pericardial fluid-derived | exosomes | promoted angiogenesis and promoted cardiac repair |
| miR-126 and miR-146a | - | exosomes encapsulated in alginate hydrogel | promoting cardiac repair |
| not specified | MSC | exosomes | partial restoration of cardiac function targeting EZH2 |
| miR-214 | bone-marrow | exosomes | regulate calcium overload |
| CXCR4 | - | exosomes | cardioprotective effect |
| not specified | MSC | exosomes | intrapericardial injection and stimulating cardiac repair |
| not specified | cardiospheres | exosomes and EVs | anti-inflammatory, anti-fibrotic, angiogenic and cardiomyogenic properties |
| miR-126 | cardiomyocyte progenitor cell | engineered small-EVs (sEV)-like vesicles (ELV) | cardiac repair post-MI by promoting angiogenesis |
| lncRNAs UCA1, MALAT1, NEAT1, KLF3-AS1 and HCP5 | - | EVs | inhibition of cardiomyocyte autophagy |
| lncRNA HCG15, miR-153-3p and miR-328-3p | - | EVs | exacerbate ischemic injury post-infarct |
| Diabetic Cardiomyopathy | |||
| Cargo | Origin | EV classification | Therapeutic outcome |
| not specified | MSC | exosomes | cardioprotective by blocking myocardial injury and fibrosis |
| not specified | HUCMSC | exosomes | beneficial cardiac function by attenuating myocardial autophagy |
| Sepsis-induced cardiomyopathy | |||
| Cargo | Origin | EV classification | Therapeutic outcome |
| miR-233 | MSC | exosomes | CMs uptake, reduction of inflammatory response and cell death |
| curcumin | self-assembled into the lipid bilayer | exosomes | anti-inflammatory and anti-fibrotic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laura Francés, J.; Pagiatakis, C.; Di Mauro, V.; Climent, M. Therapeutic Potential of EVs: Targeting Cardiovascular Diseases. Biomedicines 2023, 11, 1907. https://doi.org/10.3390/biomedicines11071907
Laura Francés J, Pagiatakis C, Di Mauro V, Climent M. Therapeutic Potential of EVs: Targeting Cardiovascular Diseases. Biomedicines. 2023; 11(7):1907. https://doi.org/10.3390/biomedicines11071907
Chicago/Turabian StyleLaura Francés, Javier, Christina Pagiatakis, Vittoria Di Mauro, and Montserrat Climent. 2023. "Therapeutic Potential of EVs: Targeting Cardiovascular Diseases" Biomedicines 11, no. 7: 1907. https://doi.org/10.3390/biomedicines11071907
APA StyleLaura Francés, J., Pagiatakis, C., Di Mauro, V., & Climent, M. (2023). Therapeutic Potential of EVs: Targeting Cardiovascular Diseases. Biomedicines, 11(7), 1907. https://doi.org/10.3390/biomedicines11071907